Post-Hatching Development of Posture and Behavior in the Barn Owl (Tyto alba): From a General Behavioral Pattern of Vertebrates to the Typical Owl Behavior
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Apparatus and Procedure
2.3. Data Acquisition and Analysis
2.4. Statistics
3. Results
3.1. Postural Development, Plumage, and Flight
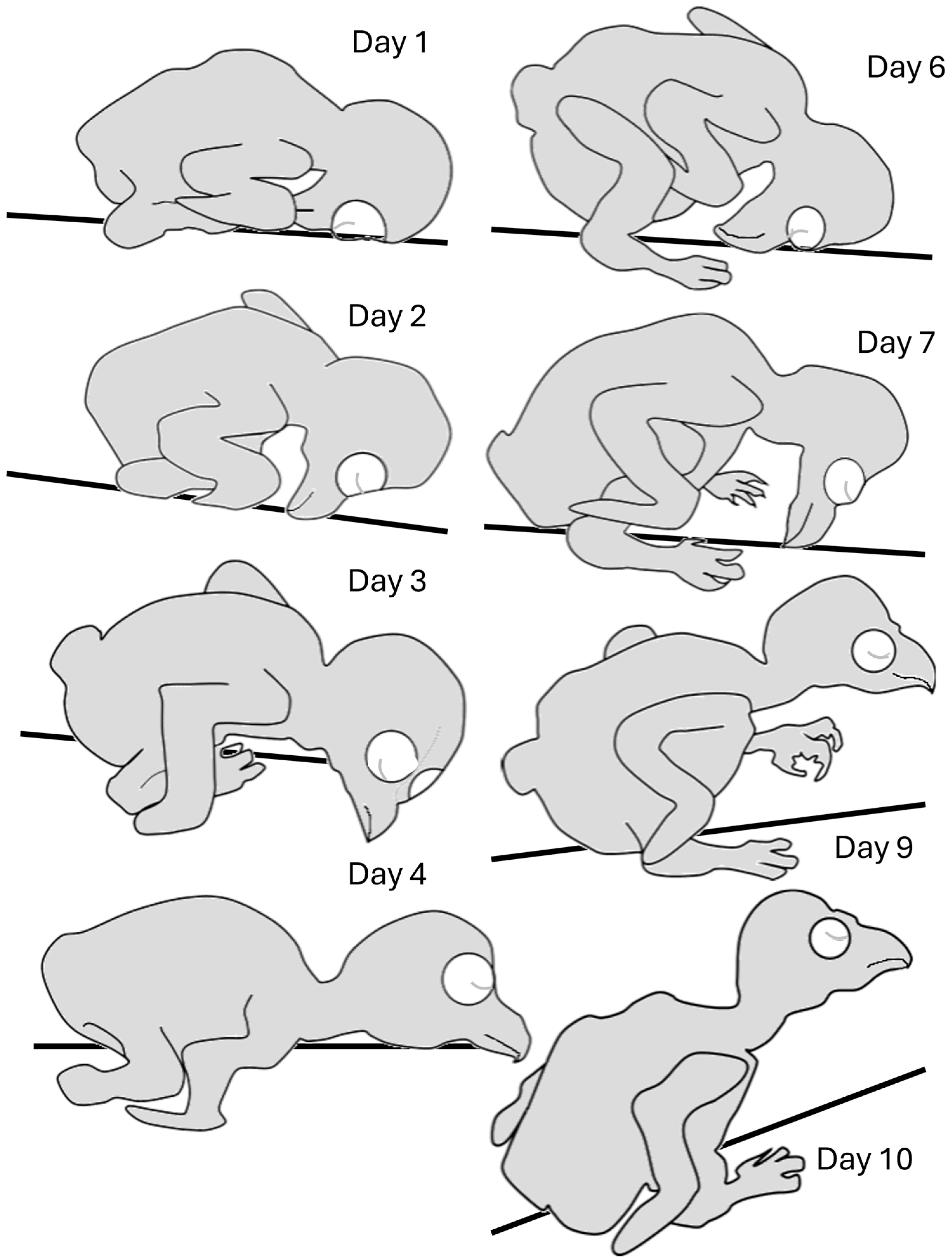
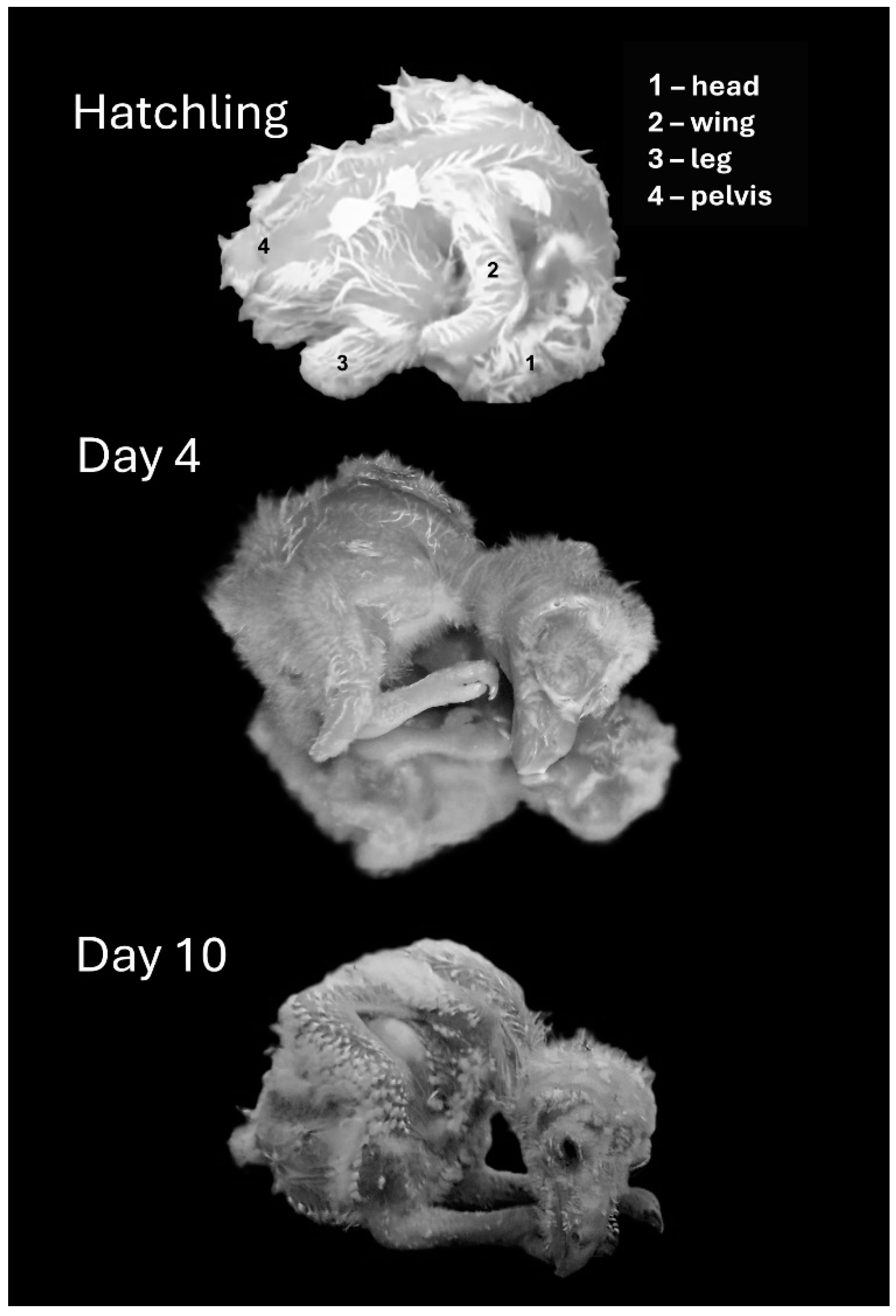
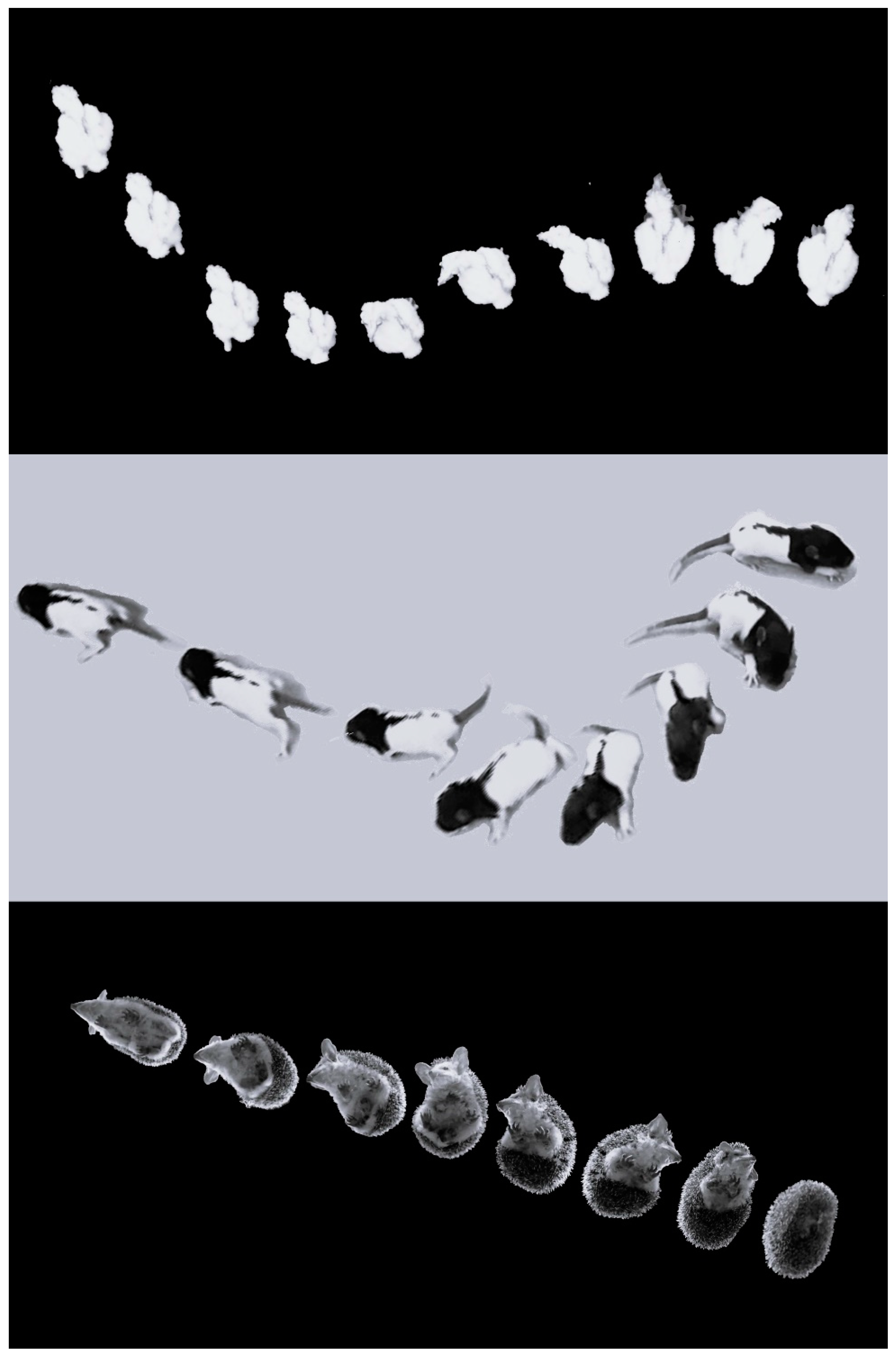
3.1.1. Days 1–10: From Hatching to Eye Opening
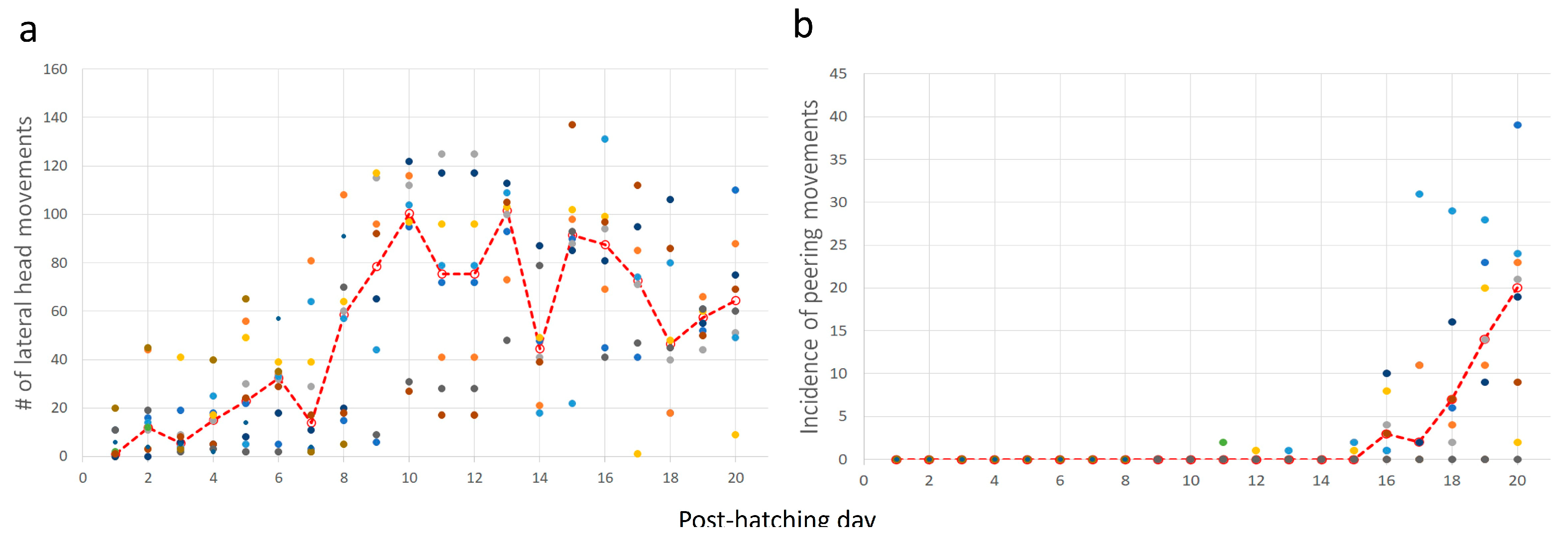
3.1.2. Days 11–20: From Eyes Opening to Peering Movements
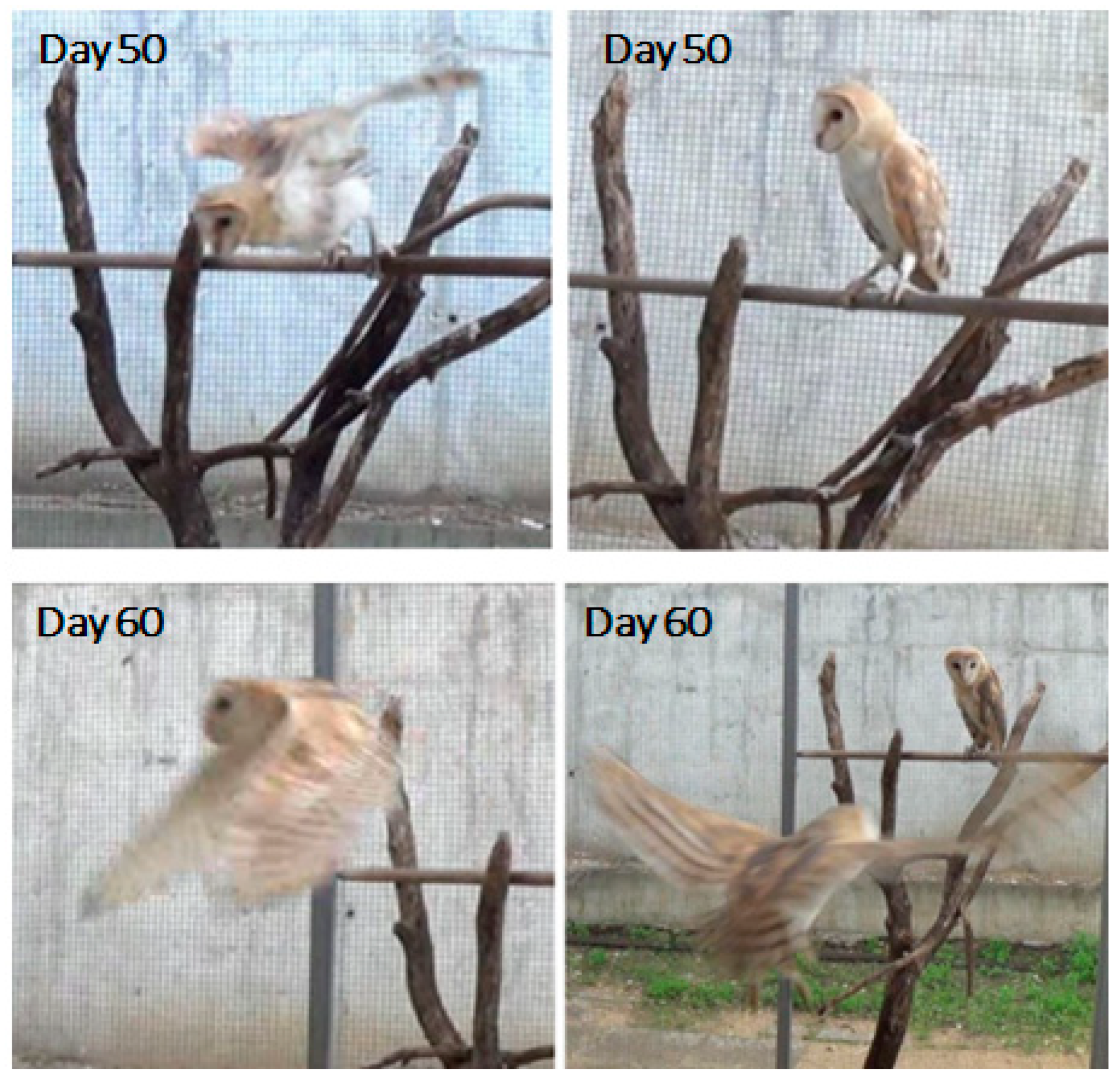
3.1.3. Days 21–60: Accomplishing Plumage, Standing, and Flying
3.2. Behavioral Development
3.2.1. From Unsighted Hatchlings to Fledglings
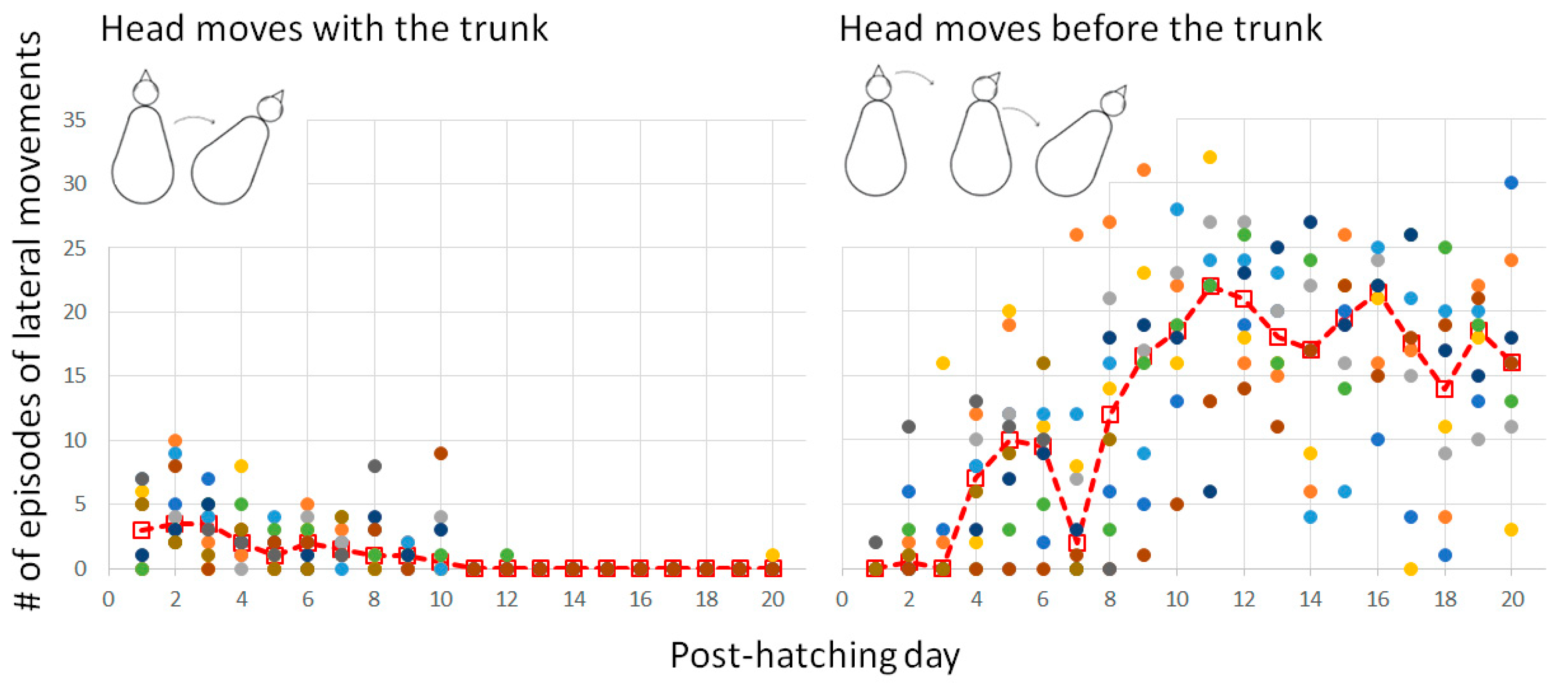
3.2.2. Warm-Up and the Emergence of Forward Progression from Pivoting
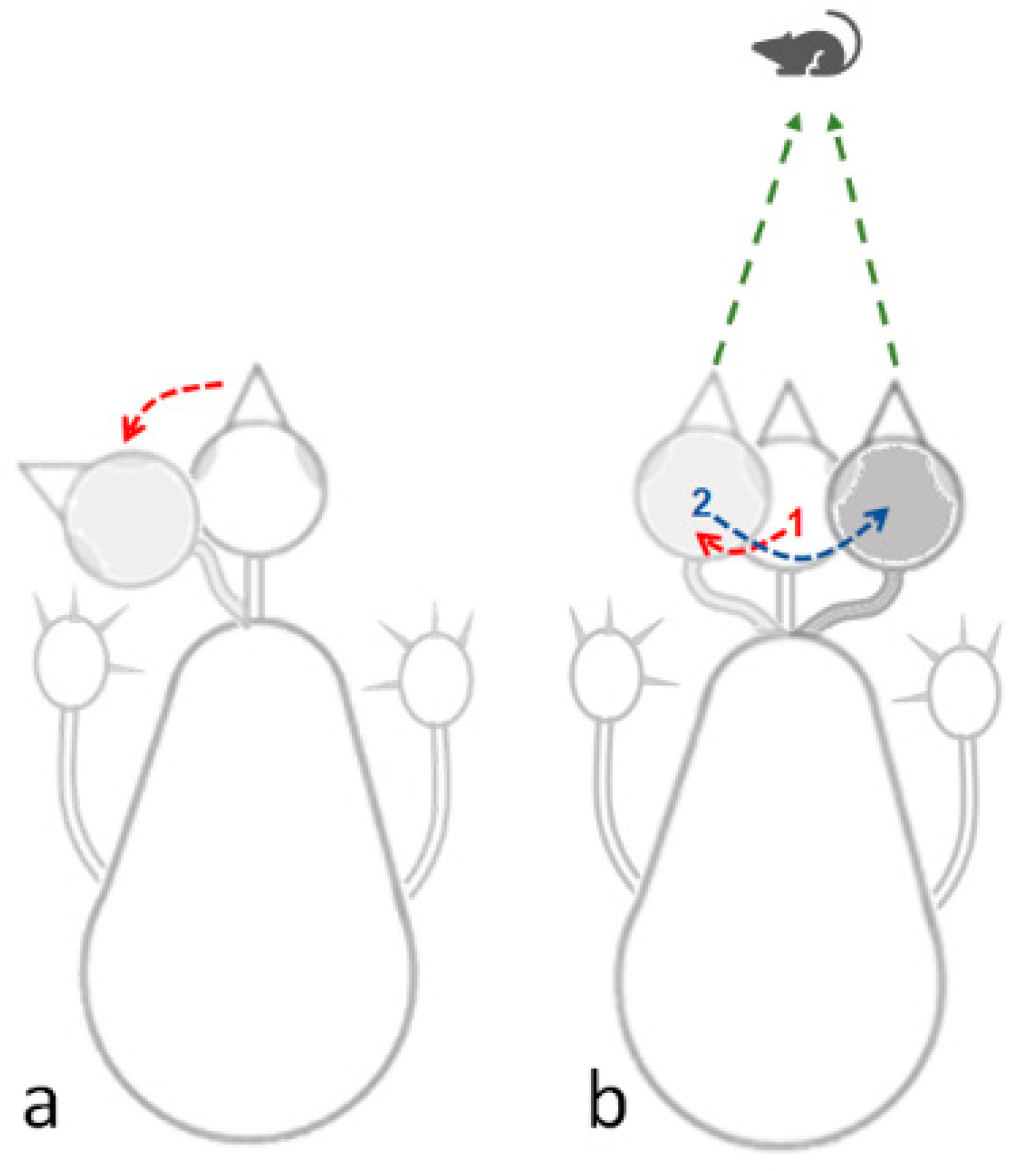
3.2.3. Pivoting
