Brain–Periphery Axes: The Potential Role of Extracellular Vesicles-Delivered miRNAs
Simple Summary
Abstract
1. Introduction
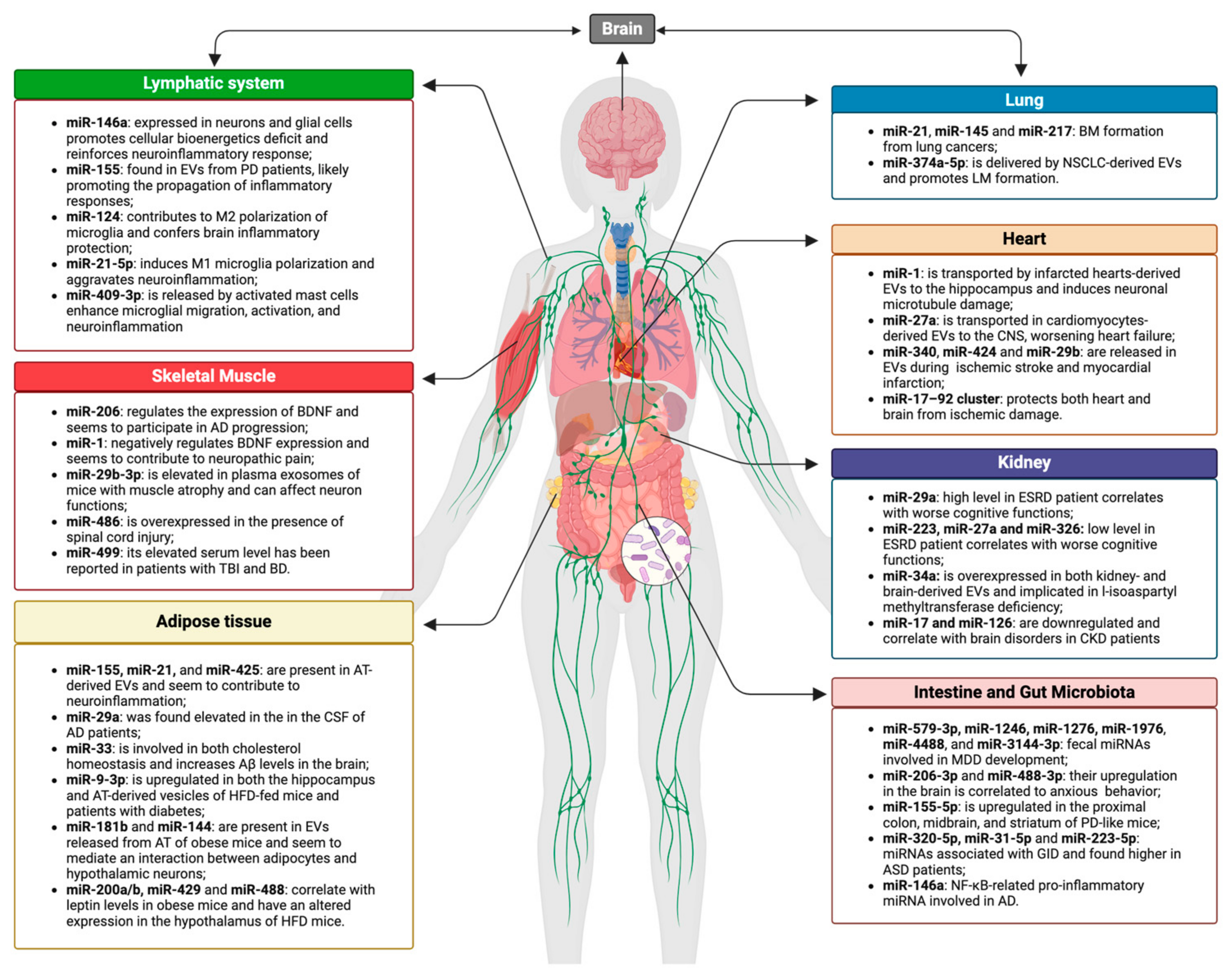
2. Gut–Brain Axis
2.1. Gut–Brain–Microbiota Axis and EVs in Neurologic Disorders
2.2. Gut–Brain–Microbiota Axis and miRNAs in Neurologic Disorders
3. Lung–Brain Axis
4. Heart–Brain Axis
5. Skeletal Muscle–Brain Axis
6. Adipose Tissue–Brain Axis
7. Kidney–Brain Axis
8. Immune–Brain Axis
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Ingolia, N.T.; Weissman, J.S.; Bartel, D.P. Mammalian microRNAs Predominantly Act to Decrease Target mRNA Levels. Nature 2010, 466, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Lytle, J.R.; Yario, T.A.; Steitz, J.A. Target mRNAs Are Repressed as Efficiently by microRNA-Binding Sites in the 5′ UTR as in the 3′ UTR. Proc. Natl. Acad. Sci. USA 2007, 104, 9667–9672. [Google Scholar] [CrossRef] [PubMed]
- Ørom, U.A.; Nielsen, F.C.; Lund, A.H. MicroRNA-10a Binds the 5′UTR of Ribosomal Protein mRNAs and Enhances Their Translation. Mol. Cell 2008, 30, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Forman, J.J.; Legesse-Miller, A.; Coller, H.A. A Search for Conserved Sequences in Coding Regions Reveals That the Let-7 microRNA Targets Dicer within Its Coding Sequence. Proc. Natl. Acad. Sci. USA 2008, 105, 14879–14884. [Google Scholar] [CrossRef]
- Jung, H.M.; Patel, R.S.; Phillips, B.L.; Wang, H.; Cohen, D.M.; Reinhold, W.C.; Chang, L.-J.; Yang, L.-J.; Chan, E.K.L. Tumor Suppressor miR-375 Regulates MYC Expression via Repression of CIP2A Coding Sequence through Multiple miRNA–mRNA Interactions. Mol. Biol. Cell 2013, 24, 1638–1648. [Google Scholar] [CrossRef]
- Place, R.F.; Li, L.-C.; Pookot, D.; Noonan, E.J.; Dahiya, R. MicroRNA-373 Induces Expression of Genes with Complementary Promoter Sequences. Proc. Natl. Acad. Sci. USA 2008, 105, 1608–1613. [Google Scholar] [CrossRef]
- Cai, R.; Qimuge, N.; Ma, M.; Wang, Y.; Tang, G.; Zhang, Q.; Sun, Y.; Chen, X.; Yu, T.; Dong, W.; et al. MicroRNA-664-5p Promotes Myoblast Proliferation and Inhibits Myoblast Differentiation by Targeting Serum Response Factor and Wnt1. J. Biol. Chem. 2018, 293, 19177–19190. [Google Scholar] [CrossRef]
- Alles, J.; Fehlmann, T.; Fischer, U.; Backes, C.; Galata, V.; Minet, M.; Hart, M.; Abu-Halima, M.; Grässer, F.A.; Lenhof, H.-P.; et al. An Estimate of the Total Number of True Human miRNAs. Nucleic Acids Res. 2019, 47, 3353–3364. [Google Scholar] [CrossRef]
- Upreti, A.; Hoang, T.V.; Li, M.; Tangeman, J.A.; Dierker, D.S.; Wagner, B.D.; Tsonis, P.A.; Liang, C.; Lachke, S.A.; Robinson, M.L. miR-26 Deficiency Causes Alterations in Lens Transcriptome and Results in Adult-Onset Cataract. Investig. Ophthalmol. Vis. Sci. 2024, 65, 42. [Google Scholar] [CrossRef]
- Stanczyk, J.; Pedrioli, D.M.; Brentano, F.; Sanchez-Pernaute, O.; Kolling, C.; Gay, R.E.; Detmar, M.; Gay, S.; Kyburz, D. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 2008, 58, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhen, Y.; Wang, R.; Li, X.; Huang, S.; Zhong, H.; Wen, H.; Sun, Q. Downregulation of miRNA miR-1305 and upregulation of miRNA miR-6785-5p may be associated with psoriasis. Front. Genet. 2022, 13, 891465. [Google Scholar] [CrossRef] [PubMed]
- Dou, P.; He, Y.; Yu, B.; Duan, J. Downregulation of microRNA-29b by DNMT3B decelerates chondrocyte apoptosis and the progression of osteoarthritis via PTHLH/CDK4/RUNX2 axis. Aging 2020, 13, 7676–7690. [Google Scholar] [CrossRef] [PubMed]
- De Santis, G.; Ferracin, M.; Biondani, A.; Caniatti, L.; Rosaria Tola, M.; Castellazzi, M.; Zagatti, B.; Battistini, L.; Borsellino, G.; Fainardi, E.; et al. Altered miRNA expression in T regulatory cells in course of multiple sclerosis. J. Neuroimmunol. 2010, 226, 165–171. [Google Scholar] [CrossRef]
- Zummo, L.; Vitale, A.M.; Caruso Bavisotto, C.; De Curtis, M.; Garbelli, R.; Giallonardo, A.T.; Di Bonaventura, C.; Fanella, M.; Conway De Macario, E.; Cappello, F.; et al. Molecular Chaperones and miRNAs in Epilepsy: Pathogenic Implications and Therapeutic Prospects. Int. J. Mol. Sci. 2021, 22, 8601. [Google Scholar] [CrossRef]
- Zhao, G.; Jing, X.; Li, Z.; Wu, X.; Gao, Z.; Ma, R. The Diagnostic and Prognostic Values of Circulating miRNA-1246 in Multiple Myeloma. Hematology 2022, 27, 778–784. [Google Scholar] [CrossRef]
- Hung, Y.H.; Sethupathy, P. Important Considerations for Studies of Circulating MicroRNAs in Clinical Samples. EBioMedicine 2017, 24, 22–23. [Google Scholar] [CrossRef]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef]
- Arroyo, J.D.; Chevillet, J.R.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.S.; Bennett, C.F.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 Complexes Carry a Population of Circulating microRNAs Independent of Vesicles in Human Plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef]
- Turchinovich, A.; Weiz, L.; Langheinz, A.; Burwinkel, B. Characterization of Extracellular Circulating microRNA. Nucleic Acids Res. 2011, 39, 7223–7233. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal Information for Studies of Extracellular Vesicles (MISEV2023): From Basic to Advanced Approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Caruso Bavisotto, C.; Marino Gammazza, A.; Rappa, F.; Fucarino, A.; Pitruzzella, A.; David, S.; Campanella, C. EXOSOMES: Can Doctors Still Ignore Their Existence? EMBJ 2013, 8, 136–139. [Google Scholar] [CrossRef]
- David, S.; Vitale, A.M.; Fucarino, A.; Scalia, F.; Vergilio, G.; Conway de Macario, E.; Macario, A.J.L.; Caruso Bavisotto, C.; Pitruzzella, A. The Challenging Riddle about the Janus-Type Role of Hsp60 and Related Extracellular Vesicles and miRNAs in Carcinogenesis and the Promises of Its Solution. Appl. Sci. 2021, 11, 1175. [Google Scholar] [CrossRef]
