Oocytes Quality Assessment—The Current Insight: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Information Source and Search Strategy
2.3. Study Selection, Data Extraction, and Risk of Bias Assessment
3. Results
3.1. Systematic Review Overview
3.1.1. Search Sequence and Quality Assessment
3.1.2. Studies Characteristics
3.1.3. Main Outcome
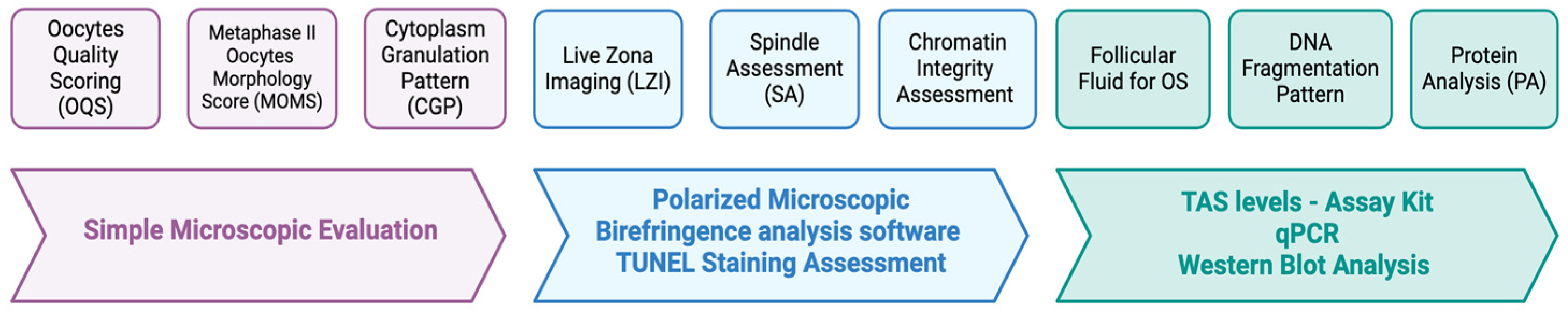
- The Oocytes Quality Scoring (OQS)
- Evolution of OQS—Metaphase II Oocytes Morphology Score (MOMS)
- Spindle Assessment (SA)
- i.
- Spindle Assessment (SA) alone
- ii.
- Combination of OQS with Spindle Assessment (SA)
- iii.
- Combination of Polar Body I Morphology (PBM) with Spindle Assessment (SA)
- iv.
- Combination of Follicular Fluid (FF) with Spindle Assessment (SA)
- Cytoplasm Granulation Pattern (CGP)
- Live Zona Imaging
- Protein Analysis and DNA Fragmentation and Chromatin Integrity Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lazzari, E.; Potancokova, M.; Sobotka, T.; Gray, E.; Chambers, G.M. Projecting the Contribution of Assisted Reproductive Technology to Completed Cohort Fertility. Popul. Res. Policy Rev. 2023, 42, 6. [Google Scholar] [CrossRef] [PubMed]
- Kamath, M.S.; Vogiatzi, P.; Sunkara, S.K.; Woodward, B. Oocyte activation for women following intracytoplasmic sperm injection (ICSI). Cochrane Database Syst. Rev. 2021, 2021. [Google Scholar] [CrossRef]
- Vo, K.C.T.; Kawamura, K. In Vitro Activation Early Follicles: From the Basic Science to the Clinical Perspectives. Int. J. Mol. Sci. 2021, 22, 3785. [Google Scholar] [CrossRef] [PubMed]
- eClinicalMedicine. The current status of IVF: Are we putting the needs of the individual first? EClinicalMedicine 2023, 65, 102343. [Google Scholar] [CrossRef]
- Satouh, Y.; Sato, K. Reorganization, specialization, and degradation of oocyte maternal components for early development. Reprod. Med. Biol. 2023, 22, e12505. [Google Scholar] [CrossRef]
- Lemseffer, Y.; Terret, M.E.; Campillo, C.; Labrune, E. Methods for Assessing Oocyte Quality: A Review of Literature. Biomedicines 2022, 10, 2184. [Google Scholar] [CrossRef]
