Effects of Nitrogen and Phosphorus in Sediment on the Occurrence of Cladophora sp. (Cladophoraceae) in Aquaculture Ponds
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Collection and Analysis
3. Results
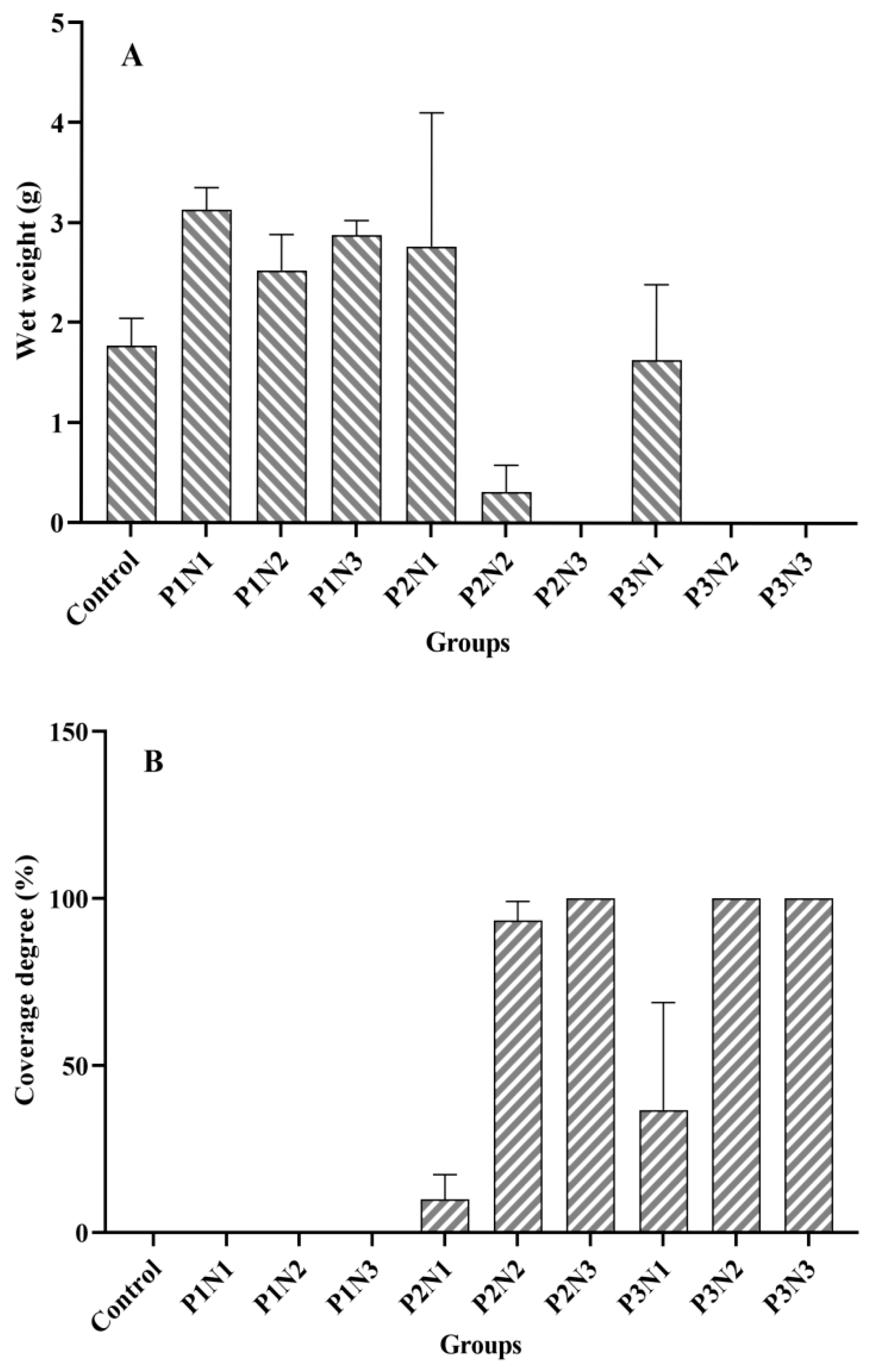
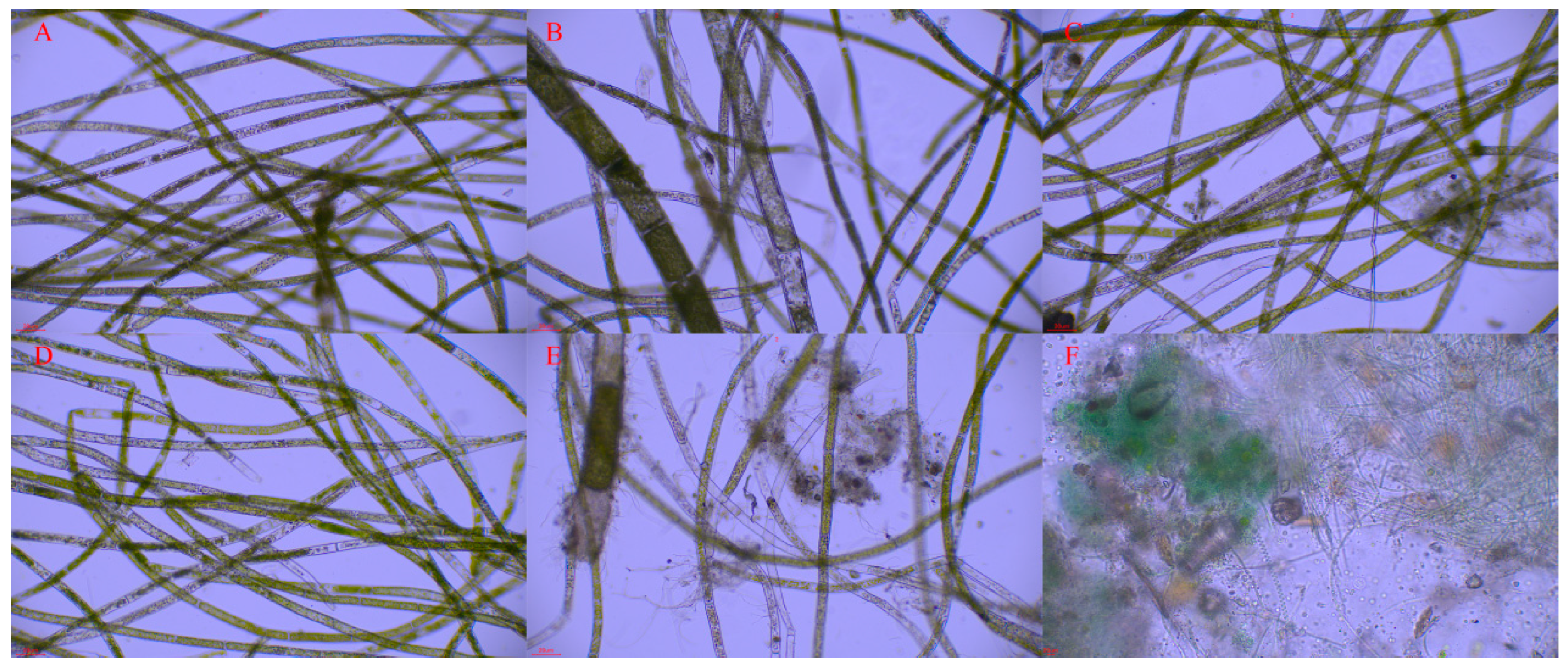
3.1. Cladophora Growth in Experiment 1
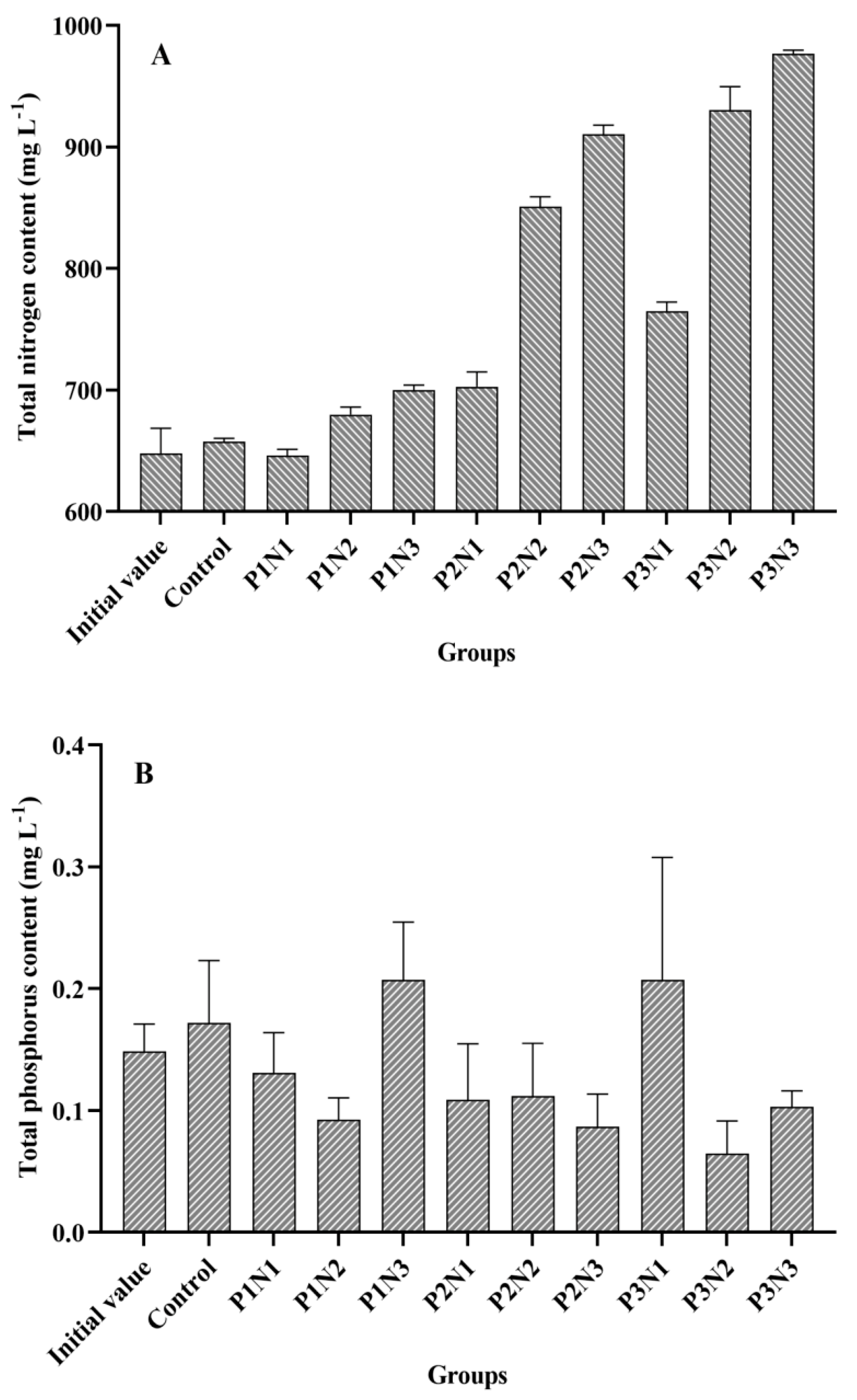
3.2. The Nitrogen and Phosphorus Content and the Biomass of Phytoplankton in the Water
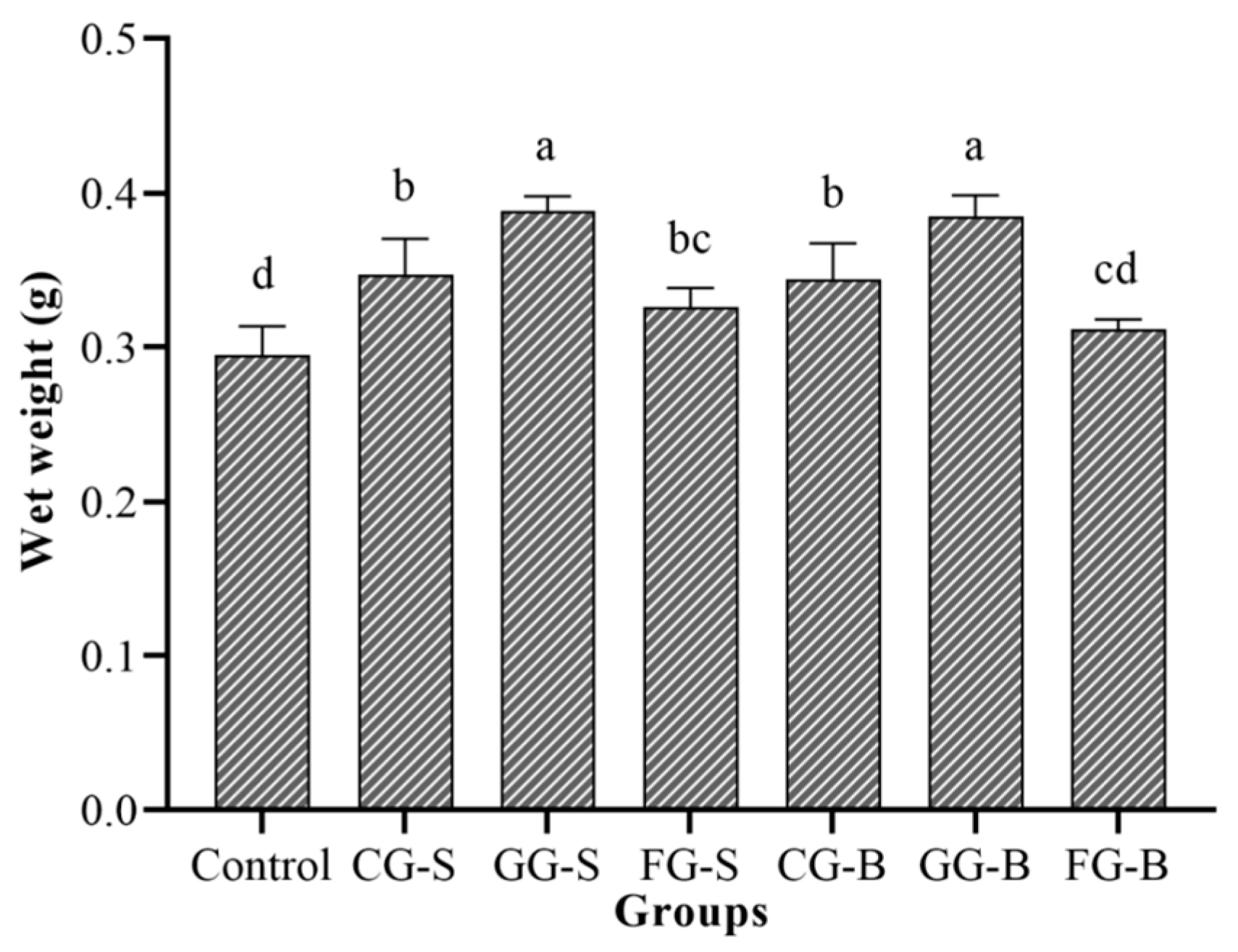
3.3. The Wet Weight Statistics of Each Experimental Group in Experiment 2
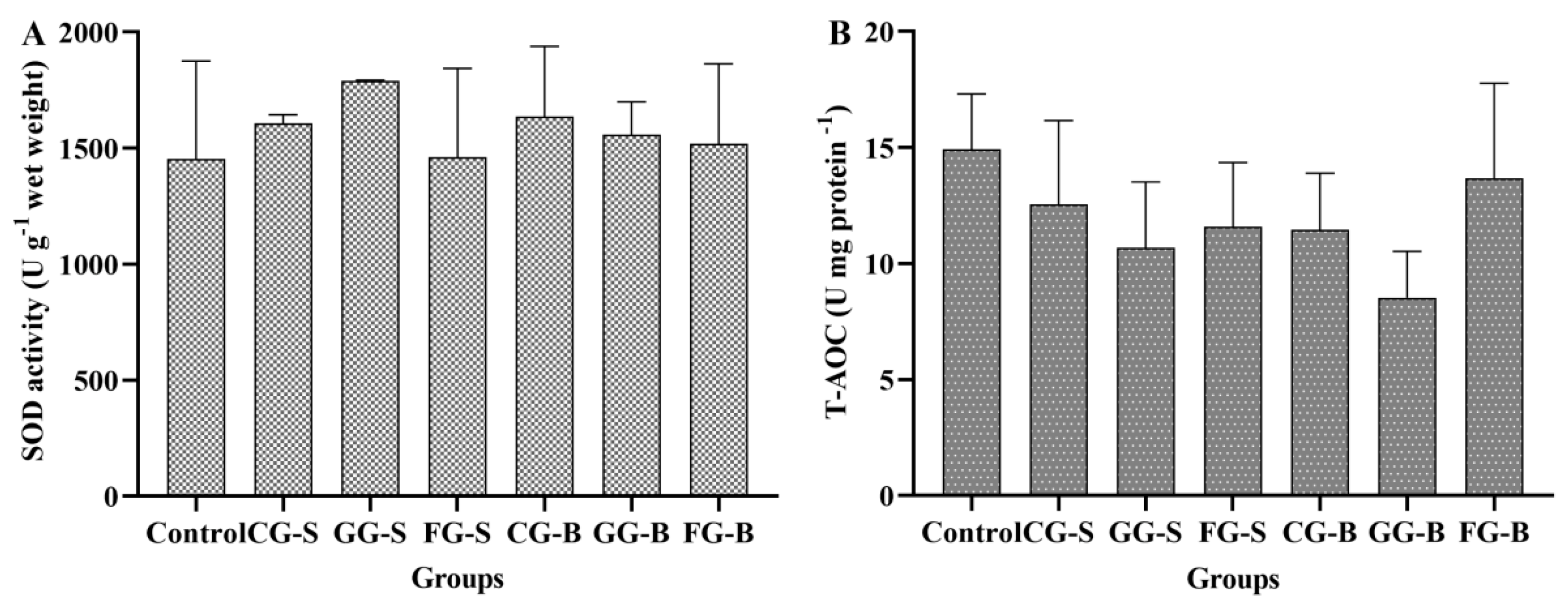
3.4. Superoxide Dismutase Activity and the Total Antioxidant Capacity of Cladophora
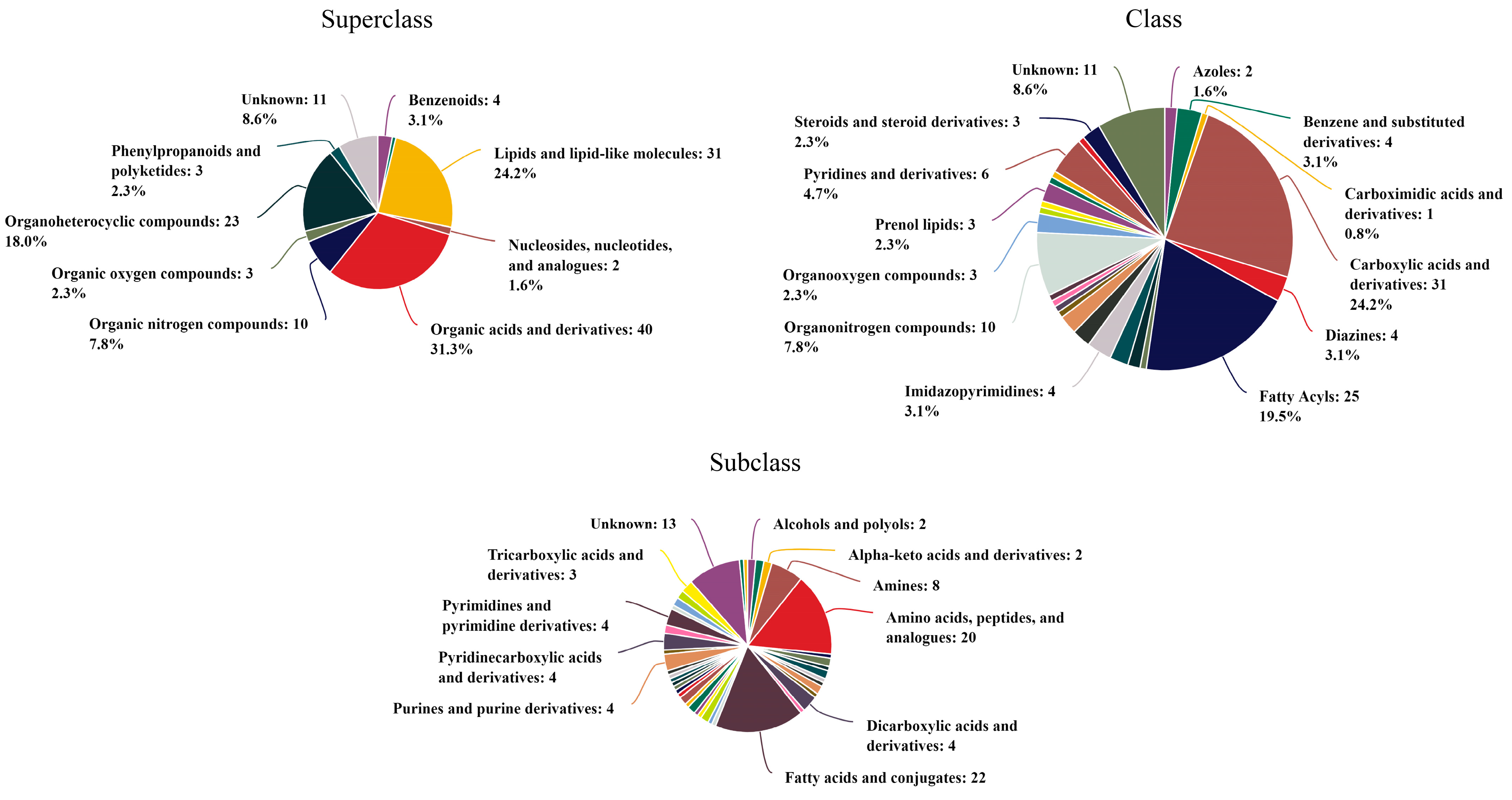
3.5. The Detection of Benthic Cyanobacterial Metabolites
4. Discussion
4.1. The Effect of the Nitrogen-Phosphorus Ratio in Sediment on Cladophora
4.2. Reasons for the Inhibition of Cladophora by Benthic Cyanobacteria
4.3. The Analysis of Benthic Cyanobacterial Metabolites
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berezina, N.A.; Tsiplenkina, I.G.; Pankova, E.S.; Gubelit, J.I. Dynamics of invertebrate communities on the stony littoral of the Neva Estuary (Baltic Sea) under macroalgal blooms and bioinvasions. Transit. Waters Bull. 2007, 1, 65–76. [Google Scholar] [CrossRef]
- Berezina, N.A.; Golubkov, S.M. Effect of drifting macroalgae Cladophoraglomerata on benthic community dynamics in the easternmost Baltic Sea. J. Mar. Syst. 2008, 74, S80–S85. [Google Scholar] [CrossRef]
- Han, W.F.; Sun, Y.F.; Liu, J.; Zhang, Y.W.; Cheng, Y.X. Effect of solid and liquid algicides on the growth of and antioxidant capacity of Cladophora. Aquacult. Rep. 2021, 20, 100736. [Google Scholar] [CrossRef]