- Vitale, A.M.; Santonocito, R.; Vergilio, G.; Marino Gammazza, A.; Campanella, C.; Conway De Macario, E.; Bucchieri, F.; Macario, A.J.L.; Caruso Bavisotto, C. Brain Tumor-Derived Extracellular Vesicles as Carriers of Disease Markers: Molecular Chaperones and MicroRNAs. Appl. Sci. 2020, 10, 6961. [Google Scholar] [CrossRef]
- Graziano, F.; Iacopino, D.G.; Cammarata, G.; Scalia, G.; Campanella, C.; Giannone, A.G.; Porcasi, R.; Florena, A.M.; Conway De Macario, E.; Macario, A.J.L.; et al. The Triad Hsp60-miRNAs-Extracellular Vesicles in Brain Tumors: Assessing Its Components for Understanding Tumorigenesis and Monitoring Patients. Appl. Sci. 2021, 11, 2867. [Google Scholar] [CrossRef]
- Fernández-Messina, L.; Rodríguez-Galán, A.; de Yébenes, V.G.; Gutiérrez-Vázquez, C.; Tenreiro, S.; Seabra, M.C.; Ramiro, A.R.; Sánchez-Madrid, F. Transfer of extracellular vesicle-microRNA controls germinal center reaction and antibody production. EMBO Rep. 2020, 21, e48925. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Karunakaran, D.; Geoffrion, M.; Cheng, H.S.; Tandoc, K.; Perisic Matic, L.; Hedin, U.; Maegdefessel, L.; Fish, J.E.; Rayner, K.J. Extracellular Vesicles Secreted by Atherogenic Macrophages Transfer MicroRNA to Inhibit Cell Migration. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 49–63. [Google Scholar] [CrossRef]
- Gong, M.; Yu, B.; Wang, J.; Wang, Y.; Liu, M.; Paul, C.; Millard, R.W.; Xiao, D.S.; Ashraf, M.; Xu, M. Mesenchymal stem cells release exosomes that transfer miRNAs to endothelial cells and promote angiogenesis. Oncotarget 2017, 8, 45200–45212. [Google Scholar] [CrossRef]
- Fon, E.A.; Edwards, R.H. Molecular mechanisms of neurotransmitter release. Muscle Nerve 2001, 24, 581–601. [Google Scholar] [CrossRef]
- Liu, Y.J.; Wang, C. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Commun. Signal. CCS 2023, 21, 77. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Wu, Z.; Huang, K.; Yang, C.; Yang, L. Brain–Heart Communication in Health and Diseases. Brain Res. Bull. 2022, 183, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Brain and Organ Communication. Effects of Crosstalk on Neurophysiology, 1st ed.; Mahajan, C., Kapoor, I., Prabhakar, H., Eds.; Academic Press: London, UK; Elsevier: London, UK, 2024. [Google Scholar]
- Zhou, W.; Zhao, L.; Mao, Z.; Wang, Z.; Zhang, Z.; Li, M. Bidirectional Communication Between the Brain and Other Organs: The Role of Extracellular Vesicles. Cell. Mol. Neurobiol. 2023, 43, 2675–2696. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, R.; Paladino, L.; Vitale, A.M.; D’Amico, G.; Zummo, F.P.; Pirrotta, P.; Raccosta, S.; Manno, M.; Accomando, S.; D’Arpa, F.; et al. Nanovesicular Mediation of the Gut–Brain Axis by Probiotics: Insights into Irritable Bowel Syndrome. Biology 2024, 13, 296. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef]
- Ramos-Zaldívar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalán, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular vesicles through the blood-brain barrier: A review. Fluids Barriers CNS 2022, 19, 60. [Google Scholar] [CrossRef]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef]
- Van Delen, M.; Derdelinckx, J.; Wouters, K.; Nelissen, I.; Cools, N. A systematic review and meta-analysis of clinical trials assessing safety and efficacy of human extracellular vesicle-based therapy. J. Extracell. Vesicles 2024, 13, e12458. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of Gut Bacteria on Human Health and Diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Bosch, T.C.G.; McFall-Ngai, M.J. Metaorganisms as the New Frontier. Zoology 2011, 114, 185–190. [Google Scholar] [CrossRef]
- Intili, G.; Paladino, L.; Rappa, F.; Alberti, G.; Plicato, A.; Calabrò, F.; Fucarino, A.; Cappello, F.; Bucchieri, F.; Tomasello, G.; et al. From Dysbiosis to Neurodegenerative Diseases through Different Communication Pathways: An Overview. Biology 2023, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Fucarino, A.; Burgio, S.; Paladino, L.; Caruso Bavisotto, C.; Pitruzzella, A.; Bucchieri, F.; Cappello, F. The Microbiota Is Not an Organ: Introducing the Muco-Microbiotic Layer as a Novel Morphofunctional Structure. Anatomia 2022, 1, 186–203. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Potential Pathways of Abnormal Tau and α-Synuclein Dissemination in Sporadic Alzheimer’s and Parkinson’s Diseases. Cold Spring Harb. Perspect. Biol. 2016, 8, a023630. [Google Scholar] [CrossRef]
- Holmqvist, S.; Chutna, O.; Bousset, L.; Aldrin-Kirk, P.; Li, W.; Björklund, T.; Wang, Z.Y.; Roybon, L.; Melki, R.; Li, J.Y. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014, 128, 805–820. [Google Scholar] [CrossRef]
- Macia, L.; Nanan, R.; Hosseini-Beheshti, E.; Grau, G.E. Host- and Microbiota-Derived Extracellular Vesicles, Immune Function, and Disease Development. Int. J. Mol. Sci. 2019, 21, 107. [Google Scholar] [CrossRef]
- Duarte-Silva, E.; Oriá, A.C.; Mendonça, I.P.; De Melo, M.G.; Paiva, I.H.R.; Maes, M.; Joca, S.R.L.; Peixoto, C.A. Tiny in Size, Big in Impact: Extracellular Vesicles as Modulators of Mood, Anxiety and Neurodevelopmental Disorders. Neurosci. Biobehav. Rev. 2022, 135, 104582. [Google Scholar] [CrossRef]
- Cuesta, C.M.; Guerri, C.; Ureña, J.; Pascual, M. Role of Microbiota-Derived Extracellular Vesicles in Gut-Brain Communication. Int. J. Mol. Sci. 2021, 22, 4235. [Google Scholar] [CrossRef]
- Gurtan, A.M.; Sharp, P.A. The Role of miRNAs in Regulating Gene Expression Networks. J. Mol. Biol. 2013, 425, 3582–3600. [Google Scholar] [CrossRef]
- Moloney, G.M.; Dinan, T.G.; Clarke, G.; Cryan, J.F. Microbial Regulation of microRNA Expression in the Brain–Gut Axis. Curr. Opin. Pharmacol. 2019, 48, 120–126. [Google Scholar] [CrossRef]
- Blume, J.; Douglas, S.D.; Evans, D.L. Immune Suppression and Immune Activation in Depression. Brain Behav. Immun. 2011, 25, 221–229. [Google Scholar] [CrossRef]
