Seasonal Water-Column Structure Drives the Trophic Niche of Fish Communities on a Temperate Continental Shelf
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
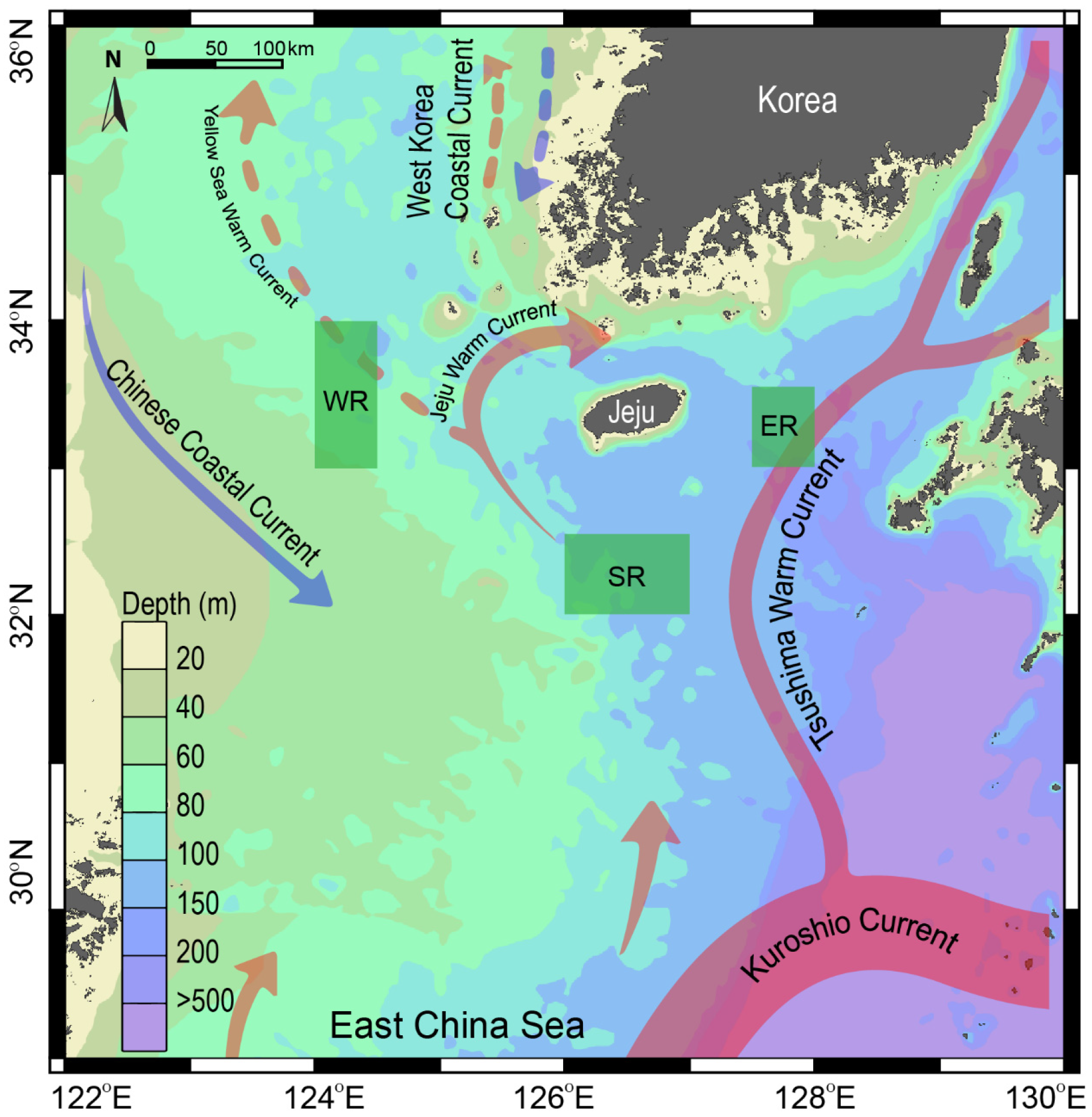
2.1. Study Area
2.2. Sample Collection, Processing, and Stable Isotope Analysis
2.3. Data Analysis and Statistics
2.4. Trophic Niche Metrics
2.5. Trophic Position and Reliance Measures
3. Results
3.1. Spatial and Seasonal Patterns in δ13C and δ15N Values
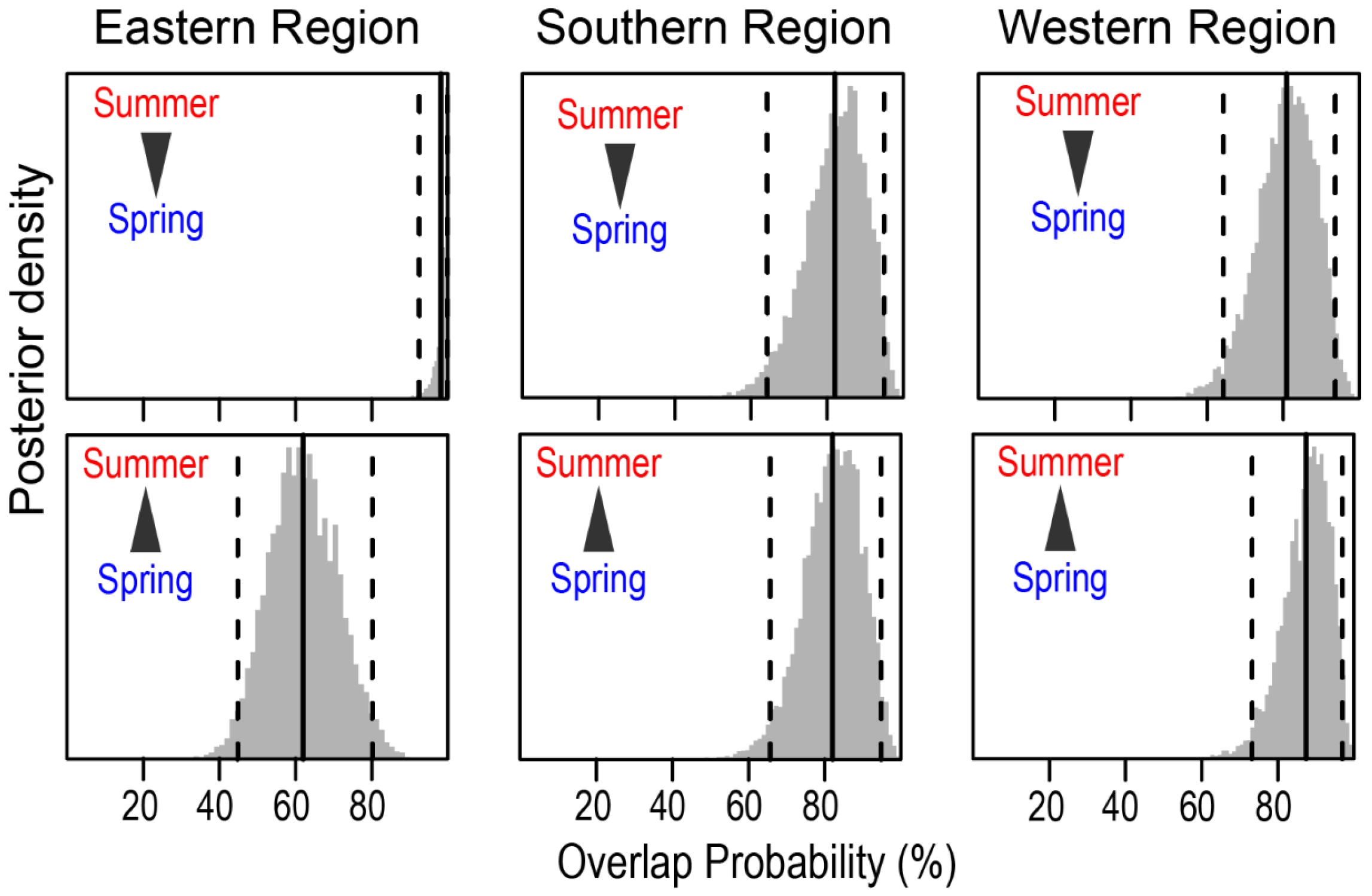
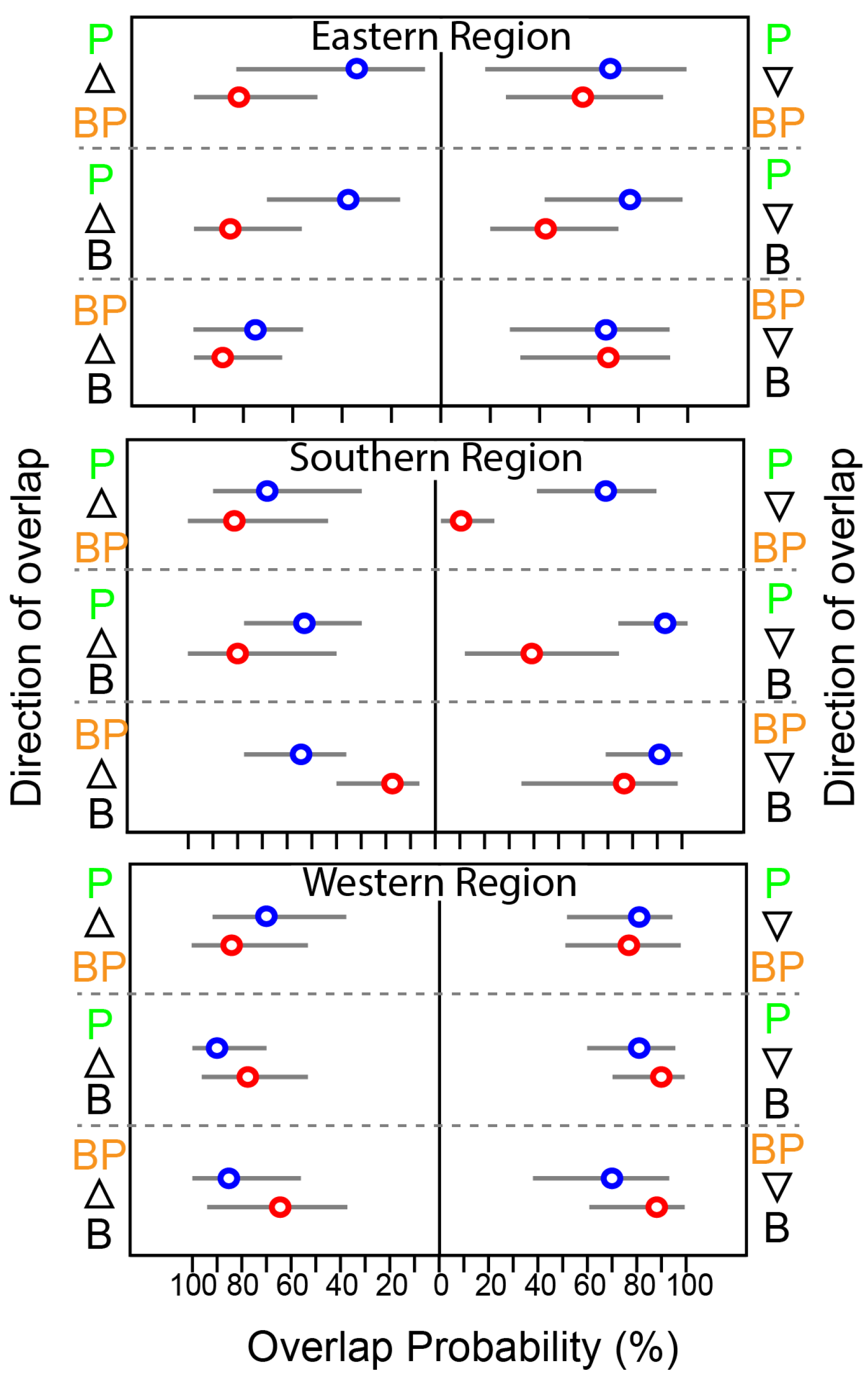
3.2. Spatial and Seasonal Pattern in Isotopic Niche of Fish
3.3. Trophic Position of Fish
3.4. Contribution of Benthic Pathway to Fish Nutrition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kortsch, S.; Primicerio, R.; Aschan, M.; Lind, S.; Dolgov, A.V.; Planque, B. Food-web structure varies along environmental gradients in a high-latitude marine ecosystem. Ecography 2019, 42, 295–308. [Google Scholar] [CrossRef]
- Cao, L.; Liu, J.; Dou, S.; Huang, W. Biomagnification of methylmercury in a marine food web in Laizhou Bay (North China) and associated potential risks to public health. Mar. Pollut. Bull. 2020, 150, 110762. [Google Scholar] [CrossRef] [PubMed]
- Frank, K.T.; Petrie, B.; Choi, J.S.; Leggett, W.C. Trophic cascades in a formerly cod-dominated ecosystem. Science 2005, 308, 1621–1623. [Google Scholar] [CrossRef]
- Kortsch, S.; Primicerio, R.; Fossheim, M.; Dolgov, A.V.; Aschan, M. Climate change alters the structure of arctic marine food webs due to poleward shifts of boreal generalists. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151546. [Google Scholar] [CrossRef]
- Maitra, S.; Harikrishnan, M.; Shibu, A.V.; Sureshkumar, S.; Ranjeet, K.; Nandan, S.B. Studies on temporal variations of exploited fishery resources and their trophic levels in a tropical estuary. Reg. Stud. Mar. Sci. 2018, 22, 61–69. [Google Scholar] [CrossRef]
