Anti-Obesity Effects Evaluation of a Blackcurrant Leaf Standardized Hydro-Alcoholic Extract in Wistar Rat Subjected to a High-Fat Diet
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Blackcurrant Extract
2.2. Experimental Animals
2.3. Experimental Procedure
2.4. Biochemical Parameters
2.4.1. Oral Glucose Tolerance Test (OGTT)
2.4.2. Determination of Plasma Insulin, Adiponectin and Leptin
2.4.3. Lipid Profile Analysis in Rat Plasma
2.4.4. Oxidant Parameters
2.4.5. Short-Chain Fatty Acid Determination
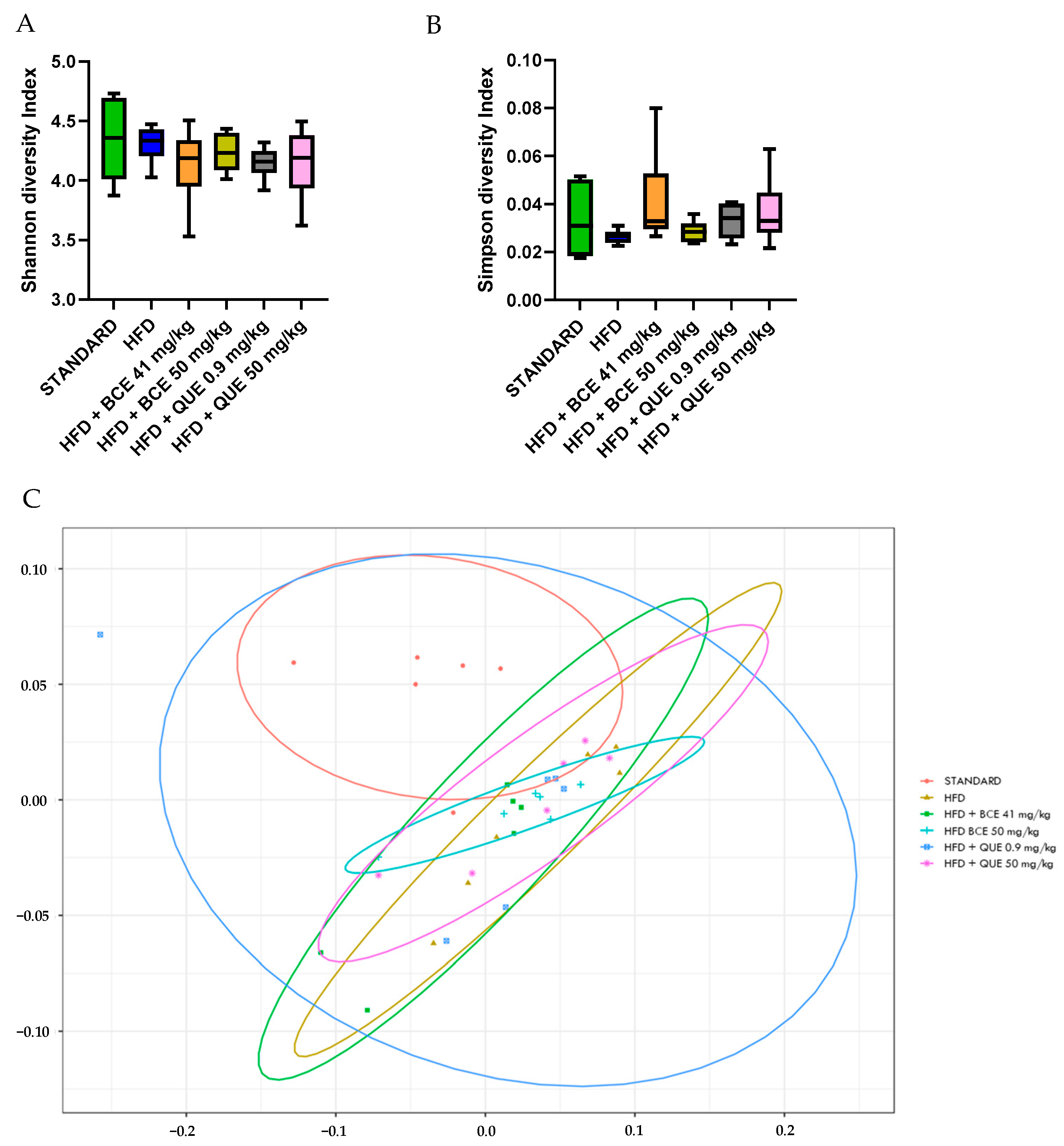
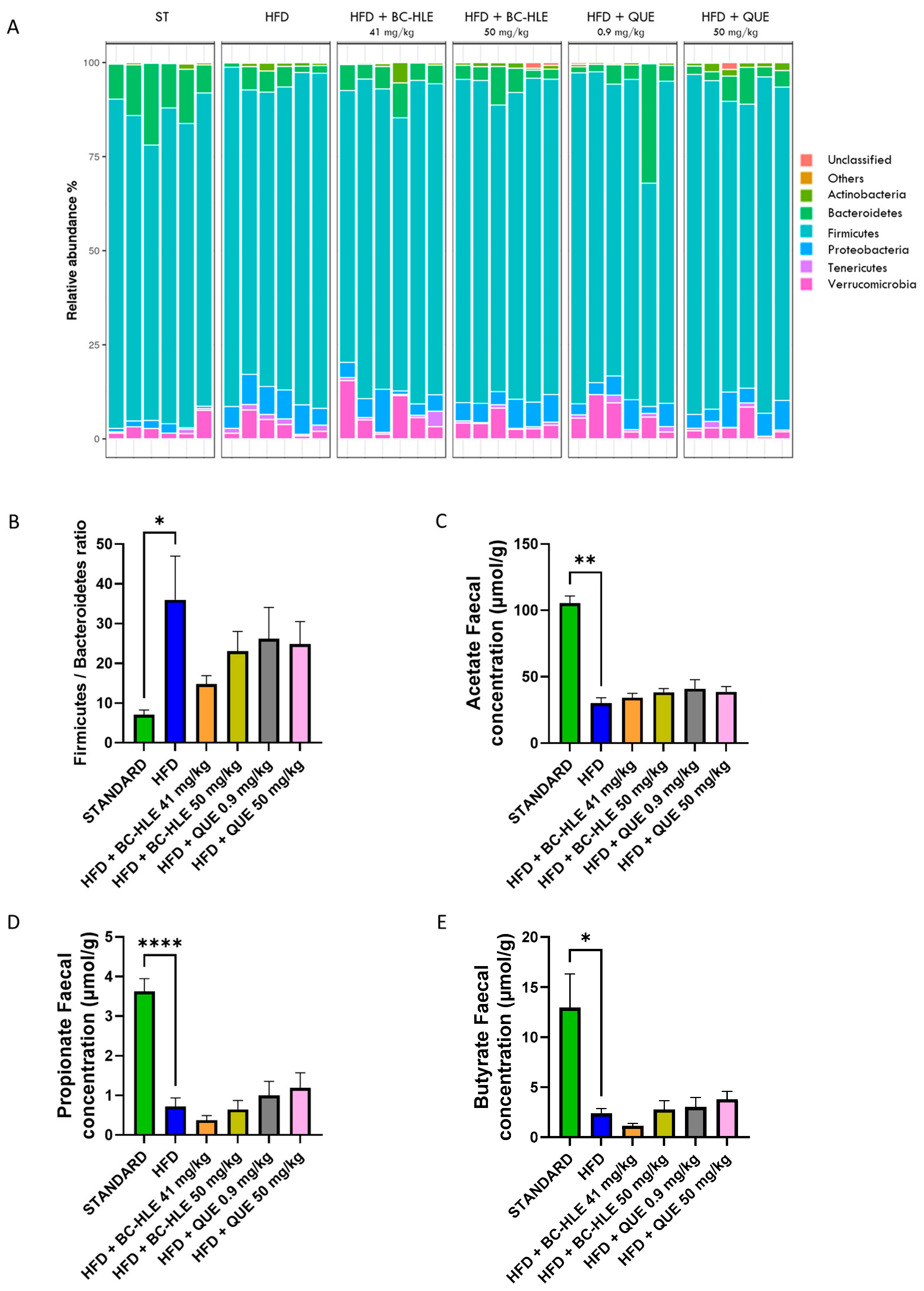
2.4.6. Microbiota
DNA Extraction
16S Metabarcoding Analysis—Library Preparation and Sequencing
16S Metabarcoding Analysis—Data Processing
2.5. Statistical Analysis
3. Results
3.1. Effect of HFD and Supplementations Consumption on Body Weight, Food/Water Intake, Index Adiposity and Abdominal Circumference
3.2. Effect of HFD and Supplementations Consumption on OGTT
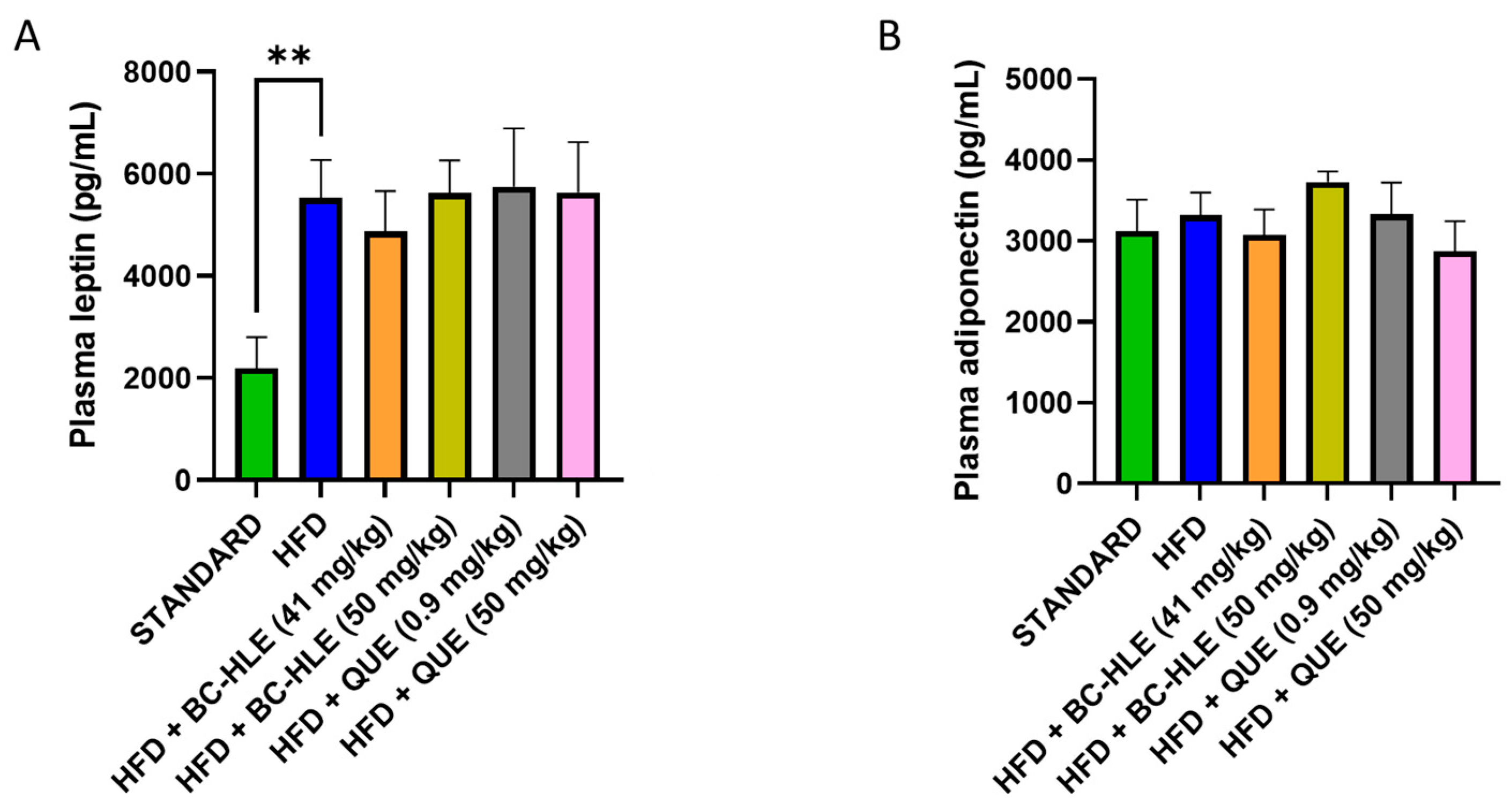
3.3. Effect of HFD and Supplementations Consumption on Plasma Lipid and Metabolic (Adiponectin, Leptin) Profiles
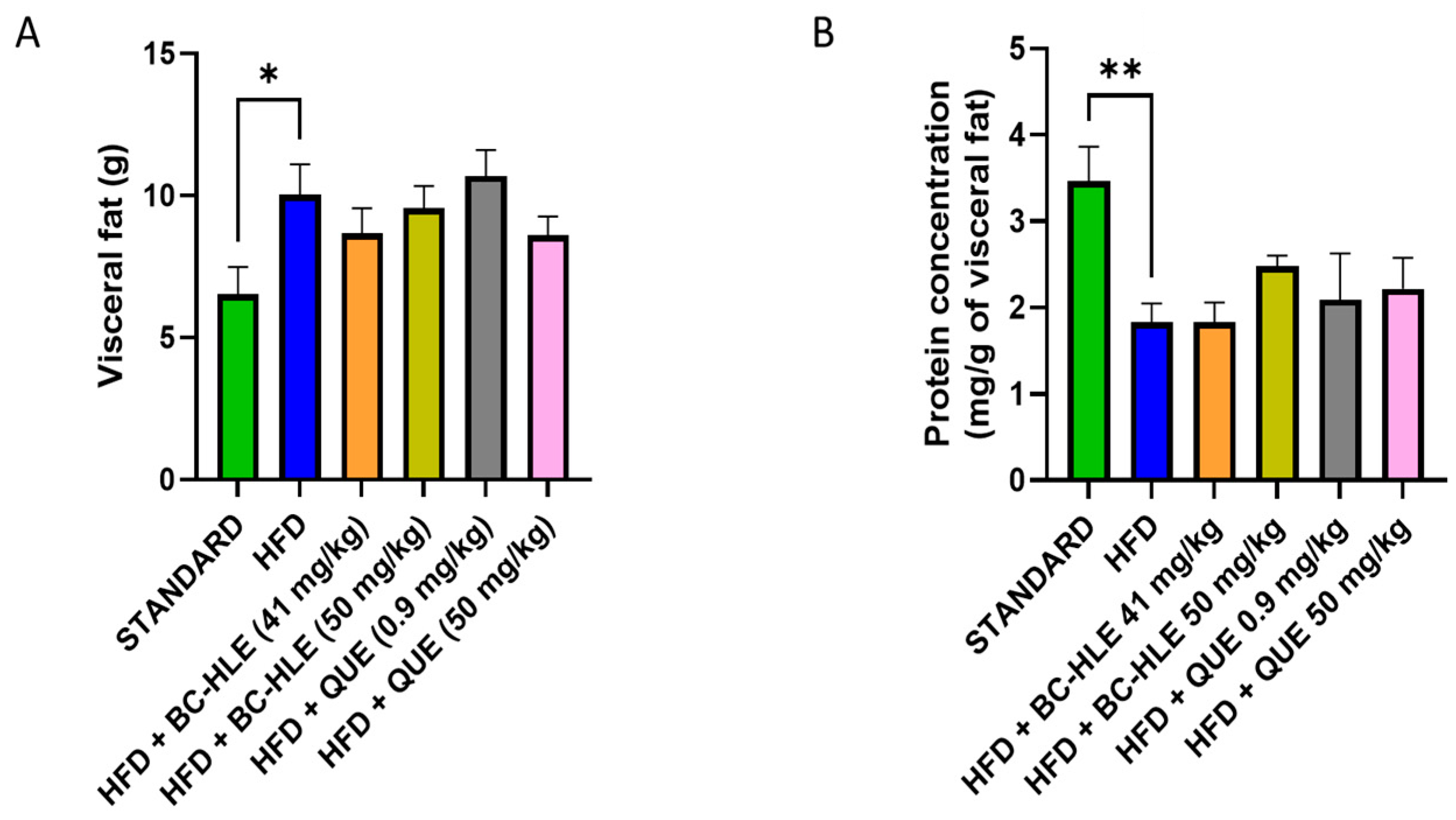
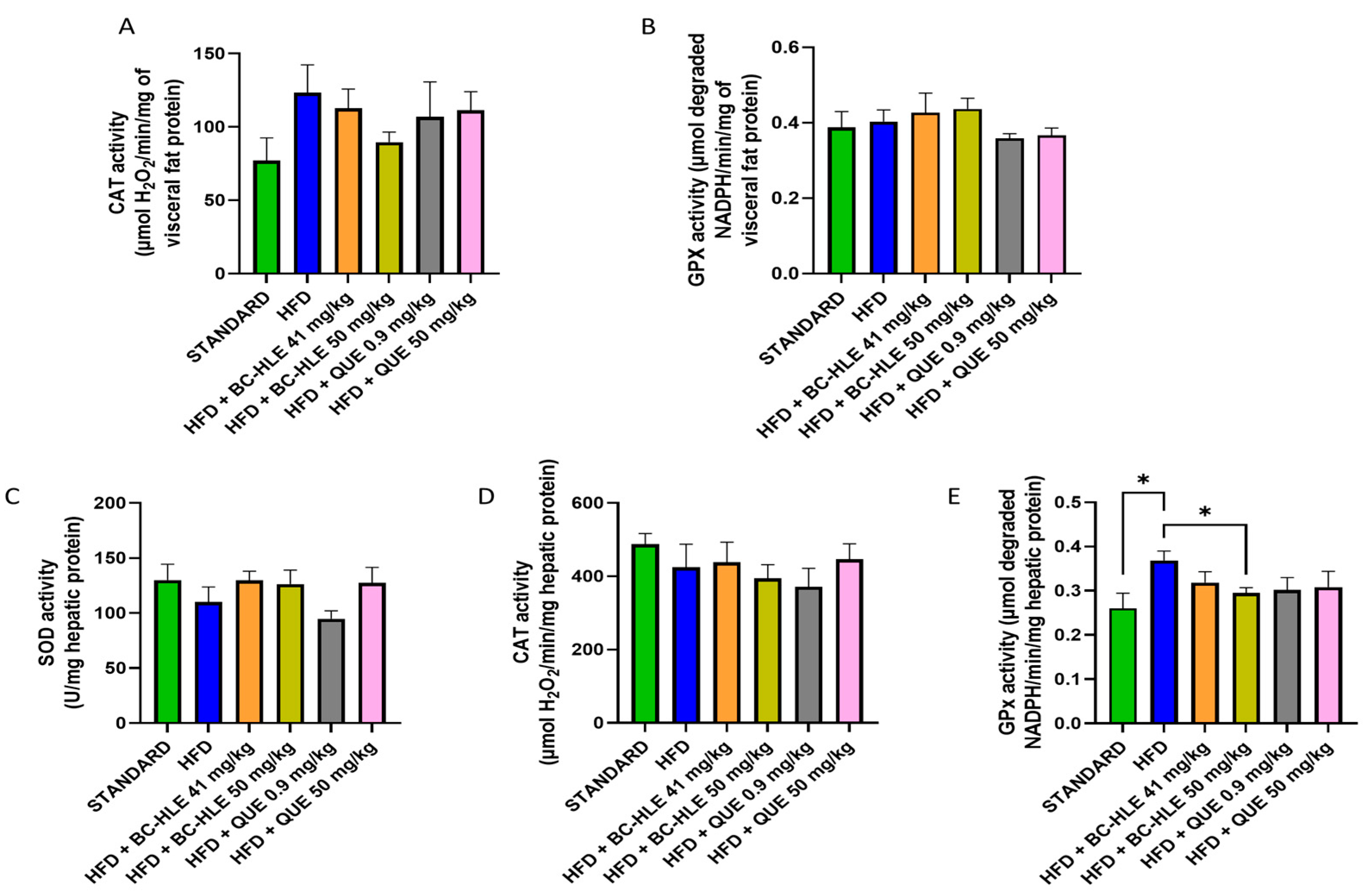
3.4. Effect of HFD and Supplementations Consumption on Visceral Adipose Tissue: Protein Expression and Oxidative Profile
3.5. Effect of HFD and Supplementations Consumption on Hepatic Tissue: Antioxidant Profile
3.6. Effect of HFD and Supplementations Consumption on Microbiota and Its Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. In Obesity and Lipotoxicity; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2017; pp. 1–17. [Google Scholar] [CrossRef]
- Astrup, A.; Dyerberg, J.; Selleck, M.; Stender, S. Nutrition transition and its relationship to the development of obesity and related chronic diseases. Obes. Rev. 2008, 9, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Hall, K.D. Did the Food Environment Cause the Obesity Epidemic? Obesity 2017, 26, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Zhai, Z.; Li, Z.; Wang, L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem. Biol. Interact. 