Proline Promotes Drought Tolerance in Maize
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Electrolyte Leakage Measurement
2.3. Measurement of Relative Water Content (RWC), Leaf Area, and Stem Diameter
2.4. Chlorophyll Content
2.5. Measurement of Hydrogen Peroxide and Lipid Peroxidation
2.6. Measurement of Antioxidant Enzyme Activities
2.7. Measurement of Proline, Total Amino Acids, and Sugar Contents
2.8. Quantification of Nutrients (N, P, and K)
2.9. Statistical Analysis
3. Results
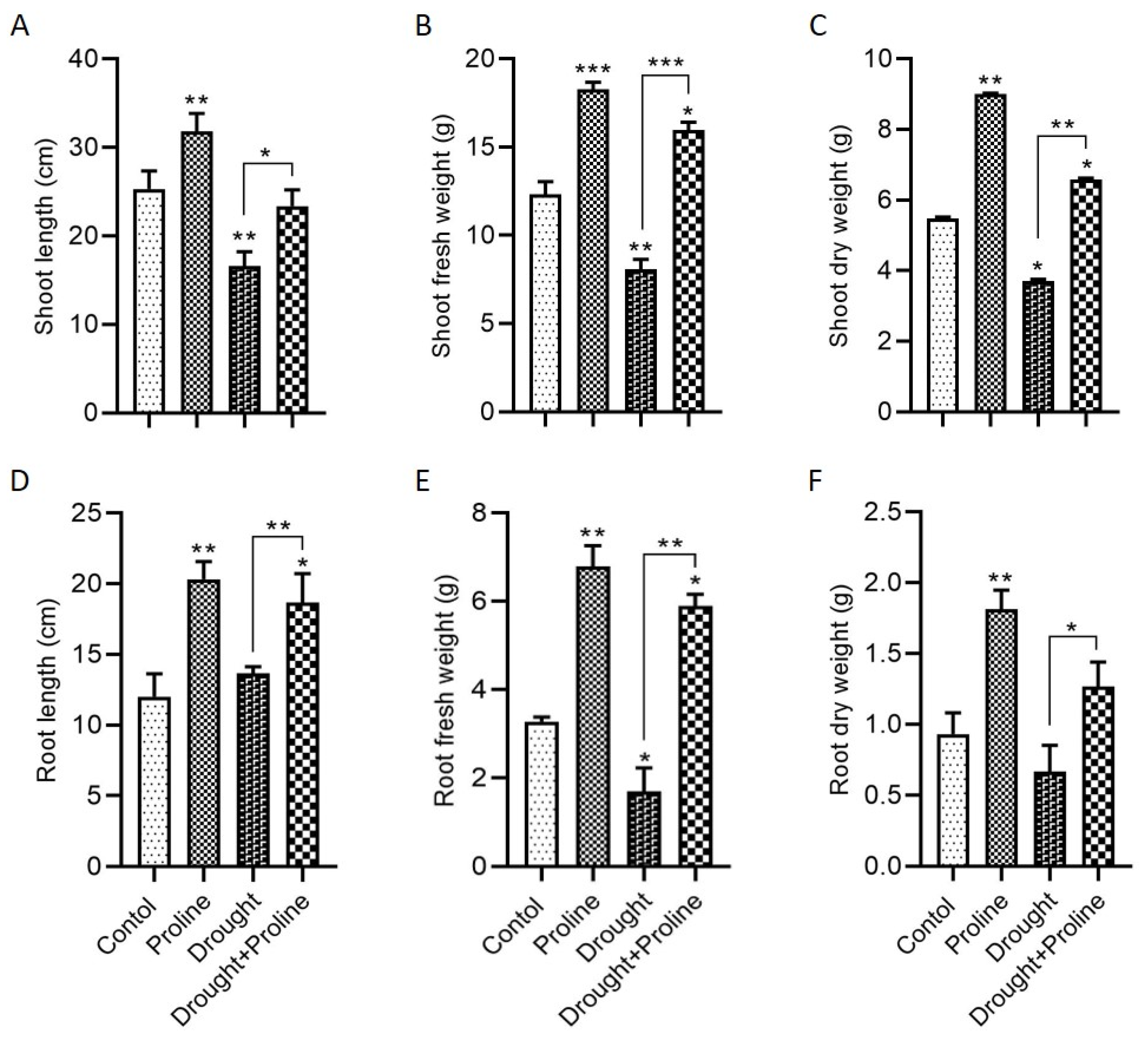
3.1. Application of Proline Enhances Maize Growth Under Drought Stress
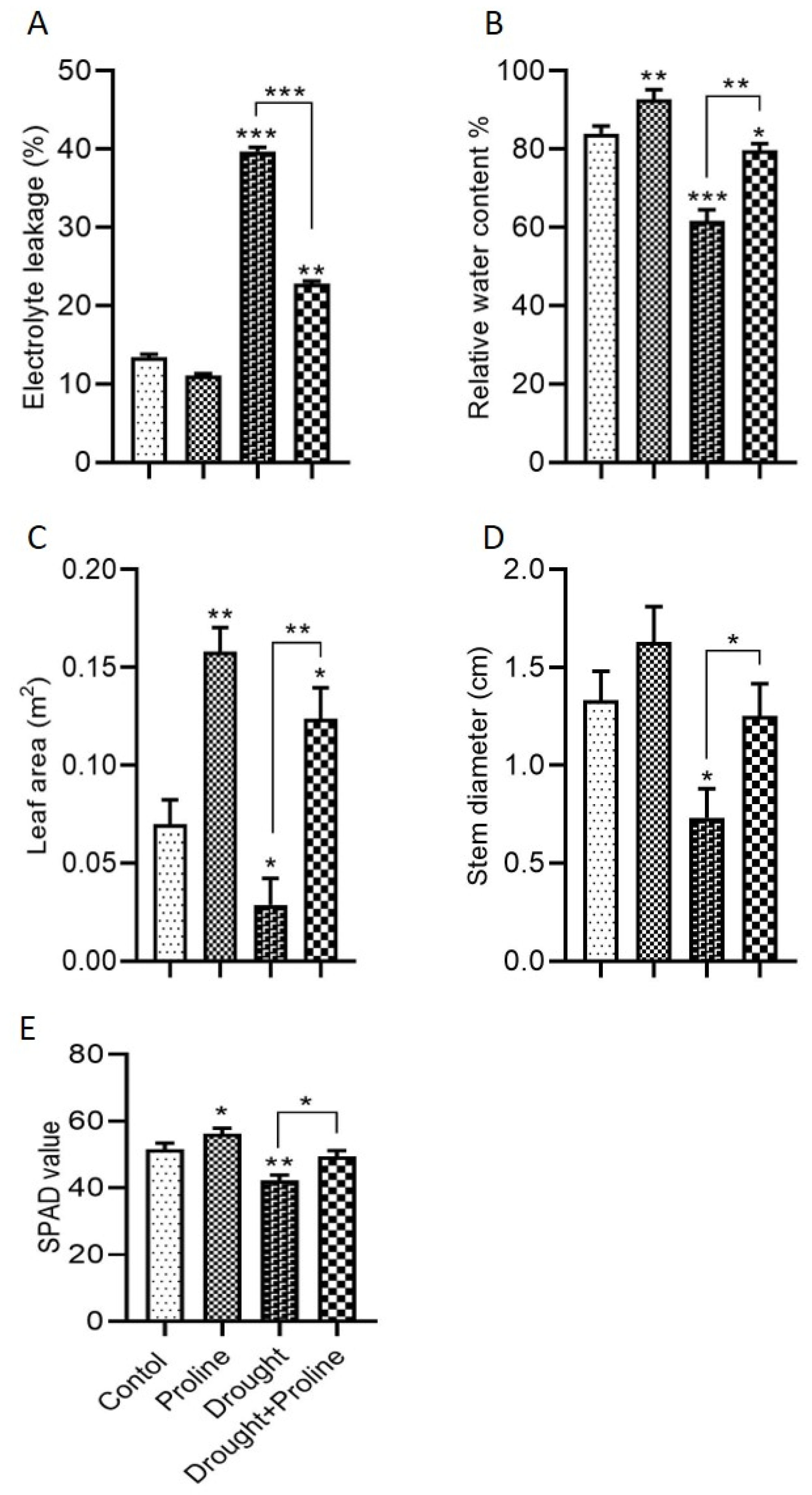
3.2. Effects of Proline on Electrolyte Leakage, Water Retention, Leaf Area, Stem Diameter, and Chlorophyll Stability
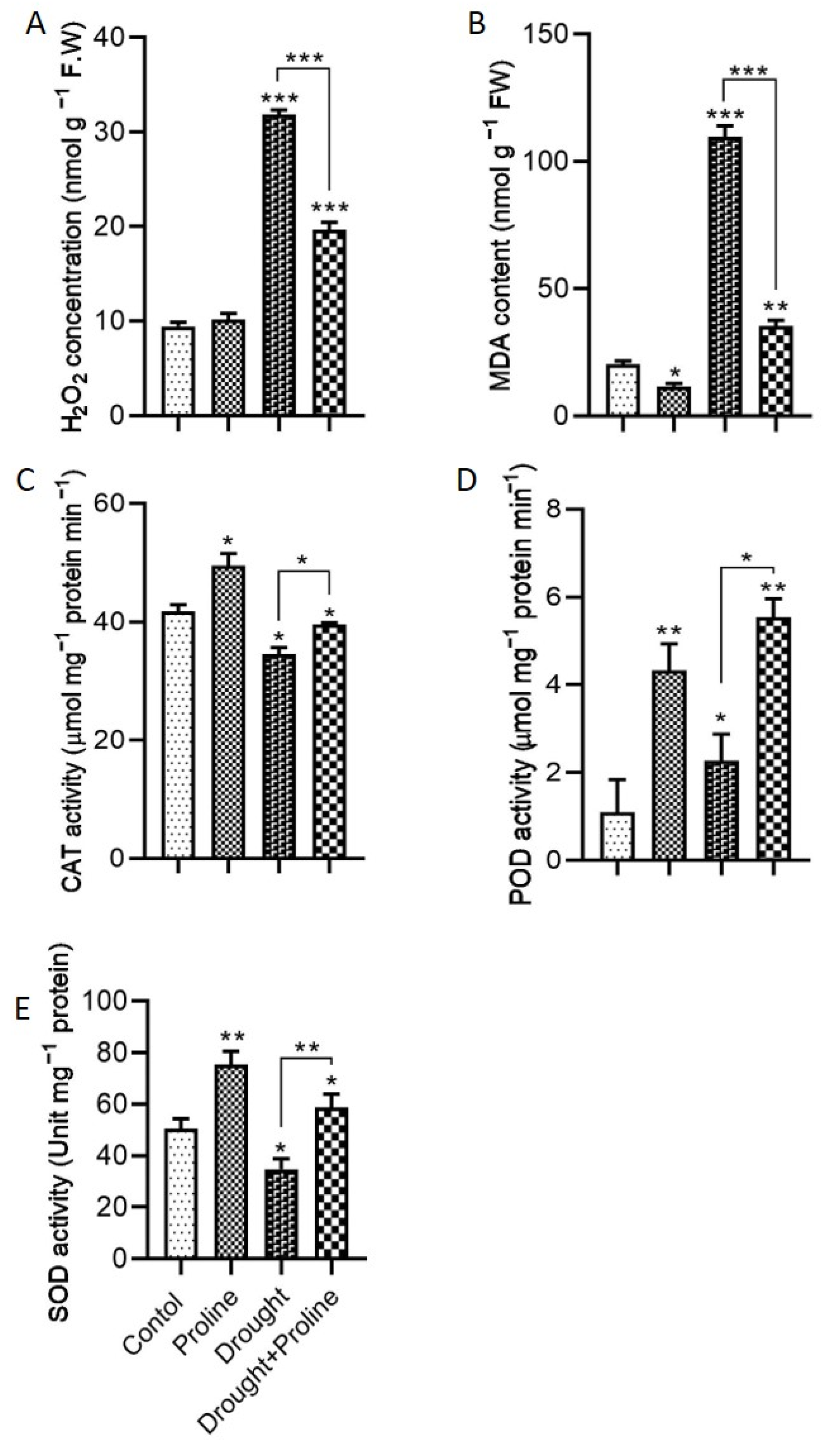
3.3. Proline Mitigates Drought-Induced Oxidative Stress by Regulating the Antioxidant System
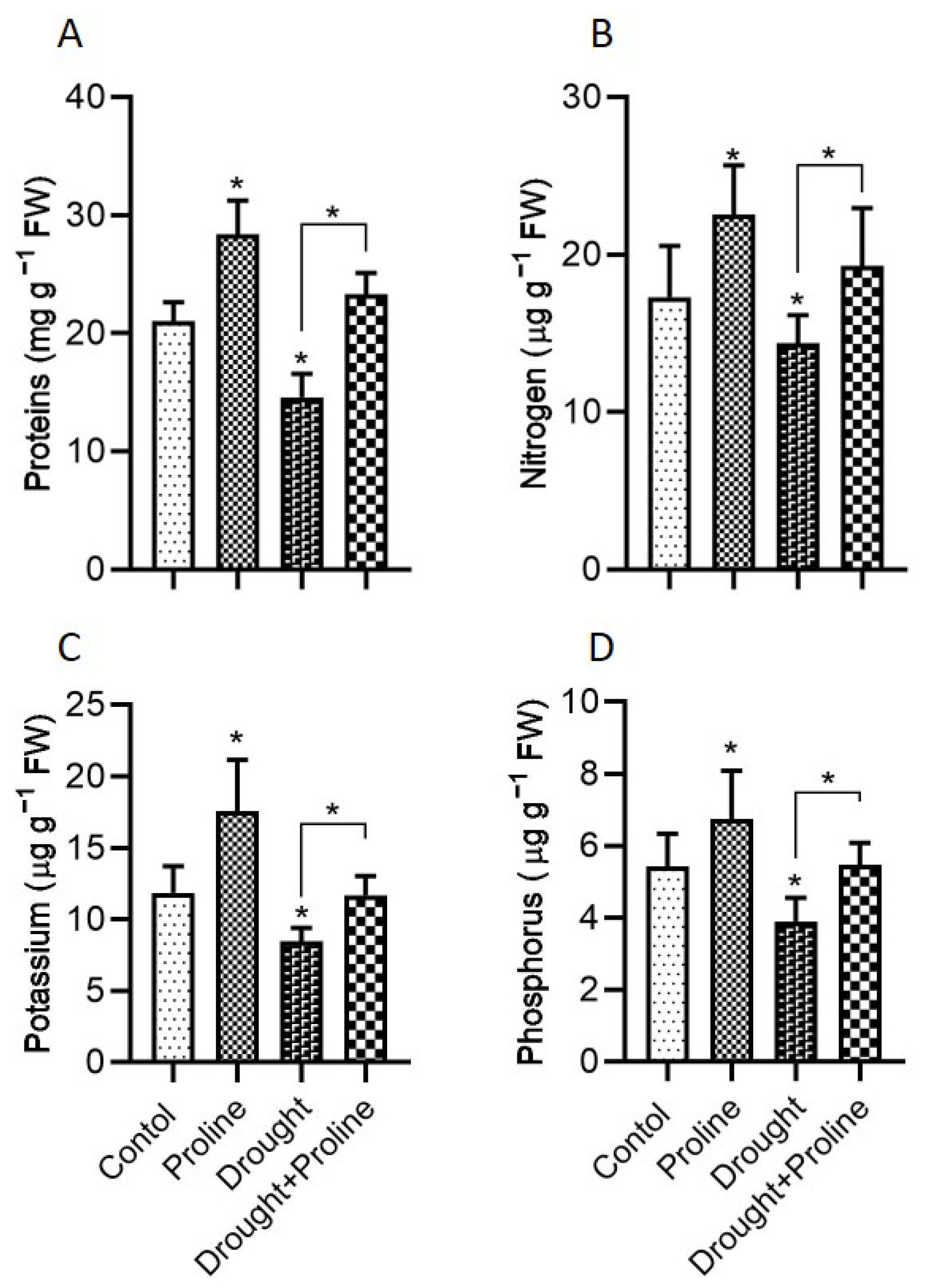
3.4. Proline Enhances Total Protein and Nutrient Contents Under Drought Stress
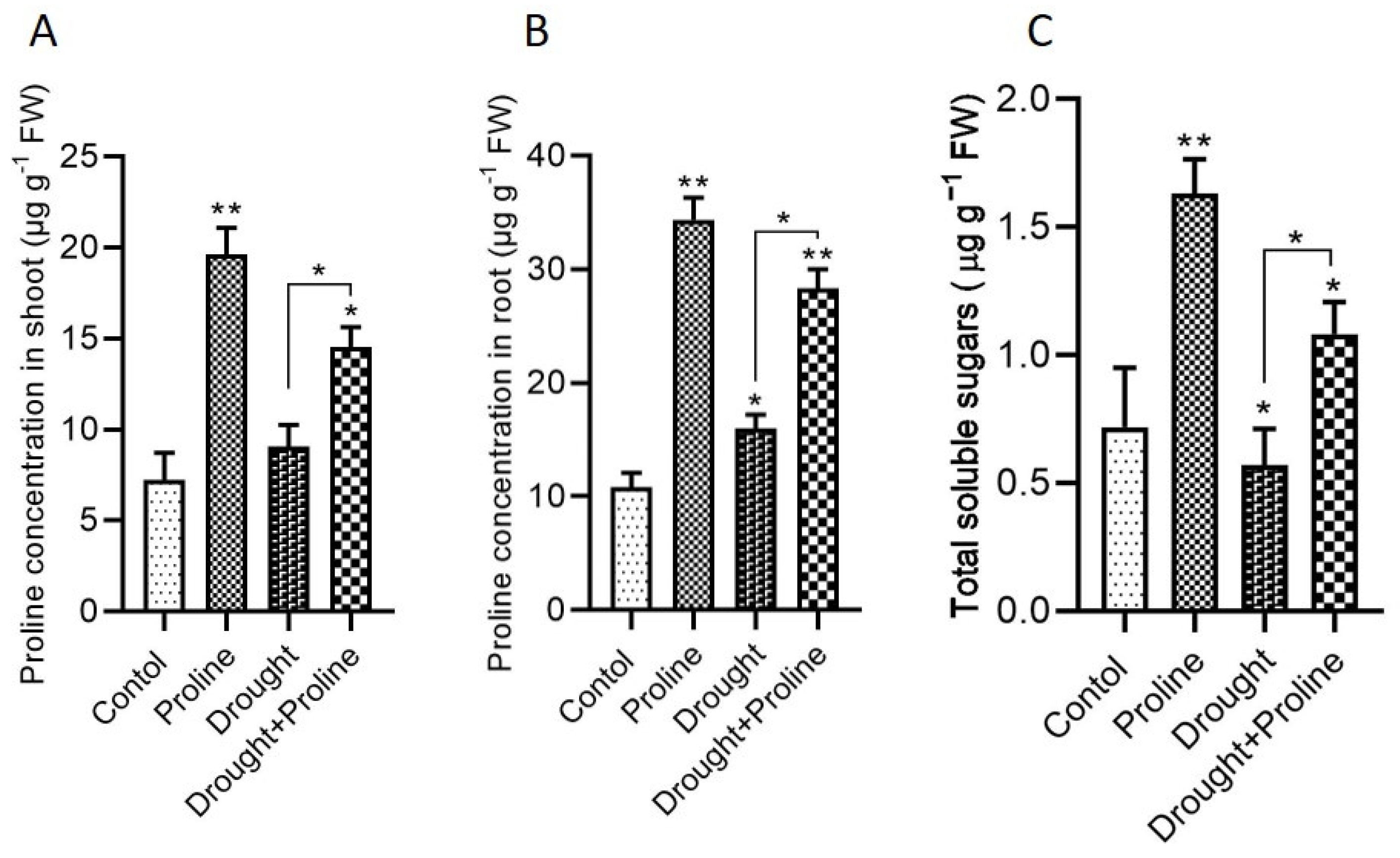
3.5. Proline and Sugar Accumulation During Drought Stress
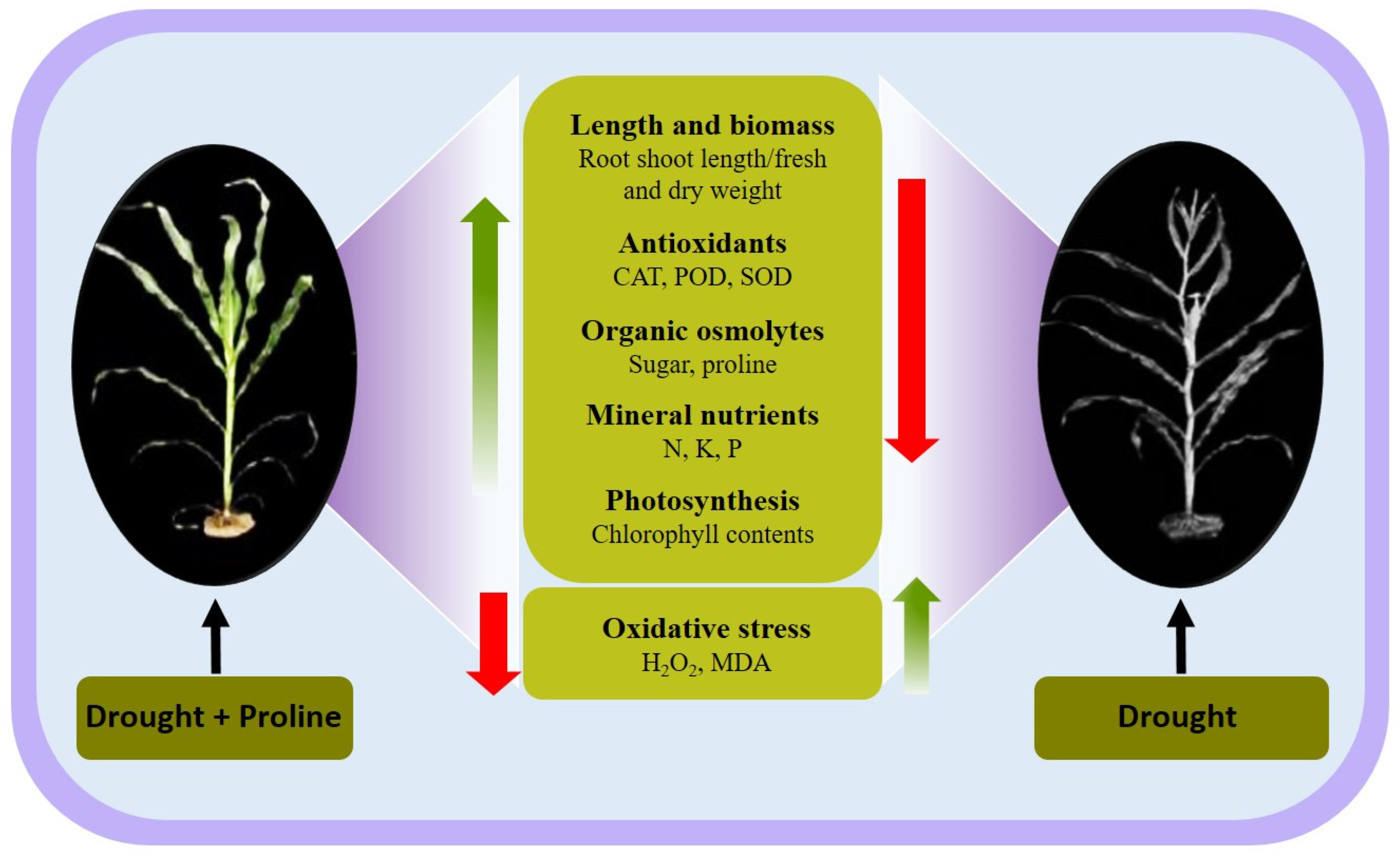
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez-Nicora, H.; Peng, D.; Saikai, K.; Rashidifard, M. Nematode problems in maize and their sustainable management. In Nematode Diseases of Crops and their Sustainable Management; Academic Press: Cambridge, MA, USA, 2023; pp. 167–181. [Google Scholar]
- Zhu, H.; Lai, R.; Chen, W.; Lu, C.; Chachar, Z.; Lu, S.; Lin, H.; Fan, L.; Hu, Y.; An, Y. Genetic dissection of maize (Zea mays L.) trace element traits using genome-wide association studies. BMC Plant Biol. 2023, 23, 631. [Google Scholar] [CrossRef]
- Gong, F.; Yang, L.; Tai, F.; Hu, X.; Wang, W. “Omics” of maize stress response for sustainable food production: Opportunities and challenges. Omics J. Integr. Biol. 2014, 18, 714–732. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Singh, N.; Pal, K. The effect of salt stress on biochemicals of chili at seedling level. Int. J. Pharma Prof. Res. 2012, 3, 572–577. [Google Scholar]
