RETRACTED: Using Stable Isotope Techniques to Analyze the Trophic Relationship between Argentine Hake (Merluccius hubbsi) and Anisakidae
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection Time and Locations
2.2. Gonadal Maturity Identification
- Stage I (Immature): Small, thin, and thread-like gonads with no visible differentiation;
- Stage II (Developing): Enlargement of gonads with visible oocytes in females and whitish testes in males;
- Stage III (Mature): Fully developed gonads with larger ovaries containing visible eggs in females and enlarged, whitish testes in males;
- Stage IV (Spawning): Maximum-size gonads with ovaries filled with mature eggs in females and large, milky testes in males.
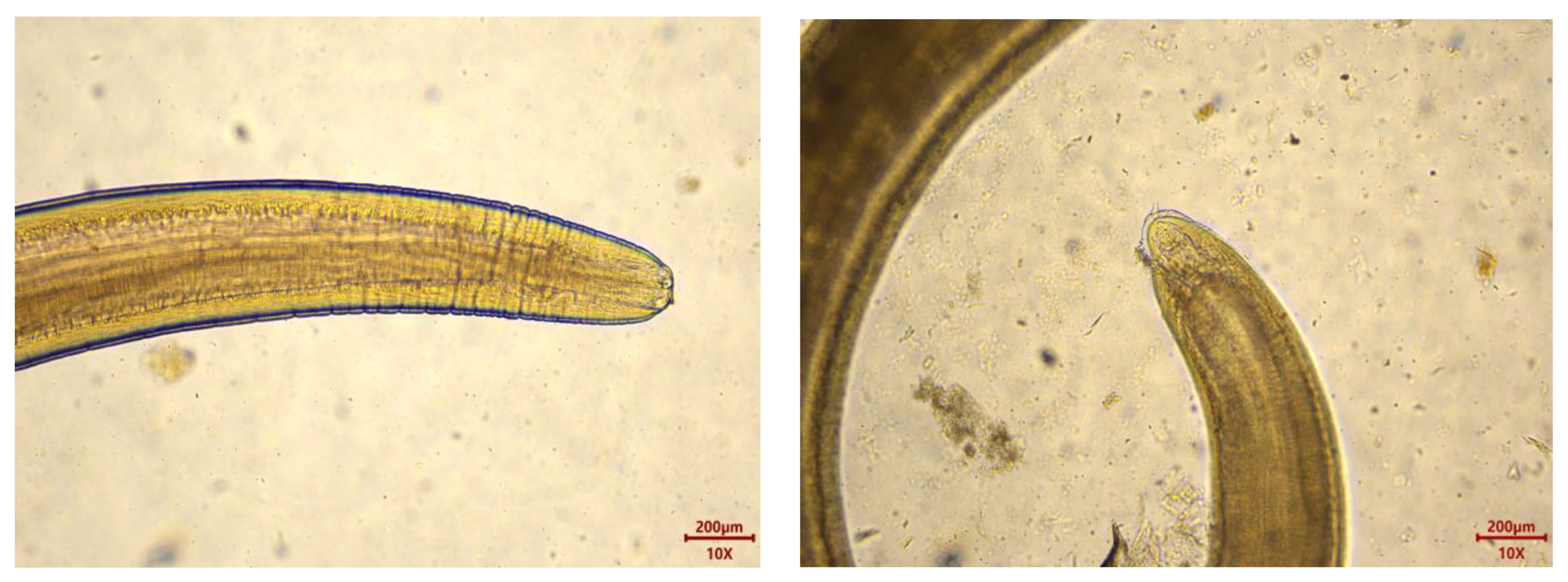
2.3. Collection and Identification of Parasites
2.4. Stable Isotope Analysis
2.5. Trophic Niche Calculation
- CR: This is the range of δ13C values within a sample, representing the difference between the maximum and minimum δ13C values. The δ13C values indicate the carbon sources consumed by an organism. A larger carbon range suggests a higher diversity of carbon sources, indicating a more varied diet and diverse nutritional resources within the population or group of individuals [20];
- NR: This is the range of δ15N values within a sample, representing the difference between the maximum and minimum δ15N values. The δ15N values reflect the trophic level at which the organism is feeding. A larger nitrogen range indicates a wider range of trophic levels within the population or group, reflecting dietary diversity [20];
- TA: This is the area of the convex hull encompassing the δ13C and δ15N values on a bivariate plot. TA represents the overall extent of trophic niches utilized by the population or group. A larger total area indicates a broader use of nutritional resources and trophic positions, suggesting higher ecological diversity and adaptability [21];
- SEA: SEA is the area of an ellipse plotted using δ13C and δ15N values that contains approximately 40% of the data points. It is a standardized measure that reduces the influence of outliers on the total area. A larger SEA indicates a broader trophic niche, demonstrating greater nutritional diversity and a variety of trophic positions within the population or group [22];
- SEAc: SEAc is the sample-size-corrected version of SEA. This correction adjusts SEA based on the sample size, allowing for more reliable and accurate comparisons between groups with different sample sizes. A larger SEAc similarly indicates a broader trophic niche and higher nutritional diversity [22].