- Lee, K.-E.; Kim, J.-K.; Han, S.-K.; Lee, D.Y.; Lee, H.-J.; Yim, S.-V.; Kim, D.-H. The Extracellular Vesicle of Gut Microbial Paenalcaligenes Hominis Is a Risk Factor for Vagus Nerve-Mediated Cognitive Impairment. Microbiome 2020, 8, 107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Chung, Y.-C.E.; Chen, H.-C.; Liu, Y.-W.; Chen, I.-M.; Lu, M.-L.; Hsiao, F.S.-H.; Chen, C.-H.; Huang, M.-C.; Shih, W.-L.; et al. Exploration of the Relationship between Gut Microbiota and Fecal microRNAs in Patients with Major Depressive Disorder. Sci. Rep. 2022, 12, 20977. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, J.; Gao, Z.; Zhang, Y.; Gu, J. Gut Microbiota-Induced microRNA-206-3p Increases Anxiety-like Behaviors by Inhibiting Expression of Cited2 and STK39. Microb. Pathog. 2023, 176, 106008. [Google Scholar] [CrossRef]
- Chen, S.; Li, M.; Tong, C.; Wang, Y.; He, J.; Shao, Q.; Liu, Y.; Wu, Y.; Song, Y. Regulation of miRNA Expression in the Prefrontal Cortex by Fecal Microbiota Transplantation in Anxiety-like Mice. Front. Psychiatry 2024, 15, 1323801. [Google Scholar] [CrossRef]
- Balestrino, R.; Schapira, A.H.V. Parkinson Disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Yang, Y.; Stewart, T.; Zhang, C.; Wang, P.; Xu, Z.; Jin, J.; Huang, Y.; Liu, Z.; Lan, G.; Liang, X.; et al. Erythrocytic α-Synuclein and the Gut Microbiome: Kindling of the Gut-Brain Axis in Parkinson’s Disease. Mov. Disord. 2024, 39, 40–52. [Google Scholar] [CrossRef]
- Lee, Y.Z.; Cheng, S.-H.; Chang, M.-Y.; Lin, Y.-F.; Wu, C.-C.; Tsai, Y.-C. Neuroprotective Effects of Lactobacillus Plantarum PS128 in a Mouse Model of Parkinson’s Disease: The Role of Gut Microbiota and MicroRNAs. Int. J. Mol. Sci. 2023, 24, 6794. [Google Scholar] [CrossRef]
- Calabrò, M.; Rinaldi, C.; Santoro, G.; Crisafulli, C. The Biological Pathways of Alzheimer Disease: A Review. AIMS Neurosci. 2020, 8, 86. [Google Scholar] [CrossRef]
- Lukiw, W.J. Bacteroides Fragilis Lipopolysaccharide and Inflammatory Signaling in Alzheimer’s Disease. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Zhao, Y.; Lukiw, W.J. Microbiome-Mediated Upregulation of MicroRNA-146a in Sporadic Alzheimer’s Disease. Front. Neurol. 2018, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Zhao, Y.; Clement, C.; Neumann, D.M.; Lukiw, W.J. HSV-1 Infection of Human Brain Cells Induces miRNA-146a and Alzheimer-Type Inflammatory Signaling. NeuroReport 2009, 20, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Bezawada, N.; Phang, T.H.; Hold, G.L.; Hansen, R. Autism Spectrum Disorder and the Gut Microbiota in Children: A Systematic Review. Ann. Nutr. Metab. 2020, 76, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; Preda, A.; Blottière, H.M.; Clarke, G.; Albani, D.; Belcastro, V.; Carotenuto, M.; Cattaneo, A.; Citraro, R.; Ferraris, C.; et al. Microbiota-Gut Brain Axis Involvement in Neuropsychiatric Disorders. Expert. Rev. Neurother. 2019, 19, 1037–1050. [Google Scholar] [CrossRef]
- Guiducci, L.; Cabiati, M.; Santocchi, E.; Prosperi, M.; Morales, M.A.; Muratori, F.; Randazzo, E.; Federico, G.; Calderoni, S.; Del Ry, S. Expression of miRNAs in Pre-Schoolers with Autism Spectrum Disorders Compared with Typically Developing Peers and Its Effects after Probiotic Supplementation. J. Clin. Med. 2023, 12, 7162. [Google Scholar] [CrossRef]
- Unlu, H.T.; Saridas, F.; Taskapilioglu, O.; Cecener, G.; Egeli, U.; Turan, O.F.; Tunca, B.; Zarifoglu, M. Investigation of miR-146a Expression Profiles in Fecal Samples of Patients With Multiple Sclerosis for Early Diagnosis and Treatment. Neurol. Sci. Neurophys. 2023, 40, 81–87. [Google Scholar] [CrossRef]
- Sarshar, M.; Scribano, D.; Ambrosi, C.; Palamara, A.T.; Masotti, A. Fecal microRNAs as Innovative Biomarkers of Intestinal Diseases and Effective Players in Host-Microbiome Interactions. Cancers 2020, 12, 2174. [Google Scholar] [CrossRef]
- Martellucci, S.; Orefice, N.S.; Angelucci, A.; Luce, A.; Caraglia, M.; Zappavigna, S. Extracellular Vesicles: New Endogenous Shuttles for miRNAs in Cancer Diagnosis and Therapy? Int. J. Mol. Sci. 2020, 21, 6486. [Google Scholar] [CrossRef]
- Du, S.; Guan, Y.; Xie, A.; Yan, Z.; Gao, S.; Li, W.; Rao, L.; Chen, X.; Chen, T. Extracellular vesicles: A rising star for therapeutics and drug delivery. J. Nanobiotechnol. 2023, 21, 231. [Google Scholar] [CrossRef]
- Azzoni, R.; Marsland, B.J. The Lung-Brain Axis: A New Frontier in Host-Microbe Interactions. Immunity 2022, 55, 589–591. [Google Scholar] [CrossRef]
- Xie, X.; Wang, L.; Dong, S.; Ge, S.; Zhu, T. Immune Regulation of the Gut-Brain Axis and Lung-Brain Axis Involved in Ischemic Stroke. Neural Regen. Res. 2024, 19, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Starke, N.; Challa, N.V.D.; Yuan, H.; Chen, S.; Duncan, M.R.; Cabrera Ranaldi, E.D.L.R.M.; De Rivero Vaccari, J.P.; Schott, A.; Aguilar, A.C.; Lee, Y.-S.; et al. Extracellular Vesicle ASC: A Novel Mediator for Lung–Brain Axis in Preterm Brain Injury. Am. J. Respir. Cell Mol. Biol. 2024, 71, 464–480. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guan, S.; Lu, P.; Li, Y.; Xu, H. Extracellular Vesicles: Critical Bilateral Communicators in Periphery-Brain Crosstalk in Central Nervous System Disorders. Biomed. Pharmacother. 2023, 160, 114354. [Google Scholar] [CrossRef]
- Chung, J.-W.; Cho, Y.H.; Ahn, M.-J.; Lee, M.J.; Kim, G.-M.; Chung, C.-S.; Bang, O.Y. Association of Cancer Cell Type and Extracellular Vesicles With Coagulopathy in Patients With Lung Cancer and Stroke. Stroke 2018, 49, 1282–1285. [Google Scholar] [CrossRef]
- Norouzi-Barough, L.; Asgari Khosroshahi, A.; Gorji, A.; Zafari, F.; Shahverdi Shahraki, M.; Shirian, S. COVID-19-Induced Stroke and the Potential of Using Mesenchymal Stem Cells-Derived Extracellular Vesicles in the Regulation of Neuroinflammation. Cell. Mol. Neurobiol. 2023, 43, 37–46. [Google Scholar] [CrossRef]