- Jagadish, S.; Septiningsih, E.; Kohli, A.; Thomson, M.; Ye, C.; Redona, E.; Kumar, A.; Gregorio, G.; Wassmann, R.; Ismail, A. Genetic advances in adapting rice to a rapidly changing climate. J. Agron. Crop Sci. 2012, 198, 360–373. [Google Scholar] [CrossRef]
- Hanif, S.; Shakoor, A.; Saleem, M.F.; Saleem, I.; Ali, S.; Ashraf, M.A.; Nadeem, M.; Shair, H.; Haq, A.; Khan, R.A.H. Exogenous application of proline to enhance rice tolerance against heat and drought stresses. Pak. J. Agric. Res. 2022, 35, 324–333. [Google Scholar] [CrossRef]
- Ali, Q.; Ashraf, M.; Athar, H.-U.-R. Exogenously applied proline at different growth stages enhances growth of two maize cultivars grown under water deficit conditions. Pak. J. Bot. 2007, 39, 1133–1144. [Google Scholar]
- Serraj, R.; Sinclair, T. Osmolyte accumulation: Can it really help increase crop yield under drought conditions? Plant Cell Environ. 2002, 25, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, G.C.; Murata, N. The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygen-evolving photosystem II complex. Photosynth. Res. 1995, 44, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, G.V.; Wheeler, R.M.; Levine, L.H.; Stutte, G.W. Glycine betaine accumulation, ionic and water relations of red-beet at contrasting levels of sodium supply. J. Plant Physiol. 2001, 158, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.W.; Storey, R.; Leigh, R.; Ahmad, N.; Pollard, A. A hypothesis on cytoplasmic osmoregulation. In the Regulation of Cell Membrane Activities in Plants. Proceedings of the International Workshop, Pallanza, Italy, 26–29 August 1976; Elsevier/North-Holland Biomedical Press: Amsterdam, The Netherlands, 1977. [Google Scholar]
- Bohnert, H.J.; Jensen, R.G. Strategies for engineering water-stress tolerance in plants. Trends Biotechnol. 1996, 14, 89–97. [Google Scholar] [CrossRef]