2.6. Calculation of Trophic Discrimination Factor (ΔTDF)
2.7. Data Processing
3. Results
3.1. Biological Characteristics of M. hubbsi
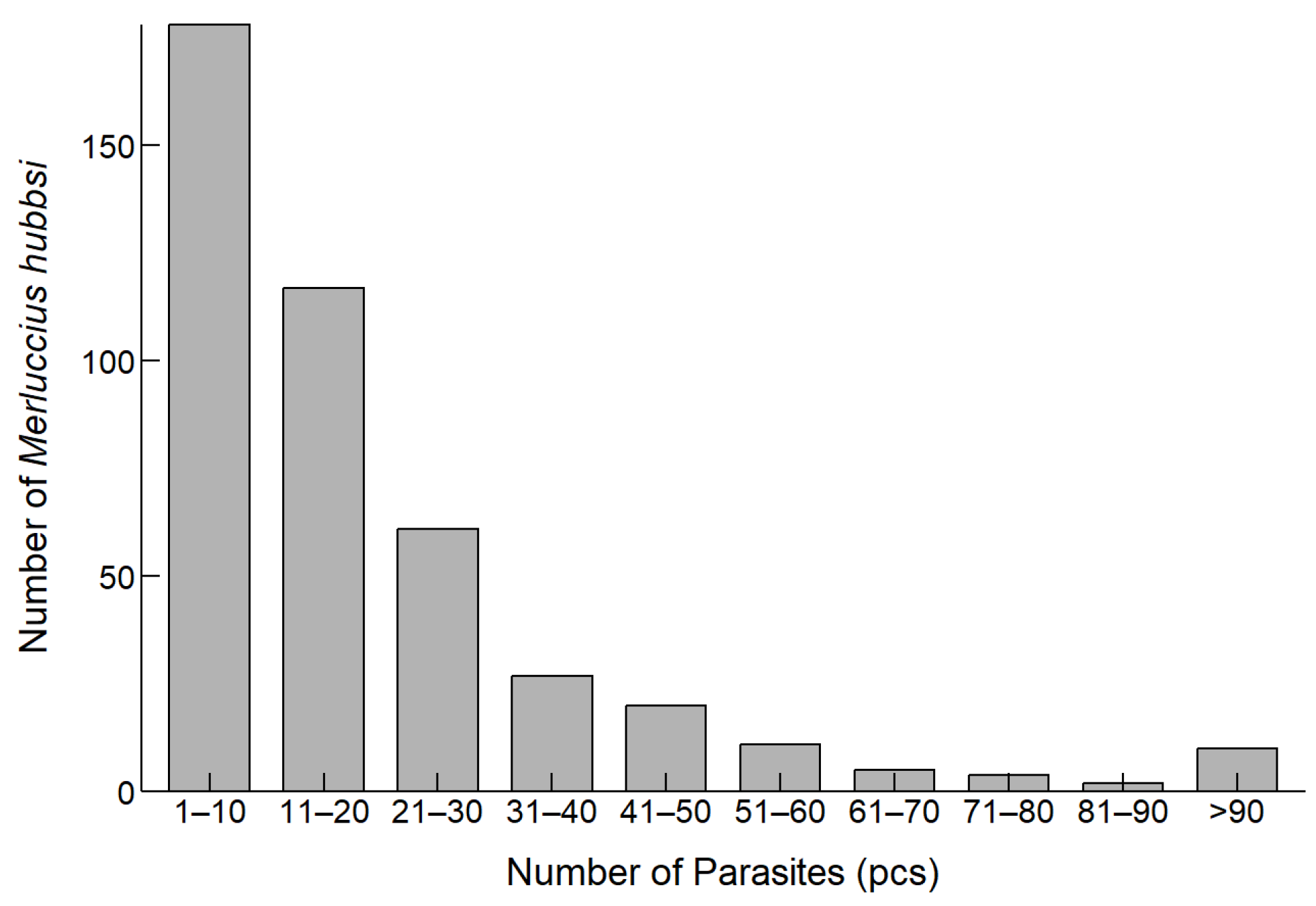
3.2. Parasite Infection in M. hubbsi
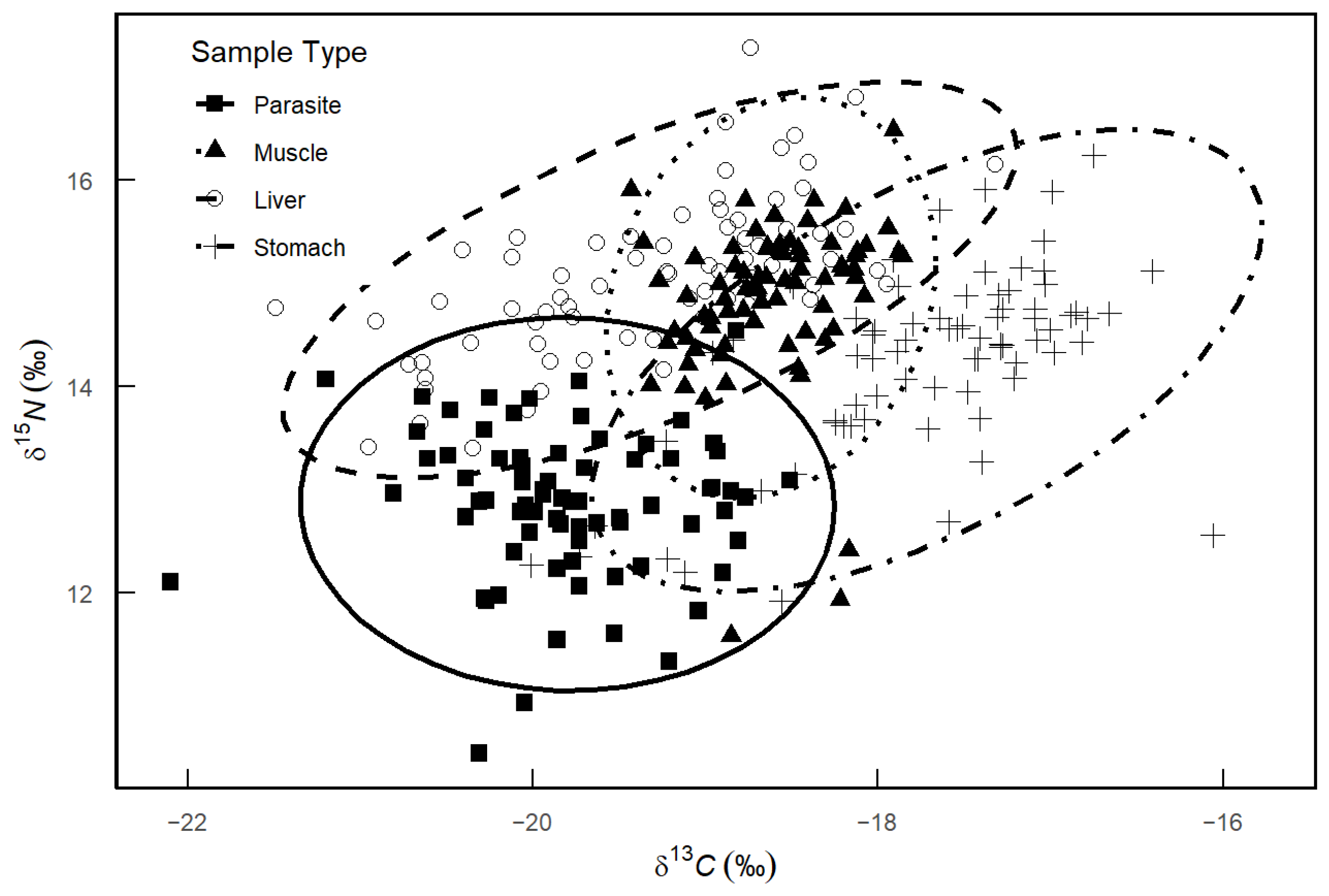
3.3. Stable Isotope Composition
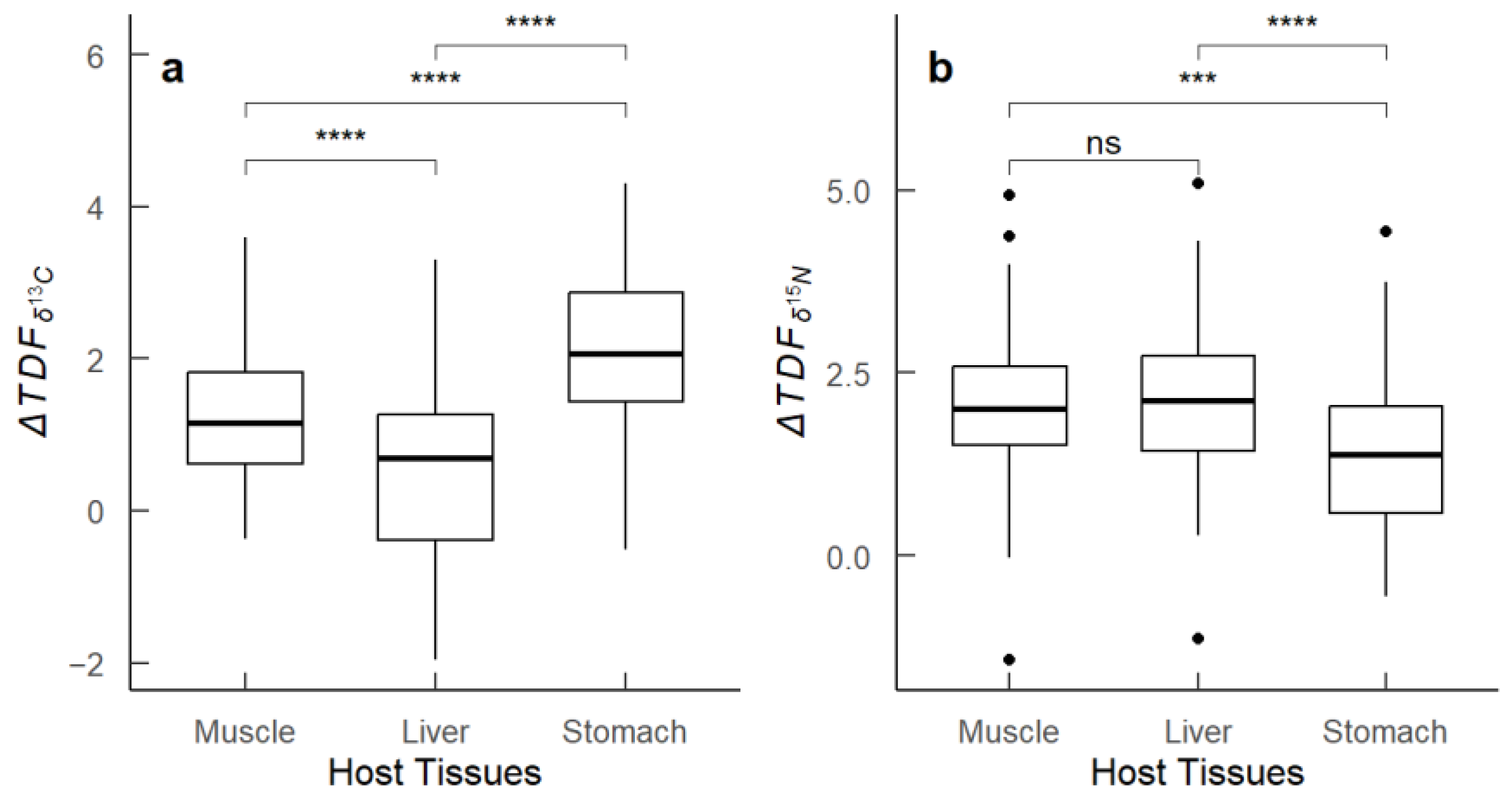
3.4. ΔTDF and Correlation Analysis
4. Discussion
4.1. Infection Status of M. hubbsi by Anisakidae
4.2. Stable Isotope Composition and Nutritional Niche Indicators
4.3. Stable Isotope Fractionation and Correlation Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macchi, G.J.; Colombo, G.Á.; Aubone, A.; Belleggia, M.; Cordo, H.; de la Garza, J.; Diodato, S.; Echave, M.; Hernandez, D.; Herrero, M. Recruitment of the Argentine hake, Merluccius hubbsi, from Patagonian stock: A review of main features affecting the reproductive potential and survival during early life stages. Mar. Fish. Sci. 2023, 36, 1–36. [Google Scholar]
- International Union of Pure and Applied Chemistry Home Page. Available online: http://www.iupac.org/dhtml_home.html (accessed on 14 November 2023).