- Kerr, N.; De Rivero Vaccari, J.P.; Dietrich, W.D.; Keane, R.W. Neural-Respiratory Inflammasome Axis in Traumatic Brain Injury. Exp. Neurol. 2020, 323, 113080. [Google Scholar] [CrossRef]
- Mascia, L. Acute Lung Injury in Patients with Severe Brain Injury: A Double Hit Model. Neurocrit. Care 2009, 11, 417–426. [Google Scholar] [CrossRef]
- Samary, C.S.; Ramos, A.B.; Maia, L.A.; Rocha, N.N.; Santos, C.L.; Magalhães, R.F.; Clevelario, A.L.; Pimentel-Coelho, P.M.; Mendez-Otero, R.; Cruz, F.F.; et al. Focal Ischemic Stroke Leads to Lung Injury and Reduces Alveolar Macrophage Phagocytic Capability in Rats. Crit. Care 2018, 22, 249. [Google Scholar] [CrossRef]
- Li, C.; Chen, W.; Lin, F.; Li, W.; Wang, P.; Liao, G.; Zhang, L. Functional Two-Way Crosstalk Between Brain and Lung: The Brain–Lung Axis. Cell. Mol. Neurobiol. 2023, 43, 991–1003. [Google Scholar] [CrossRef]
- Morgan, A.D.; Sharma, C.; Rothnie, K.J.; Potts, J.; Smeeth, L.; Quint, J.K. Chronic Obstructive Pulmonary Disease and the Risk of Stroke. Ann. Am. Thorac. Soc. 2017, 14, 754. [Google Scholar] [CrossRef]
- Groot, M.; Lee, H. Sorting Mechanisms for MicroRNAs into Extracellular Vesicles and Their Associated Diseases. Cells 2020, 9, 1044. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martínez, M.; Lorente-Sorolla, C.; Naharro, S.; Rodrigo-Muñoz, J.M.; Del Pozo, V. Advances and Highlights of miRNAs in Asthma: Biomarkers for Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 1628. [Google Scholar] [CrossRef]
- He, L.; Liu, J.; Wang, X.; Wang, Y.; Zhu, J.; Kang, X. Identifying a Novel Serum microRNA Biomarker Panel for the Diagnosis of Childhood Asthma. Exp. Biol. Med. 2022, 247, 1732–1740. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, C.; Dewanjee, S.; Bhattacharya, H.; Chakraborty, P.; Jha, N.K.; Gangopadhyay, M.; Jha, S.K.; Liu, Q. Tumor-Derived Small Extracellular Vesicles in Cancer Invasion and Metastasis: Molecular Mechanisms, and Clinical Significance. Mol. Cancer 2024, 23, 18. [Google Scholar] [CrossRef]
- Siegl, F.; Vecera, M.; Roskova, I.; Smrcka, M.; Jancalek, R.; Kazda, T.; Slaby, O.; Sana, J. The Significance of MicroRNAs in the Molecular Pathology of Brain Metastases. Cancers 2022, 14, 3386. [Google Scholar] [CrossRef]
- Jin, J.; Cui, Y.; Niu, H.; Lin, Y.; Wu, X.; Qi, X.; Bai, K.; Zhang, Y.; Wang, Y.; Bu, H. NSCLC Extracellular Vesicles Containing miR-374a-5p Promote Leptomeningeal Metastasis by Influencing Blood–Brain Barrier Permeability. Mol. Cancer Res. 2024, 22, 699–710. [Google Scholar] [CrossRef]
- Hudson, K.; Mondia, M.W.; Zhang, Y.; Saha, S.; Gibert, M.K.; Dube, C.; Sun, Y.; Marcinkiewicz, P.; Fadul, C.; Abounader, R. The Role of microRNAs in Brain Metastasis. J. Neurooncol. 2024, 166, 231–241. [Google Scholar] [CrossRef]
- Bautista-Sánchez, D.; Arriaga-Canon, C.; Pedroza-Torres, A.; De La Rosa-Velázquez, I.A.; González-Barrios, R.; Contreras-Espinosa, L.; Montiel-Manríquez, R.; Castro-Hernández, C.; Fragoso-Ontiveros, V.; Álvarez-Gómez, R.M.; et al. The Promising Role of miR-21 as a Cancer Biomarker and Its Importance in RNA-Based Therapeutics. Mol. Ther. Nucleic Acids 2020, 20, 409–420. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Z.; Gu, T.; Xu, S.-F.; Dong, L.-X.; Li, X.; Fu, B.-H.; Fu, Z.-Z. The Role of microRNA-21 in Predicting Brain Metastases from Non-Small Cell Lung Cancer. OncoTargets Ther. 2016, 10, 185. [Google Scholar] [CrossRef]
- Zhu, Z.; Li, Q.; Xu, M.; Qi, Z. Effect of Whole-Brain and Intensity-Modulated Radiotherapy on Serum Levels of miR-21 and Prognosis for Lung Cancer Metastatic to the Brain. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e924640. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, Y.; Zhang, Y.; Tan, W.; Xue, J.; Yang, Z.; Zhang, Y.; Lu, Y.; Hu, X. Downregulation of miR-145 Contributes to Lung Adenocarcinoma Cell Growth to Form Brain Metastases. Oncol. Rep. 2013, 30, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Hou, L.; Wei, J.; Du, Y.; Zhao, Y.; Deng, X.; Lin, X. Hsa-miR-217 Inhibits the Proliferation, Migration, and Invasion in Non-Small Cell Lung Cancer Cells Via Targeting SIRT1 and P53/KAI1 Signaling. Balk. Med. J. 2020, 37, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zheng, H.; Xiong, J.; Huang, Y.; Li, H.; Jin, H.; Ai, S.; Wang, Y.; Su, T.; Sun, G.; et al. miR-596-3p suppresses brain metastasis of non-small cell lung cancer by modulating YAP1 and IL-8. Cell Death Dis. 2022, 13, 699. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, L.; Wang, Z.; Yan, K.; Zhao, L.; Xiao, W. Research Progress on Transorgan Regulation of the Cardiovascular and Motor System through Cardiogenic Exosomes. Int. J. Mol. Sci. 2022, 23, 5765. [Google Scholar] [CrossRef]
- Saheera, S.; Jani, V.P.; Witwer, K.W.; Kutty, S. Extracellular Vesicle Interplay in Cardiovascular Pathophysiology. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1749–H1761. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, G.; Liang, Y.; He, S.; Lyu, M.; Zhu, Y. Heart–Brain Interaction in Cardiogenic Dementia: Pathophysiology and Therapeutic Potential. Front. Cardiovasc. Med. 2024, 11, 1304864. [Google Scholar] [CrossRef]
- Ciesla, M.; Skrzypek, K.; Kozakowska, M.; Loboda, A.; Jozkowicz, A.; Dulak, J. MicroRNAs as Biomarkers of Disease Onset. Anal. Bioanal. Chem. 2011, 401, 2051–2061. [Google Scholar] [CrossRef]
- Sun, L.-L.; Duan, M.-J.; Ma, J.-C.; Xu, L.; Mao, M.; Biddyut, D.; Wang, Q.; Yang, C.; Zhang, S.; Xu, Y.; et al. Myocardial Infarction-Induced Hippocampal Microtubule Damage by Cardiac Originating microRNA-1 in Mice. J. Mol. Cell. Cardiol. 2018, 120, 12–27. [Google Scholar] [CrossRef]
- Tian, C.; Gao, L.; Rudebush, T.L.; Yu, L.; Zucker, I.H. Extracellular Vesicles Regulate Sympatho-Excitation by Nrf2 in Heart Failure. Circ. Res. 2022, 131, 687–700. [Google Scholar] [CrossRef]