2020, 330, 109199. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory Links Between High Fat Diets and Diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, G.; Herbst, K.L.; Donato, K.; Dhuli, K.; Kiani, A.K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Bertelli, M. Dietary supplements for obesity. J. Prev. Med. Hyg. 2022, 63 (Suppl. S3), E160–E168. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Mokdad, A.H. Fruit and Vegetable Consumption and Diabetes Mellitus Incidence Among U.S. Adults. Prev. Med. 2001, 32, 33–39. [Google Scholar] [CrossRef]
- Vioque, J.; Weinbrenner, T.; Castelló, A.; Asensio, L.; De la Hera, M.G. Intake of Fruits and Vegetables in Relation to 10-year Weight Gain Among Spanish Adults. Obesity 2008, 16, 664–670. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural Colorants with Health-Promoting Properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Zin, C.A.J.C.M.; Mohamed, W.M.I.W.; Khan, N.A.K.; Ishak, W.R.W. Effects of Fruit and Vegetable Polyphenols on the Glycemic Control and Metabolic Parameters in Type 2 Diabetes Mellitus: A Review. Prev. Nutr. Food Sci. 2022, 27, 257–264. [Google Scholar] [CrossRef]
- Staszowska-Karkut, M.; Materska, M. Phenolic Composition, Mineral Content, and Beneficial Bioactivities of Leaf Extracts from Black Currant (Ribes nigrum L.), Raspberry (Rubus idaeus), and Aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, T.; Wu, X.; Kim, M.; Kim, Y.; Yang, E.; Yoon, Y.; Park, S. Aqueous Blackcurrant Extract Improves Insulin Sensitivity and Secretion and Modulates the Gut Microbiome in Non-Obese Type 2 Diabetic Rats. Antioxidants 2021, 10, 756. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kho, M.C.; Kim, H.Y.; Ahn, Y.M.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Blackcurrant Suppresses Metabolic Syndrome Induced by High-Fructose Diet in Rats. Evid.-Based Complement. Altern. Med. 2015, 2015, 385976. [Google Scholar] [CrossRef] [PubMed]