- Costa, P.A.S.; Braga, A.C.; Malavolti, G.S.; Vieira, J.P.; Coelho, L.C.; Martins, A.S.; Rodrigues, S.C.; Tavares, M. Feeding habits and trophic status of Merluccius hubbsi along the northernmost limit of its distribution in the South-western Atlantic. J. Mar. Biol. Assoc. U. K. 2019, 99, 1399–1408. [Google Scholar] [CrossRef]
- Diaz, M.V.; Olivar, M.P.; Macchi, G.J. Larval condition of Merluccius hubbsi (Marini, 1933) in the northern Patagonian spawning ground. Fish. Res. 2014, 160, 60–68. [Google Scholar] [CrossRef]
- Bas, M.; Salemme, M.; Green, E.J.; Santiago, F.; Speller, C. Predicting habitat use by the Argentine hake Merluccius hubbsi in a warmer world: Inferences from the Middle Holocene. Oecologia 2020, 193, 461–474. [Google Scholar] [CrossRef] [PubMed]
- Mehrdana, F.; Buchmann, K. Excretory/secretory products of anisakid nematodes: Biological and pathological roles. Acta Vet. Scand. 2017, 59, 42. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.J.; Castro, R.; Cavaleiro, F.; Rangel, L. Comparison of anisakid infection levels between two species of Atlantic mackerel (Scomber colias and S. scombrus) off the Atlantic Portuguese coast. Sci. Mar. 2017, 81, 179–185. [Google Scholar] [CrossRef]
- Cai, W.W.; Lin, C.X.; Zheng, D.; Xie, H.G. Prevalence of anisakise infections in marine fishes in eastern Fujian fishing ground of Fujian Province. Chin. J. Schistosomiasis Control 2023, 35, 78–81. [Google Scholar]
- Hemmingsen, W.; MacKenzie, K.; Curtis, M.A. Growth of Atlantic cod Gadus morhua L. infected with anisakid larvae. J. Fish Biol. 2005, 66, 731–742. [Google Scholar]
- Levsen, A.; Berland, B.; Svendsen, E. Anisakis simplex third stage larvae in the flesh of locally caught cod. Dis. Aquat. Organ. 2008, 81, 37–46. [Google Scholar]
- Scholz, T.; Garcia-Prieto, L.; Vidal-Martinez, V.M. Parasites as biological tags for stock discrimination of marine fish: A brief review. Folia Parasitol. 2001, 48, 161–163. [Google Scholar]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Kanaya, G.; Solovyev, M.M.; Shikano, S.; Okano, J.; Kikuchi, E.; Doi, H. Application of stable isotopic analyses for fish host–parasite systems: An evaluation tool for parasite-mediated material flow in aquatic ecosystems. Aquat. Ecol. 2019, 53, 217–232. [Google Scholar] [CrossRef]
- Pinnegar, J.K.; Campbell, N.; Polunin, N.V.C. Unusual stable isotope fractionation patterns observed for fish host–parasite trophic relationships. J. Fish Biol. 2001, 59, 494–503. [Google Scholar]
- Porter, M.; Barton, D.P.; Shamsi, S.; Noga, E.J.; Nowak, B.F. Deciphering the complex trophic relationship of the black-spotted croaker (Teleostei: Sciaenidae) and its parasites using stable isotope analysis. Can. J. Zool. 2023, 101, 385–392. [Google Scholar] [CrossRef]
- Honj, R.M.; Vaz-dos-Santos, A.M.; Rossi-Wongtschowsk, C.L.D.B. Identification of the stages of ovarian maturation of the Argentine hake Merluccius hubbsi Marini, 1933 (Teleostei: Merlucciidae): Advantages and disadvantages of the use of the macroscopic and microscopic scales. Neotrop. Ichthyol. 2006, 4, 329–337. [Google Scholar] [CrossRef]