- Cury, P.M.; Shin, Y.-J.; Planque, B.; Durant, J.M.; Fromentin, J.-M.; Kramer-Schadt, S.; Stenseth, N.C.; Travers, M.; Grimm, V. Ecosystem oceanography for global change in fisheries. Trends Ecol. Evol. 2008, 23, 338–346. [Google Scholar] [CrossRef]
- Brodziak, J.; Link, J. Ecosystem-based fishery management: What is it and how can we do it? Bull. Mar. Sci. 2002, 70, 589–611. [Google Scholar]
- Sporta Caputi, S.; Careddu, G.; Calizza, E.; Fiorentino, F.; Maccapan, D.; Rossi, L.; Costantini, M.L. Seasonal food web dynamics in the Antarctic benthos of Tethys Bay (Ross Sea): Implications for biodiversity persistence under different seasonal sea-ice coverage. Front. Mar. Sci. 2020, 7, 1046. [Google Scholar] [CrossRef]
- Mantua, N.J.; Hare, S.R.; Zhang, Y.; Wallace, J.M.; Francis, R.C. A Pacific interdecadal climate oscillation with impacts on salmon production. Bull. Am. Meteorol. Soc. 1997, 78, 1069–1079. [Google Scholar] [CrossRef]
- Park, T.H.; Lee, C.I.; Kang, C.K.; Kwak, J.H.; Lee, S.H.; Park, H.J. Seasonal variation in food web structure and fish community composition in the East/Japan Sea. Estuaries Coasts 2020, 43, 615–629. [Google Scholar] [CrossRef]
- Greenstreet, S.P.R.; Rogers, S.I. Indicators of the health of the North Sea fish community: Identifying reference levels for an ecosystem approach to management. ICES J. Mar. Sci. 2006, 63, 573–593. [Google Scholar] [CrossRef]
- Brodeur, R.D. Interannual variations in zooplankton biomass in the Gulf of Alaska, and covariation with California Current zooplankton biomass. CalCOFI Rep. 1996, 37, 80–99. [Google Scholar]