- Bartolacci, A.; Intra, G.; Coticchio, G.; dell’Aquila, M.; Patria, G.; Borini, A. Does morphological assessment predict oocyte developmental competence? A systematic review and proposed score. J. Assist. Reprod. Genet. 2022, 39, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Giovanni Coticchio, A.A.; Arroyo, G.; Balaban, B.; Campbell, A.; De, M.J.; Los Santos, T.E.; Gardner, D.; Kovačič, B.; Lundin, K.; Magli, C.; et al. The Istanbul Consensus Update: A Revised ESHRE/ALPHA Consensus on Oocyte and Embryo Static and Dynamic Morphological Assessment; European Society of Human Reproduction and Embryology: Strombeek, Belgium, 2024; pp. 1–80. [Google Scholar]
- Rienzi, L.; Vajta, G.; Ubaldi, F. Predictive value of oocyte morphology in human IVF: A systematic review of the literature. Hum. Reprod. Update 2011, 17, 34–45. [Google Scholar] [CrossRef]
- Rienzi, L.; Balaban, B.; Ebner, T.; Mandelbaum, J. The oocyte. Hum. Reprod. 2012, 27 (Suppl. S1), i2–i21. [Google Scholar] [CrossRef]
- Atzmon, Y.; Michaeli, M.; Aslih, N.; Ruzov, O.; Rotfarb, N.; Shoshan-Karchovsky, E.; Shalom-Paz, E. Degenerative Oocytes in the Aspirated Cohort Are Not Due to the Aspirating Needle: A Prospective Randomized Pilot Study with Sibling Oocytes. Reprod. Sci. 2021, 28, 1882–1889. [Google Scholar] [CrossRef]
- Lazzaroni-Tealdi, E.; Barad, D.H.; Albertini, D.F.; Yu, Y.; Kushnir, V.A.; Russell, H.; Wu, Y.G.; Gleicher, N. Oocyte Scoring Enhances Embryo-Scoring in Predicting Pregnancy Chances with IVF Where It Counts Most. PLoS ONE 2015, 10, e0143632. [Google Scholar] [CrossRef] [PubMed]
- Lazzaroni, E.; Gleicher, N.; Yu, Y.; Kushnir, V.A.; Shohat-Tal, A.; Barad, D.H. A new oocyte scoring system with better predictability for clinical pregnancy than day 3 embryo quality. Fertil. Steril. 2013, 100, S502. [Google Scholar] [CrossRef]
- Chamayou, S.; Ragolia, C.; Alecci, C.; Storaci, G.; Maglia, E.; Russo, E.; Guglielmino, A. Meiotic spindle presence and oocyte morphology do not predict clinical ICSI outcomes: A study of 967 transferred embryos. Reprod. Biomed. Online 2006, 13, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Rienzi, L.; Ubaldi, F.M.; Iacobelli, M.; Minasi, M.G.; Romano, S.; Ferrero, S.; Sapienza, F.; Baroni, E.; Litwicka, K.; Greco, E. Significance of metaphase II human oocyte morphology on ICSI outcome. Fertil. Steril. 2008, 90, 1692–1700. [Google Scholar] [CrossRef]
- Rajani, S.; Chattopadhyay, R.; Goswami, S.K.; Ghosh, S.; Sharma, S.; Chakravarty, B. Assessment of oocyte quality in polycystic ovarian syndrome and endometriosis by spindle imaging and reactive oxygen species levels in follicular fluid and its relationship with IVF-ET outcome. J. Hum. Reprod. Sci. 2012, 5, 187–193. [Google Scholar] [CrossRef]