- Franchina, G.; Tripodi, V.F.; Mazzeo, A.T. Crosstalk between brain and the heart. In Brain and Organ Communication. Effects of Crosstalk on Neurophysiology, 1st ed.; Mahajan, C., Kapoor, I., Prabhakar, H., Eds.; Academic Press: London, UK; Elsevier: London, UK, 2024; Chapter 3; pp. 45–64. [Google Scholar]
- Otero-Ortega, L.; Alonso-López, E.; Pérez-Mato, M.; Laso-García, F.; Gómez-de Frutos, M.C.; Diekhorst, L.; García-Bermejo, M.L.; Conde-Moreno, E.; Fuentes, B.; Alonso De Leciñana, M.; et al. Similarities and Differences in Extracellular Vesicle Profiles between Ischaemic Stroke and Myocardial Infarction. Biomedicines 2020, 9, 8. [Google Scholar] [CrossRef]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N.E.; Timmers, L.; van Rijen, H.V.; Doevendans, P.A.; Pasterkamp, G.; et al. Mesenchymal Stem Cell-Derived Exosomes Increase ATP Levels, Decrease Oxidative Stress and Activate PI3K/Akt Pathway to Enhance Myocardial Viability and Prevent Adverse Remodeling after Myocardial Ischemia/Reperfusion Injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, W.; Wani, M.; Yu, X.; Ashraf, M. Ischemic Preconditioning Potentiates the Protective Effect of Stem Cells through Secretion of Exosomes by Targeting Mecp2 via miR-22. PLoS ONE 2014, 9, e88685. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Kim, H.W.; Gong, M.; Wang, J.; Millard, R.W.; Wang, Y.; Ashraf, M.; Xu, M. Exosomes Secreted from GATA-4 Overexpressing Mesenchymal Stem Cells Serve as a Reservoir of Anti-Apoptotic microRNAs for Cardioprotection. Int. J. Cardiol. 2015, 182, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Z.-P.; Seok, H.Y.; Ding, J.; Kataoka, M.; Zhang, Z.; Hu, X.; Wang, G.; Lin, Z.; Wang, S.; et al. Mir-17–92 Cluster Is Required for and Sufficient to Induce Cardiomyocyte Proliferation in Postnatal and Adult Hearts. Circ. Res. 2013, 112, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Chopp, M.; Pang, H.; Zhang, Z.G.; Mahmood, A.; Xiong, Y. MiR-17-92 Cluster-Enriched Exosomes Derived from Human Bone Marrow Mesenchymal Stromal Cells Improve Tissue and Functional Recovery in Rats after Traumatic Brain Injury. J. Neurotrauma 2021, 38, 1535–1550. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Cai, J.; Tang, Y.; Zhao, Q. MiR-17-92 Cluster Is a Novel Regulatory Gene of Cardiac Ischemic/Reperfusion Injury. Med. Hypotheses 2013, 81, 108–110. [Google Scholar] [CrossRef]
- Frontera, W.R.; Ochala, J. Skeletal Muscle: A Brief Review of Structure and Function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle-Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Carson, B.P. The Potential Role of Contraction-Induced Myokines in the Regulation of Metabolic Function for the Prevention and Treatment of Type 2 Diabetes. Front. Endocrinol. 2017, 8, 97. [Google Scholar] [CrossRef]
- García-Mesa, Y.; López-Ramos, J.C.; Giménez-Llort, L.; Revilla, S.; Guerra, R.; Gruart, A.; Laferla, F.M.; Cristòfol, R.; Delgado-García, J.M.; Sanfeliu, C. Physical Exercise Protects against Alzheimer’s Disease in 3xTg-AD Mice. J. Alzheimers Dis. 2011, 24, 421–454. [Google Scholar] [CrossRef]
- Consorti, A.; Di Marco, I.; Sansevero, G. Physical Exercise Modulates Brain Physiology Through a Network of Long- and Short-Range Cellular Interactions. Front. Mol. Neurosci. 2021, 14, 710303. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Khan, R.; Cortes, C.J. Forgot to Exercise? Exercise Derived Circulating Myokines in Alzheimer’s Disease: A Perspective. Front. Neurol. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.J.; Erickson, K.I.; Raz, N.; Webb, A.G.; Cohen, N.J.; McAuley, E.; Kramer, A.F. Aerobic Fitness Reduces Brain Tissue Loss in Aging Humans. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Berchtold, N.C. Physical Activity and the Maintenance of Cognition: Learning from Animal Models. Alzheimers Dement. 2007, 3, S30–S37. [Google Scholar] [CrossRef] [PubMed]
- Marosi, K.; Bori, Z.; Hart, N.; Sárga, L.; Koltai, E.; Radák, Z.; Nyakas, C. Long-Term Exercise Treatment Reduces Oxidative Stress in the Hippocampus of Aging Rats. Neuroscience 2012, 226, 21–28. [Google Scholar] [CrossRef]
- Steiner, J.L.; Murphy, E.A.; McClellan, J.L.; Carmichael, M.D.; Davis, J.M. Exercise Training Increases Mitochondrial Biogenesis in the Brain. J. Appl. Physiol. 2011, 111, 1066–1071. [Google Scholar] [CrossRef]
- Han, X.; Ashraf, M.; Tipparaju, S.M.; Xuan, W. Muscle–Brain Crosstalk in Cognitive Impairment. Front. Aging Neurosci. 2023, 15. [Google Scholar] [CrossRef]
- Tastan, B.; Tarakcioglu, E.; Birinci, Y.; Park, Y.; Genc, S. Role of Exosomal MicroRNAs in Cell-to-Cell Communication. Methods Mol. Biol. 2022, 2257, 269–292. [Google Scholar] [CrossRef]
- Tsujimura, K.; Shiohama, T.; Takahashi, E. microRNA Biology on Brain Development and Neuroimaging Approach. Brain Sci. 2022, 12, 1366. [Google Scholar] [CrossRef]
- Panaro, M.A.; Benameur, T.; Porro, C. Extracellular Vesicles miRNA Cargo for Microglia Polarization in Traumatic Brain Injury. Biomolecules 2020, 10, 901. [Google Scholar] [CrossRef]
- McCarthy, J.J. MicroRNA-206: The Skeletal Muscle-Specific myomiR. Biochim. Biophys. Acta 2008, 1779, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Margolis, L.M.; McClung, H.L.; Murphy, N.E.; Carrigan, C.T.; Pasiakos, S.M. Skeletal Muscle myomiR Are Differentially Expressed by Endurance Exercise Mode and Combined Essential Amino Acid and Carbohydrate Supplementation. Front. Physiol. 2017, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, S.; Marcuzzo, S.; Malacarne, C.; Giagnorio, E.; Masson, R.; Zanin, R.; Arnoldi, M.T.; Andreetta, F.; Simoncini, O.; Venerando, A.; et al. Circulating MyomiRs as Potential Biomarkers to Monitor Response to Nusinersen in Pediatric SMA Patients. Biomedicines 2020, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, K.; Chen, J.; Guo, J.; Yin, Y.; Cai, X.; Guo, X.; Wang, G.; Yang, R.; Zhu, L.; et al. In Vitro Evidence Suggests That miR-133a-Mediated Regulation of Uncoupling Protein 2 (UCP2) Is an Indispensable Step in Myogenic Differentiation. J. Biol. Chem. 2009, 284, 5362–5369. [Google Scholar] [CrossRef]