- Arfan, M.; Athar, H.R.; Ashraf, M. Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? J. Plant Physiol. 2007, 164, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Aty, M.S.; Kamara, M.M.; Elgamal, W.H.; Mesbah, M.I.; Abomarzoka, E.A.; Alwutayd, K.M.; Mansour, E.; Abdelmalek, I.B.; Behiry, S.I.; Almoshadak, A.S. Exogenous application of nano-silicon, potassium sulfate, or proline enhances physiological parameters, antioxidant enzyme activities, and agronomic traits of diverse rice genotypes under water deficit conditions. Heliyon 2024, 10, e26077. [Google Scholar] [CrossRef] [PubMed]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of exogenous proline to abiotic stresses tolerance in plants: A review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-L.; Lan, S.-S.; Gong, M. Hydrogen peroxide-induced proline and metabolic pathway of its accumulation in maize seedlings. J. Plant Physiol. 2009, 166, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Ihtisham, M.; Hasanuzzaman, M.; El-Sappah, A.H.; Zaman, F.; Khan, N.; Raza, A.; Sarraf, M.; Khan, S.; Abbas, M.; Hassan, M.J. Primary plant nutrients modulate the reactive oxygen species metabolism and mitigate the impact of cold stress in overseeded perennial ryegrass. Front. Plant Sci. 2023, 14, 1149832. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, J.; Singh, V.; Prasad, S. Proline and salinity tolerance in plants. Biochem. Pharmacol 2014, 3, e170. [Google Scholar] [CrossRef]
- Aslam, M.; Saeed, M.S.; Sattar, S.; Sajad, S.; Sajjad, M.; Adnan, M.; Iqbal, M.; Sharif, M.T. Specific role of proline against heavy metals toxicity in plants. Int. J. Pure Appl. Biosci. 2017, 5, 27–34. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Y.; Han, W.; Feng, R.; Hu, Y.; Guo, J.; Gong, H. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant Sci. 2016, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Shahbaz, M.; Ashraf, M.; Akram, N.A. Alleviation of drought-induced adverse effects in spring wheat (Triticum aestivum L.) using proline as a pre-sowing seed treatment. Pak. J. Bot. 2009, 41, 621–632. [Google Scholar]