- De Santis, L.; Cino, I.; Rabellotti, E.; Calzi, F.; Persico, P.; Borini, A.; Coticchio, G. Polar body morphology and spindle imaging as predictors of oocyte quality. Reprod. Biomed. Online 2005, 11, 36–42. [Google Scholar] [CrossRef]
- Karabulut, S.; Korkmaz, O.; Kutlu, P.; Gozel, H.E.; Keskin, I. Effects o follicular fluid oxidative status on human mural granulosa cells, oocyte competency and ICSI parameters. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 127–136. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef]
- Ten, J.; Mendiola, J.; Vioque, J.; de Juan, J.; Bernabeu, R. Donor oocyte dysmorphisms and their influence on fertilization and embryo quality. Reprod. Biomed. Online 2007, 14, 40–48. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Boumerdassi, Y.; Labrosse, J.; Hammami, F.; Dahoun, M.; Bouyer, J.; O’Neill, L.; Sarandi, S.; Peigne, M.; Cedrin, I.; Grynberg, M.; et al. Impact of oxygen tension during in vitro maturation: A sibling-oocyte prospective double-blinded study. Fertil. Steril. 2024, 121, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Robin, C.; Uk, A.; Decanter, C.; Behal, H.; Collinet, P.; Rubod, C.; Barbotin, A.L.; Robin, G. Impact of endometriosis on oocyte morphology in IVF-ICSI: Retrospective study of a cohort of more than 6000 mature oocytes. Reprod. Biol. Endocrinol. 2021, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Molinari, E.; Darmon, S.; Zhang, L.; Patrizio, P.; Barad, D.H.; Gleicher, N. Predictive value of cytoplasmic granulation patterns during in vitro fertilization in metaphase II oocytes: Part I, poor-prognosis patients. Fertil. Steril. 2021, 116, 431–443. [Google Scholar] [CrossRef]
- Montag, M.; Schimming, T.; Koster, M.; Zhou, C.; Dorn, C.; Rosing, B.; van der Ven, H.; Ven der Ven, K. Oocyte zona birefringence intensity is associated with embryonic implantation potential in ICSI cycles. Reprod. Biomed. Online 2008, 16, 239–244. [Google Scholar] [CrossRef]
- Montag, M.; Schimming, T.; van der Ven, H. Spindle imaging in human oocytes: The impact of the meiotic cell cycle. Reprod. Biomed. Online 2006, 12, 442–446. [Google Scholar] [CrossRef]
- Sigala, J.; Sifer, C.; Dewailly, D.; Robin, G.; Bruyneel, A.; Ramdane, N.; Lefebvre-Khalil, V.; Mitchell, V.; Decanter, C. Is polycystic ovarian morphology related to a poor oocyte quality after controlled ovarian hyperstimulation for intracytoplasmic sperm injection? Results from a prospective, comparative study. Fertil. Steril. 2015, 103, 112–118. [Google Scholar] [CrossRef]