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Payne, R.S. Acoustic location of prey by barn owls (Tyto alba). J. Exp. Biol. 1971, 54, 535–573. [Google Scholar] [CrossRef]
- Konishi, M. Coding of auditory space. Ann. Rev. Neurosci. 2003, 26, 31–55. [Google Scholar] [CrossRef]
- Knudsen, E.I. The hearing of the barn owl. Sci. Am. 1981, 245, 113–125. [Google Scholar] [CrossRef]
- Collett, T.S. Peering—A locust behavior pattern for obtaining motion parallax information. J. Exp. Biol. 1978, 76, 237–241. [Google Scholar] [CrossRef]
- Wallace, G.K. Visual scanning in the desert locust Schistocerca gregaria, Forskal. J. Exp. Biol. 1959, 36, 512–525. [Google Scholar] [CrossRef]
- van der Willigen, R.F.; Frost, B.J.; Wagner, H. Depth generalization from stereo to motion parallax in the owl. J. Comp. Physiol. A 2002, 187, 997–1007. [Google Scholar] [CrossRef]
- Hataji, Y.; Kuroshima, H.; Fujita, K. Motion parallax via head movements modulates visuo-motor control in pigeons. J. Exp. Biol. 2021, 224, eb236547. [Google Scholar] [CrossRef]