- Ali, Q.; Ashraf, M.; Shahbaz, M.; Humera, H. Ameliorating effect of foliar applied proline on nutrient uptake in water stressed maize (Zea mays L.) plants. Pak. J. Bot. 2008, 40, 211–219. [Google Scholar]
- Ahmad, Z.; Anjum, S.; Waraich, E.A.; Ayub, M.A.; Ahmad, T.; Tariq, R.M.S.; Ahmad, R.; Iqbal, M.A. Growth, physiology, and biochemical activities of plant responses with foliar potassium application under drought stress—a review. J. Plant Nutr. 2018, 41, 1734–1743. [Google Scholar] [CrossRef]
- Kaya, C.; Tuna, L.; Higgs, D. Effect of silicon on plant growth and mineral nutrition of maize grown under water-stress conditions. J. Plant Nutr. 2006, 29, 1469–1480. [Google Scholar] [CrossRef]
- Mustamu, N.E.; Tampubolon, K.; Basyuni, M.; Al-Taey, D.K.; Jawad Kadhim Al Janabi, H.; Mehdizadeh, M. Drought stress induced by polyethylene glycol (PEG) in local maize at the early seedling stage. Heliyon 2023, 9, e20209. [Google Scholar] [CrossRef] [PubMed]
- Elings, A. Estimation of leaf area in tropical maize. Agron. J. 2000, 92, 436–444. [Google Scholar] [CrossRef]
- Tran, C.T.M.; Al Azzawi, T.N.I.; Khan, M.; Ali, S.; Moon, Y.-S.; Yun, B.-W. Brevundimonas vesicularis (S1T13) Mitigates Drought-Stress-Associated Damage in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 16590. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Khan, M.A.; Asaf, S.; Lubna; Lee, I.-J.; Kim, K.-M. Over-expression of chorismate mutase enhances the accumulation of salicylic acid, lignin, and antioxidants in response to the white-backed planthopper in rice plants. Antioxidants 2021, 10, 1680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, B.; Li, Z. Inhibition of NADPH oxidase increases defense enzyme activities and improves maize seed germination under Pb stress. Ecotoxicol. Environ. Saf. 2018, 158, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Ullah, I.; Khan, A.L.; Hong, S.-J.; Waqas, M.; Park, G.-S.; Kwak, Y.; Choi, J.; Jung, B.-K.; Park, M. Phytostabilization and physicochemical responses of Korean ecotype Solanum nigrum L. to cadmium contamination. Water Air Soil Pollut. 