- Otsuki, J.; Okada, A.; Morimoto, K.; Nagai, Y.; Kubo, H. The relationship between pregnancy outcome and smooth endoplasmic reticulum clusters in MII human oocytes. Hum. Reprod. 2004, 19, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, M.; Shi, H.; Yi, S.; Li, Q.; Su, Y.; Guo, Y.; Hu, L.; Sun, J.; Sun, Y.P. Causes and Effects of Oocyte Retrieval Difficulties: A Retrospective Study of 10,624 Cycles. Front. Endocrinol. 2021, 12, 564344. [Google Scholar] [CrossRef]
- Aziz, N.; Biljan, M.M.; Taylor, C.T.; Manasse, P.R.; Kingsland, C.R. Effect of aspirating needle calibre on outcome of in-vitro fertilization. Hum. Reprod. 1993, 8, 1098–1100. [Google Scholar] [CrossRef]
- Chamayou, S.; Giacone, F.; Cannarella, R.; Guglielmino, A. What Does Intracytoplasmic Sperm Injection Change in Embryonic Development? The Spermatozoon Contribution. J. Clin. Med. 2023, 12, 671. [Google Scholar] [CrossRef] [PubMed]
- Ruder, E.H.; Hartman, T.J.; Goldman, M.B. Impact of oxidative stress on female fertility. Curr. Opin. Obstet. Gynecol. 2009, 21, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Cho, M.; Chun, S.; Park, T.W.; Joo, J.H.; Koo, Y.H.; Lee, Y.C. Clinical effectiveness of spindle-view intracytoplasmic sperm injection compared to conventional intracytoplasmic sperm injection in patients with poor ovarian response and previous implantation failure. Obstet. Gynecol. Sci. 2024, 67, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Kilani, S.; Cooke, S.; Tilia, L.; Chapman, M. Does meiotic spindle normality predict improved blastocyst development, implantation and live birth rates? Fertil. Steril. 2011, 96, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Revelli, A.; Delle Piane, L.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 2009, 7, 40. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Giorgi, V.S.I.; Wang, F.; Keefe, D.L.; Albertini, D.; Navarro, P.A. Influence of follicular fluid and cumulus cells on oocyte quality: Clinical implications. J. Assist. Reprod. Genet. 2018, 35, 735–751. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Xu, X.; Li, J.; Yuan, F.; Bo, S.; Qiao, J.; Xia, G.; Su, Y.; Zhang, M. Transforming growth factor-beta is involved in maintaining oocyte meiotic arrest by promoting natriuretic peptide type C expression in mouse granulosa cells. Cell Death Dis. 2019, 10, 558. [Google Scholar] [CrossRef]


