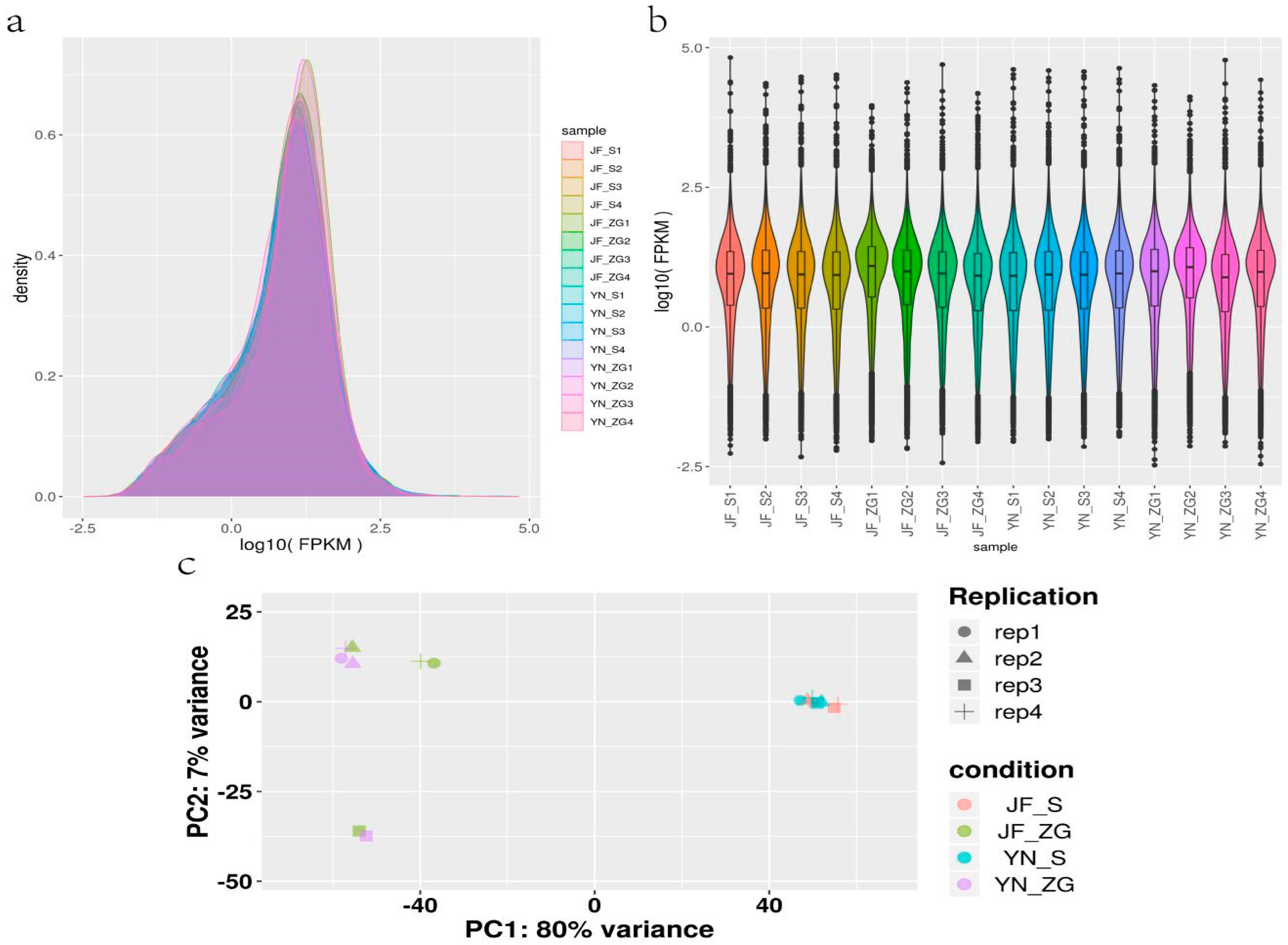
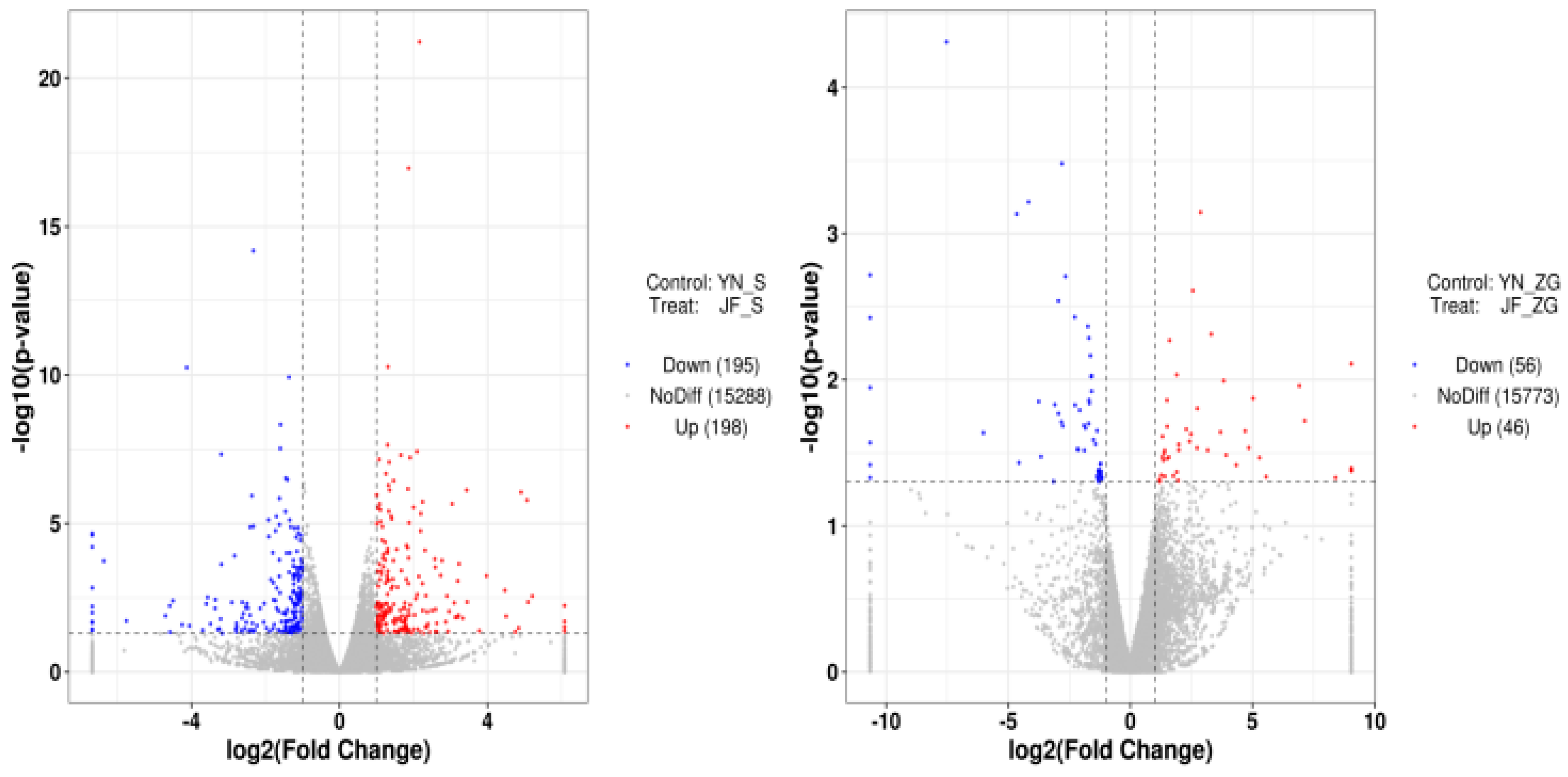
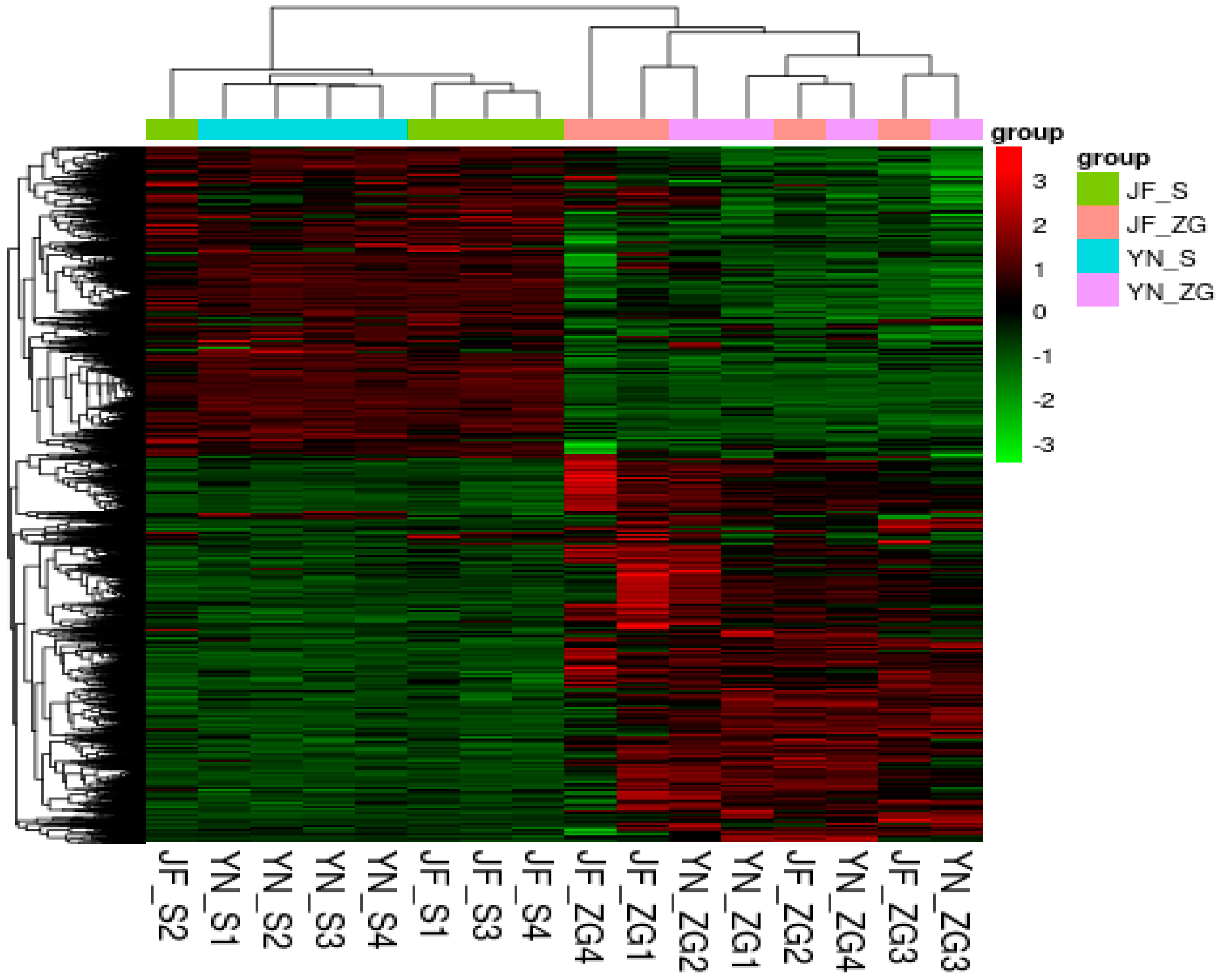
Eggshell quality assessment is significant for a variety of purposes, including chicken farming, food safety, and genetic research [
23]. Eggshell quality, along with other essential production factors such as egg size or lay rate, is critical in the chicken breeding selection phase [
24]. Better eggshell quality indicates that chickens have efficient calcium metabolism, which translates to good utilization of dietary calcium [
25]. So far, eggshell quality assessment and the identification of associated genetic markers have enabled breeding programs focused on boosting shell strength, thickness, and overall durability. It is commonly understood that eggshell gives calcium and other nutrients to the developing embryo [
26]. As a result, poor eggshell quality can impede embryo development and reduce hatchability rates in breeding operations. Furthermore, stronger and more homogeneous eggshells are less likely to break or become contaminated, extending the shelf life of eggs and increasing consumer safety [
27]. The eggshell also acts as the first line of defense against bacterial contamination, notably diseases like Salmonella [