2014, 225, 2147. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.-J. Plant growth-promoting endophytic bacteria versus pathogenic infections: An example of Bacillus amyloliquefaciens RWL-1 and Fusarium oxysporum f. sp. lycopersici in tomato. PeerJ 2017, 5, e3107. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Khan, M.A.; Asaf, S.; Lubna; Lee, I.-J.; Kim, K.M. Metal resistant endophytic bacteria reduces cadmium, nickel toxicity, and enhances expression of metal stress related genes with improved growth of Oryza sativa, via regulating its antioxidant machinery and endogenous hormones. Plants 2019, 8, 363. [Google Scholar] [CrossRef]
- Bhatta, D.; Adhikari, A.; Kang, S.-M.; Kwon, E.-H.; Jan, R.; Kim, K.-M.; Lee, I.-J. Hormones and the antioxidant transduction pathway and gene expression, mediated by Serratia marcescens DB1, lessen the lethality of heavy metals (As, Ni, and Cr) in Oryza sativa L. Ecotoxicol. Environ. Saf. 2023, 263, 115377. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Kishor, P.K.; Hong, Z.; Miao, G.-H.; Hu, C.-A.A.; Verma, D.P.S. Overexpression of [delta]-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants. Plant Physiol. 1995, 108, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Chrominski, A.; Halls, S.; Weber, D.; Smith, B. Proline affects ACC to ethylene conversion under salt and water stresses in the halophyte, Allenrolfea occidentalis. Environ. Exp. Bot. 1989, 29, 359–363. [Google Scholar] [CrossRef]
- Alam, R.; Das, D.; Islam, M.; Murata, Y.; Hoque, M. Exogenous proline enhances nutrient uptake and confers tolerance to salt stress in maize (Zea mays L.). Progress. Agric. 2016, 27, 409–417. [Google Scholar] [CrossRef]
- Ehsanpour, A.; Fatahian, N. Effects of salt and proline on Medicago sativa callus. Plant Cell Tissue Organ Cult. 2003, 73, 53–56. [Google Scholar] [CrossRef]
