Effects of Different Salinity Stress on the Transcriptomic Responses of Freshwater Crayfish (Procambarus clarkii, Girard, 1852)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animal
2.2. Exposure and Sample Collection
2.3. RNA Extraction, Library Preparation, and Sequencing
2.4. Data Analysis
2.4.1. Gene Differential Expression and Functional Annotation
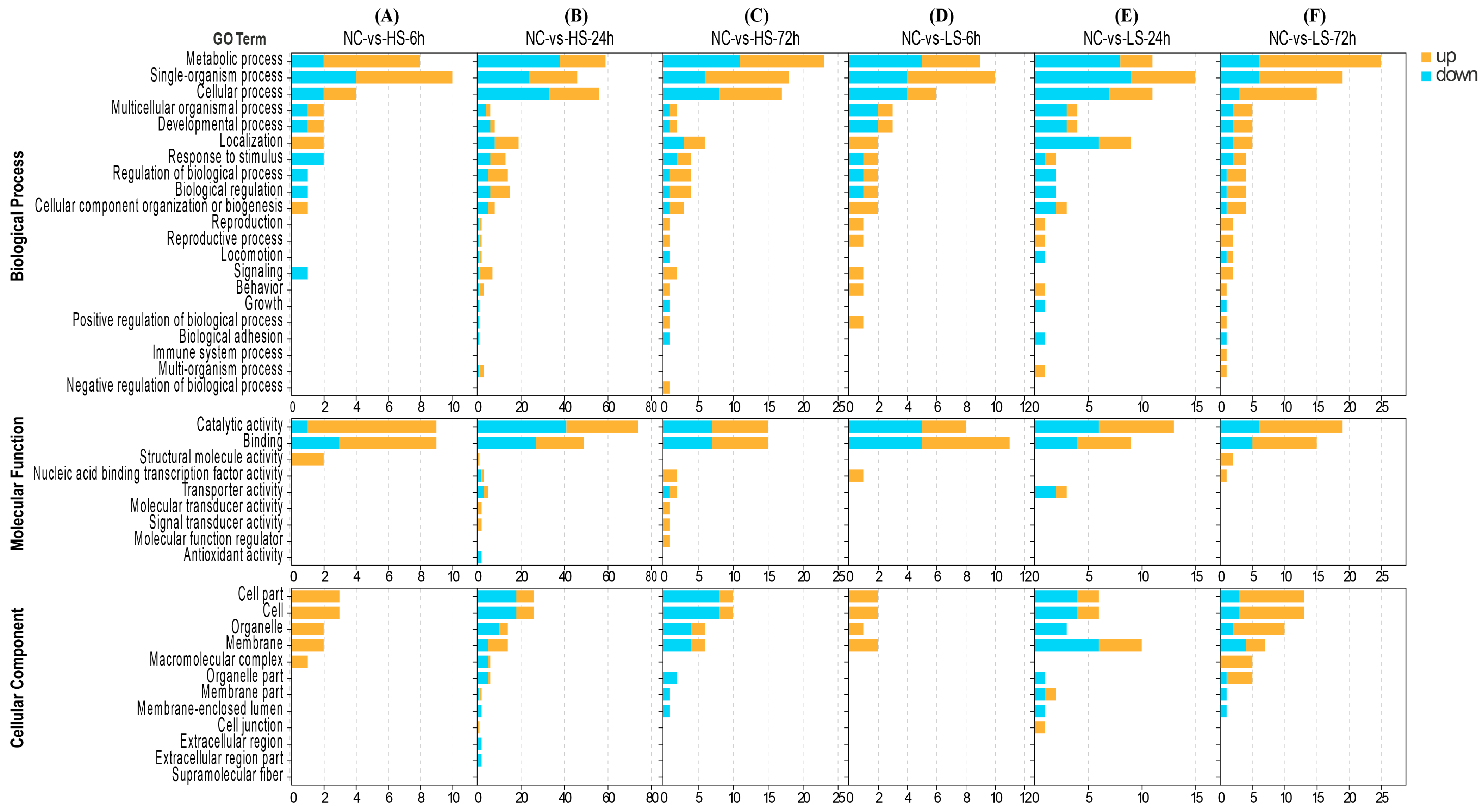
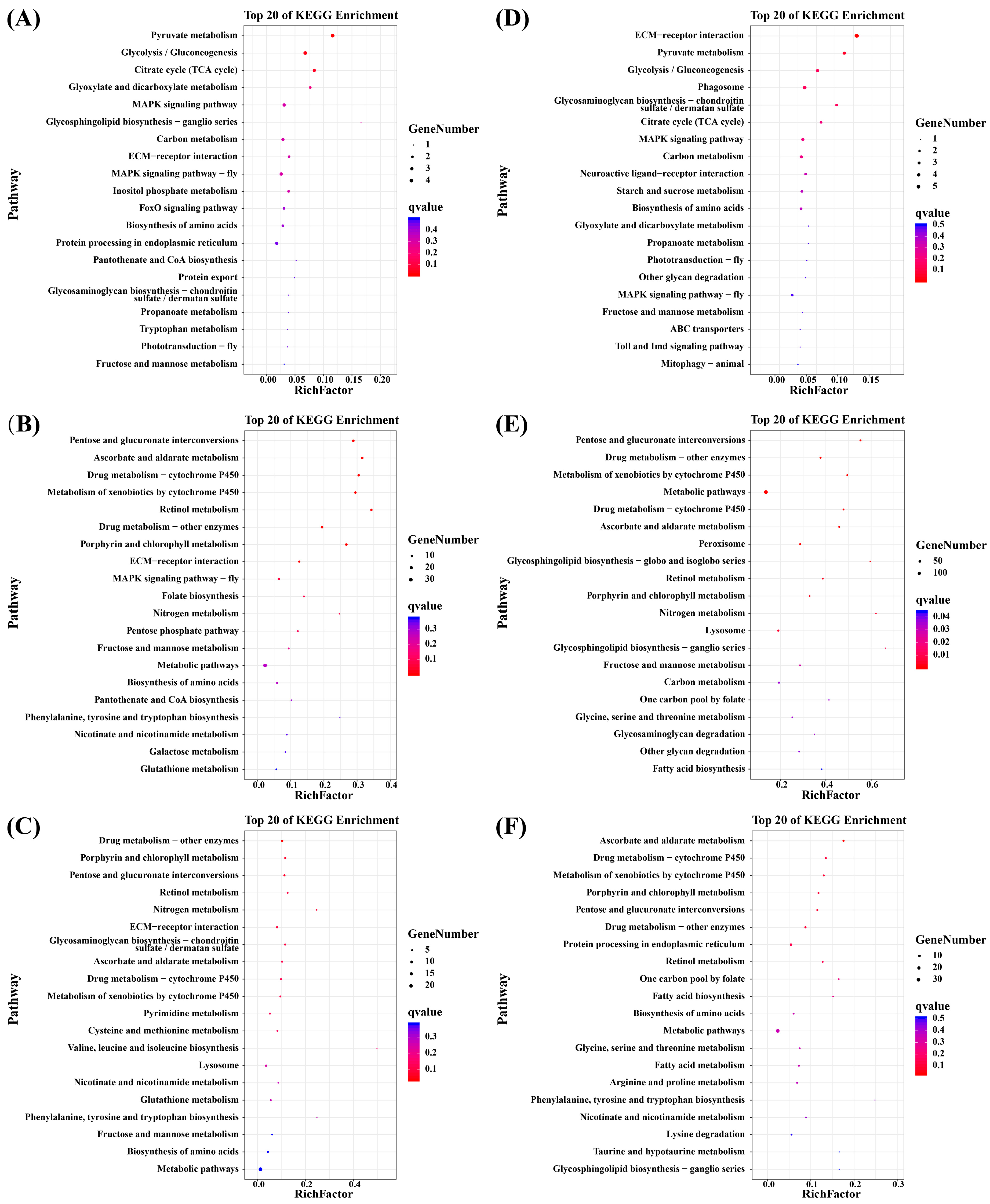
2.4.2. GO, KEGG Enrichment, and Gene Expression Pattern Analysis
2.4.3. Ion Transport-Related Gene Selection and Function Prediction
2.5. Verification by Quantitative Real-Time PCR (qRT-PCR)
2.6. Statistical Analysis
3. Results
3.1. RNA-Seq Data
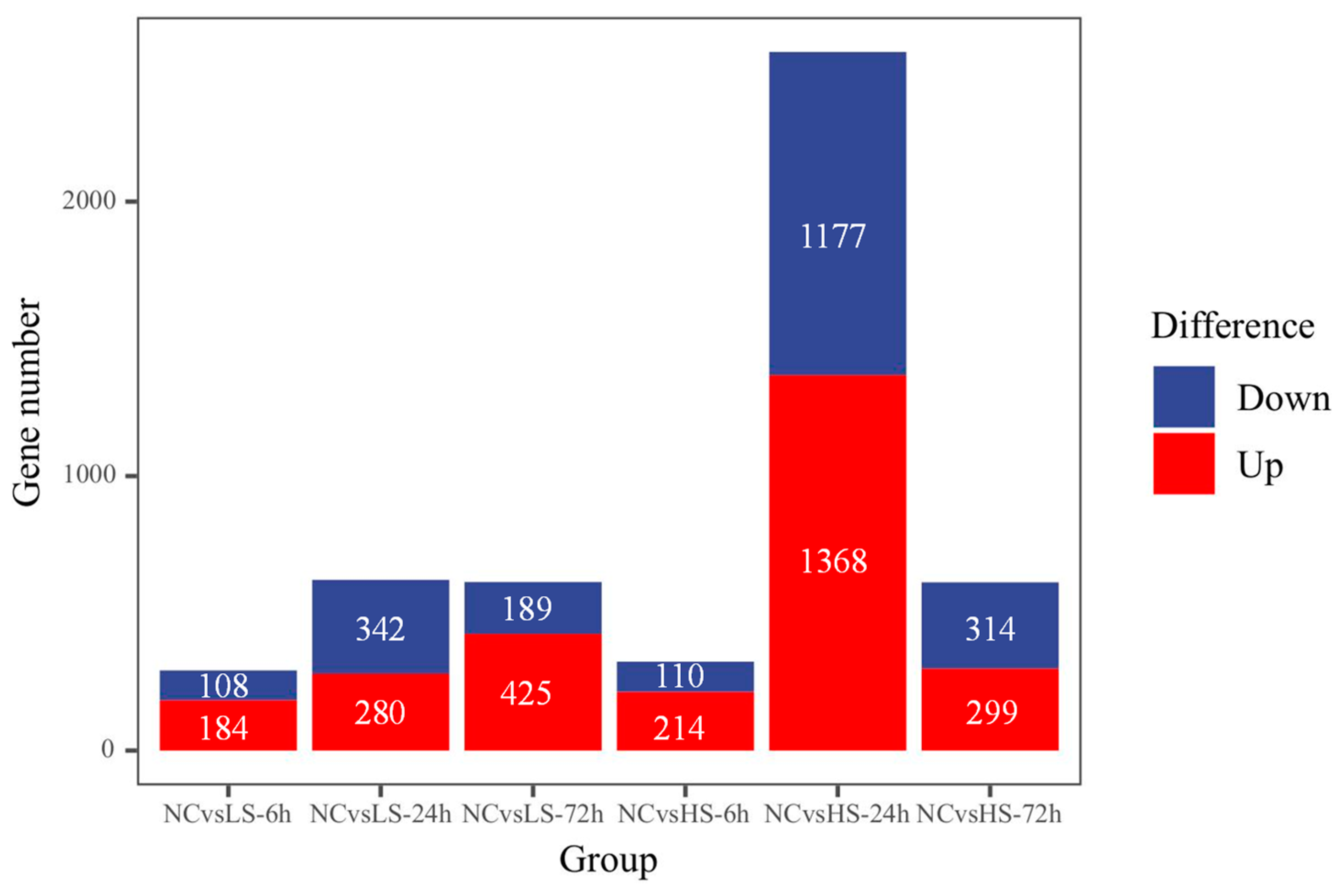
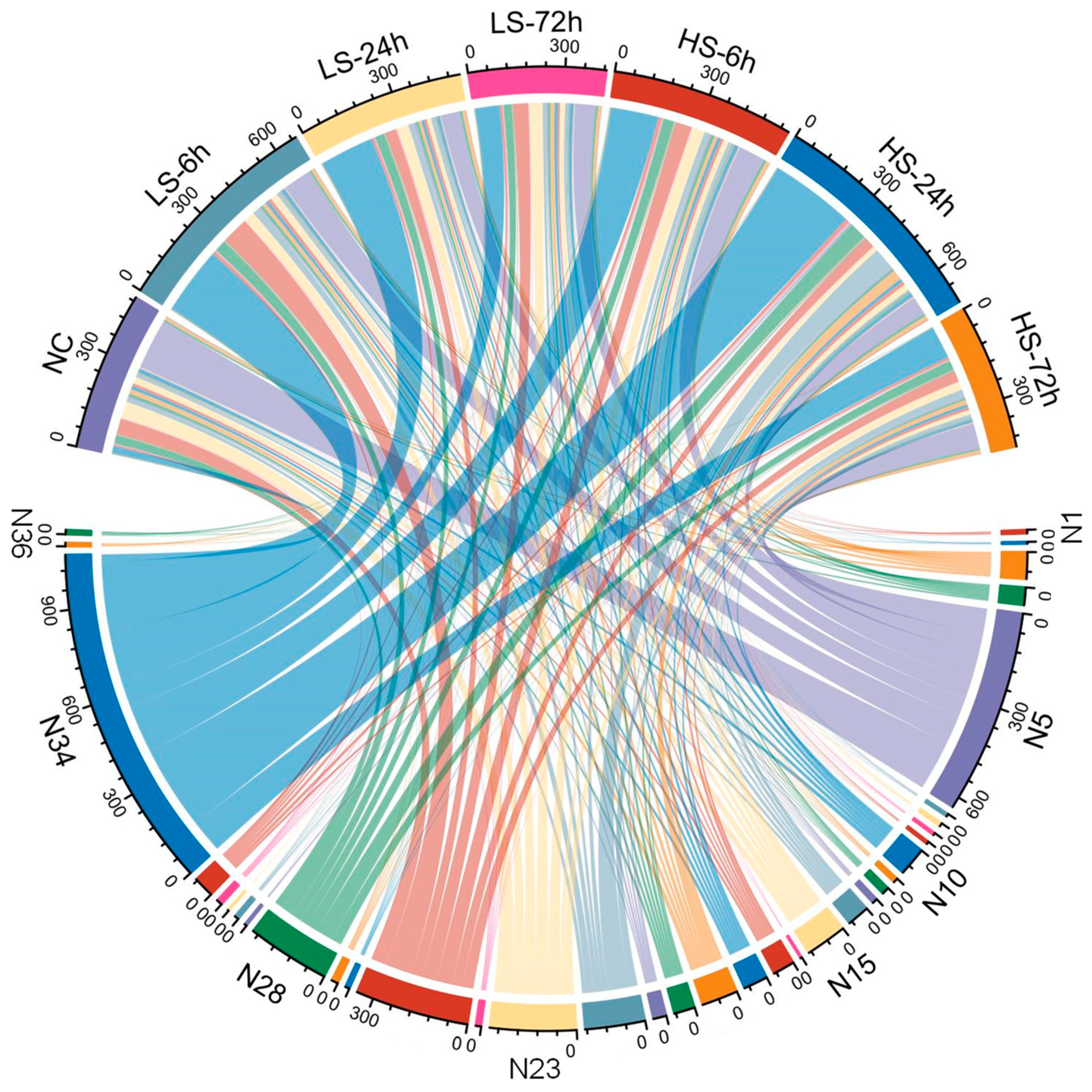
3.2. Differential Expression Responses of P. clarkii Exposed to Different Salinity Gradients
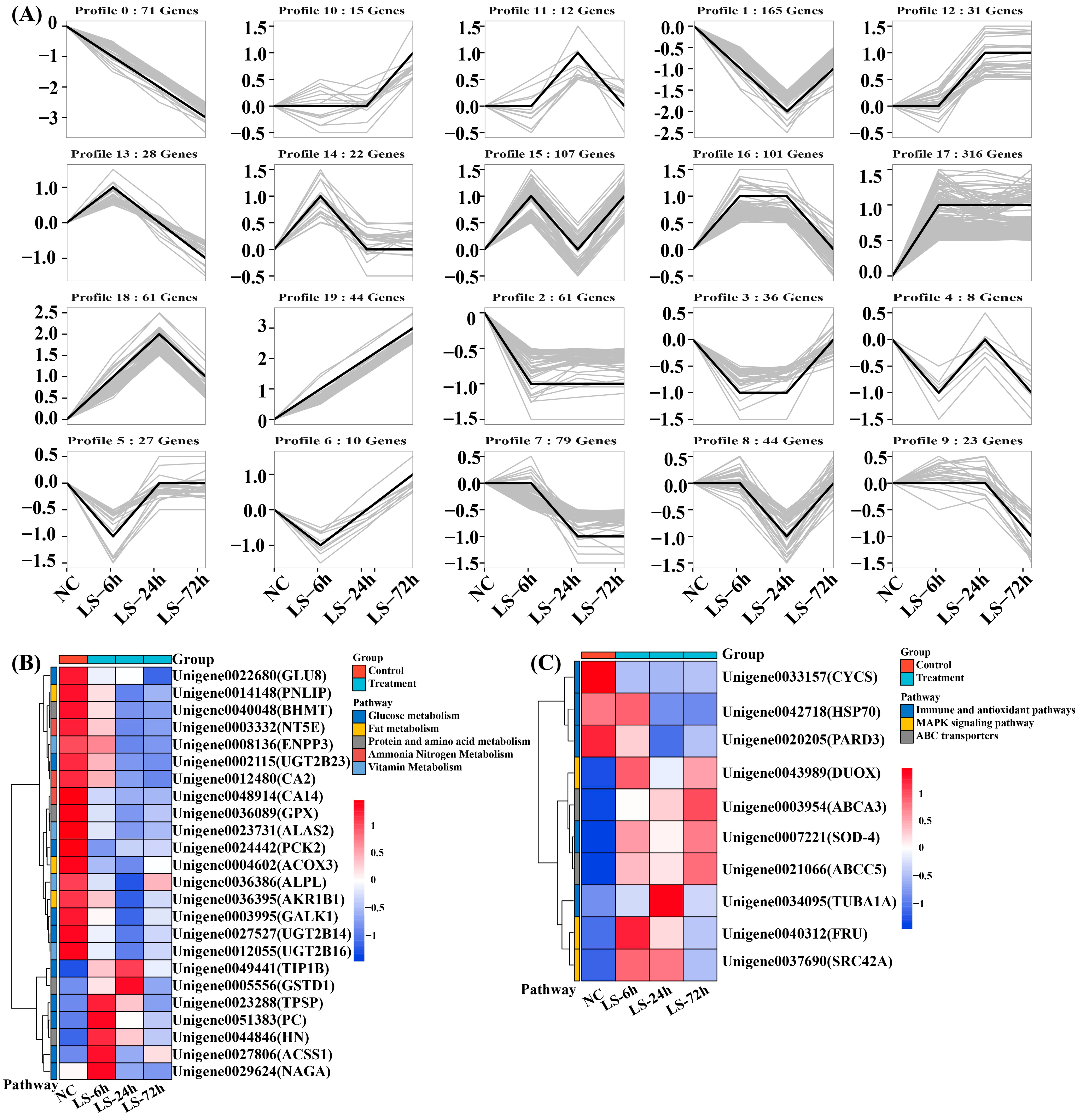
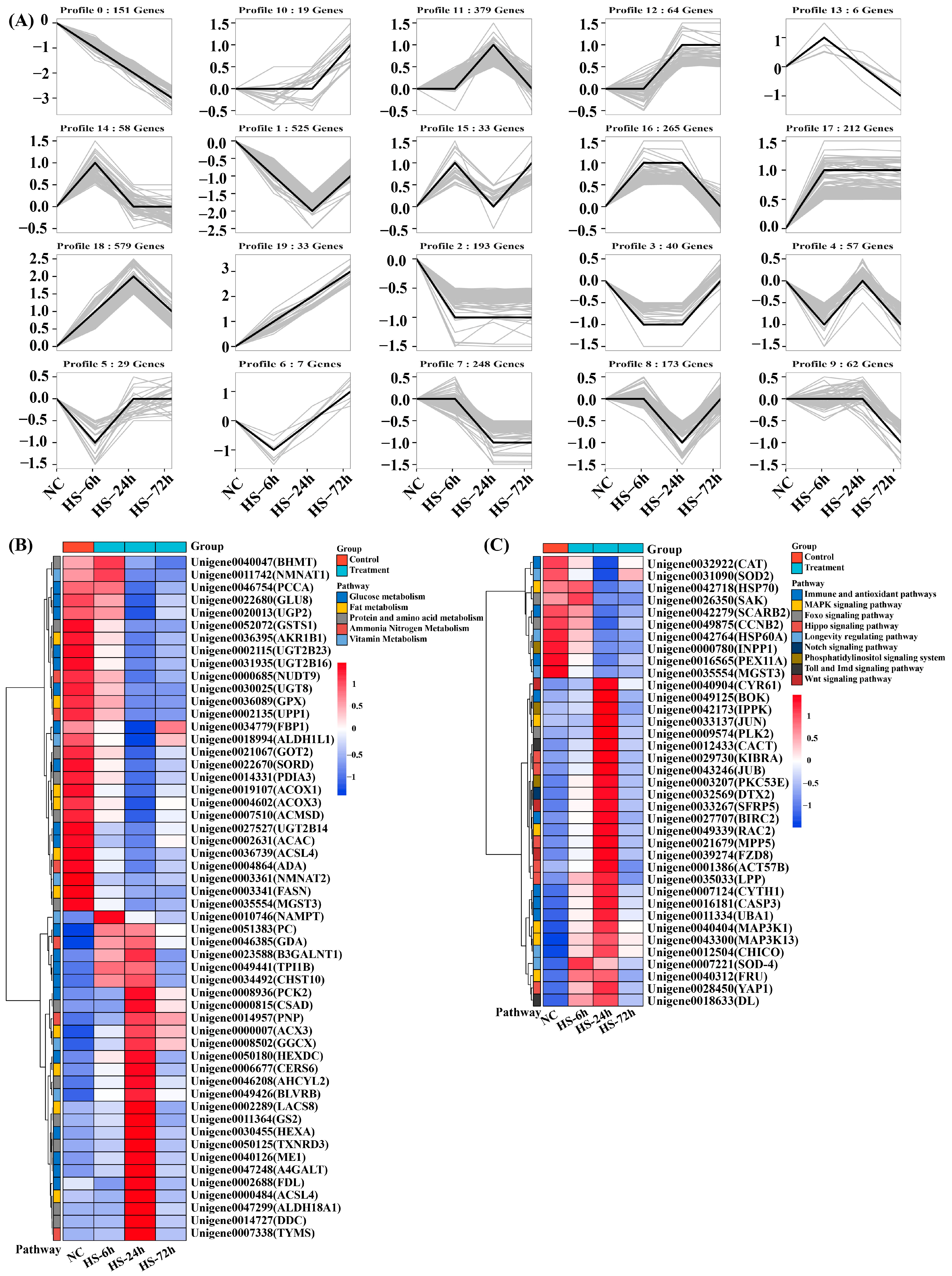
3.3. Trend Analysis of DEGs under Salinity Treatment
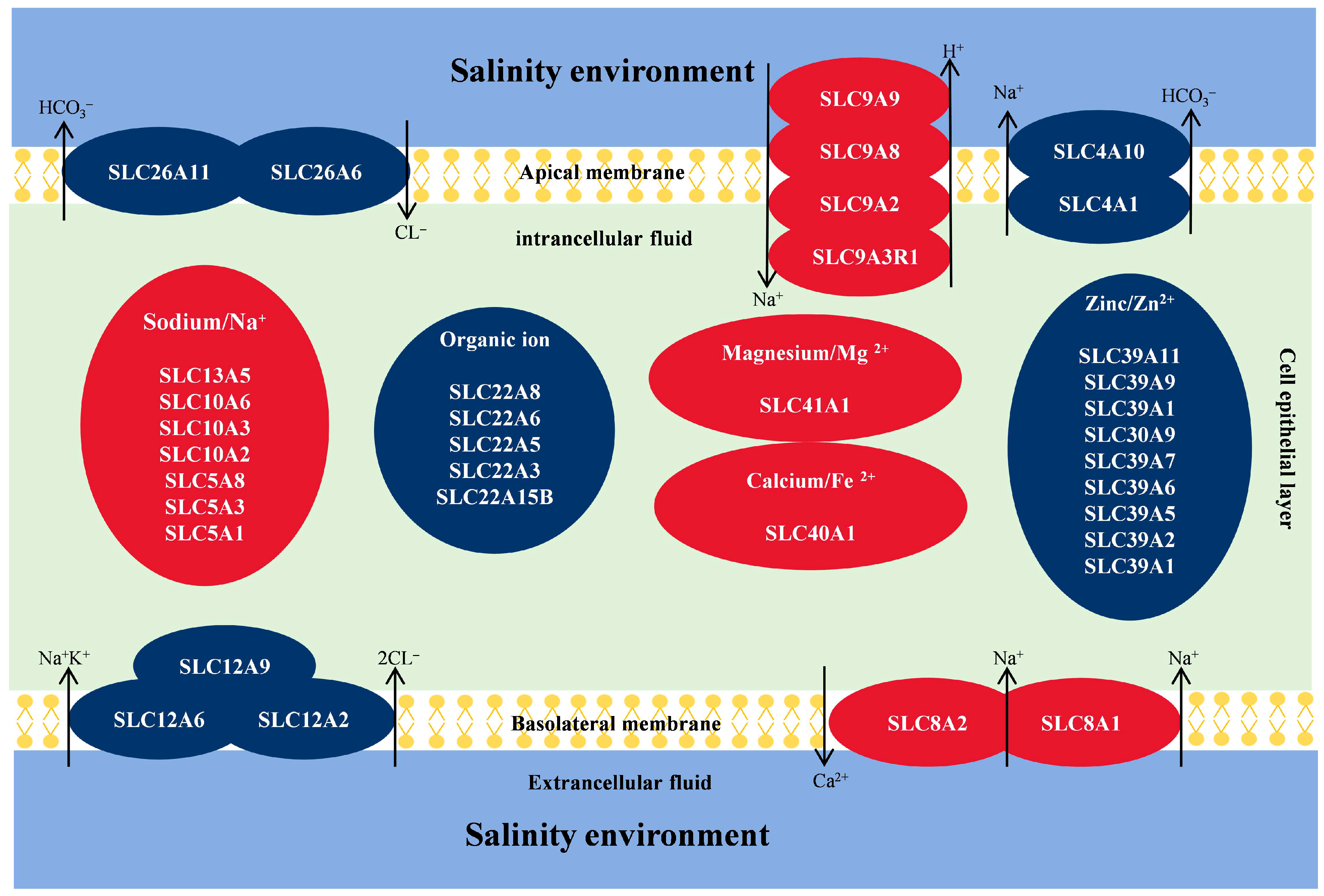
3.4. Analysis of Genes Related to Ion Exchange in P. clarkii
3.5. Quantitative Real-Time PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D. Multiple Threats Imperil Freshwater Biodiversity in the Anthropocene. Curr. Biol. 2019, 29, R960–R967. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging Threats and Persistent Conservation Challenges for Freshwater Biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, E.; Søndergaard, M.; Pedersen, A.R.; Jürgens, K.; Strzelczak, A.; Lauridsen, T.L.; Johansson, L.S. Salinity Induced Regime Shift in Shallow Brackish Lagoons. Ecosystems 2007, 10, 47–57. [Google Scholar] [CrossRef]
- Gardner, A.S.; Moholdt, G.; Cogley, J.G.; Wouters, B.; Arendt, A.A.; Wahr, J.; Berthier, E.; Hock, R.; Pfeffer, W.T.; Kaser, G.; et al. A Reconciled Estimate of Glacier Contributions to Sea Level Rise: 2003 to 2009. Science 2013, 340, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Gächter, R. Increasing Chloride Concentrations in Lake Constance: Characterization of Sources and Estimation of Loads. Aquat. Sci. 2012, 74, 101–112. [Google Scholar] [CrossRef]
- Cañedo-Argüelles, M.; Kefford, B.J.; Piscart, C.; Prat, N.; Schäfer, R.B.; Schulz, C.J. Salinisation of Rivers: An Urgent Ecological Issue. Environ. Pollut. 2013, 173, 157–167. [Google Scholar] [CrossRef]
- Kaushal, S.S.; Likens, G.E.; Mayer, P.M.; Shatkay, R.R.; Shelton, S.A.; Grant, S.B.; Utz, R.M.; Yaculak, A.M.; Maas, C.M.; Reimer, J.E.; et al. The Anthropogenic Salt Cycle. Nat. Rev. Earth Environ. 2023, 4, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Cañedo-Argüelles, M.; Kefford, B.; Schäfer, R. Salt in Freshwaters: Causes, Effects and Prospects—Introduction to the Theme Issue. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20180002. [Google Scholar] [CrossRef]
- Arora, S.; Singh, A.K.; Singh, Y.P. Bioremediation of Salt Affected Soils: An Indian Perspective; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783319482576. [Google Scholar]
- Rind, K.; Beyrend, D.; Blondeau-Bidet, E.; Charmantier, G.; Cucchi, P.; Lignot, J.H. Effects of Different Salinities on the Osmoregulatory Capacity of Mediterranean Sticklebacks Living in Freshwater. J. Zool. 2017, 303, 270–280. [Google Scholar] [CrossRef]