- Giraldo, C.; Ernande, B.; Cresson, P.; Kopp, D.; Cachera, M.; Travers-Trolet, M.; Lefebvre, S. Depth gradient in the resource use of a fish community from a semi-enclosed sea. Limnol. Oceanogr. 2017, 62, 2213–2226. [Google Scholar] [CrossRef]
- Woodland, R.J.; Secor, D.H. Benthic-pelagic coupling in a temperate inner continental shelf fish assemblage. Limnol. Oceanogr. 2013, 58, 966–976. [Google Scholar] [CrossRef]
- Duffill Telsnig, J.I.; Jennings, S.; Mill, A.C.; Walker, N.D.; Parnell, A.C.; Polunin, N.V.C. Estimating contributions of pelagic and benthic pathways to consumer production in coupled marine food webs. J. Anim. Ecol. 2019, 88, 405–415. [Google Scholar] [CrossRef]
- Skyllas, N.; Bintanja, R.; Buma, A.G.J.; Brussaard, C.P.D.; Gröger, M.; Hieronymus, J.; van de Poll, W.H. Validation of stratification-driven phytoplankton biomass and nutrient concentrations in the Northeast Atlantic Ocean as simulated by EC-Earth. Geosciences 2019, 9, 450. [Google Scholar] [CrossRef]
- Shokri, M.; Cozzoli, F.; Basset, A. Metabolic rate and foraging behaviour: A mechanistic link acrossbody size and temperature gradients. Oikos 2024, 2024, e10817. [Google Scholar] [CrossRef]
- Loick-Wilde, N.; Fernández-Urruzola, I.; Eglite, E.; Liskow, I.; Nausch, M.; Schulz-Bull, D.; Wodarg, D.; Wasmund, N.; Mohrholz, V. Stratification, nitrogen fixation, and cyanobacterial bloom stage regulate the planktonic food web structure. Glob. Change Biol. 2019, 25, 794–810. [Google Scholar] [CrossRef]
- Pinnegar, J.K.; Polunin, N.V.C. Contributions of stable-isotope data to elucidating food webs of Mediterranean rocky littoral fishes. Oecologia 2000, 122, 399–409. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2012; FAO: Rome, Italy, 2012. [Google Scholar]