- Boonman, A.; Zadicario, P.; Mazon, Y.; Rabi, C.; Eilam, D. The sounds of silence: Barn owl noise in landing and taking off. Behav. Process 2018, 157, 484–488. [Google Scholar] [CrossRef]
- Edut, S.; Eilam, D. Protean behavior under barn-owl attack: Voles alternate between freezing and fleeing and spiny mice flee in alternating patterns. Behav. Brain Res. 2004, 155, 207–216. [Google Scholar] [CrossRef]
- Eilam, D. Die hard: A blend of freezing and fleeing as a dynamic defense—Implications for the control of defensive behavior. Neurosci. Biobehav. Rev. 2005, 29, 1181–1191. [Google Scholar] [CrossRef]
- Fux, M.; Eilam, D. How barn owls (Tyto alba) visually follow moving voles (Microtus socialis) before attacking them. Physiol. Behav. 2009, 98, 359–366. [Google Scholar] [CrossRef]
- Shifferman, E.; Eilam, D. Movement and direction of movement of a simulated prey affect the success rate in barn owl (Tyto alba) attack. J. Avian Biol. 2004, 35, 111–116. [Google Scholar] [CrossRef]
- Schalcher, K.; Milliet, E.; Séchaud, R.; Bühler, R.; Almasi, B.; Potier, S.; Becciu, P.; Roulin, A.; Shepard, E.L.C. Landing force reveals new form of motion-induced sound camouflage in a wild predator. eLife 2024, 12, RP87775. [Google Scholar] [CrossRef]
- Pickwell, G. Barn owl growth and behaviorism. Auk 1948, 65, 359–373. [Google Scholar] [CrossRef]
- Wuntke, B. Zur Entwicklung der Tagesrhythmik bei Schleiereulen (Tyto alba). J. Ornithol. 2003, 144, 81–85. [Google Scholar] [CrossRef]
- Foreman, N.; Altaha, M. The development of exploration and spontaneous alternation in hooded rat pups: Effects of unusually early eyelid opening. Dev. Psychobiol. 1991, 24, 521–537. [Google Scholar] [CrossRef]
- Durant, J.M.; Handrich, Y. Growth and food requirement flexibility in captive chicks of the European barn owl (Tyto alba). J. Zool. 1998, 245, 137–145. [Google Scholar] [CrossRef]