- Haswell, C.; Ali, A.; Page, R.; Hurst, R.; Rutherfurd-Markwick, K. Potential of Beetroot and Blackcurrant Compounds to Improve Metabolic Syndrome Risk Factors. Metabolites 2021, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Tabart, J.; Franck, T.; Kevers, C.; Pincemail, J.; Serteyn, D.; Defraigne, J.; Dommes, J. Antioxidant and anti-inflammatory activities of Ribes nigrum extracts. Food Chem. 2012, 131, 1116–1122. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schäfer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2017, 62, 1700447. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J. Flavonols and cardiovascular disease. Mol. Aspects Med. 2010, 31, 478–494. [Google Scholar] [CrossRef]
- D’Andrea, G. Quercetin: A flavonol with multifaceted therapeutic applications? Fitoterapia 2015, 106, 256–271. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Brown, L. Quercetin Ameliorates Cardiovascular, Hepatic, and Metabolic Changes in Diet-Induced Metabolic Syndrome in Rats. J. Nutr. 2012, 142, 1026–1032. [Google Scholar] [CrossRef]
- Peng, L.; Zhang, Q.; Zhang, Y.; Yao, Z.; Song, P.; Wei, L.; Zhao, G.; Yan, Z. Effect of tartary buckwheat, rutin, and quercetin on lipid metabolism in rats during high dietary fat intake. Food Sci. Nutr. 2019, 8, 199–213. [Google Scholar] [CrossRef]
- Dessouroux, A.; Seyrig, C.; Leclerc, C. Point sur la qualité des extraits fluides glycérinés de plantes fraîches standardisés (EPS) et leur intérêt pharmacologique. Phytothérapie 2011, 9, 249–254. [Google Scholar] [CrossRef]