- Tang, Y.T.; Wang, C.; Cheng, Y.X.; Sun, Y.F.; Zhao, L.J.; Qian, C.; Yang, Y.F. Investigation on the key factors of filamentous algae occurrence in aquaculture ponds. J. Fish. China. 2024, 48, 049119. (In Chinese) [Google Scholar] [CrossRef]
- Lehvo, A.; Back, S. Survey of macroalgal mats in the Gulf of Finland, Baltic Sea. Aquat. Conserv. 2001, 11, 11–18. [Google Scholar] [CrossRef]
- Meng, F.L.; Rong, X.J.; Li, B.; Chen, Z.Y.; Liao, M.J.; Wang, L.; Zhang, Z.; Chen, G.P.; Wang, Y.G.; Fan, R.Y. Investigation and prevention and control strategy of a common Cladophora in a pond of sea cucumber. Fish. Sci. Technol. Inf. 2013, 40, 179–182. (In Chinese) [Google Scholar] [CrossRef]
- Tang, Y.T.; Wang, L.P.; Zhao, L.J.; Qian, C.; Lun, F.; Wang, C.; Zheng, H.; Tang, B.P.; Cheng, Y.X.; Guo, X.S. Inhibitory effects and oxidative damages in Cladophora sp. (Cladophoraceae) exposed to berberine. Aquacult. Rep. 2022, 27, 101357. [Google Scholar] [CrossRef]
- Li, H. The Study of Toxic Effect, Enrichment and Torance Mechanism between Heavy Metal and Cladophora; Anhui Agriculture University: Hefei, China, 2013. [Google Scholar]
- Higgins, S.N.; Malkin, S.Y.; Howell, E.T.; Guildford, S.J.; Hecky, R.E. An ecological review of Cladophora glomerata (Chlorophyta) in the Laurentian Great Lakes. J. Phycol. 2010, 44, 839–854. [Google Scholar] [CrossRef]
- Çelekli, A.; Arslanargun, H.; Soysal, Ç.; Gültekin, E.; Bozkurt, H. Biochemical responses of filamentous algae in different aquatic ecosystems in South East Turkey and associated water quality parameters. Ecotoxicol. Environ. Saf. 2016, 133, 403–412. [Google Scholar] [CrossRef]
- Flores-Morales, G.; Díaz, M.; Arancibia-Avila, P.; Muñoz-Carrascod, M.; Jara-Zapata, P.; Toledo-Montiel, F.; Vega-Román, E. Removal of nutrients from Organic Liquid Agricultural Waste using filamentous algae. Braz. J. Biol. 2020, 81, 544–550. [Google Scholar] [CrossRef]