- Belkin, I.M. Rapid warming of large marine ecosystems. Prog. Oceanogr. 2009, 81, 207–221. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Olson, D.; Yu, N.; Chen, L. Evaluating ecosystem structure and functioning of the East China Sea Shelf ecosystem, China. Hydrobiologia 2009, 636, 331–351. [Google Scholar] [CrossRef]
- Takahashi, D.; Morimoto, A. Mean field and annual variation of surface flow in the East China Sea as revealed by combining satellite altimeter and drifter data. Prog. Oceanogr. 2013, 111, 125–139. [Google Scholar] [CrossRef]
- Takikawa, T.; Yoon, J.H. Volume transport through the Tsushima Straits estimated from sea level difference. J. Oceanogr. 2005, 61, 699–708. [Google Scholar] [CrossRef]
- Chang, P.H.; Isobe, A. A numerical study on the Changjiang diluted water in the Yellow and East China Seas. J. Geophys. Res. Oceans 2003, 108, 3299. [Google Scholar] [CrossRef]
- Shon, D.H.; Shin, K.S.; Jang, P.G.; Kim, Y.O.; Chang, M.; Kim, W.S. Effect of thermal stratification and mixing on phytoplankton community structure in the western channel of the Korea Strait. Ocean Polar Res. 2008, 30, 261–275. [Google Scholar] [CrossRef]
- Xu, Q.; Sukigara, C.; Goes, J.I.; do Rosario Gomes, H.; Zhu, Y.; Wang, S.; Shen, A.; de Raús Maúre, E.; Matsuno, T.; Watanabe, Y.; et al. Interannual changes in summer phytoplankton community composition in relation to water mass variability in the East China Sea. J. Oceanogr. 2019, 75, 61–79. [Google Scholar] [CrossRef]
- Xie, S.P.; Hafner, J.; Tanimoto, Y.; Liu, W.T.; Tokinaga, H.; Xu, H. Bathymetric effect on the winter sea surface temperature and climate of the Yellow and East China Seas. Geophys. Res. Lett. 2002, 29, 81-1–81-4. [Google Scholar] [CrossRef]
- Michener, R.H.; Kaufman, L. Stable isotope ratios as tracers in marine food webs: An update. In Stable Isotopes in Ecology and Environmental Science, 2nd ed.; Michener, R.H., Lajtha, K., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 238–282. [Google Scholar]