- Thomas, D.; Rekha, M.U.; Jani, J.R.; Sreekanth, G.B.; Thiagarajan, G.; Subburaj, R.; Kailasam, M.; Vijayan, K.K. Effects of Salinity Amendments on the Embryonic and Larval Development of a Tropical Brackishwater Ornamental Silver Moony Fish, Monodactylus argenteus (Linnaeus, 1758). Aquaculture 2021, 544, 737073. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, S.; Huang, J.; Zhou, F.; Yang, Q.; Jiang, S.; Yang, L. C-Type Lectin Response to Bacterial Infection and Ammonia Nitrogen Stress in Tiger Shrimp (Penaeus Monodon). Fish Shellfish Immunol. 2019, 90, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, F.; Huang, J.; Jiang, S. Ammonia and salinity tolerance of Penaeus monodon across eight breeding families. SpringerPlus 2016, 5, 171. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, Y.; Xu, W.; Chen, D.; Li, B.; Cheng, Y.; Guo, X. The Effects of Salinities Stress on Histopathological Changes, Serum Biochemical Index, Non-Specific Immune and Transcriptome Analysis in Red Swamp Crayfish Procambarus clarkii. Sci. Total Environ. 2022, 840, 156502. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Luo, L.; Liu, D.L.; Huang, J.H.; Jiang, S.G.; Zhou, F.L.; Yang, Q.B.; Jiang, S.; Li, Y.D.; Tan, L.Q.; et al. Effect of Acute Salinity Stress on Metabolism, Antioxidant Status, and Histological Structure of Procambarus clarkii. Aquac. Res. 2023, 2023, 2748257. [Google Scholar] [CrossRef]
- Jacquin, L.; Petitjean, Q.; Côte, J.; Laffaille, P.; Jean, S. Effects of Pollution on Fish Behavior, Personality, and Cognition: Some Research Perspectives. Front. Ecol. Evol. 2020, 8, 86. [Google Scholar] [CrossRef]
- Bal, A.; Panda, F.; Pati, S.G.; Das, K.; Agrawal, P.K.; Paital, B. Modulation of Physiological Oxidative Stress and Antioxidant Status by Abiotic Factors Especially Salinity in Aquatic Organisms: Redox Regulation under Salinity Stress. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 241, 108971. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Gao, T.; Han, Z. International Journal of Biological Macromolecules Effect of Salinity Fl Uctuation on the Transcriptome of the Japanese Mantis Shrimp Oratosquilla oratoria. Int. J. Biol. Macromol. 2019, 140, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Sánchez, A.; Hose, G.C.; Reboleira, A.S.P.S. Salinity and Temperature Increase Impact Groundwater Crustaceans. Sci. Rep. 2020, 10, 12328. [Google Scholar] [CrossRef]
- Pan, L.Q.; Zhang, L.J.; Liu, H.Y. Effects of Salinity and PH on Ion-Transport Enzyme Activities, Survival and Growth of Litopenaeus vannamei Postlarvae. Aquaculture 2007, 273, 711–720. [Google Scholar] [CrossRef]
- Fabri, L.M.; Moraes, C.M.; Costa, M.I.C.; Garçon, D.P.; Fontes, C.F.L.; Pinto, M.R.; McNamara, J.C.; Leone, F.A. Salinity-Dependent Modulation by Protein Kinases and the FXYD2 Peptide of Gill (Na+, K+)-ATPase Activity in the Freshwater Shrimp Macrobrachium amazonicum (Decapoda, Palaemonidae). Biochim. Biophys. Acta-Biomembr. 2022, 1864, 183982. [Google Scholar] [CrossRef] [PubMed]