- Çelekli, A.; Bozkurt, H. Assessing biochemical responses of filamentous algae integrated with surface waters in Yavuzeli-Araban catchment. Ecol. Eng. 2021, 159, 106126. [Google Scholar] [CrossRef]
- Liu, J.Z.; Vyverman, W. Differences in nutrient uptake capacity of the benthic filamentous algae Cladophora sp., Klebsormidium sp. and Pseudanabaena sp. under varying N/P conditions. Bioresour. Technol. 2015, 179, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Juijuljerm, R.; Vanijajiva, O.; Chittapun, S. The potential of using akinetes as seed starters for Cladophora glomerata cultivation: Germination and growth of akinetes under different light intensities and humic concentrations. Algal Res. 2021, 60, 102478. [Google Scholar] [CrossRef]
- Zhao, W. Hydrobiology; China Agriculture Press: Beijing, China, 2005. [Google Scholar]
- Feng, M.W.; Wu, Y.H.; Feng, S.X.; Wu, Y.Y. Effect of different N/P ratios on algal growth. Ecol. Environ. 2008, 17, 1759–1763. (In Chinese) [Google Scholar] [CrossRef]
- Gugger, M.; Lenoir, S.; Berger, C.; Ledreux, A.; Druart, J.C.; Humbert, J.; Guette, C.; Bernard, C. First report in a river in France of the benthic cyanobacterium Phormidium favosum producing anatoxin-a associated with dog neurotoxicosis. Toxicon 2005, 45, 919–928. [Google Scholar] [CrossRef]
- Cirés, S.; Alvarez-Roa, C.; Wood, S.A.; Puddick, J.; Loza, V.; Heimann, K. First report of microcystin-producing Fischerella sp. (Stigonematales, Cyanobacteria) in tropical Australia. Toxicon 2014, 88, 62–66. [Google Scholar] [CrossRef]
- Gaget, V.; Humpage, A.R.; Huang, Q.; Monis, P.; Brookes, J.D. Benthic cyanobacteria: A source of cylindrospermopsin and microcystin in Australian drinking water reservoirs. Water Res. 2017, 124, 454–464. [Google Scholar] [CrossRef]
- Trout-Haney, J.V.; Ritger, A.L.; Cottingham, K.L. Benthic cyanobacteria of the genus Nostoc are a source of microcystins in Greenlandic lakes and ponds. Freshw. Biol. 2020, 66, 266–277. [Google Scholar] [CrossRef]