- Huang, Y.; Bie, Z.; Liu, Z.; Zhen, A.; Wang, W. Protective role of proline against salt stress is partially related to the improvement of water status and peroxidase enzyme activity in cucumber. Soil Sci. Plant Nutr. 2009, 55, 698–704. [Google Scholar] [CrossRef]
- Iqbal, A.; Iftikhar, I.; Nawaz, H.; Nawaz, M. Role of proline to induce salinity tolerance in Sunflower (helianthus annusl.). Sci. Technol. Dev. 2014, 33, 88–93. [Google Scholar]
- Nounjan, N.; Nghia, P.T.; Theerakulpisut, P. Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J. Plant Physiol. 2012, 169, 596–604. [Google Scholar] [CrossRef]
- Rady, M.M.; Kuşvuran, A.; Alharby, H.F.; Alzahrani, Y.; Kuşvuran, S. Pretreatment with proline or an organic bio-stimulant induces salt tolerance in wheat plants by improving antioxidant redox state and enzymatic activities and reducing the oxidative stress. J. Plant Growth Regul. 2019, 38, 449–462. [Google Scholar] [CrossRef]
- Roy, D.; Basu, N.; Bhunia, A.; Banerjee, S. Counteraction of exogenous L-proline with NaCl in salt-sensitive cultivar of rice. Biol. Plant. 1993, 35, 69–72. [Google Scholar] [CrossRef]
- Okuma, E.; Soeda, K.; Tada, M.; Murata, Y. Exogenous proline mitigates the inhibition of growth of Nicotiana tabacum cultured cells under saline conditions. Soil Sci. Plant Nutr. 2000, 46, 257–263. [Google Scholar] [CrossRef]
- HuR, A. Photosynthesis under drought stress. Handb. Photosynth 2005, 793–809. [Google Scholar]
- Sharkey, T.D.; Bernacchi, C.J.; Farquhar, G.D.; Singsaas, E.L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ. 2007, 30, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Uzal, O. Effects of proline treatments on plant growth, lipid peroxidation and antioxidant enzyme activities of tomato (Solanum lycopersicum L.) seedlings under chilling stress. Gesunde Pflanz. 2022, 74, 729–736. [Google Scholar] [CrossRef]
- Mohammadrezakhani, S.; Hajilou, J.; Rezanejad, F.; Zaare-Nahandi, F. Assessment of exogenous application of proline on antioxidant compounds in three Citrus species under low temperature stress. J. Plant Interact. 2019, 14, 347–358. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Suravajhala, P.; Rathnagiri, P.; Sreenivasulu, N. Intriguing role of proline in redox potential conferring high temperature stress tolerance. Front. Plant Sci. 2022, 13, 867531. [Google Scholar] [CrossRef]