| Author, Year | Title, Cohort, and Conclusion | Method of OQ Assessment | |
|---|---|---|---|
| Degenerative Oocytes in the Aspirated Cohort Are Not Due to the Aspirating Needle: A Prospective Randomized Pilot Study with Sibling Oocytes Cohort: 580 oocytes from 43 women
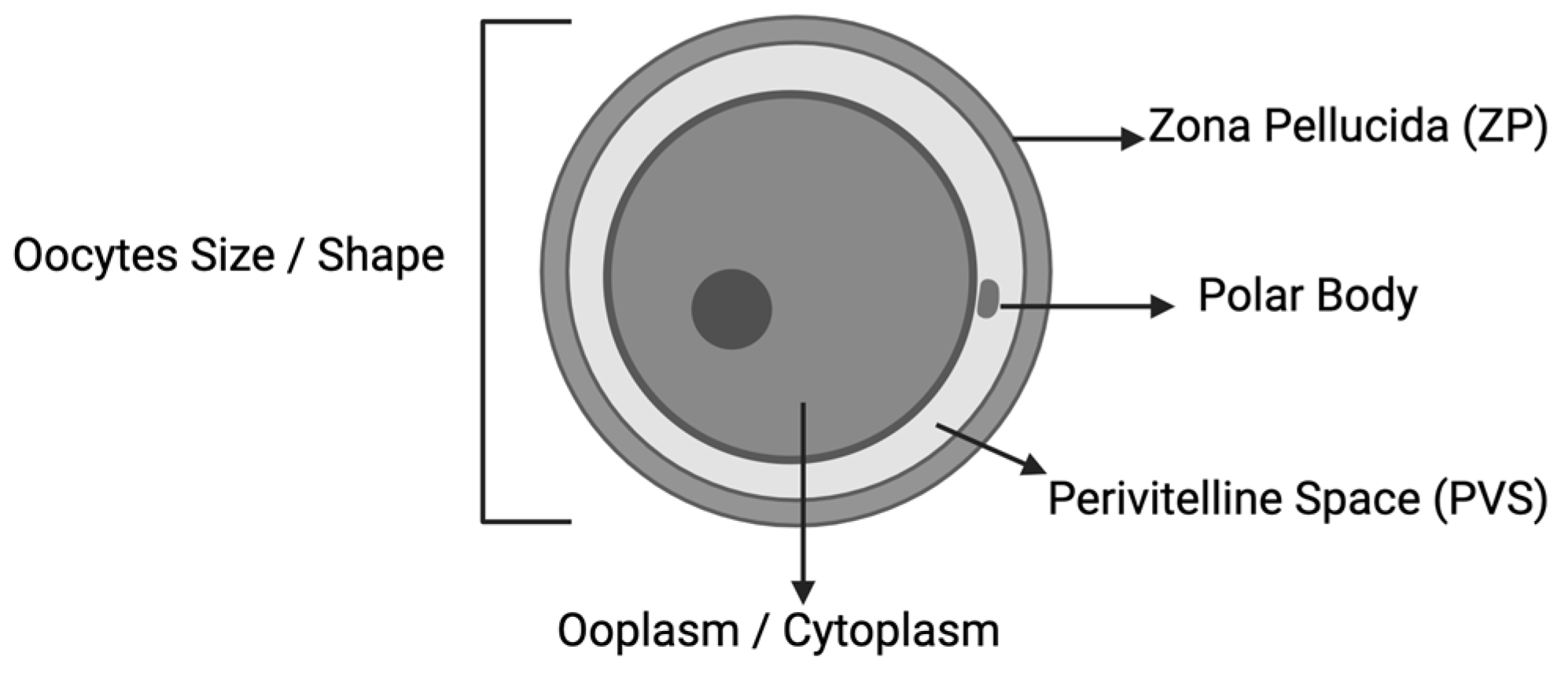
| The five standard parameters for oocyte quality are as follows: (1) size and symmetry of the perivitelline space structure; (2) color and integrity of the cytoplasm; (3) intactness of zona pellucida; (4) polar body morphology; (5) presence of vacuoles. Score: =0 if normal =−1 if abnormal All the negative parameters were summed. Scores from 0 to −5. A total score of 0 was considered the best oocyte quality. Degenerative oocytes—cytoplasm dark in color and shrunken—(DEG) | |
| Impact Of Oxygen Tension During In Vitro Maturation: A Sibling-Oocyte Prospective Double-Blinded Study Cohort: In vitro maturation (IVM) culture among fertility preservation (FP)—161 IVM cycles n = 500 (group 5% O2), 491 (group 20% O2) Conclusion: Culture under low O2 tension (5% O2) improves oocyte morphology IVM, suggests that culture under hypoxia should be standardized. | Total oocytes quality TOS scoring—the oocytes were evaluated based on 6 parameters: (i) oocyte shape; (ii) oocyte size; (iii) ooplasm characteristics. [19] structure of the perivitelline space (PVS); (v) zona pellucida (ZP); (vi) polar body morphology. Each parameter was graded as worst (−1), average (0), or best (1) The maximal TOS of an oocyte, therefore, could be a +6, the lowest a −6 | |
| Assessment Of Oocyte Quality In Polycystic Ovarian Syndrome And Endometriosis By Spindle Imaging And Reactive Oxygen Species Levels In Follicular Fluid And Its Relationship With IVF-ET Outcome Cohort FF assessment; n = 637
Cohort for MS (Meiotic Spindle); n = 637
Conclusion: Good correlation between spindle imaging and ROS levels as reliable predictors of oocyte assessment. | Follicular fluid (FF) assessment for reactive oxidation stress (ROS)
| |
| Meiotic Spindle Presence And Oocyte Morphology Do Not Predict Clinical ICSI Outcomes: A Study Of 967 Transferred Embryos Author Links Open Overlay Panel Cohort: 404 infertile women; n = 967 oocytes Conclusion: No relationship was found between oocyte morphology or meiotic spindle presence or absence and clinical pregnancy per transfer and implantation rates after ICSI. | The parameters for oocyte quality