27]. The eggshell structure-associated parameters can directly influence mechanical properties and quality assessment parameters. Different methods including scanning using electron microscopy to examine the eggshells being used across the world for both commercial as well as research purposes [
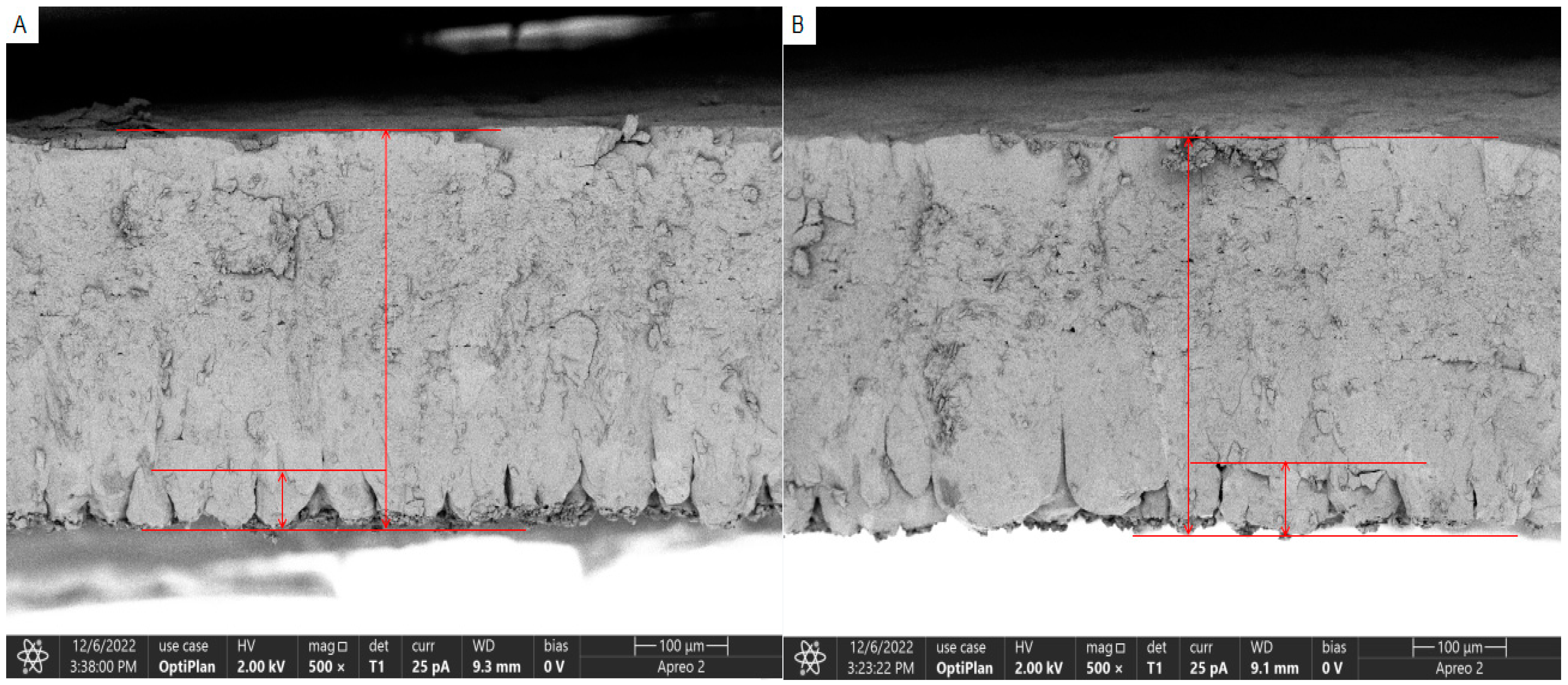
23]. In this study, the eggshell thickness and strength of Yunnong chickens were found to be greater than those of Jingfen chickens. Ultrastructural cross-section observations revealed that the mammillary layer of Yunnong chickens was thinner compared to that of Jingfen chickens. Additionally, internal ultrastructural analysis indicated that the mammillary density in Yunnong chickens was higher than that in Jingfen chickens. In summary, Yunnong chickens demonstrate superior eggshell thickness and strength compared to Jingfen chickens. Earlier research established that the mammillary layer was the most common site of structural alteration in cracked eggs [
28]. Additionally, a study by Liao and Qiao [
4] reported a significant positive correlation between the percentage thickness of the mammillary layer and eggshell strength. However, Bain [