- Duan, Z.P.; Tan, X.; Shi, L.; Zeng, Q.F.; Ali, I.; Zhu, R.; Chen, H.M.; Parajuli, K. Phosphorus Accumulation in Extracellular Polymeric Substances (EPS) of Colony-Forming Cyanobacteria Challenges Imbalanced Nutrient Reduction Strategies in Eutrophic Lakes. Environ. Sci. Technol. 2023, 57, 1600–1612. [Google Scholar] [CrossRef]
- Pei, G.F.; Liu, G.X.; Hu, Z.Y. The role of benthic algae in phosphorous retention in Donghu Lake, Wuhan. Acta Sci. Circumst. 2009, 29, 840–845. (In Chinese) [Google Scholar] [CrossRef]
- Song, Y.Z.; Zhang, Y.D.; Zheng, J.W.; Gao, Y.X. Periphytic algae ecology in freshwater lake. Chin. J. Ecol. 2016, 35, 534–541. (In Chinese) [Google Scholar] [CrossRef]
- Levich, A.P. The role of nitrogen-phosphorus ratio in selecting for dominance of phytoplankton by cyanobacteria or green algae and its application to reservoir management. J. Aquat. Ecosyst. Health 1996, 5, 55–61. [Google Scholar] [CrossRef]
- Paerl, H.W.; Xu, H.; Mccarthy, M.J.; Zhu, G.W.; Qin, B.Q.; Li, Y.P.; Gardner, W.S. Controlling harmful cyanobacterial blooms in a hyper-eutrophic lake (Lake Taihu, China): The need for a dual nutrient (N & P) management strategy. Water Res. 2011, 45, 1973–1983. [Google Scholar] [CrossRef]
- Grover, J.P.; Scott, J.T.; Roelke, D.L.; Brooks, B.W. Competitive superiority of N-fixing cyanobacteria when fixed N is scarce: Reconsiderations based on a model with heterocyst differentiation. Ecol. Model. 2022, 466, 109904. [Google Scholar] [CrossRef]
- Smith, G.D.; Doan, N.T. Cyanobacterial metabolites with bioactivity against photosynthesis in cyanobacteria, algae and higher plant. J. Appl. Phycol. 1999, 11, 337–344. [Google Scholar] [CrossRef]
- Suikkanen, S. Allelopathic effects of filamentous cyanobacteria on phytoplankton in the Baltic Sea. J. Appl. Spectrosc. 2008, 14, 114–116. [Google Scholar] [CrossRef]