- Wilson, R.T.; Wilson, M.P.; Durkin, J.W. Growth of nestling barn owls Tyto alba in central Mali. Ibis 1987, 129, 305–318. [Google Scholar] [CrossRef]
- Karl, K. Side-to-side head movements to obtain motion depth cues:: A short review of research on the praying mantis. Behav. Process 1998, 43, 71–77. [Google Scholar] [CrossRef]
- Karl, K. Behavioural–analytical studies of the role of head movements in depth perception in insects, birds and mammals. Behav. Process 2003, 64, 1–2. [Google Scholar] [CrossRef]
- Ohayon, S.; van der Willigen, R.F.; Wagner, H.; Katsman, I.; Rivlin, E. On the barn owl’s visual pre-attack behavior: I. Structure of head movements and motion patterns. Comp. Physiol. A 2006, 192, 927–940. [Google Scholar] [CrossRef]
- Eilam, D.; Golani, I. The ontogeny of exploratory behavior in the house rat (Rattus rattus): The mobility gradient. Dev. Psychobiol. 1988, 21, 679–710. [Google Scholar] [CrossRef]
- Golani, I.; Wolgin, D.; Teitelbaum, P. A proposed natural geometry of recovery fromakinesia in the lateral hypothalamic rat. Brain Res. 1979, 164, 237–267. [Google Scholar] [CrossRef]
- Golani, I.; Bronchti, G.; Moualem, D.; Teitelbaum, P. “Warm-up” along dimensions ofmovement in the ontogeny of exploration in rats and other infant mammals. Proc. Natl. Acad. Sci. USA 1981, 78, 7226–7229. [Google Scholar] [CrossRef]
- Golani, I. A mobility gradient in the organization of vertebrate movement: The perception of movement through symbolic language. Behav. Brain Sci. 1992, 15, 249–266. [Google Scholar] [CrossRef]
- Golani, I. The developmental dynamics of behavioral growth processes in rodent egocentric and allocentric space. Behav. Brain Res. 2012, 23, 309–316. [Google Scholar] [CrossRef]
- Eilam, D. The mobility gradient from a comparative phylogenetic perspective. Behav. Brain Sci. 1992, 15, 274–275. [Google Scholar] [CrossRef]
- Eilam, D. Comparative morphology of locomotion in vertebrates. J. Mot. Behav. 1995, 27, 100–111. [Google Scholar] [CrossRef]