29] suggested that the role of the mammillary layer in shell strength is bidirectional. Furthermore, pigment deposition is another factor influencing eggshell strength [
30]. Pigment granules stored in the shell gland epithelial cells reach their maximum deposition rate at the end of eggshell calcification [
31], which may impair the deposition of the vertical crystal layer, leading to reduced density and a more porous structure. As mineral deposition in the eggshell decreases, the eggshell matrix proteins may be upregulated, potentially affecting eggshell quality and reducing eggshell strength. Moreover, trace elements such as zinc, manganese, and copper serve as cofactors for certain enzymes and can influence the mechanical properties of eggshells by affecting the formation of calcite crystals and altering the crystalline structure of the eggshell [
32]. Early experiments indicated that hens fed a manganese-deficient diet produced thinner eggshells, with alterations in the microstructure of the mammillary layer and reduced levels of hexosamines and hexuronic acids in the organic matrix [
33]. This study also found that Yunnong chickens exhibited highly significant positive correlations between various metrics, including the egg shape index and eggshell strength, eggshell strength and eggshell thickness, eggshell strength and the Haugh unit, and egg white height and the Haugh unit. These findings align with the results of Bogdanski and Silveira [
34] and Wang and Ruan [
35].
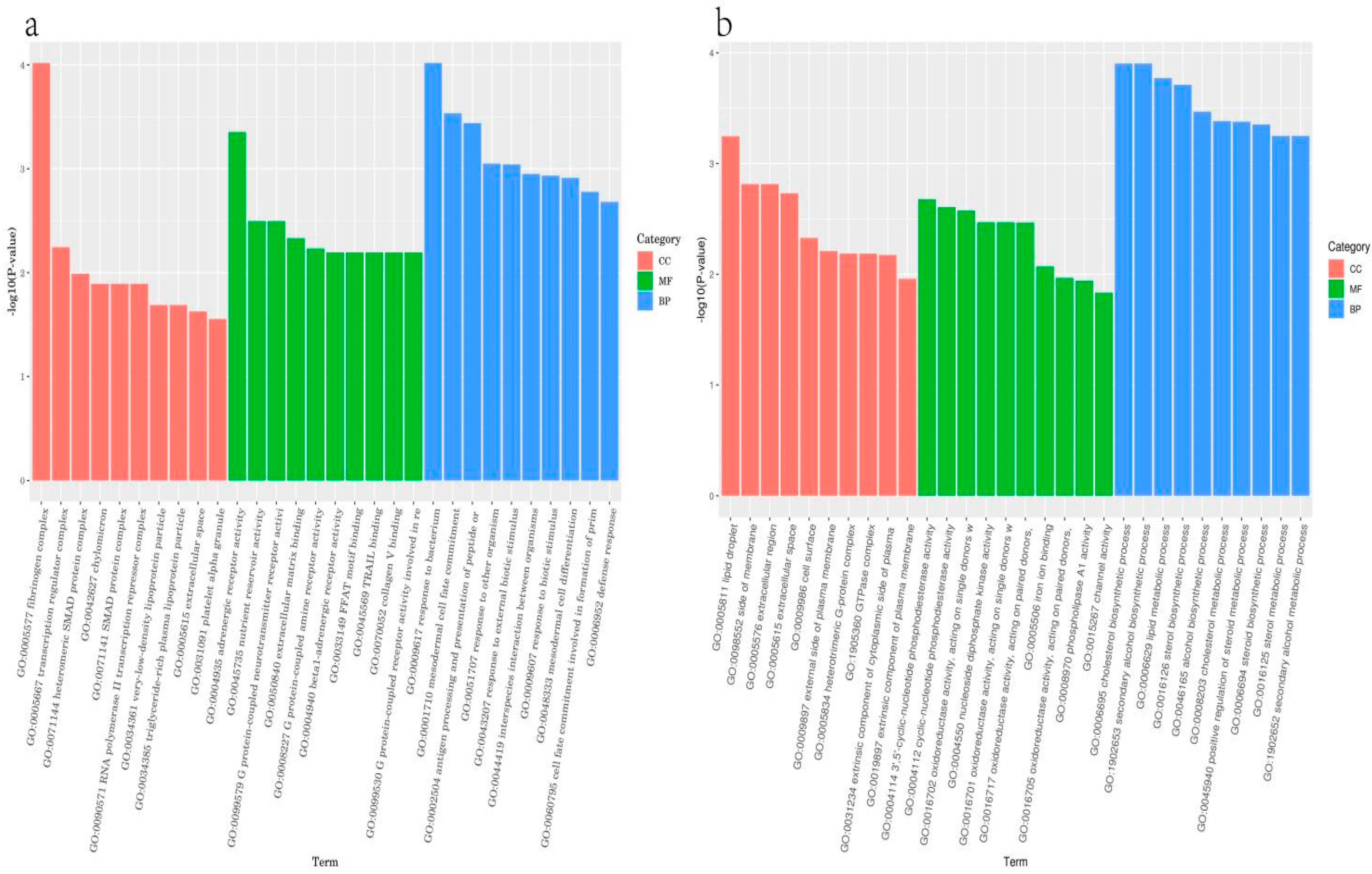
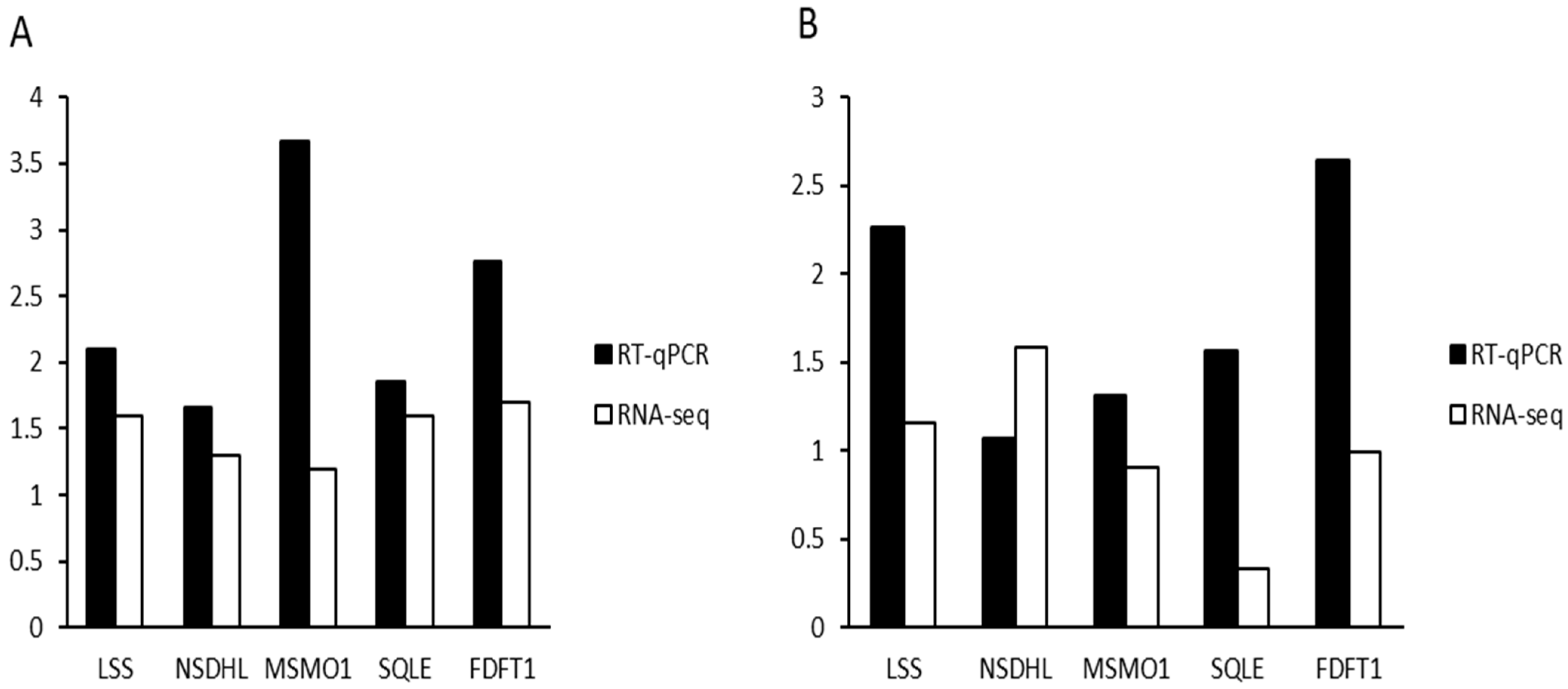
Several genes have been implicated in the biomechanical properties and formation of eggshells. For example, single nucleotide polymorphisms (SNPs) in the ovocleidin-116 (OC-116) gene are associated with the modulus and thickness of eggshells [
36]. The ovocleidin-17 (OC-17) gene is predicted to catalyze the conversion of amorphous calcium carbonate into calcite, the primary component of mineralized eggshells [
37]. Additionally, SNPs in the OCX-32 gene have been shown to significantly correlate with the thickness of the mammillary layer in birds, while osteopontin (OPN) is associated with eggshell fracture toughness [
38]. In this study, candidate differential genes related to eggshells were identified through transcriptome sequencing analysis, revealing significantly enriched KEGG pathways, including steroid biosynthesis, light signal transduction, TGF-beta signaling, ether lipid metabolism, and