- Layman, C.A.; Araújo, M.S.; Boucek, R.; Hammerschlag-Peyer, C.M.; Harrison, E.; Jud, Z.R.; Matich, P.; Rosenblatt, A.E.; Vaudo, J.J.; Yeager, L.A.; et al. Applying stable isotopes to examine food-web structure: An overview of analytical tools. Biol. Rev. 2012, 87, 545–562. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Kim, D.W.; Park, Y.J.; Jeong, J.Y.; Jo, Y.H. Estimation of hourly sea surface salinity in the East China Sea using Geostationary Ocean Color Imager measurements. Remote Sens. 2020, 12, 755. [Google Scholar] [CrossRef]
- Rebstock, G.A.; Kang, Y.S. A comparison of three marine ecosystems surrounding the Korean peninsula: Responses to climate change. Prog. Oceanogr. 2003, 59, 357–379. [Google Scholar] [CrossRef]
- Chen, C.T.A.; Lin, C.M.; Huang, B.T.; Chang, L.F. Stoichiometry of carbon, hydrogen, nitrogen, sulfur and oxygen in the particulate matter of the western North Pacific marginal seas. Mar. Chem. 1996, 54, 179–190. [Google Scholar] [CrossRef]
- Kim, I.S.; Choi, Y.; Lee, C.L.; Lee, Y.J.; Kim, B.J.; Kim, J.H. Illustrated Book of Korean Fishes; Kyo-Hak Publishing Co.: Seoul, Republic of Korea, 2005; p. 615. [Google Scholar]
- Choi, Y.; Kim, J.H.; Park, J.Y. Marine Fishes of Korea; Kyo-Hak Publishing Co.: Seoul, Republic of Korea, 2002; p. 646. [Google Scholar]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Focken, U.; Becker, K. Metabolic fractionation of stable carbon isotopes: Implications of different proximate compositions for studies of the aquatic food webs using δ13C data. Oecologia 1998, 115, 337–343. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase; World Wide Web Electronic Publication, 2017. Available online: http://www.fishbase.org (accessed on 15 November 2022).