- AlKahtani, M.D.; Hafez, Y.M.; Attia, K.; Rashwan, E.; Husnain, L.A.; AlGwaiz, H.I.; Abdelaal, K.A. Evaluation of silicon and proline application on the oxidative machinery in drought-stressed sugar beet. Antioxidants 2021, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Dien, D.C.; Mochizuki, T.; Yamakawa, T. Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in Rice (Oryza sativa L.) varieties. Plant Prod. Sci. 2019, 22, 530–545. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.-S. Proline metabolism and molecular cloning of AmP5CS in the mangrove Avicennia marina under heat stress. Ecotoxicology 2020, 29, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Semida, W.M.; Abdelkhalik, A.; Rady, M.O.; Marey, R.A.; Abd El-Mageed, T.A. Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Sci. Hortic. 2020, 272, 109580. [Google Scholar] [CrossRef]
- Gelaw, T.A.; Sanan-Mishra, N. Molecular priming with H2O2 and proline triggers antioxidant enzyme signals in maize seedlings during drought stress. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2024, 1868, 130633. [Google Scholar] [CrossRef]
- Gondim, F.A.; Gomes-Filho, E.; Costa, J.H.; Alencar, N.L.M.; Prisco, J.T. Catalase plays a key role in salt stress acclimation induced by hydrogen peroxide pretreatment in maize. Plant Physiol. Biochem. 2012, 56, 62–71. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Wang, C.; Han, K.; Hu, L.; Niu, T.; Yang, Y.; Chang, Y.; Xie, J. Exogenous proline enhances systemic defense against salt stress in celery by regulating photosystem, phenolic compounds, and antioxidant system. Plants 2023, 12, 928. [Google Scholar] [CrossRef] [PubMed]
- Burritt, D.J. Proline and the cryopreservation of plant tissues: Functions and practical applications. Curr. Front. Cryopreserv. 2012, 10, 36249. [Google Scholar]