vascular smooth muscle contraction. The five most representative genes identified were LSS (lanosterol synthase), NSDHL (NAD(P)-dependent steroid dehydrogenase-like), MSMO1 (methylsterol monooxygenase 1), SALE (squalene epoxidase), and FDFT1 (farnesyl diphosphate farnesyltransferase 1). However, research on the roles of LSS, NSDHL, and FDFT1 in animals is relatively scarce. Liu and Liu [
39] identified key genes related to steroid metabolism, such as LSS, by analyzing the pectoral muscle of chickens from high-triglyceride-content (HTG) and low-triglyceride-content (LTG) groups. In humans, mutations in the LSS gene can lead to congenital cataracts and alopecia [
40], while its effects in animals are typically associated with fat deposition [
41]. Zhang and Li [
42] utilized quantitative real-time PCR to confirm that NSDHL knockdown increased the proliferation of 3T3-L1 preadipocytes while diminishing their differentiation, suggesting that NSDHL is a novel regulator of lipogenesis. Iannaccone and Elgendy [
43] reported that the expression of genes such as NSDHL was lower in Friesian calves fed diets supplemented with grape pomace (GPO) compared to those not receiving GPO, correlating with reduced cholesterol levels in the blood and showcasing antioxidant activity. While NSDHL is implicated in cancer development in humans, research on this gene in livestock and poultry remains limited. Guo and Wei [
44] employed RNA-seq technology to identify differentially expressed genes in the subcutaneous adipose tissue of Muscovy ducks at three developmental stages (12 weeks, 35 weeks, and 66 weeks). Compared to the 12-week stage, the subcutaneous adipose tissue at 35 weeks exhibited upregulation of genes related to cholesterol and fatty acid biosynthesis, such as HSD17B7 and MSMO1, providing potential candidate genes and pathways involved in fat deposition in ducks and enhancing understanding of the molecular mechanisms governing fat deposition during development. Guo and Wei [
44] investigated the effects of ovariectomy on meat quality in broilers; they found that ovariectomized birds showed significant increases in abdominal fat weight (AFW) and abdominal fat percentage (AFP) at 14 and 19 weeks post-surgery compared to the sham operation group. Genes such as CETP, DGAT2, DHCR24, HSD17B7, and MSMO1 were significantly upregulated following ovariectomy. In humans, mutations in the MSMO1 gene have been linked to diseases such as pancreatic cancer [