- Leão, P.N.; Pereira, A.R.; Liu, W.T.; Ng, J.; Pevzner, P.A.; Dorrestein, P.C.; Koenig, G.M.; Vasconcelos, V.M.; Gerwick, W.H. Synergistic allelochemicals from a freshwater cyanobacterium. Proc. Natl. Acad. Sci. USA 2010, 107, 11183–11188. [Google Scholar] [CrossRef]
- Faassen, E.J.; Harkema, L.; Begeman, L.; Lurling, M. First report of (homo) anatoxin-a and dog neurotoxicosis after ingestion of benthic cyanobacteria in The Netherlands. Toxicon 2012, 60, 378–384. [Google Scholar] [CrossRef]
- Tian, X.M.; Pei, H.Y.; Xu, H.Z. A review on the prevalence and features of toxic benthic cyanobacteria in freshwater. Chin. J. Ecol. 2020, 39, 2070–2085. (In Chinese) [Google Scholar] [CrossRef]
- Leão, P.N.; Engene, N.; Antunes, A.; Gerwick, W.H.; Vasconcelos, V. The chemical ecology of cyanobacteria. Nat. Prod. Rep. 2012, 29, 372–391. [Google Scholar] [CrossRef]
- Śliwińska-Wilczewska, S.; Wiśniewska, K.; Konarzewska, Z.; Cieszyńska, A.; Felpeto, A.B.; Lewandowska, A.U.; Latała, A. The current state of knowledge on taxonomy, modulating factors, ecological roles, and mode of action of phytoplankton allelochemicals. Sci. Total Environ. 2021, 773, 145681. [Google Scholar] [CrossRef]
- Schlegel, I.; Doan, N.T.; Chazal, N.D.; Smith, G.D. Antibiotic activity of new cyanobacterial isolates from Australia and Asia against green algae and cyanobacteria. J. Appl. Phycol. 1998, 10, 471–479. [Google Scholar] [CrossRef]
- Heath, M.; Wood, S.A.; Young, R.G.; Ryan, K.G. The role of nitrogen and phosphorus in regulating Phormidium sp. (Cyanobacteria) growth and anatoxin production. FEMS Microbiol. Ecol. 2016, 92, fiw021. [Google Scholar] [CrossRef]
- Kurmayer, R. The toxic Cyanobacterium Nostoc sp. strain 152 produces highest amounts of microcystin and nostophycin under stress conditions. J. Phycol. 2011, 47, 200–207. [Google Scholar] [CrossRef]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef]