- Feng, Y.; Niu, L.-L.; Wei, W.; Zhang, W.-Y.; Li, X.-Y.; Cao, J.-H.; Zhao, S.-H. A Feedback Circuit between miR-133 and the ERK1/2 Pathway Involving an Exquisite Mechanism for Regulating Myoblast Proliferation and Differentiation. Cell Death Dis. 2013, 4, e934. [Google Scholar] [CrossRef]
- Lee, S.-T.; Chu, K.; Jung, K.-H.; Kim, J.H.; Huh, J.-Y.; Yoon, H.; Park, D.-K.; Lim, J.-Y.; Kim, J.-M.; Jeon, D.; et al. miR-206 Regulates Brain-Derived Neurotrophic Factor in Alzheimer Disease Model. Ann. Neurol. 2012, 72, 269–277. [Google Scholar] [CrossRef]
- Grieb, A.; Schmitt, A.; Fragasso, A.; Widmann, M.; Mattioni Maturana, F.; Burgstahler, C.; Erz, G.; Schellhorn, P.; Nieß, A.M.; Munz, B. Skeletal Muscle MicroRNA Patterns in Response to a Single Bout of Exercise in Females: Biomarkers for Subsequent Training Adaptation? Biomolecules 2023, 13, 884. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Li, P.; Bai, F.; Liu, C.; Huang, X. Serum Exosomes miR-206 and miR-549a-3p as Potential Biomarkers of Traumatic Brain Injury. Sci. Rep. 2024, 14, 10082. [Google Scholar] [CrossRef]
- Shi, G.; Zeng, P.; Zhao, Q.; Zhao, J.; Xie, Y.; Wen, D.; Yan, L.; Gu, H.; Ma, S.; Cai, X. The Regulation of miR-206 on BDNF: A Motor Function Restoration Mechanism Research on Cerebral Ischemia Rats by Meridian Massage. Evid. Based Complement. Altern. Med. 2022, 2022, 8172849. [Google Scholar] [CrossRef]
- Coull, J.A.M.; Beggs, S.; Boudreau, D.; Boivin, D.; Tsuda, M.; Inoue, K.; Gravel, C.; Salter, M.W.; De Koninck, Y. BDNF from Microglia Causes the Shift in Neuronal Anion Gradient Underlying Neuropathic Pain. Nature 2005, 438, 1017–1021. [Google Scholar] [CrossRef]
- Varendi, K.; Kumar, A.; Härma, M.-A.; Andressoo, J.-O. miR-1, miR-10b, miR-155, and miR-191 Are Novel Regulators of BDNF. Cell. Mol. Life Sci. 2014, 71, 4443–4456. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.-X.; Lin, Q.-X.; Deng, C.-Y.; Zhu, J.-N.; Mai, L.-P.; Liu, J.-L.; Fu, Y.-H.; Liu, X.-Y.; Li, Y.-X.; Zhang, Y.-Y.; et al. miR-1/miR-206 Regulate Hsp60 Expression Contributing to Glucose-Mediated Apoptosis in Cardiomyocytes. FEBS Lett. 2010, 584, 3592–3600. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-P.; Yang, W.-S.; Wong, Y.-H.; Wang, K.-H.; Teng, Y.-C.; Chang, M.-H.; Liao, K.-H.; Nian, F.-S.; Chao, C.-C.; Tsai, J.-W.; et al. Muscle Atrophy-Related Myotube-Derived Exosomal microRNA in Neuronal Dysfunction: Targeting Both Coding and Long Noncoding RNAs. Aging Cell 2020, 19, e13107. [Google Scholar] [CrossRef] [PubMed]
- Samani, A.; Hightower, R.M.; Reid, A.L.; English, K.G.; Lopez, M.A.; Doyle, J.S.; Conklin, M.J.; Schneider, D.A.; Bamman, M.M.; Widrick, J.J.; et al. miR-486 Is Essential for Muscle Function and Suppresses a Dystrophic Transcriptome. Life Sci. Alliance 2022, 5, e202101215. [Google Scholar] [CrossRef]
- Jee, M.K.; Jung, J.S.; Choi, J.I.; Jang, J.A.; Kang, K.S.; Im, Y.B.; Kang, S.K. MicroRNA 486 Is a Potentially Novel Target for the Treatment of Spinal Cord Injury. Brain 2012, 135, 1237–1252. [Google Scholar] [CrossRef]
- Uittenbogaard, M.; Baxter, K.K.; Chiaramello, A. The Neurogenic Basic Helix-Loop-Helix Transcription Factor NeuroD6 Confers Tolerance to Oxidative Stress by Triggering an Antioxidant Response and Sustaining the Mitochondrial Biomass. ASN Neuro 2010, 2, e00034. [Google Scholar] [CrossRef]
- Wu, J.; Yue, B.; Lan, X.; Wang, Y.; Fang, X.; Ma, Y.; Bai, Y.; Qi, X.; Zhang, C.; Chen, H. MiR-499 Regulates Myoblast Proliferation and Differentiation by Targeting Transforming Growth Factor β Receptor 1. J. Cell. Physiol. 2019, 234, 2523–2536. [Google Scholar] [CrossRef]
- Yang, T.; Song, J.; Bu, X.; Wang, C.; Wu, J.; Cai, J.; Wan, S.; Fan, C.; Zhang, C.; Wang, J. Elevated Serum miR-93, miR-191, and miR-499 Are Noninvasive Biomarkers for the Presence and Progression of Traumatic Brain Injury. J. Neurochem. 2016, 137, 122–129. [Google Scholar] [CrossRef]
- Martins, H.C.; Gilardi, C.; Sungur, A.Ö.; Winterer, J.; Pelzl, M.A.; Bicker, S.; Gross, F.; Kisko, T.M.; Malikowska-Racia, N.; Braun, M.D.; et al. Bipolar-Associated miR-499-5p Controls Neuroplasticity by Downregulating the Cav1.2 Subunit CACNB2. EMBO Rep. 2022, 23, e54420. [Google Scholar] [CrossRef]
- Koutsoulidou, A.; Kyriakides, T.C.; Papadimas, G.K.; Christou, Y.; Kararizou, E.; Papanicolaou, E.Z.; Phylactou, L.A. Elevated Muscle-Specific miRNAs in Serum of Myotonic Dystrophy Patients Relate to Muscle Disease Progress. PLoS ONE 2015, 10, e0125341. [Google Scholar] [CrossRef]
- Tasca, E.; Pegoraro, V.; Merico, A.; Angelini, C. Circulating microRNAs as Biomarkers of Muscle Differentiation and Atrophy in ALS. Clin. Neuropathol. 2016, 35, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Pivonello, C.; Patalano, R.; Simeoli, C.; Montò, T.; Negri, M.; Amatrudo, F.; Paola, N.D.; Larocca, A.; Crescenzo, E.M.; Pirchio, R.; et al. Circulating myomiRNAs as Biomarkers in Patients with Cushing’s Syndrome. J. Endocrinol. Investig. 2023, 47, 655. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Flier, J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine Dysregulation and Adipose Tissue Inflammation in Human Obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Chen, J.; He, Y.; Ma, W.; Liu, X.; Sun, X. Effects of Multi-Organ Crosstalk on the Physiology and Pathology of Adipose Tissue. Front. Endocrinol. 2023, 14. [Google Scholar] [CrossRef]
- Sun, L.; Laurila, S.; Lahesmaa, M.; Rebelos, E.; Virtanen, K.A.; Schnabl, K.; Klingenspor, M.; Nummenmaa, L.; Nuutila, P. Secretin Modulates Appetite via Brown Adipose Tissue-Brain Axis. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 1597–1606. [Google Scholar] [CrossRef]