- Anderson, M.J.; Clarke, K.R.; Gorley, R.N. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Swanson, H.K.; Lysy, M.; Power, M.; Stasko, A.D.; Johnson, J.D.; Reist, J.D. A new probabilistic method for quantifying n-dimensional ecological niches and niche overlap. Ecology 2015, 96, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Quezada-Romegialli, C.; Jackson, A.L.; Hayden, B.; Kahilainen, K.K.; Lopes, C.; Harrod, C. tRophicPosition, an R package for the Bayesian estimation of trophic position from consumer stable isotope ratios. Methods Ecol. Evol. 2018, 9, 1592–1599. [Google Scholar] [CrossRef]
- McCutchan, J.H., Jr.; Lewis, W.M., Jr.; Kendall, C.; McGrath, C.C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 2003, 102, 378–390. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows, version 21.0; IBM Corp.: Armonk, NY, USA, 2012. [Google Scholar]
- Gearing, J.N.; Gearing, P.J.; Rudnick, D.T.; Requejo, A.G.; Hutchins, M.J. Isotopic variability of organic carbon in a phytoplankton-based, temperate estuary. Geochim. Cosmochim. Acta 1984, 48, 1089–1098. [Google Scholar] [CrossRef]
- Cartes, J.E.; Fanelli, E.; Papiol, V.; Zucca, L. Distribution and diversity of open-ocean, near-bottom macroplankton in the western Mediterranean: Analysis at different spatio-temporal scales. Deep Sea Res. Part I Oceanogr. Res. Pap. 2010, 57, 1485–1498. [Google Scholar]
- Brutemark, A.; Lindehoff, E.; Granéli, E.; Granéli, W. Carbon isotope signature variability among cultured microalgae: Influence of species, nutrients and growth. J. Exp. Mar. Biol. Ecol. 2009, 372, 98–105. [Google Scholar] [CrossRef]
- Ho, P.C.; Okuda, N.; Yeh, C.F.; Wang, P.L.; Gong, G.C.; Hsieh, C.H. Carbon and nitrogen isoscape of particulate organic matter in the East China Sea. Prog. Oceanogr. 2021, 197, 102667. [Google Scholar] [CrossRef]
- Lara, R.J.; Alder, V.; Franzosi, C.A.; Kattner, G. Characteristics of suspended particulate organic matter in the southwestern Atlantic: Influence of temperature, nutrient and phytoplankton features on the stable isotope signature. J. Mar. Syst. 2010, 79, 199–209. [Google Scholar] [CrossRef]
- Kurle, C.M.; McWhorter, J.K. Spatial and temporal variability within marine isoscapes: Implications for interpreting stable isotope data from marine systems. Mar. Ecol. Prog. Ser. 2017, 568, 31–45. [Google Scholar] [CrossRef]
- Peterson, B.J.; Fry, B. Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Kürten, B.; Frutos, I.; Struck, U.; Painting, S.J.; Polunin, N.V.C.; Middelburg, J.J. Trophodynamics and functional feeding groups of North Sea fauna: A combined stable isotope and fatty acid approach. Biogeochemistry 2013, 113, 189–212. [Google Scholar] [CrossRef]
- Kong, C.E.; Yoo, S.; Jang, C.J. East China Sea ecosystem under multiple stressors: Heterogeneous responses in the sea surface chlorophyll-a. Deep Sea Res. Part I Oceanogr. Res. Pap. 2019, 151, 103078. [Google Scholar] [CrossRef]