- Cuffaro, B.; Assohoun, A.; Boutillier, D.; Peucelle, V.; Desramaut, J.; Boudebbouze, S.; Croyal, M.; Waligora-Dupriet, A.; Rhimi, M.; Grangette, C.; et al. Identification of New Potential Biotherapeutics from Human Gut Microbiota-Derived Bacteria. Microorganisms 2021, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Ansorge, R.; Birolo, G.; James, S.A.; Telatin, A. Dadaist2: A Toolkit to Automate and Simplify Statistical Analysis and Plotting of Metabarcoding Experiments. Int. J. Mol. Sci. 2021, 22, 5309. [Google Scholar] [CrossRef] [PubMed]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef] [PubMed]
- López-Jaramillo, P.; Gómez-Arbeláez, D.; López-López, J.; López-López, C.; Martínez-Ortega, J.; Gómez-Rodríguez, A.; Triana-Cubillos, S. The role of leptin/adiponectin ratio in metabolic syndrome and diabetes. Horm. Mol. Biol. Clin. Investig. 2013, 18, 37–45. [Google Scholar] [CrossRef]
- Bergman, R.N.; Van Citters, G.W.; Mittelman, S.D.; Dea, M.K.; Hamilton-Wessler, M.; Kim, S.P.; Ellmerer, M. Central Role of the Adipocyte in the Metabolic Syndrome. J. Investig. Med. 2001, 49, 119–126. [Google Scholar] [CrossRef]
- Boden, G. Obesity, insulin resistance and free fatty acids. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 139–143. [Google Scholar] [CrossRef]
- Buettner, R.; Newgard, C.B.; Rhodes, C.J.; O’Doherty, R.M. Correction of diet-induced hyperglycemia, hyperinsulinemia, and skeletal muscle insulin resistance by moderate hyperleptinemia. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E563–E569. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef]
- Gaballah, H.H.; Zakaria, S.S.; Mwafy, S.E.; Tahoon, N.M.; Ebeid, A.M. Mechanistic insights into the effects of quercetin and/or GLP-1 analogue liraglutide on high-fat diet/streptozotocin-induced type 2 diabetes in rats. Biomed. Pharmacother. 2017, 92, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yu, Y.; Dai, S.; Zhang, C.; Xue, X.; Zhou, M.; Yao, C.; Li, Y. Kaempferol efficacy in metabolic diseases: Molecular mechanisms of action in diabetes mellitus, obesity, non-alcoholic fatty liver disease, steatohepatitis, and atherosclerosis. Biomed. Pharmacother. 2024, 175, 116694. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Morand, C.; Manach, C.; Crespy, V.; Remesy, C. Quercetin 3-O-β-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic. Res. 2000, 33, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.; Clifton, P. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.V.A.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef]
- Hanhineva, K.; Törrönen, R.; Bondia-Pons, I.; Pekkinen, J.; Kolehmainen, M.; Mykkänen, H.; Poutanen, K. Impact of Dietary Polyphenols on Carbohydrate Metabolism. Int. J. Mol Sci. 2010, 11, 1365–1402. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Chee, K.; Lee, B.H. Anti- and prooxidant effects of chronic quercetin administration in rats. Eur. J. Pharmacol. 2003, 482, 281–285. [Google Scholar] [CrossRef]
- Papiez, M.; Cierniak, A.; Krzysciak, W.; Bzowska, M.; Taha, H.; Jozkowicz, A.; Piskula, M. The changes of antioxidant defense system caused by quercetin administration do not lead to DNA damage and apoptosis in the spleen and bone marrow cells of rats. Food Chem. Toxicol. 2008, 46, 3053–3058. [Google Scholar] [CrossRef]
- Vincent, H.K.; Innes, K.E.; Vincent, K.R. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes. Metab. 2007, 9, 813–839. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Mechanistic Insight into Oxidative Stress-Triggered Signaling Pathways and Type 2 Diabetes. Molecules 2022, 27, 950. [Google Scholar] [CrossRef]
- Galinier, A.; Carrière, A.; Fernandez, Y.; Carpéné, C.; André, M.; Caspar-Bauguil, S.; Thouvenot, J.; Périquet, B.; Pénicaud, L.; Casteilla, L. Adipose Tissue Proadipogenic Redox Changes in Obesity. J. Biol. Chem. 2006, 281, 12682–12687. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, H.S.; Bhandari, U.; Khanna, G. Preventive Effect of Embelin fromEmbelia ribeson Lipid Metabolism and Oxidative Stress in High-Fat Diet-Induced Obesity in Rats. Planta Med. 2012, 78, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Ambrożewicz, E.; Augustyniak, A.; Gęgotek, A.; Bielawska, K.; Skrzydlewska, E. Black-Currant