- Koyama, H.; Kamiya, K.; Sasaki, Y.; Yamakawa, R.; Kuniyoshi, H.; Piyapattanakorn, S.; Watabe, S. Cloning of Glutamine Synthetase Gene from Abdominal Muscle of Kuruma Shrimp Marsupenaeus japonicus and Its Expression Profile. Fish. Sci. 2023, 89, 215–222. [Google Scholar] [CrossRef]
- Jiang, J.L.; Xu, J.; Ye, L.; Sun, M.L.; Jiang, Z.Q.; Mao, M.G. Identification of Differentially Expressed Genes in Gills of Tiger Puffer (Takifugu rubripes) in Response to Low-Salinity Stress. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 243, 110437. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ma, A.; Yuan, C.; Zhao, T.; Chang, H.; Zhang, J. Transcriptome Analysis of Liver Lipid Metabolism Disorders of the Turbot Scophthalmus maximus in Response to Low Salinity Stress. Aquaculture 2021, 534, 736273. [Google Scholar] [CrossRef]
- Lou, F.; Gao, T.; Han, Z. Transcriptome Analyses Reveal Alterations in Muscle Metabolism, Immune Responses and Reproductive Behavior of Japanese Mantis Shrimp (Oratosquilla oratoria) at Different Cold Temperature. Comp. Biochem. Physiol.-Part D Genom. Proteom. 2019, 32, 100615. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tang, S.; Cai, F.; Lin, Y.; Wu, Z. Microsatellite Evidence of Dispersal Mechanism of Red Swamp Crayfish (Procambarus clarkii) in the Pearl River Basin and Implications for Its Management. Sci. Rep. 2017, 7, 8272. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture and Rural Affairs of China. China Fishery Statistical Yearbook; Ministry of Agriculture and Rural Affairs of China: Beijing, China, 2023; p. 34. (In Chinese) [Google Scholar]
- Li, H.T.; Zhou, W.Z.; Gao, H.L.; Zhang, G. Combined toxicity test of salinity and alkalinity on Procambarus clarkii. Aquaculture 2006, 27, 1. (In Chinese) [Google Scholar]
- Liu, D.L.; Huang, J.H.; Yang, L.S.; Tan, L.Q. Effects of multiple environmental factors on molting death of Procambarus clarkii and countermeasures. S. China Fish. Sci. 2020, 16, 29–35. (In Chinese) [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast All-in-One FASTQ Preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-Length Transcriptome Assembly from RNA-Seq Data without a Reference Genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and Quantifying Mammalian Transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, K.; Li, E.; Li, T.; Xu, C.; Wang, X.; Lin, H.; Qin, J.G.; Chen, L. Transcriptome and Molecular Pathway Analysis of the Hepatopancreas in the Pacific White Shrimp Litopenaeus vannamei under Chronic Low-Salinity Stress. PLoS ONE 2015, 10, 0131503. [Google Scholar] [CrossRef] [PubMed]
- Bissattini, A.M.; Traversetti, L.; Bellavia, G.; Scalici, M. Tolerance of Increasing Water Salinity in the Red Swamp Crayfish Procambarus clarkii (Girard, 1852). J. Crustac. Biol. 2015, 35, 682–685. [Google Scholar] [CrossRef]
- Luo, L.; Huang, J.H.; Liu, D.L.; Jiang, S.G.; Zhou, F.L.; Jiang, S.; Yang, Q.B.; Li, Y.D.; Li, T.; Tan, L.Q.; et al. Transcriptome Reveals the Important Role of Metabolic Imbalances, Immune Disorders and Apoptosis in the Treatment of Procambarus clarkii at Super High Temperature. Comp. Biochem. Physiol.-Part D Genom. Proteom. 2021, 37, 100781. [Google Scholar] [CrossRef]