45], whereas in livestock, MSMO1 appears to influence fat deposition in chickens [
39] and ducks [
46]. Zhang and Deng [
47] transfected an SQLE overexpression vector into bovine skeletal muscle-derived mesenchymal stem/stromal cells (MSCs) and found that SQLE significantly promoted MSC differentiation and apoptosis while inhibiting proliferation, revealing its role in myoblast differentiation and providing new insights into the regulatory network of muscle development in cattle. To investigate the biological functions and regulatory roles of gga-miR-221-5p in chicken liver, Zhang and Wang [
48] discovered that the highly conserved gga-miR-221-5p directly targets ELOVL6 and SQLE mRNAs, influencing intracellular triglyceride and total cholesterol levels. Furthermore, 7β-estradiol was shown to suppress the expression of gga-miR-221-5p while upregulating ELOVL6 and SQLE expression, thereby promoting triglyceride synthesis and cholesterol levels in the ovaries of egg-laying hens. In humans, SQLE is associated with the inhibition of colorectal cancer, and in livestock, it influences muscle development in cattle, with its expression in the chicken liver potentially impacting cholesterol deposition in the ovaries during the laying period. Huang and Zhang [
49] identified FDFT1 as a potential biomarker associated with ferroptosis in kidney cancer (KIRC), finding that FDFT1 upregulation inhibited cell proliferation, migration, and invasion, with potential antitumor effects occurring via the AKT signaling pathway. Nie and Shan [
50] analyzed the role of ferroptosis in the prognosis of colorectal cancer (CC) through RNA-seq, establishing a prognostic model composed of five ferroptosis-related genes (AKR1C1, ALOX12, FDFT1, ATP5MC3, CARS1), concluding that ferroptosis-related features could partially predict the survival rates of CC patients. In this study, LSS, NSDHL, MSMO1, SQLE, and FDFT1 were identified as significant genes derived from the steroid biosynthesis signaling pathway. Through transcriptome analysis, this study found that all five genes within the steroid biosynthesis signaling pathway were significantly upregulated and that this pathway was related to the synthesis of vitamin D3. Furthermore, the results were validated by quantitative real-time PCR, corroborating the findings from transcriptome sequencing. The expression levels of LSS, NSDHL, MSMO1, SQLE, and FDFT1 may be positively correlated with eggshell formation.