- Sinnamon, H.M.; Karvosky, M.E.; Ilch, C.P. Locomotion and head scanning initiated by hypothalamic stimulation are inversely related. Behv Brain Res. 1999, 99, 219–229. [Google Scholar] [CrossRef]
- Gomez-Marin, A.; Oron, E.; Gakamsky, A.; Valente, D.; Benjamini, Y.; Golani, I. Searching for behavioral homologies: Shared generative rules for expansion and narrowing down of the locomotor repertoire in arthropods and vertebrates. arXiv 2015, arXiv:1507.07270. [Google Scholar]
- May, C.J. Modeling the behavior of infant Norway rats (Rattus norvegicus). Diss. Abstr. Int. Sect. B Sci. Eng. 2008, 68, 6383. [Google Scholar]
- May, C.J.; Schank, J.C. The interaction of body morphology, directional kinematics, and environmental structure in the generation of neonatal rat (Rattus norvegicus) locomotor behavior. Ecol. Psychol. 2009, 21, 308–333. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eilam, D.; Hagbi, Z. Post-Hatching Development of Posture and Behavior in the Barn Owl (Tyto alba): From a General Behavioral Pattern of Vertebrates to the Typical Owl Behavior. Biology 2024, 13, 834. https://doi.org/10.3390/biology13100834
Eilam D, Hagbi Z. Post-Hatching Development of Posture and Behavior in the Barn Owl (Tyto alba): From a General Behavioral Pattern of Vertebrates to the Typical Owl Behavior. Biology. 2024; 13(10):834. https://doi.org/10.3390/biology13100834
Chicago/Turabian StyleEilam, David, and Zohar Hagbi. 2024. "Post-Hatching Development of Posture and Behavior in the Barn Owl (Tyto alba): From a General Behavioral Pattern of Vertebrates to the Typical Owl Behavior" Biology 13, no. 10: 834. https://doi.org/10.3390/biology13100834
APA StyleEilam, D., & Hagbi, Z. (2024). Post-Hatching Development of Posture and Behavior in the Barn Owl (Tyto alba): From a General Behavioral Pattern of Vertebrates to the Typical Owl Behavior. Biology, 13(10), 834. https://doi.org/10.3390/biology13100834