- Krüger, T.; Hölzel, N.; Luckas, B. Influence of cultivation parameters on growth and microcystin production of Microcystis aeruginosa (Cyanophyceae) isolated from Lake Chao (China). Microb. Ecol. 2012, 63, 199–209. [Google Scholar] [CrossRef]
- Legrand, C.; Rengefors, K.; Fistarol, G.O.; Graneli, E. Allelopathy in phytoplankton-biochemical, ecological and evolutionary aspects. Phycologia 2003, 42, 406–419. [Google Scholar] [CrossRef]
- Inderjit. Inderjit. Experimental complexities in evaluating the allelopathic activities in laboratory bioassays: A case study. Soil Biol. Biochem 2006, 38, 256–262. [Google Scholar] [CrossRef]


| Available Phosphorus Content | N:P | Available Nitrogen Content | Groups |
|---|---|---|---|
| P1 (13.44 mg/kg) | 10:1 | N1 (134.4 mg/kg) | P1N1 |
| 40:1 | N2 (537.6 mg/kg) | P1N2 | |
| 80:1 | N3 (1075.2 mg/kg) | P1N3 | |
| P2 (107.52 mg/kg) | 10:1 | N1 (1075.2 mg/kg) | P2N1 |
| 40:1 | N2 (4300.8 mg/kg) | P2N2 | |
| 80:1 | N3 (8601.6 mg/kg) | P2N3 | |
| P3 (215.04 mg/kg) | 10:1 | N1 (2150.4 mg/kg) | P3N1 |
| 40:1 | N2 (8601.6 mg/kg) | P3N2 | |
| 80:1 | N3 (17,203.2 mg/kg) | P3N3 |
| Attachment Location | Experimental Groups | Culture Mediums and Volume (mL) | Additives and Weight (g) | |
|---|---|---|---|---|
| Control group (Control) | BG11 medium (200 mL) | Ultrapure water (100 mL) | - | |
| Sidewall | Coculture group (CG-S) | BG11 medium (200 mL) | Sidewall epiphytic algae culture medium (100 mL) | - |
| Ground group (GG-S) | BG11 medium (200 mL) | Ultrapure water (100 mL) | Ground sidewall epiphytic algae (0.1 g) | |
| Filtration group (FG-S) | BG11 medium (200 mL) | Filtered sidewall epiphytic algae culture medium (0.22 μm) (100 mL) | - | |
| Bottom | Coculture group (CG-B) | BG11 medium (200 mL) | Bottom epiphytic algae culture medium (100 mL) | - |