- Li, D.; Ji, F.Y.; Wang, L.J.; Wang, Y.; Li, Y.; Yan, G.; Xu, Y.; Bu, X. Anisakis infection in marine fishes and resident awareness of anisakiasis in Qingdao in 2021. Chin. J. Parasitol. Parasit. Dis. 2023, 41, 52–58. [Google Scholar]
- Wu, S.Q. Aquatic Animal Parasitology; China Agriculture Press: Beijing, China, 2015. [Google Scholar]
- Zhao, Z.F.; Hu, G.Y.; Chen, X.J. Studies on monthly difference of fatty acid composition and dietary indicator of Dosidicus gigas in offshore waters of Peru. Trans. Oceanol. Limnol. 2022, 44, 98–105. [Google Scholar]
- Layman, C.A.; Arrington, D.A.; Montana, C.G.; Post, D.M. Can stable isotope ratios provide quantitative measures of trophic diversity within food webs? Ecology 2007, 88, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.X. Stable Isotope-Based Community Trophic Structure of Fishery Organisms in the Offshore Waters of Central and Southern Zhejiang. Doctoral Dissertation, Shanghai Ocean University, Shanghai, China, 2020. [Google Scholar]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Piola, A.R.; Rivas, A.L. Currents of the continental shelf. El Mar Argent. Y Sus Recur. Pesq. 1997, 1, 119–132. [Google Scholar]
- Timi, J.T.; Paoletti, M.; Cimmaruta, R.; Lanfranchi, A.L.; Alarcos, A.J.; Garbin, L.; González, A. Molecular identification, morphological characterization and new insights into the ecology of larval Pseudoterranova cattani in fishes from the Argentine coast with its differentiation from the Antarctic species, P. decipiens sp. E (Nematoda: Anisakidae). Vet. Parasitol. 2014, 199, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Cantatore, D.M.P.; Timi, J.T. Marine parasites as biological tags in South American Atlantic waters, current status and perspectives. Parasitology 2015, 142, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, K.; Mehrdana, F. Effects of anisakid nematodes Anisakis simplex (s.l.), Pseudoterranova decipiens (s.l.) and Contracaecum osculatum (s.l.) on fish and consumer health. Food Waterborne Parasitol. 2016, 4, 13–22. [Google Scholar] [CrossRef]
- Klemme, I.; Debes, P.V.; Primmer, C.R.; Vasemägi, A. Host developmental stage effects on parasite resistance and tolerance. Am. Nat. 2022, 200, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Chaves-Pozo, E.; Cabas, I.; García-Ayala, A. Sex steroids modulate fish immune response. In Sex Steroids; InTech: Rijeka, Croatia, 2012; pp. 199–220. [Google Scholar]
- Valero, A.; García-Lavia, T.; Navarro, M.C. Spatial distribution of Anisakis simplex s.l. in European hake (Merluccius merluccius) from the North-east Atlantic. J. Fish Biol. 2006, 69, 104–113. [Google Scholar]
- Llarena-Reino, M.; Abollo, E.; Pascual, S. Intra-individual anatomical distribution of Anisakis simplex s.s. and Anisakis pegreffii larvae in fresh fish. Int. J. Food Microbiol. 2013, 167, 269–276. [Google Scholar]
- D’Amelio, S.; Mathiopoulos, K.D.; Santos, C.P.; Pugachev, O.N.; Webb, S.C.; Picanco, M.; Paggi, L. Genetic markers in ribosomal DNA for the identification of members of the genus Anisakis (Nematoda: Ascaridoidea). J. Parasitol. 1997, 83, 197–201. [Google Scholar]
- DeNiro, M.J.; Epstein, S. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 1978, 42, 495–506. [Google Scholar] [CrossRef]