- Ye, L.; Jiang, S.; Zhu, X.; Yang, Q.; Wen, W.; Wu, K. Effects of salinity on growth and energy budget of juvenile Penaeus monodon. Aquaculture 2009, 290, 140–144. [Google Scholar] [CrossRef]
- Luo, L.; Huang, J.H.; Liu, D.L.; Jiang, S.G.; Zhou, F.L.; Jiang, S.; Yang, Q.B.; Li, Y.D.; Li, T.; Tan, L.Q.; et al. Comparative Transcriptome Analysis of Differentially Expressed Genes and Pathways in Procambarus clarkii (Louisiana Crawfish) at Different Acute Temperature Stress. Genomics 2022, 114, 110415. [Google Scholar] [CrossRef]
- Wang, H.; Tang, L.; Wei, H.; Lu, J.; Mu, C.; Wang, C. Transcriptomic Analysis of Adaptive Mechanisms in Response to Sudden Salinity Drop in the Mud Crab, Scylla paramamosain. BMC Genom. 2018, 19, 421. [Google Scholar] [CrossRef]
- Lee, M.H.; Atkinson, S.; Murphy, G. Identification of the Extracellular Matrix (ECM) Binding Motifs of Tissue Inhibitor of Metalloproteinases (TIMP)-3 and Effective Transfer to TIMP-1. J. Biol. Chem. 2007, 282, 6887–6898. [Google Scholar] [CrossRef] [PubMed]
- Freedman, B.R.; Bade, N.D.; Riggin, C.N.; Zhang, S.; Haines, P.G.; Ong, K.L.; Janmey, P.A. The (Dys)Functional Extracellular Matrix. Biochim. Biophys. Acta-Mol. Cell Res. 2015, 1853, 3153–3164. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Wang, H.; Fan, C.; Sun, Y.; Li, J.; Cheng, J.; Chu, P.; Yin, S. Environmental Salinity Influences the Branchial Expression of TCR Pathway Related Genes Based on Transcriptome of a Catadromous Fish. Comp. Biochem. Physiol.-Part D Genom. Proteom. 2021, 38, 100815. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Shou, C.; Han, Z. Transcriptome Analysis of Marbled Rockfish Sebastiscus marmoratus under Salinity Stress. Animals 2023, 13, 400. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Richardson, B.C. Biological Effects of the MAPK Pathways. Lancet Oncol. 2005, 6, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.N.; Liu, Y.; Cai, D.X.; Li, J.Z.; Wang, A.L. Two Types of ATPases from the Pacific White Shrimp, Litopenaeus vannamei in Response to Environmental Stress. Mol. Biol. Rep. 2012, 39, 6427–6438. [Google Scholar] [CrossRef] [PubMed]
- Gutekunst, J.; Andriantsoa, R.; Falckenhayn, C.; Hanna, K.; Stein, W.; Rasamy, J.; Lyko, F. Clonal Genome Evolution and Rapid Invasive Spread of the Marbled Crayfish. Nat. Ecol. Evol. 2018, 2, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Ma, D.; Fan, J.; Zhong, Z.; Li, Y.; Zhu, H. Metabolism Response Mechanism in the Gill of Oreochromis Mossambicus under Salinity, Alkalinity and Saline-Alkalinity Stresses. Ecotoxicol. Environ. Saf. 2023, 251, 114523. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Hu, Y.; Lan, T.; Guan, K.L.; Luo, T.; Luo, M. The Hippo Signalling Pathway and Its Implications in Human Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 376. [Google Scholar] [CrossRef]
- Bray, S.J. Notch Signalling: A Simple Pathway Becomes Complex. Nat. Rev. Mol. Cell Biol. 2006, 7, 678–689. [Google Scholar] [CrossRef]