- Sommer, U.; Stibor, H.; Katechakis, A.; Sommer, F.; Hansen, T. Pelagic food web configurations at different levels of nutrient richness and their implications for the ratio fish production: Primary production. In Sustainable Increase of Marine Harvesting: Fundamental Mechanisms and New Concepts; Canfield, D.E., Ed.; Springer: Dordrecht, The Netherlands, 2002; pp. 11–20. [Google Scholar]
- Le Loc’h, F.; Hily, C.; Grall, J. Benthic community and food web structure on the continental shelf of the Bay of Biscay (North Eastern Atlantic) revealed by stable isotopes analysis. J. Mar. Syst. 2008, 72, 17–34. [Google Scholar] [CrossRef]
- Boyle, M.D.; Ebert, D.A.; Cailliet, G.M. Stable-isotope analysis of a deep-sea benthic-fish assemblage: Evidence of an enriched benthic food web. J. Fish Biol. 2012, 80, 1485–1507. [Google Scholar] [CrossRef]
- Vander Zanden, J.M.; Fetzer, W.W. Global patterns of aquatic food chain length. Oikos 2007, 116, 1378–1388. [Google Scholar] [CrossRef]
- Kang, H.Y.; Kim, C.; Kim, D.; Lee, Y.J.; Park, H.J.; Kundu, G.K.; Bibi, R.; Jang, J.; Lee, K.H.; Kim, H.W.; et al. Identifying patterns in the multitrophic community and food-web structure of a low-turbidity temperate estuarine bay. Sci. Rep. 2020, 10, 20448. [Google Scholar] [CrossRef]
- Layman, C.A.; Arrington, D.A.; Montana, C.G.; Post, D.M. Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 2007, 88, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Rigolet, C.; Thiébaut, E.; Brind’Amour, A.; Dubois, S.F. Investigating isotopic functional indices to reveal changes in the structure and functioning of benthic communities. Funct. Ecol. 2015, 29, 1350–1360. [Google Scholar] [CrossRef]
- Shokri, M.; Lezzi, L.; Basset, A. The seasonal response of metabolic rate to projected climate change scenarios in aquatic amphipods. J. Therm. Biol. 2024, 124, 103941. [Google Scholar] [CrossRef] [PubMed]
- Gauzens, B.; Rosenbaum, B.; Kalinkat, G.; Boy, T.; Jochum, M.; Kortsch, S.; O’Gorman, E.J.; Brose, U. Flexible foraging behaviour increases predator vulnerability to climate change. Nat. Clim. Change 2024, 14, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.L.; Lin, F.S.; Lee, Y.C.; Gong, G.C.; Hsieh, C.H. Trophic structure of the pelagic food web in the East China Sea. Zool. Stud. 2015, 54, 7. [Google Scholar] [CrossRef]
- Chen, W.Y.; Lee, M.A.; Lan, K.W.; Gong, G.C. Distributions and assemblages of larval fish in the East China Sea during the northeasterly and southwesterly monsoon seasons of 2008. Biogeosciences 2014, 11, 547–561. [Google Scholar] [CrossRef]
- Davis, J.M.; Rosemond, A.D.; Eggert, S.L.; Cross, W.F.; Wallace, J.B. Long-term nutrient enrichment decouples predator and prey production. Proc. Natl. Acad. Sci. USA 2010, 107, 121–126. [Google Scholar] [CrossRef]
- Planas, M. Was that my meal? Uncertainty from source sampling period in diet reconstruction based on stable isotopes in a syngnathid fish. Front. Mar. Sci. 2022, 9, 982883. [Google Scholar]