- Poulin, R.; Thomas, F. The distribution of parasite burden among host populations: The role of immune function. Parasitology 1999, 119, 405–411. [Google Scholar]
- Dalton, J.P.; Skelly, P.; Halton, D.W. Role of the tegument and gut in nutrient uptake by parasitic platyhelminths. Can. J. Zool. 2004, 82, 211–232. [Google Scholar] [CrossRef]
- Saunders, E.C.; de Souza, D.P.; Chambers, J.M.; Sernee, M.F.; Ralton, J.E.; Doyle, M.A.; Macrae, J.I.; McConville, M.J. Use of 13C stable isotope labelling for pathway and metabolic flux analysis in Leishmania parasites. In Parasite Genomics Protocols; Springer: Berlin/Heidelberg, Germany, 2015; pp. 281–296. [Google Scholar]
- Krishnan, A.; Soldati-Favre, D. Amino acid metabolism in apicomplexan parasites. Metabolites 2021, 11, 61. [Google Scholar] [CrossRef]
- Lafferty, K.D.; Kuris, A.M. Trophic strategies, animal diversity and body size. Trends Ecol. Evol. 2002, 17, 507–513. [Google Scholar] [CrossRef]
- Chang, J.C.H.; Wu, S.M.; Tseng, Y.C.; Lee, Y.C.; Lee, M.F.; Hwang, P.P. Regulation of glycogen metabolism in gills and liver of the euryhaline tilapia (Oreochromis mossambicus) during acclimation to seawater. J. Exp. Biol. 2007, 210, 3494–3504. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.P.; Chang, H.; Ni, Y.Y.; Li, C.; Chen, L.; Hou, M.; Ji, M.J. Schistosoma japonicum infection causes a reprogramming of glycolipid metabolism in the liver. Parasites Vectors 2019, 12, 388. [Google Scholar] [CrossRef]
- Wilkie, M.P.; Claude, J.F.; Cockshutt, A.; Wood, C.M.; Wang, Y.S. Shifting patterns of nitrogen excretion and amino acid catabolism capacity during the life cycle of the sea lamprey (Petromyzon marinus). Physiol. Biochem. Zool. 2006, 79, 885–898. [Google Scholar] [CrossRef]
- Marcogliese, D.J.; Cone, D.K. Food Web Interactions and Parasites in Freshwater Systems; Springer: Berlin/Heidelberg, Germany, 1997; pp. 197–221. [Google Scholar]
- Beninger, P.G.; Le Pennec, G.; Le Pennec, M. Demonstration of nutrient pathway from the digestive system to oocytes in the gonad intestinal loop of the scallop Pecten maximus L. Biol. Bull. 2003, 205, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Pulkkinen, K.; Aalto, S.L.; Nykänen, H. Parasite infection alters host stable-isotope composition under controlled feeding. Freshw. Biol. 2016, 61, 1981–1990. [Google Scholar] [CrossRef]
- Yohannes, E.; Grimm, C.; Rothhaupt, K.O.; Boix-Hinzen, C.; Traugott, M. The effect of parasite infection on stable isotope turnover rates of δ15N, δ13C and δ34S in multiple tissues of Eurasian perch (Perca fluviatilis). PLoS ONE 2017, 12, e0169058. [Google Scholar] [CrossRef]
- Scharnweber, K.; Andersson, M.L.; Chaguaceda, F.; Mehner, T. Intraspecific differences in metabolic rates shape carbon stable isotope trophic discrimination factors of muscle tissue in the common teleost Eurasian perch (Perca fluviatilis). Ecol. Evol. 2021, 11, 9804–9814. [Google Scholar] [CrossRef]