- Powis, G.; Phil, D. Inhibitors of Phosphatidylinositol Signalling as Antiproliferative Agents. Cancer Metastasis Rev. 1994, 13, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, K.M.; Connon, R.E.; Verhille, C.E.; Dabruzzi, T.F.; Britton, M.T.; Durbin-Johnson, B.P.; Fangue, N.A. Divergent Transcriptomic Signatures in Response to Salinity Exposure in Two Populations of an Estuarine Fish. Evol. Appl. 2019, 12, 1212–1226. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.L.; Wang, L.Y.; Zhao, X.L.; Yang, Y.S.; Ma, Q.; Chen, G. Effects of Salinity Acclimation on Histological Characteristics and MiRNA Expression Profiles of Scales in Juvenile Rainbow Trout (Oncorhynchus mykiss). BMC Genom. 2022, 23, 300. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Shi, X.; Guo, H.; Bai, Y.; Shen, C.; Zhang, Y.; Wang, Z. Comparative Transcriptome Analysis of the Gills of Procambarus clarkii Provides Novel Insights into the Immune-Related Mechanism of Copper Stress Tolerance. Fish Shellfish Immunol. 2020, 96, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Simon, M.; Zhao, Y.; Ablaeva, J.; Corson, N.; Choi, Y.; Yamada, K.L.Y.H.; Schork, N.J.; Hood, W.R.; Hill, G.E.; et al. Comparative Transcriptomics Reveals Circadian and Pluripotency Networks as Two Pillars of Longevity Regulation. Cell Metab. 2022, 34, 836–856.e5. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.l.; Cao, Z.h.; He, C.l.; Zhong, Y.c.; Liu, W.y.; Zhang, P.; Yang, F.; Xu, Y.j. Ferric Ion Induction of Triggering Receptor Expressed in Myeloid Cells-2 Expression and PI3K/Akt Signaling Pathway in Preosteoclast Cells to Promote Osteoclast Differentiation. Orthop. Surg. 2020, 12, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Prymaczok, N.C.; Pasqualino, V.M.; Viau, V.E.; Rodríguez, E.M.; Medesani, D.A. Involvement of the Crustacean Hyperglycemic Hormone (CHH) in the Physiological Compensation of the Freshwater Crayfish Cherax quadricarinatus to Low Temperature and High Salinity Stress. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2016, 186, 181–191. [Google Scholar] [CrossRef]
- Xu, Z.; Gan, L.; Li, T.; Xu, C.; Chen, K.; Wang, X.; Qin, J.G.; Chen, L.; Li, E. Transcriptome Profiling and Molecular Pathway Analysis of Genes in Association with Salinity Adaptation in Nile Tilapia Oreochromis niloticus. PLoS ONE 2015, 10, e0136506. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The Multifunctional Fish Gill: Dominant Site of Gas Exchange, Osmoregulation, Acid-Base Regulation, and Excretion of Nitrogenous Waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Velotta, J.P.; McCormick, S.D.; O’Neill, R.J.; Schultz, E.T. Relaxed Selection Causes Microevolution of Seawater Osmoregulation and Gene Expression in Landlocked Alewives. Oecologia 2014, 175, 1081–1092. [Google Scholar] [CrossRef]
- Arroyo, J.P.; Kahle, K.T.; Gamba, G. The SLC12 Family of Electroneutral Cation-Coupled Chloride Cotransporters. Mol. Asp. Med. 2013, 34, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ghishan, F.K.; Kiela, P.R. SLC9 Gene Family: Function, Expression, and Regulation. Compr. Physiol. 2018, 8, 555–583. [Google Scholar] [CrossRef] [PubMed]