| |
| Polar Body Morphology And Spindle Imaging As Predictors Of Oocyte Quality Cohort: 382 infertile women (n = 873 oocytes) Conclusion: PBI morphology not indicative for development potential and no significant relationship between average spindle retardance of oocytes with embryo quality. | Polar body I (PBI) morphology assessment using an inverted microscope (1 × 70 Olympus, Hamburg, Germany)
| |
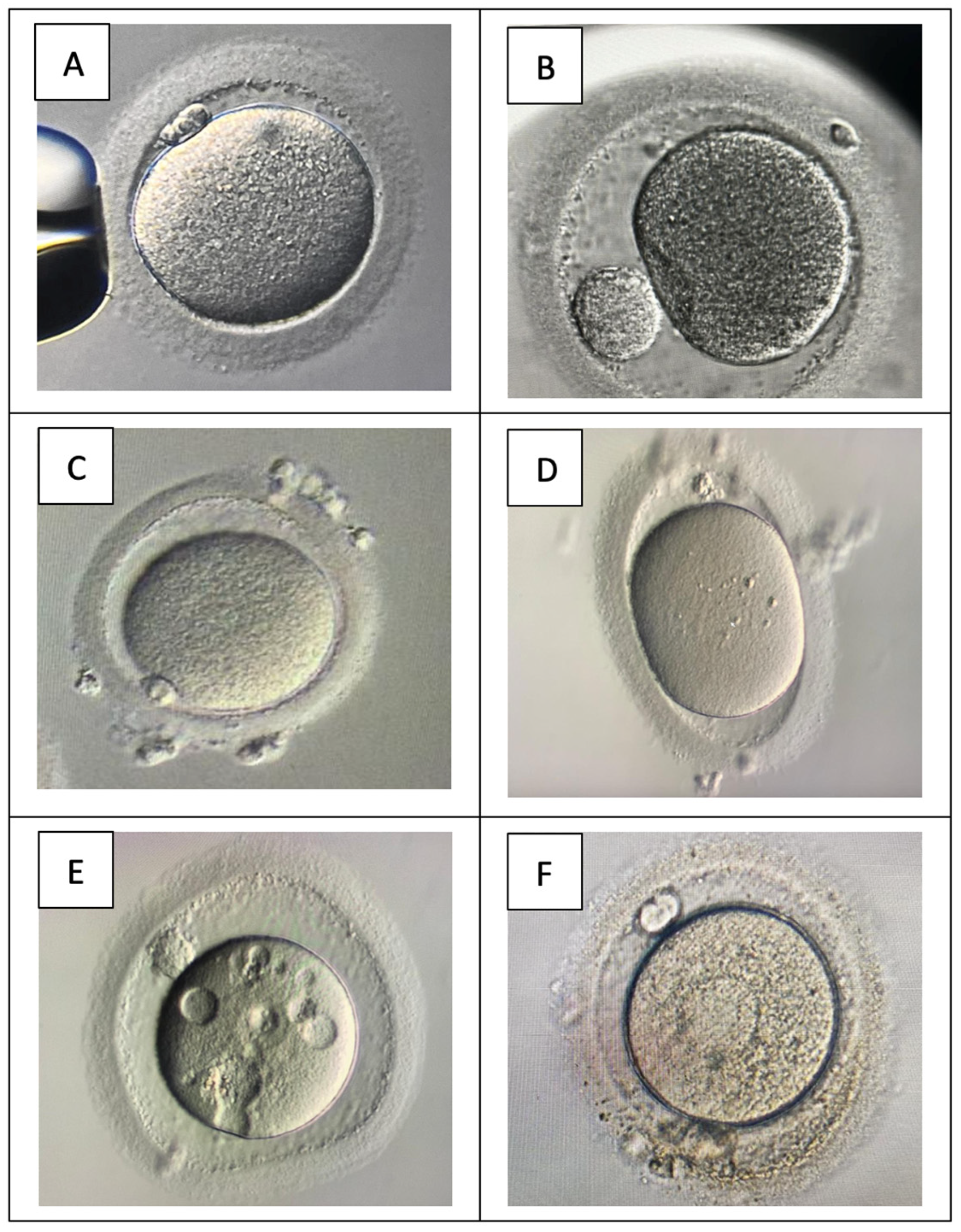
| Predictive Value Of Cytoplasmic Granulation Patterns During In Vitro Fertilization In Metaphase II Oocytes: Part I, Poor-Prognosis Patients Cohort: poor-prognosis infertile women (elderly and low AMH level, n = 2690 oocytes) Conclusion: The four distinct cytoplasmic granulation patterns in metaphase II oocytes had a predictive value for fertilization, pregnancy, and live birth outcomes in the in vitro fertilization cycles of poor-prognosis patients. | Cytoplasm granulation pattern for OQ The granulation type
| |
| Effects Of Follicular Fluid Oxidative Status On Human Mural Granulosa Cells, Oocyte Competency And ICSI Parameters Cohort: 166 infertile women (elderly and low AMH level, n = 2690 oocytes) Conclusion: Oxidative stress in FF adversely affects fertilization rates post-ICSI, but has no effect on embryo quality, pregnancy, and implantation rates. The DNA damage and chromatin integrity were increased, whereas Hsp70 and Tgf-ß decreased in the mural granulosa cells in cases of oxidative stress which may indirectly reflect the oocyte competency and may be used as biomarkers for ICSI outcome measures. | Protein analysis by immunocytochemistry and immunofluorescence Immunofluorescence analysis—to determine the expression levels by measuring relative staining intensity. The Western blot confirmed the results. To analyze the effects of oxidative stress on mural granulosa cells:
Total antioxidant status (TAS) and total oxidant status [8] levels in follicular fluids were determined by using Rel assay kits (Rel Assay Diagnostics, Gaziantep, Turkey). TAS Assay Kit was used and the absorbances were measured using a spectrophotometer (Molecular Devices SpectraMax i3 Multi-Mode Microplate reader San Jose, CA, USA). The intraassay %CV values for the TAS measurement were 2.36% for the 0.50 (0.35–0.65) mmol Trolox equiv/L and 2.24% for the 2.0 (1.7–2.3) mmol Trolox equiv/L. The intraassay CV% values for the TOS measurements were 3.57% for 5.5 (3.0–8.0) μmol/L and 5.17% for 19.5 (16–23) μmol/L. Oxidative stress index [26] = total oxidant status [8]/total antioxidant level (TAS) Oocytes quality assessment Good OQ:
DNA fragmentation and chromatin integrity assessment:
| |
| Oocyte Scoring Enhances Embryo-Scoring In Predicting Pregnancy Chances With IVF Where It Counts Most Cohort: 94 infertile women (n = 594 oocytes) Conclusion: Oocyte scoring thus provides useful clinical information, especially in patients with good prognosis and large numbers of high quality embryos. | Total oocytes quality [8]—TOS scoring (Lazzaroni-Tealdi et al., 2015) [12] The oocytes were evaluated based on 6 parameters: (i) Morphology
The maximal TOS of an oocyte, therefore, could be a +6, the lowest a −6. | |
| Spindle Imaging In Human Oocytes: The Impact Of The Meiotic Cell Cycle Cohort: infertile women (n = 113 oocytes) Conclusion: Spindle imaging is a technique that can potentially improve treatment of patients in assisted reproduction that may be of clinical importance. The timing of ICSI can be fine-tuned especially in patients with difficult ovarian stimulation and/or patients who present with oocytes of different maturational stages. | Spindle assessment Non-invasively on a Nikon Eclipse TE-2000 Phihong Enterprise (Taiwan) inverted microscope
| |
| Oocyte Zona Birefringence Intensity Is Associated With Embryonic Implantation Potential In ICSI Cycles Cohort: 124 infertile women (n = 1029 oocytes) Conclusion: Overall, the embryo development was superior in embryos derived from HZB oocytes. This study concludes that oocyte zona birefringence is a good selection criterion and a good predictive criterion for embryo implantation potential. | Live zone imaging Nikon Eclipse TE-2000 inverted microscope with ×10, ×20 and ×40 Hoffmann interference optics, ×20 and ×40 stain-free objectives, a circular polarization filter and liquid crystal analyser optics. The birefringence analysis—autocalibration was fully controlled by a polarization imaging software module (OCTAX ICSI GuardTM, OCTAX Microscience GmbH, Altdorf, Germany) with imaging software system (OCTAX EyewareTM). The microscope-motorized stage (OCTAX) containing a fully heated ceramic plate with a glass insert in the objective pathway.