| Ground group (GG-B) | BG11 medium (200 mL) | Ultrapure water (100 mL) | Ground bottom epiphytic algae (0.1 g) | |
| Filtration group (FG-B) | BG11 medium (200 mL) | Filtered bottom epiphytic algae culture medium (0.22 μm) (100 mL) | - | |
| Metabolite | Proportion (‰) | Superclass | Class | Subclass |
|---|---|---|---|---|
| Cis,cis-muconic acid | 32.48 | Lipids and lipid-like molecules | Fatty Acyls | Fatty acids and conjugates |
| Erucamide | 9.52 | Lipids and lipid-like molecules | Fatty Acyls | Fatty amides |
| Phosphoric acid | 6.97 | Homogeneous nonmetal compounds | Nonmetal oxoanionic compounds | Nonmetal phosphates |
| Fenpropidin | 6.53 | Benzenoids | Benzene and substituted derivatives | Phenylpropanes |
| Propionic acid | 5.16 | Organic acids and derivatives | Carboxylic acids and derivatives | Carboxylic acids |
| Phytosphingosine | 2.58 | Organic nitrogen compounds | Organonitrogen compounds | Amines |
| Pro-Trp | 1.94 | Organic nitrogen compounds | Organonitrogen compounds | Amines |
| Citrate | 1.79 | Organic acids and derivatives | Carboxylic acids and derivatives | Tricarboxylic acids and derivatives |
| Tuberostemonine | 1.69 | Alkaloids and derivatives | Stemona alkaloids | Stemoamide-type alkaloids |
| 2,4,6-trichlorophenol | 1.52 | Benzenoids | Phenols | Halophenols |
| Chenodeoxycholate | 1.47 | Lipids and lipid-like molecules | Steroids and steroid derivatives | Bile acids, alcohols and derivatives |
| Glufosinate | 1.01 | Organic acids and derivatives | Carboxylic acids and derivatives | Amino acids, peptides, and analogs |
| Palmitic acid | 1.00 | Lipids and lipid-like molecules | Fatty Acyls | Fatty acids and conjugates |
| Caffeate | 0.99 | Phenylpropanoids and polyketides | Cinnamic acids and derivatives | Hydroxycinnamic acids and derivatives |