- Kamiya, E.; Urabe, M.; Okuda, N. Does atypical 15N and 13C enrichment in parasites result from isotope ratio variation of host tissues they are infected? Limnology 2020, 21, 139–149. [Google Scholar] [CrossRef]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Milovanović, I.; Busarčević, M.; Trbovich, A.; Ivović, V.; Vasilev, D.; Klun, I. Evidence for host genetic regulation of altered lipid metabolism in experimental toxoplasmosis supported with gene data mining results. PLoS ONE 2017, 12, e0176700. [Google Scholar] [CrossRef] [PubMed]
- Doi, H.; Yurlova, N.I.; Vodyanitskaya, S.N.; Kikuchi, E.; Shikano, S. Parasite-induced changes in nitrogen isotope signatures of host tissues. J. Parasitol. 2008, 94, 292–295. [Google Scholar] [CrossRef] [PubMed]

| Sampling Date | Coordinates (Longitude, Latitude) | Sample Size (n) | Length Range (mm) | Weight Range (g) |
|---|---|---|---|---|
| 26 October 2022 | 60°09′ W, 45°53′ S | 102 | 237–560 | 100–1370 |
| 17 November 2022 | 60°10′ W, 45°57′ S | 89 | 217–495 | 115–1258 |
| 16 December 2022 | 60°45′ W, 45°33′ S | 99 | 233–442 | 85–945 |
| 13 January 2023 | 60°30′ W, 45°19′ S | 70 | 239–430 | 121–1141 |
| 2 February 2023 | 60°38′ W, 45°41′ S | 75 | 230–521 | 130–1244 |
| Gonad Maturity | Sample Size (n) | Parasite Count (Mean ± SD) (pcs.) |
|---|---|---|
| I | 46 | 9.89 ± 10.67 |
| II | 241 | 13.83 ± 14.74 |
| III | 115 | 25.86 ± 22.17 |
| IV | 33 | 50.61 ± 56.78 |
| Sample Type | CR | NR | TA | SEA | SEAc |
|---|---|---|---|---|---|
| Parasite | 3.88 | 4.17 | 9.38 | 1.41 | 1.43 |
| Muscle from M. hubbsi | 4.9 | 1.57 | 5.44 | 0.92 | 0.93 |
| Liver from M. hubbsi | 4.32 | 3.95 | 8.27 | 1.54 | 1.56 |
| Stomach from M. hubbsi | 4.09 | 3.59 | 10.22 | 1.78 | 1.80 |
| Gonad Maturity | Sample Size (n) | (Host Muscle–Anisakidae) | (Host Muscle–Anisakidae) | (Host Liver–Anisakidae) | (Host Liver–Anisakidae) | (Host Stomach–Anisakidae) | (Host Stomach–Anisakidae) |
|---|---|---|---|---|---|---|---|
| I | 4 | 2.33 ± 0.94 | 1.82 ± 0.51 | 1.96 ± 0.78 | 2.08 ± 0.71 | 3.33 ± 0.62 | 1.56 ± 0.89 |
| II | 30 | 1.49 ± 0.78 | 1.82 ± 1.80 | 0.97 ± 1.12 | 2.26 ± 0.89 | 2.48 ± 1.05 | 1.31 ± 1.09 |
| III | 26 | 0.96 ± 0.65 | 2.09 ± 1.17 | 0.11 ± 0.98 | 2.22 ± 1.05 | 1.83 ± 0.87 | 1.52 ± 0.92 |
| IV | 15 | 0.96 ± 0.76 | 2.04 ± 1.03 | 0.08 ± 1.10 | 1.96 ± 1.30 | 1.73 ± 1.18 | 1.28 ± 1.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shu, Y.; Wu, F.; Fang, Z. RETRACTED: Using Stable Isotope Techniques to Analyze the Trophic Relationship between Argentine Hake (Merluccius hubbsi) and Anisakidae. Biology 2024, 13, 515. https://doi.org/10.3390/biology13070515
Shu Y, Wu F, Fang Z. RETRACTED: Using Stable Isotope Techniques to Analyze the Trophic Relationship between Argentine Hake (Merluccius hubbsi) and Anisakidae. Biology. 2024; 13(7):515. https://doi.org/10.3390/biology13070515
Chicago/Turabian StyleShu, Yue, Feiyu Wu, and Zhou Fang. 2024. "RETRACTED: Using Stable Isotope Techniques to Analyze the Trophic Relationship between Argentine Hake (Merluccius hubbsi) and Anisakidae" Biology 13, no. 7: 515. https://doi.org/10.3390/biology13070515
APA StyleShu, Y., Wu, F., & Fang, Z. (2024). RETRACTED: Using Stable Isotope Techniques to Analyze the Trophic Relationship between Argentine Hake (Merluccius hubbsi) and Anisakidae. Biology, 13(7), 515. https://doi.org/10.3390/biology13070515