| |
| Significance Of Metaphase II Human Oocyte Morphology On ICSI Outcome Cohort: 516 infertile women (n = 1191 oocytes) Conclusion: A significant relationship was found between MOMS and female age, female basal FSH, and clinical outcome. The morphologic evaluation before ICSI helps to identify MII oocytes with higher developmental potential. | Oocyte morphology assessment The oocyte’s morphologic characteristics were classified as extracytoplasmic abnormalities
Relative mark was given to each analyzed oocyte
| |
| Parameter | Points | ||
| Extracytoplasmic abnormalities | |||
| Abnormal PB I | 2.0 | ||
| Large PVS | 1.4 | ||
| Cytoplasmic Features | |||
| Granular cytoplasm | 1.4 | ||
| Centrally located granular area | 2.7 | ||
| Vacuoles | 2.1 | ||
| Impact Of Endometriosis On Oocyte Morphology In IVF-ICSI: Retrospective Study Of A Cohort Of More Than 6000 Mature Oocytes Cohort: 596 women—195 endometriosis vs. 401 control (n = 2016 MII endometriosis vs. 4073 MII control) Conclusion: Endometriosis does not have a negative impact on oocytes’ morphology in IVF-ICSI. | Oocyte morphology assessment Two oocyte morphology scores:
The average MOMS scores of the oocytes collected per attempt were then calculated by the ratio of the sum of the MOMS scores of the oocytes to the number of MII oocytes collected on the attempt. | |
| Donor Oocyte Dysmorphisms And Their Influence On Fertilization And Embryo Quality Cohort: 126 donor women (n = 1622 MII) Conclusion: The oocytes dysmorphisms (OD) found in the oocytes from proven fertile patients did not affect fertilization rates after ICSI. However, OD may significantly decrease (or increase) the chance of having good quality embryos. | Oocyte morphology assessment The oocyte dysmorphisms (OD) evaluated via direct comparison with normal oocytes:
| |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.F.; Elias, M.H.; Mat Jin, N.; Abu, M.A.; Syafruddin, S.E.; Zainuddin, A.A.; Suzuki, N.; Abdul Karim, A.K. Oocytes Quality Assessment—The Current Insight: A Systematic Review. Biology 2024, 13, 978. https://doi.org/10.3390/biology13120978
Ahmad MF, Elias MH, Mat Jin N, Abu MA, Syafruddin SE, Zainuddin AA, Suzuki N, Abdul Karim AK. Oocytes Quality Assessment—The Current Insight: A Systematic Review. Biology. 2024; 13(12):978. https://doi.org/10.3390/biology13120978
Chicago/Turabian StyleAhmad, Mohd Faizal, Marjanu Hikmah Elias, Norazilah Mat Jin, Muhammad Azrai Abu, Saiful Effendi Syafruddin, Ani Amelia Zainuddin, Nao Suzuki, and Abdul Kadir Abdul Karim. 2024. "Oocytes Quality Assessment—The Current Insight: A Systematic Review" Biology 13, no. 12: 978. https://doi.org/10.3390/biology13120978
APA StyleAhmad, M. F., Elias, M. H., Mat Jin, N., Abu, M. A., Syafruddin, S. E., Zainuddin, A. A., Suzuki, N., & Abdul Karim, A. K. (2024). Oocytes Quality Assessment—The Current Insight: A Systematic Review. Biology, 13(12), 978. https://doi.org/10.3390/biology13120978