- Zhang, Y.; Chua, S. Leptin Function and Regulation. Compr. Physiol. 2017, 8, 351–369. [Google Scholar] [CrossRef]
- Lechan, R.M.; Fekete, C. The TRH Neuron: A Hypothalamic Integrator of Energy Metabolism. Prog. Brain Res. 2006, 153, 209–235. [Google Scholar] [CrossRef]
- Mastorakos, G.; Zapanti, E. The Hypothalamic-Pituitary-Adrenal Axis in the Neuroendocrine Regulation of Food Intake and Obesity: The Role of Corticotropin Releasing Hormone. Nutr. Neurosci. 2004, 7, 271–280. [Google Scholar] [CrossRef]
- Blandin, A.; Amosse, J.; Froger, J.; Hilairet, G.; Durcin, M.; Fizanne, L.; Ghesquière, V.; Prieur, X.; Chaigneau, J.; Vergori, L.; et al. Extracellular vesicles are carriers of adiponectin with insulin-sensitizing and anti-inflammatory properties. Cell Rep. 2023, 42, 112866. [Google Scholar] [CrossRef]
- Makrygianni, E.A.; Chrousos, G.P. Extracellular Vesicles and the Stress System. Neuroendocrinology 2023, 113, 120–167. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, X.; Gu, Y.; Zhou, G. Adipocyte-Derived Extracellular Vesicles: Small Vesicles with Big Impact. Front. Biosci. 2023, 28, 149. [Google Scholar] [CrossRef] [PubMed]
- Bond, S.T.; Calkin, A.C.; Drew, B.G. Adipose-Derived Extracellular Vesicles: Systemic Messengers and Metabolic Regulators in Health and Disease. Front. Physiol. 2022, 13, 837001. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, L.; Zhang, Z.; Zhang, X.; Zhu, Y.; Zhang, C.; Bi, Y. Extracellular Vesicles Mediate the Communication of Adipose Tissue with Brain and Promote Cognitive Impairment Associated with Insulin Resistance. Cell Metab. 2022, 34, 1264–1279.e8. [Google Scholar] [CrossRef]
- Deng, Z.; Poliakov, A.; Hardy, R.W.; Clements, R.; Liu, C.; Liu, Y.; Wang, J.; Xiang, X.; Zhang, S.; Zhuang, X.; et al. Adipose Tissue Exosome-like Vesicles Mediate Activation of Macrophage-Induced Insulin Resistance. Diabetes 2009, 58, 2498–2505. [Google Scholar] [CrossRef]
- Perdoncin, M.; Konrad, A.; Wyner, J.R.; Lohana, S.; Pillai, S.S.; Pereira, D.G.; Lakhani, H.V.; Sodhi, K. A Review of miRNAs as Biomarkers and Effect of Dietary Modulation in Obesity Associated Cognitive Decline and Neurodegenerative Disorders. Front. Mol. Neurosci. 2021, 14. [Google Scholar] [CrossRef]
- Payet, T.; Gabinaud, E.; Landrier, J.-F.; Mounien, L. Role of Micro-RNAs Associated with Adipose-Derived Extracellular Vesicles in Metabolic Disorders. Obes. Rev. 2024, 25, e13755. [Google Scholar] [CrossRef]
- Benavides-Aguilar, J.A.; Torres-Copado, A.; Isidoro-Sánchez, J.; Pathak, S.; Duttaroy, A.K.; Banerjee, A.; Paul, S. The Regulatory Role of MicroRNAs in Obesity and Obesity-Derived Ailments. Genes 2023, 14, 2070. [Google Scholar] [CrossRef]
- Müller, M.; Jäkel, L.; Bruinsma, I.B.; Claassen, J.A.; Kuiperij, H.B.; Verbeek, M.M. MicroRNA-29a Is a Candidate Biomarker for Alzheimer’s Disease in Cell-Free Cerebrospinal Fluid. Mol. Neurobiol. 2016, 53, 2894–2899. [Google Scholar] [CrossRef]
- Montesinos, J.; Pera, M.; Larrea, D.; Guardia-Laguarta, C.; Agrawal, R.R.; Velasco, K.R.; Yun, T.D.; Stavrovskaya, I.G.; Xu, Y.; Koo, S.Y.; et al. The Alzheimer’s Disease-Associated C99 Fragment of APP Regulates Cellular Cholesterol Trafficking. EMBO J. 2020, 39, e103791. [Google Scholar] [CrossRef]
- Gao, J.; Li, X.; Wang, Y.; Cao, Y.; Yao, D.; Sun, L.; Qin, L.; Qiu, H.; Zhan, X. Adipocyte-Derived Extracellular Vesicles Modulate Appetite and Weight through mTOR Signalling in the Hypothalamus. Acta Physiol. 2020, 228, e13339. [Google Scholar] [CrossRef] [PubMed]
- Crépin, D.; Benomar, Y.; Riffault, L.; Amine, H.; Gertler, A.; Taouis, M. The Over-Expression of miR-200a in the Hypothalamus of Ob/Ob Mice Is Linked to Leptin and Insulin Signaling Impairment. Mol. Cell. Endocrinol. 2014, 384, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sangiao-Alvarellos, S.; Pena-Bello, L.; Manfredi-Lozano, M.; Tena-Sempere, M.; Cordido, F.; Sangiao-Alvarellos, S.; Pena-Bello, L.; Manfredi-Lozano, M.; Tena-Sempere, M.; Cordido, F. Perturbation of Hypothalamic Microrna Expression Patterns in Male Rats after Metabolic Distress: Impact of Obesity and Conditions of Negative Energy Balance. Endocrinology 2014, 155, 1838–1850. [Google Scholar] [CrossRef] [PubMed]
- Derghal, A.; Djelloul, M.; Trouslard, J.; Mounien, L. An Emerging Role of Micro-RNA in the Effect of the Endocrine Disruptors. Front. Neurosci. 2016, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Okusa, M.D. Crosstalk between the Nervous System and the Kidney. Kidney Int. 2020, 97, 466–476. [Google Scholar] [CrossRef]
- Chen, X.; Yang, D.; Zhao, H.; Zhang, H.; Hong, P. Stroke-Induced Renal Dysfunction: Underlying Mechanisms and Challenges of the Brain–Kidney Axis. CNS Neurosci. Ther. 2024, 30, e70114. [Google Scholar] [CrossRef]
- Karpman, D.; Ståhl, A.; Arvidsson, I. Extracellular Vesicles in Renal Disease. Nat. Rev. Nephrol. 2017, 13, 545–562. [Google Scholar] [CrossRef]
- Lv, L.-L.; Feng, Y.; Wen, Y.; Wu, W.-J.; Ni, H.-F.; Li, Z.-L.; Zhou, L.-T.; Wang, B.; Zhang, J.-D.; Crowley, S.D.; et al. Exosomal CCL2 from Tubular Epithelial Cells Is Critical for Albumin-Induced Tubulointerstitial Inflammation. J. Am. Soc. Nephrol. 2018, 29, 919–935. [Google Scholar] [CrossRef]
- Bijkerk, R.; Kallenberg, M.H.; Zijlstra, L.E.; Van Den Berg, B.M.; De Bresser, J.; Hammer, S.; Bron, E.E.; Achterberg, H.; Van Buchem, M.A.; Berkhout-Byrne, N.C.; et al. Circulating Angiopoietin-2 and Angiogenic microRNAs Associate with Cerebral Small Vessel Disease and Cognitive Decline in Older Patients Reaching End-Stage Renal Disease. Nephrol. Dial. Transplant. 2022, 37, 498–506. [Google Scholar] [CrossRef]
- Su, Z.; Ren, N.; Ling, Z.; Sheng, L.; Zhou, S.; Guo, C.; Ke, Z.; Xu, T.; Qin, Z. Differential Expression of microRNAs Associated with Neurodegenerative Diseases and Diabetic Nephropathy in Protein l -isoaspartyl Methyltransferase-deficient Mice. Cell Biol. Int. 2021, 45, 2316–2330. [Google Scholar] [CrossRef]