- Khananshvili, D. The SLC8 Gene Family of Sodium-Calcium Exchangers (NCX)-Structure, Function, and Regulation in Health and Disease. Mol. Asp. Med. 2013, 34, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Remigante, A.; Spinelli, S.; Pusch, M.; Sarikas, A.; Morabito, R.; Marino, A.; Dossena, S. Role of SLC4 and SLC26 Solute Carriers during Oxidative Stress. Acta Physiol. 2022, 235, e13796. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.H.; Lui, E.Y.; Li, Z.; Cai, S.; Sung, W.K.; Mathavan, S.; Lam, T.J.; Ip, Y.K. Differential Transcriptomic Analyses Revealed Genes and Signaling Pathways Involved in Iono-Osmoregulation and Cellular Remodeling in the Gills of Euryhaline Mozambique Tilapia, Oreochromis mossambicus. BMC Genom. 2014, 15, 921. [Google Scholar] [CrossRef] [PubMed]
- Vallaeys, L.; Van Biervliet, S.; De Bruyn, G.; Loeys, B.; Moring, A.S.; Van Deynse, E.; Cornette, L. Congenital Glucose-Galactose Malabsorption: A Novel Deletion within the SLC5A1 Gene. Eur. J. Pediatr. 2013, 172, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Marshall, W.S. Mechanosensitive Signalling in Fish Gill and Other Ion Transporting Epithelia. Acta Physiol. 2011, 202, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Feeney, G.P.; Zheng, D.; Kille, P.; Hogstrand, C. The Phylogeny of Teleost ZIP and ZnT Zinc Transporters and Their Tissue Specific Expression and Response to Zinc in Zebrafish. Biochim. Biophys. Acta-Gene Struct. Expr. 2005, 1732, 88–95. [Google Scholar] [CrossRef]
- Voss, J.G. Effect of Inorganic Cations on Bactericidal Activity of Anionic. J. Bacteriol. 1963, 86, 207–211. [Google Scholar] [CrossRef]

| Sample | Raw Data | Clean Data (%) | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|
| NC-1 | 43,079,546 | 42,982,162 (99.77%) | 96.51% | 90.75% | 51.68% |
| NC-2 | 38,593,886 | 38,496,476 (99.75%) | 96.17% | 90.06% | 51.31% |
| NC-3 | 43,843,540 | 43,725,484 (99.73%) | 96.80% | 91.58% | 51.91% |
| LS-6h-1 | 44,961,252 | 44,810,234 (99.66%) | 96.41% | 90.82% | 51.87% |
| LS-6h-2 | 40,666,280 | 40,580,188 (99.79%) | 96.61% | 91.15% | 52.15% |
| LS-6h-3 | 45,471,462 | 45,335,054 (99.70%) | 96.56% | 91.11% | 50.69% |
| LS-24h-1 | 36,343,018 | 36,273,260 (99.81%) | 96.75% | 91.39% | 52.11% |
| LS-24h-2 | 37,333,288 | 37,262,908 (99.81%) | 96.61% | 91.05% | 51.67% |
| LS-24h-3 | 43,514,756 | 43,385,520 (99.70%) | 96.48% | 91.04% | 50.90% |
| LS-72h-1 | 41,937,250 | 41,860,300 (99.82%) | 96.72% | 91.26% | 50.97% |
| LS-72h-2 | 39,298,830 | 39,218,856 (99.80%) | 96.96% | 91.88% | 52.03% |
| LS-72h-3 | 39,176,456 | 39,097,798 (99.80%) | 96.30% | 90.59% | 52.02% |
| HS-6h-1 | 45,496,514 | 45,380,462 (99.74%) | 96.26% | 90.52% | 50.76% |
| HS-6h-2 | 37,971,054 | 37,894,032 (99.80%) | 96.69% | 91.36% | 53.46% |
| HS-6h-3 | 44,680,448 | 44,583,988 (99.78%) | 96.63% | 90.88% | 51.51% |
| HS-24h-1 | 40,967,824 | 40,872,802 (99.77%) | 96.24% | 90.51% | 52.03% |
| HS-24h-2 | 41,390,576 | 41,287,814 (99.75%) | 96.22% | 90.49% | 52.74% |
| HS-24h-3 | 46,509,192 | 46,401,214 (99.77%) | 98.00% | 94.48% | 52.18% |
| HS-72h-1 | 41,216,070 | 41,140,894 (99.82%) | 96.42% | 90.79% | 52.28% |
| HS-72h-2 | 48,341,970 | 48,254,082 (99.82%) | 96.89% | 91.75% | 52.41% |
| HS-72h-3 | 43,796,998 | 43,709,326 (99.80%) | 96.70% | 91.35% | 52.01% |
| Genes Number | GC (%) | N50 Number | N50 Length | Max Length | Min Length | Average Length | Total Bases |