| 2-palmitoyl-rac-glycerol | 0.95 | Lipids and lipid-like molecules | Glycerolipids | Monoradylglycerols |
| 3,4-dihydroxyhydrocinnamic acid | 0.78 | Unknown | Unknown | Unknown |
| Heptadecasphinganine | 0.63 | Organic nitrogen compounds | Organonitrogen compounds | Amines |
| 1-stearoyl-rac-glycerol | 0.57 | Lipids and lipid-like molecules | Glycerolipids | Monoradylglycerols |
| Dl-malic acid | 0.55 | Organic acids and derivatives | Hydroxy acids and derivatives | Beta hydroxy acids and derivatives |
| Octadecanoic acid | 0.54 | Lipids and lipid-like molecules | Fatty Acyls | Fatty acids and conjugates |
| C17-sphinganine | 0.51 | Organic nitrogen compounds | Organonitrogen compounds | Amines |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, K.; Lv, J.; Peng, X.; Tang, Y.; Zhao, L.; Cheng, Y.; Liu, Q. Effects of Nitrogen and Phosphorus in Sediment on the Occurrence of Cladophora sp. (Cladophoraceae) in Aquaculture Ponds. Biology 2024, 13, 739. https://doi.org/10.3390/biology13090739
Zhang Y, Liu K, Lv J, Peng X, Tang Y, Zhao L, Cheng Y, Liu Q. Effects of Nitrogen and Phosphorus in Sediment on the Occurrence of Cladophora sp. (Cladophoraceae) in Aquaculture Ponds. Biology. 2024; 13(9):739. https://doi.org/10.3390/biology13090739
Chicago/Turabian StyleZhang, Yuanyuan, Kaifang Liu, Jun Lv, Xinliang Peng, Yongtao Tang, Liangjie Zhao, Yongxu Cheng, and Qigen Liu. 2024. "Effects of Nitrogen and Phosphorus in Sediment on the Occurrence of Cladophora sp. (Cladophoraceae) in Aquaculture Ponds" Biology 13, no. 9: 739. https://doi.org/10.3390/biology13090739
APA StyleZhang, Y., Liu, K., Lv, J., Peng, X., Tang, Y., Zhao, L., Cheng, Y., & Liu, Q. (2024). Effects of Nitrogen and Phosphorus in Sediment on the Occurrence of Cladophora sp. (Cladophoraceae) in Aquaculture Ponds. Biology, 13(9), 739. https://doi.org/10.3390/biology13090739