protection against oxidative stress formation. J. Toxicol. Environ. Health 2013, 76, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Feng, J.; Zhang, J.; Kang, X.; Qian, D. Quercetin modulates AMPK/SIRT1/NF-κB signaling to inhibit inflammatory/oxidative stress responses in diabetic high fat diet-induced atherosclerosis in the rat carotid artery. Exp. Ther. Med. 2020, 20, 1. [Google Scholar] [CrossRef]
- Dzah, C.S.; Zhang, H.; Gobe, V.; Asante-Donyinah, D.; Duan, Y. Anti- and pro-oxidant properties of polyphenols and their role in modulating glutathione synthesis, activity and cellular redox potential: Potential synergies for disease management. Adv. Redox Res. 2024, 11, 100099. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Lee, S.G.; Melough, M.M.; Sakaki, J.R.; Maas, K.R.; Koo, S.I.; Chun, O.K. Long-Term Blackcurrant Supplementation Modified Gut Microbiome Profiles in Mice in an Age-Dependent Manner: An Exploratory Study. Nutrients 2020, 12, 290. [Google Scholar] [CrossRef]
- Crovesy, L.; Masterson, D.; Rosado, E.L. Profile of the gut microbiota of adults with obesity: A systematic review. Eur. J. Clin. Nutr. 2020, 74, 1251–1262. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.; Portillo, M.; Martínez, J.; Milagro, F. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Jurgoński, A.; Fotschki, B.; Juśkiewicz, J. Disparate metabolic effects of blackcurrant seed oil in rats fed a basal and obesogenic diet. Eur. J. Nutr. 2014, 54, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, S.; Yamauchi, T.; Oshima, Y.; Tsukamoto, Y.; Kadowaki, T. Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A(y) mice. Biochem. Biophys. Res. Commun. 2006, 344, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-Chain Fatty Acids Stimulate Glucagon-Like Peptide-1 Secretion via the G-Protein–Coupled Receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Demigné, C.; Morand, C.; Levrat, M.; Besson, C.; Moundras, C.; Rémésy, C. Effect of propionate on fatty acid and cholesterol synthesis and on acetate metabolism in isolated rat hepatocytes. Br. J. Nutr. 1995, 74, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Chambers, E.S.; Morrison, D.J.; Frost, G. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int. J. Obes. 2015, 39, 1331–1338. [Google Scholar] [CrossRef]
- Jurgoński, A.; Juśkiewicz, J.; Zduńczyk, Z.; Matusevicius, P.; Kołodziejczyk, K. Polyphenol-rich extract from blackcurrant pomace attenuates the intestinal tract and serum lipid changes induced by a high-fat diet in rabbits. Eur. J. Nutr. 2014, 53, 1603–1613. [Google Scholar] [CrossRef]
- Jakobsdottir, G.; Blanco, N.; Xu, J.; Ahrné, S.; Molin, G.; Sterner, O.; Nyman, M.E.G. Formation of Short-Chain Fatty Acids, Excretion of Anthocyanins, and Microbial Diversity in Rats Fed Blackcurrants, Blackberries, and Raspberries. J. Nutr. Metab. 2013, 2013, 202534. [Google Scholar] [CrossRef]
- Paturi, G.; Butts, C.A.; Monro, J.A.; Hedderley, D. Effects of Blackcurrant and Dietary Fibers on Large Intestinal Health Biomarkers in Rats. Plant Foods Hum. Nutr. 2018, 73, 54–60. [Google Scholar] [CrossRef] [PubMed]
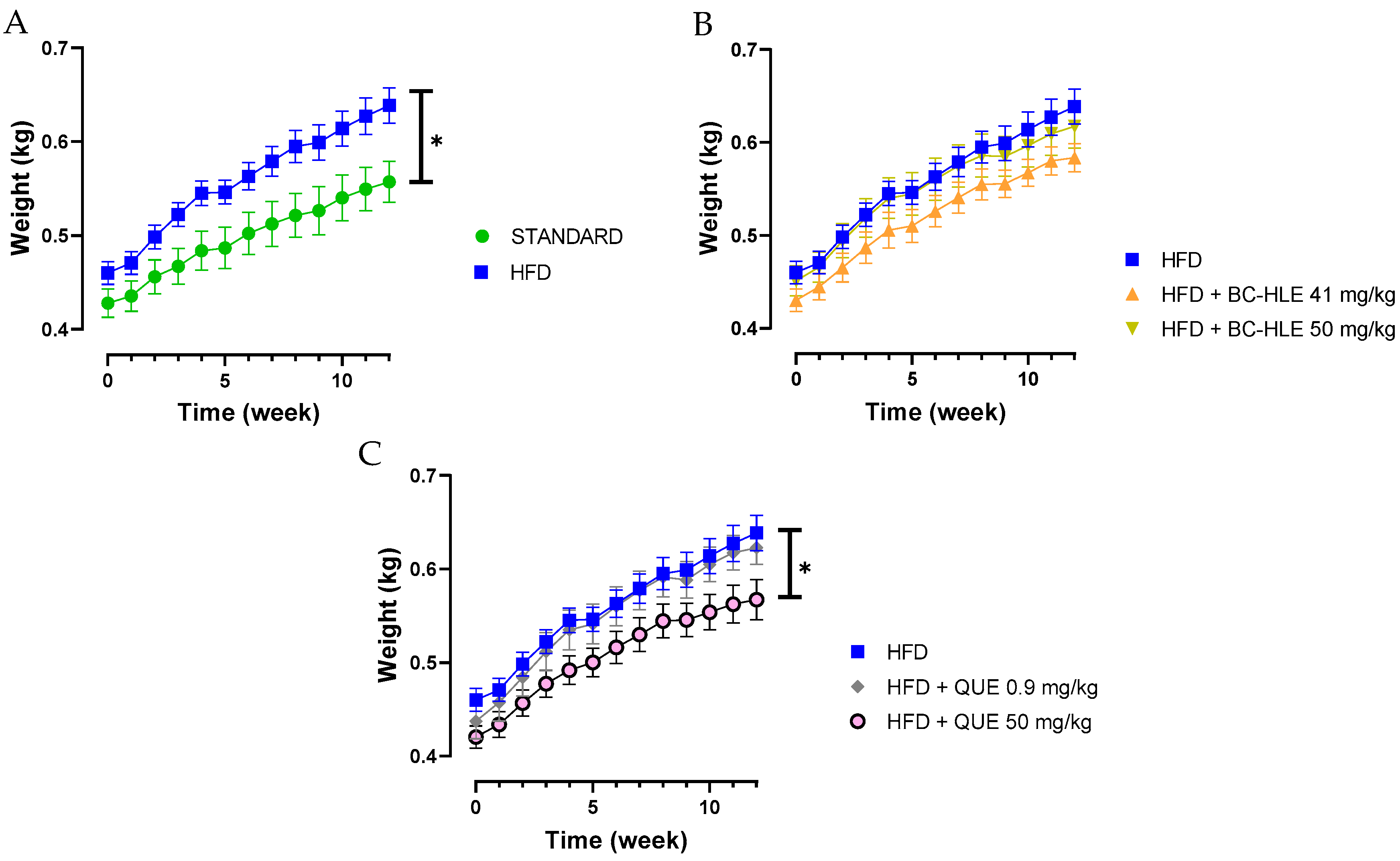

| Group | Body Weight Gain (g/12 Weeks) | Food Intake (g/Day) | Caloric Intake (Kcal/Day) | Food Efficiency (%) | Abdominal Circumference (cm) | Adiposity Index |
|---|---|---|---|---|---|---|
| Standard | 130 ± 8 | 27 ± 1 | 84 ± 3 | 6 | 19.4 ± 0.4 | 3.7 ± 0.3 |
| HFD | 178 ± 9 ** | 20 ± 1 *** | 96 ± 5 ** | 10 **** | 21.5 ± 0.4 ** | 5.2 ± 0.3 * |
| HFD + BC-HLE (41 mg/kg) | 154 ± 8 | 19 ± 1 | 89 ± 5 | 10 ± 1 | 21.3 ± 0.5 | 4.7 ± 0.1 |
| HFD + BC-HLE (50 mg/kg) | 166 ± 14 | 20 ± 1 | 94 ± 6 | 10 ± 1 | 21.4 ± 0.3 | 5.1 ± 0.4 |
| HFD + QUE (0.9 mg/kg) | 185 ± 10 | 20 ± 1 | 95 ± 6 | 11 ± 1 | 21.3 ± 0.3 | 5.5 ± 0.4 |
| HFD + QUE (50 mg/kg) | 147 ± 12 | 18 ± 1 # | 86 ± 6 # | 9 ± 1 | 20.6 ± 0.5 | 5.3 ± 0.4 |