|---|---|---|---|---|---|---|---|
| 52,533 | 43.18 | 8596 | 1688 | 32,394 | 201 | 1000 | 52,553,090 |
| Number | Symbol | Description | Rpkm (Mean) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NC | LS- 6h | LS- 24h | LS- 72h | HS- 6h | HS- 24h | HS- 72h | |||
| 1 | SLC41A1 | Solute carrier family 41 member 1 (Magnesium ion transporter) | 1.21 | 1.11 | 1.96 | 0.96 | 1.12 | 3.69 | 0.83 |
| 2 | SLC40A1 | Solute carrier family 40 member 1 (Iron ion transporter) | 1.50 | 0.76 | 0.39 | 0.86 | 0.89 | 0.61 | 0.68 |
| 3 | SLC39A9 | Solute carrier family 39 member 9 (Zinc transporter ZIP9) | 9.99 | 11.04 | 11.65 | 11.11 | 12.65 | 12.88 | 13.12 |
| 4 | SLC39A11 | Solute carrier family 39 member 11 (Zinc transporter ZIP11) | 6.87 | 7.49 | 8.40 | 8.30 | 8.70 | 8.62 | 6.25 |
| 5 | SLC39A1 | Solute carrier family 39 member 1 (Zinc transporter ZIP1) | 169.84 | 96.51 | 63.39 | 68.11 | 90.07 | 62.21 | 83.81 |
| 6 | SLC30A9 | Solute carrier family 30 member 9 (Zinc transporter 9) | 1.37 | 1.12 | 1.24 | 1.58 | 1.24 | 1.10 | 1.10 |
| 7 | SLC30A7 | Solute carrier family 30 member 7 (Zinc transporter 7) | 1.96 | 1.11 | 1.14 | 1.67 | 1.46 | 2.03 | 1.44 |
| 8 | SLC30A6 | Solute carrier family 30 member 6 (Zinc transporter 6) | 0.89 | 1.05 | 1.06 | 0.94 | 1.11 | 1.38 | 0.93 |
| 9 | SLC30A5 | Solute carrier family 30 member 5 (Zinc transporter 5) | 0.85 | 1.20 | 1.20 | 0.94 | 1.31 | 1.30 | 1.36 |
| 10 | SLC30A2 | Solute carrier family 30 member 2 (Zinc transporter 2) | 14.44 | 12.71 | 10.37 | 12.75 | 14.66 | 13.42 | 9.99 |
| 11 | SLC30A1 | Solute carrier family 30 member 1 (Zinc transporter 1) | 2.88 | 2.39 | 1.52 | 1.82 | 2.47 | 1.63 | 1.70 |
| 12 | SLC26A6 | Solute carrier family 26 member 6 (chloride-bicarbonate exchanger) | 2.50 | 2.82 | 2.77 | 2.97 | 4.08 | 4.83 | 2.83 |
| 13 | SLC26A11 | Solute carrier family 26 member 11 (chloride-bicarbonate exchanger) | 1.89 | 2.31 | 2.34 | 2.35 | 3.34 | 2.39 | 1.55 |
| 14 | SLC22A8 | Solute carrier family 22 member 8 (Organic cation transporter) | 9.04 | 10.59 | 12.87 | 8.80 | 11.79 | 4.89 | 11.21 |
| 15 | SLC22A6 | Solute carrier family 22 member 6 (Organic cation transporter) | 22.11 | 24.18 | 14.19 | 15.98 | 17.75 | 19.86 | 14.23 |
| 16 | SLC22A5 | Solute carrier family 22 member 5 (Organic cation transporter) | 0.79 | 0.86 | 0.76 | 0.63 | 0.68 | 0.54 | 0.47 |
| 17 | SLC22A3 | Solute carrier family 22 member 3 (Organic cation transporter) | 8.86 | 11.13 | 8.70 | 6.63 | 9.70 | 12.58 | 10.05 |
| 18 | SLC22A15B | Solute carrier family 22 member 15B (Organic ion transporter) | 11.15 | 11.08 | 9.40 | 11.31 | 11.34 | 10.87 | 10.04 |
| 19 | SLC13A5 | Solute carrier family 13 member 5 (Na+-dependent carboxylate and sulfate transporter) | 15.67 | 12.67 | 9.84 | 13.95 | 11.28 | 34.18 | 15.09 |
| 20 | SLC12A9 | Solute carrier family 12 member 9 (Cation-coupled Cl− cotransporter) | 10.11 | 9.59 | 10.29 | 9.13 | 11.05 | 8.53 | 6.02 |
| 21 | SLC12A6 | Solute carrier family 12 member 6 (Cation-coupled Cl− cotransporter) | 5.00 | 4.57 | 8.13 | 5.15 | 7.42 | 5.91 | 4.65 |
| 22 | SLC12A2 | Solute carrier family 12 member 2 (Na+/K+/2Cl− cotransporter) | 13.86 | 25.66 | 17.36 | 11.79 | 17.32 | 74.29 | 26.06 |