- Metzinger, L. microRNAs Are Dysregulated in the Cerebral Microvasculature of CKD Mice. Front. Biosci. 2014, E6, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Zhou, Z.; Fu, P.; Jin, C.; Wu, P.; Ji, C.; Shan, Y.; Shi, L.; Xu, M.; Qian, H. Roles of Extracellular Vesicles in Ageing-Related Chronic Kidney Disease: Demon or Angel. Pharmacol. Res. 2023, 193, 106795. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.; Banks, W.A. Brain-immune communication pathways. Brain Behav. Immun. 2007, 21, 727–735. [Google Scholar] [CrossRef]
- Delpech, J.C.; Herron, S.; Botros, M.B.; Ikezu, T. Neuroimmune Crosstalk through Extracellular Vesicles in Health and Disease. Trends Neurosci. 2019, 42, 361–372. [Google Scholar] [CrossRef]
- Cabrera-Pastor, A. Extracellular Vesicles as Mediators of Neuroinflammation in Intercellular and Inter-Organ Crosstalk. Int. J. Mol. Sci. 2024, 25, 7041. [Google Scholar] [CrossRef]
- Kim, S.J.; Russell, A.E.; Wang, W.; Gemoets, D.E.; Sarkar, S.N.; Simpkins, J.W.; Brown, C.M. miR-146a Dysregulates Energy Metabolism During Neuroinflammation. J. Neuroimmune Pharmacol. 2022, 17, 228–241. [Google Scholar] [CrossRef]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An Important Regulator of Neuroinflammation. Int. J. Mol. Sci. 2021, 23, 90. [Google Scholar] [CrossRef]
- Thome, A.D.; Harms, A.S.; Volpicelli-Daley, L.A.; Standaert, D.G. microRNA-155 Regulates Alpha-Synuclein-Induced Inflammatory Responses in Models of Parkinson Disease. J. Neurosci. 2016, 36, 2383–2390. [Google Scholar] [CrossRef]
- Anastasi, F.; Masciandaro, S.M.; Carratore, R.D.; Dell’Anno, M.T.; Signore, G.; Falleni, A.; McDonnell, L.A.; Bongioanni, P. Proteomics Profiling of Neuron-Derived Small Extracellular Vesicles from Human Plasma: Enabling Single-Subject Analysis. Int. J. Mol. Sci. 2021, 22, 2951. [Google Scholar] [CrossRef]
- Yu, A.; Zhang, T.; Duan, H.; Pan, Y.; Zhang, X.; Yang, G.; Wang, J.; Deng, Y.; Yang, Z. MiR-124 contributes to M2 polarization of microglia and confers brain inflammatory protection via the C/EBP-α pathway in intracerebral hemorrhage. Immunol. Lett 2017, 182, 1–11. [Google Scholar] [CrossRef]
- Fang, M.; Wang, J.; Zhang, X.; Geng, Y.; Hu, Z.; Rudd, J.A.; Ling, S.; Chen, W.; Han, S. The miR-124 regulates the expression of BACE1/β-secretase correlated with cell death in Alzheimer’s disease. Toxicol. Lett. 2012, 209, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhu, Z.; Wu, J.; Zhang, Y.; Zhang, H.; Sun, X.; Qian, C.; Wang, B.; Xie, L.; Zhang, S.; et al. MicroRNA-124 regulates the expression of p62/p38 and promotes autophagy in the inflammatory pathogenesis of Parkinson’s disease. FASEB J. 2019, 33, 8648–8665. [Google Scholar] [CrossRef] [PubMed]
- Chivero, E.T.; Liao, K.; Niu, F.; Tripathi, A.; Tian, C.; Buch, S.; Hu, G. Engineered Extracellular Vesicles Loaded With miR-124 Attenuate Cocaine-Mediated Activation of Microglia. Front. Cell Dev. Biol. 2020, 8, 573. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Li, Y.; Chen, X.; Yang, S.; Zhang, J. Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain Res. 2009, 1284, 191–201. [Google Scholar] [CrossRef]
- Yin, Z.; Han, Z.; Hu, T.; Zhang, S.; Ge, X.; Huang, S.; Wang, L.; Yu, J.; Li, W.; Wang, Y.; et al. Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain Behav. Immun. 2020, 83, 270–282. [Google Scholar] [CrossRef]
- Hu, L.; Si, L.; Dai, X.; Dong, H.; Ma, Z.; Sun, Z.; Li, N.; Sha, H.; Chen, Y.; Qian, Y.; et al. Exosomal miR-409-3p secreted from activated mast cells promotes microglial migration, activation and neuroinflammation by targeting Nr4a2 to activate the NF-κB pathway. J. Neuroinflammation 2021, 18, 68. [Google Scholar] [CrossRef]
- De Sousa, K.P.; Rossi, I.; Abdullahi, M.; Ramirez, M.I.; Stratton, D.; Inal, J.M. Isolation and Characterization of Extracellular Vesicles and Future Directions in Diagnosis and Therapy. WIREs Nanomed. Nanobiotechnol. 2023, 15, e1835. [Google Scholar] [CrossRef]
| Target Region | Effect on Target Genes Expression | References |
|---|---|---|
| Target-mRNAs 3′ UTR | Target-mRNAs destabilization and translation inhibition | [2] |
| Target-mRNAs 5′ UTR | Target-mRNAs destabilization and translation inhibition Enhancement of target mRNAs stability and translation | [3,4] |
| Target-mRNAs coding sequence | Target-mRNAs translation inhibition | [5,6] |
| Target-genes promoters | Target-genes transcription activation | [7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amico, G.; Carista, A.; Manna, O.M.; Paladino, L.; Picone, D.; Sarullo, S.; Sausa, M.; Cappello, F.; Vitale, A.M.; Caruso Bavisotto, C. Brain–Periphery Axes: The Potential Role of Extracellular Vesicles-Delivered miRNAs. Biology 2024, 13, 1056. https://doi.org/10.3390/biology13121056
D’Amico G, Carista A, Manna OM, Paladino L, Picone D, Sarullo S, Sausa M, Cappello F, Vitale AM, Caruso Bavisotto C. Brain–Periphery Axes: The Potential Role of Extracellular Vesicles-Delivered miRNAs. Biology. 2024; 13(12):1056. https://doi.org/10.3390/biology13121056
Chicago/Turabian StyleD’Amico, Giuseppa, Adelaide Carista, Olga Maria Manna, Letizia Paladino, Domiziana Picone, Silvia Sarullo, Martina Sausa, Francesco Cappello, Alessandra Maria Vitale, and Celeste Caruso Bavisotto. 2024. "Brain–Periphery Axes: The Potential Role of Extracellular Vesicles-Delivered miRNAs" Biology 13, no. 12: 1056. https://doi.org/10.3390/biology13121056
APA StyleD’Amico, G., Carista, A., Manna, O. M., Paladino, L., Picone, D., Sarullo, S., Sausa, M., Cappello, F., Vitale, A. M., & Caruso Bavisotto, C. (2024). Brain–Periphery Axes: The Potential Role of Extracellular Vesicles-Delivered miRNAs. Biology, 13(12), 1056. https://doi.org/10.3390/biology13121056