- Sporta Caputi, S.; Kabala, J.P.; Rossi, L.; Careddu, G.; Calizza, E.; Ventura, M.; Costantini, M.L. Individual diet variability shapes the architecture of Antarctic benthic food webs. Sci. Rep. 2024, 14, 12333. [Google Scholar] [CrossRef]




| Source | df | SS | R2 | Pseudo-F | p (perm) | p (Adjusted) |
|---|---|---|---|---|---|---|
| a. δ13C values: Main test | ||||||
| Region | 2 | 14.616 | 0.087 | 11.178 | 0.000 | - |
| Season | 1 | 5.751 | 0.034 | 8.796 | 0.003 | - |
| Feeding-zone | 2 | 5.748 | 0.034 | 4.396 | 0.015 | - |
| Region × Season | 2 | 3.092 | 0.018 | 2.365 | 0.094 | - |
| Region × Feeding zone | 4 | 3.370 | 0.020 | 1.289 | 0.272 | - |
| Seson × Feeding zone | 2 | 0.684 | 0.004 | 0.523 | 0.591 | - |
| Region × Season×Feeding zone | 4 | 4.046 | 0.024 | 1.548 | 0.192 | - |
| Residual | 200 | 130.758 | 0.778 | - | - | - |
| Total | 217 | 168.065 | 1 | - | - | - |
| b. δ13C values: Pairwise tests | ||||||
| WR vs. ER | 1 | 11.963 | 0.104 | 16.459 | 0.001 | 0.003 |
| WR vs. SR | 1 | 2.706 | 0.021 | 3.083 | 0.083 | 0.249 |
| ER vs. SR | 1 | 3.374 | 0.040 | 5.926 | 0.019 | 0.057 |
| Pelagic vs. Benthopelagic | 1 | 0.546 | 0.007 | 0.601 | 0.418 | 1.000 |
| Pelagic vs. Benthic | 1 | 6.585 | 0.053 | 9.790 | 0.001 | 0.003 |
| Benthopelagic vs. Benthic | 1 | 2.239 | 0.178 | 3.021 | 0.087 | 0.261 |
| c. δ15N values: Main test | ||||||
| Region | 2 | 1.860 | 0.005 | 0.495 | 0.613 | |
| Season | 1 | 4.600 | 0.011 | 2.457 | 0.117 | |
| Feeding-zone | 2 | 2.630 | 0.006 | 0.702 | 0.504 | |
| Region × Season | 2 | 7.030 | 0.017 | 1.877 | 0.152 | |
| Region × Feeding zone | 4 | 11.780 | 0.029 | 1.572 | 0.178 | |
| Seson × Feeding zone | 2 | 0.900 | 0.002 | 0.239 | 0.781 | |
| Region × Season×Feeding zone | 4 | 6.370 | 0.015 | 0.850 | 0.504 | |
| Residual | 200 | 374.690 | 0.914 | - | - | |
| Total | 217 | 409.860 | 1 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kundu, G.K.; Kim, C.; Jang, J.; Lee, C.I.; Kim, D.; Lim, W.-A.; Choi, J.H.; Kang, C.-K. Seasonal Water-Column Structure Drives the Trophic Niche of Fish Communities on a Temperate Continental Shelf. Biology 2024, 13, 1041. https://doi.org/10.3390/biology13121041
Kundu GK, Kim C, Jang J, Lee CI, Kim D, Lim W-A, Choi JH, Kang C-K. Seasonal Water-Column Structure Drives the Trophic Niche of Fish Communities on a Temperate Continental Shelf. Biology. 2024; 13(12):1041. https://doi.org/10.3390/biology13121041
Chicago/Turabian StyleKundu, Goutam Kumar, Changseong Kim, Jaebin Jang, Chung Il Lee, Dongyoung Kim, Weol-Ae Lim, Jung Hwa Choi, and Chang-Keun Kang. 2024. "Seasonal Water-Column Structure Drives the Trophic Niche of Fish Communities on a Temperate Continental Shelf" Biology 13, no. 12: 1041. https://doi.org/10.3390/biology13121041
APA StyleKundu, G. K., Kim, C., Jang, J., Lee, C. I., Kim, D., Lim, W.-A., Choi, J. H., & Kang, C.-K. (2024). Seasonal Water-Column Structure Drives the Trophic Niche of Fish Communities on a Temperate Continental Shelf. Biology, 13(12), 1041. https://doi.org/10.3390/biology13121041