| Group | TG (mmol/L) | TC (mmol/L) | HDL-C (mmol/L) | LDL-C (mmol/L) | NEFA (mmol/L) |
|---|---|---|---|---|---|
| Standard | 0.93 ± 0.11 | 1.71 ± 0.08 | 1.16 ± 0.06 | 0.13 ± 0.04 | 0.37 ± 0.04 |
| HFD | 0.84 ± 0.07 | 1.61 ± 0.08 | 1.07 ± 0.08 | 0.17 ± 0.02 | 0.21 ± 0.03 * |
| HFD + BC-HLE (41 mg/kg) | 1.19 ± 0.16 | 1.88 ± 0.17 | 1.23 ± 0.11 | 0.14 ± 0.03 | 0.30 ± 0.03 |
| HFD + BC-HLE (50 mg/kg) | 0.79 ± 0.04 | 1.70 ± 0.11 | 1.14 ± 0.07 | 0.20 ± 0.04 | 0.19 ± 0.04 |
| HFD + QUE (0.9 mg/kg) | 0.81 ± 0.09 | 1.62 ± 0.12 | 1.11 ± 0.08 | 0.18 ± 0.05 | 0.20 ± 0.05 |
| HFD + QUE (50 mg/kg) | 0.93 ± 0.09 | 1.52 ± 0.09 | 0.98 ± 0.07 | 0.13 ± 0.03 | 0.33 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bréger, G.; André, A.; Cotte, C.; Hammaidi, A.; Amérand, A.; Faivre, C.; Martignat, L.; Mallem, M.Y. Anti-Obesity Effects Evaluation of a Blackcurrant Leaf Standardized Hydro-Alcoholic Extract in Wistar Rat Subjected to a High-Fat Diet. Biology 2024, 13, 999. https://doi.org/10.3390/biology13120999
Bréger G, André A, Cotte C, Hammaidi A, Amérand A, Faivre C, Martignat L, Mallem MY. Anti-Obesity Effects Evaluation of a Blackcurrant Leaf Standardized Hydro-Alcoholic Extract in Wistar Rat Subjected to a High-Fat Diet. Biology. 2024; 13(12):999. https://doi.org/10.3390/biology13120999
Chicago/Turabian StyleBréger, Gwendoline, Agnès André, César Cotte, Abderrahim Hammaidi, Aline Amérand, Claude Faivre, Lionel Martignat, and Mohamed Yassine Mallem. 2024. "Anti-Obesity Effects Evaluation of a Blackcurrant Leaf Standardized Hydro-Alcoholic Extract in Wistar Rat Subjected to a High-Fat Diet" Biology 13, no. 12: 999. https://doi.org/10.3390/biology13120999
APA StyleBréger, G., André, A., Cotte, C., Hammaidi, A., Amérand, A., Faivre, C., Martignat, L., & Mallem, M. Y. (2024). Anti-Obesity Effects Evaluation of a Blackcurrant Leaf Standardized Hydro-Alcoholic Extract in Wistar Rat Subjected to a High-Fat Diet. Biology, 13(12), 999. https://doi.org/10.3390/biology13120999