- Hossain, M.A.; Kumar, V.; Burritt, D.J.; Fujita, M.; Mäkelä, P. Osmoprotectant-mediated abiotic stress tolerance in plants. In Proline Metabolism and Its Functions in Development and Stress Tolerance; Springer Nature: Cham, Switzerland, 2019; pp. 41–72. [Google Scholar]
- Hoque, M.A.; Okuma, E.; Banu, M.N.A.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. J. Plant Physiol. 2007, 164, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Bajji, M.; Lutts, S.; Kinet, J.-M. Physiological changes after exposure to and recovery from polyethylene glycol-induced water deficit in callus cultures issued from durum wheat (Triticum durum Desf.) cultivars differing in drought resistance. J. Plant Physiol. 2000, 156, 75–83. [Google Scholar] [CrossRef]
- Lahaye, P.A.; Epstein, E. Calcium and salt toleration by bean plants. Physiol. Plant. 1971, 25, 213–218. [Google Scholar] [CrossRef]
- Alam, S.M. Nutrient uptake by plants under stress conditions. Handb. Plant Crop Stress 1999, 2, 285–313. [Google Scholar]
- Pessarakli, M. Handbook of Plant and Crop Stress; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Keller, M. Deficit irrigation and vine mineral nutrition. Am. J. Enol. Vitic. 2005, 56, 267–283. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, P.; Abdelbacki, A.M.M.; Albaqami, M.; Jan, R.; Kim, K.-M. Proline Promotes Drought Tolerance in Maize. Biology 2025, 14, 41. https://doi.org/10.3390/biology14010041
Khan P, Abdelbacki AMM, Albaqami M, Jan R, Kim K-M. Proline Promotes Drought Tolerance in Maize. Biology. 2025; 14(1):41. https://doi.org/10.3390/biology14010041
Chicago/Turabian StyleKhan, Pirzada, Ashraf M. M. Abdelbacki, Mohammed Albaqami, Rahmatullah Jan, and Kyung-Min Kim. 2025. "Proline Promotes Drought Tolerance in Maize" Biology 14, no. 1: 41. https://doi.org/10.3390/biology14010041
APA StyleKhan, P., Abdelbacki, A. M. M., Albaqami, M., Jan, R., & Kim, K.-M. (2025). Proline Promotes Drought Tolerance in Maize. Biology, 14(1), 41. https://doi.org/10.3390/biology14010041