| 23 | SLC10A6 | Solute carrier family 10 member 6 (Ileal sodium/bile acid cotransporter) | 56.69 | 37.14 | 40.56 | 46.31 | 43.40 | 16.17 | 27.24 |
| 24 | SLC10A3 | Solute carrier family 10 member 3 (Ileal sodium/bile acid cotransporter) | 3.08 | 2.52 | 3.42 | 1.81 | 2.65 | 2.11 | 1.50 |
| 25 | SLC10A2 | Solute carrier family 10 member 2 (Sodium/bile acid cotransporter) | 51.12 | 89.37 | 36.31 | 50.32 | 54.28 | 34.86 | 36.86 |
| 26 | SLC9A8 | Solute carrier family 9 member 8 (Sodium/hydrogen exchanger 8) | 1.12 | 1.71 | 1.74 | 1.75 | 1.84 | 3.16 | 1.95 |
| 27 | SLC9A9 | Solute carrier family 9 member 9 (Sodium/hydrogen exchanger 9) | 2.64 | 2.88 | 3.02 | 2.51 | 3.28 | 4.20 | 2.28 |
| 28 | SLC9A3R1 | Solute carrier family 9 member 3 regulator 1 (Na+/H+exchange regulatory cofactor NHE-RF1) | 29.13 | 40.50 | 32.40 | 28.00 | 37.29 | 58.53 | 32.90 |
| 29 | SLC9A2 | Solute carrier family 9 member 2 (Na+/H+ exchanger) | 0.13 | 0.34 | 0.27 | 0.16 | 0.54 | 5.04 | 0.56 |
| 30 | SLC8A2 | Solute carrier family 8 member 2 (Sodium-calcium exchanger) | 0.68 | 1.60 | 1.72 | 1.22 | 2.14 | 4.33 | 1.48 |
| 31 | SLC8A1 | Solute carrier family 8 member 1 (Sodium-calcium exchanger) | 0.31 | 0.63 | 0.38 | 0.62 | 0.46 | 0.50 | 0.46 |
| 32 | SLC5A8 | Solute carrier family 5 member 8 (Sodium-dependent vitamin transporter) | 2.32 | 2.59 | 2.65 | 2.35 | 2.78 | 3.90 | 1.77 |
| 33 | SLC5A3 | Solute carrier family 5 member 3 (Sodium/myo-inositol cotransporter) | 5.42 | 10.56 | 8.26 | 7.93 | 11.63 | 12.35 | 8.77 |
| 34 | SLC5A1 | Solute carrier family 5 member 1 (Sodium/glucose cotransporter) | 23.56 | 227.19 | 181.73 | 82.43 | 160.33 | 283.00 | 110.62 |
| 35 | SLC4A1 | Solute carrier family 4 member 1 (Sodium bicarbonate cotransporter) | 1.68 | 1.79 | 1.72 | 1.50 | 1.84 | 1.56 | 1.00 |
| 36 | SLC4A10 | Solute carrier family 4 member 10 (Sodium bicarbonate cotransporter) | 2.04 | 2.40 | 2.23 | 1.81 | 2.60 | 1.29 | 1.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, L.; Yang, L.-S.; Huang, J.-H.; Jiang, S.-G.; Zhou, F.-L.; Li, Y.-D.; Jiang, S.; Yang, Q.-B. Effects of Different Salinity Stress on the Transcriptomic Responses of Freshwater Crayfish (Procambarus clarkii, Girard, 1852). Biology 2024, 13, 530. https://doi.org/10.3390/biology13070530
Luo L, Yang L-S, Huang J-H, Jiang S-G, Zhou F-L, Li Y-D, Jiang S, Yang Q-B. Effects of Different Salinity Stress on the Transcriptomic Responses of Freshwater Crayfish (Procambarus clarkii, Girard, 1852). Biology. 2024; 13(7):530. https://doi.org/10.3390/biology13070530
Chicago/Turabian StyleLuo, Lei, Li-Shi Yang, Jian-Hua Huang, Shi-Gui Jiang, Fa-Lin Zhou, Yun-Dong Li, Song Jiang, and Qi-Bin Yang. 2024. "Effects of Different Salinity Stress on the Transcriptomic Responses of Freshwater Crayfish (Procambarus clarkii, Girard, 1852)" Biology 13, no. 7: 530. https://doi.org/10.3390/biology13070530
APA StyleLuo, L., Yang, L.-S., Huang, J.-H., Jiang, S.-G., Zhou, F.-L., Li, Y.-D., Jiang, S., & Yang, Q.-B. (2024). Effects of Different Salinity Stress on the Transcriptomic Responses of Freshwater Crayfish (Procambarus clarkii, Girard, 1852). Biology, 13(7), 530. https://doi.org/10.3390/biology13070530






