Potential Ecological Distribution of the Beetle Agrilus mali Matsumura (Coleoptera: Buprestidae) in China under Three Climate Change Scenarios, with Consequences for Commercial and Wild Apple Forests
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Historic and Current Locations of A. mali
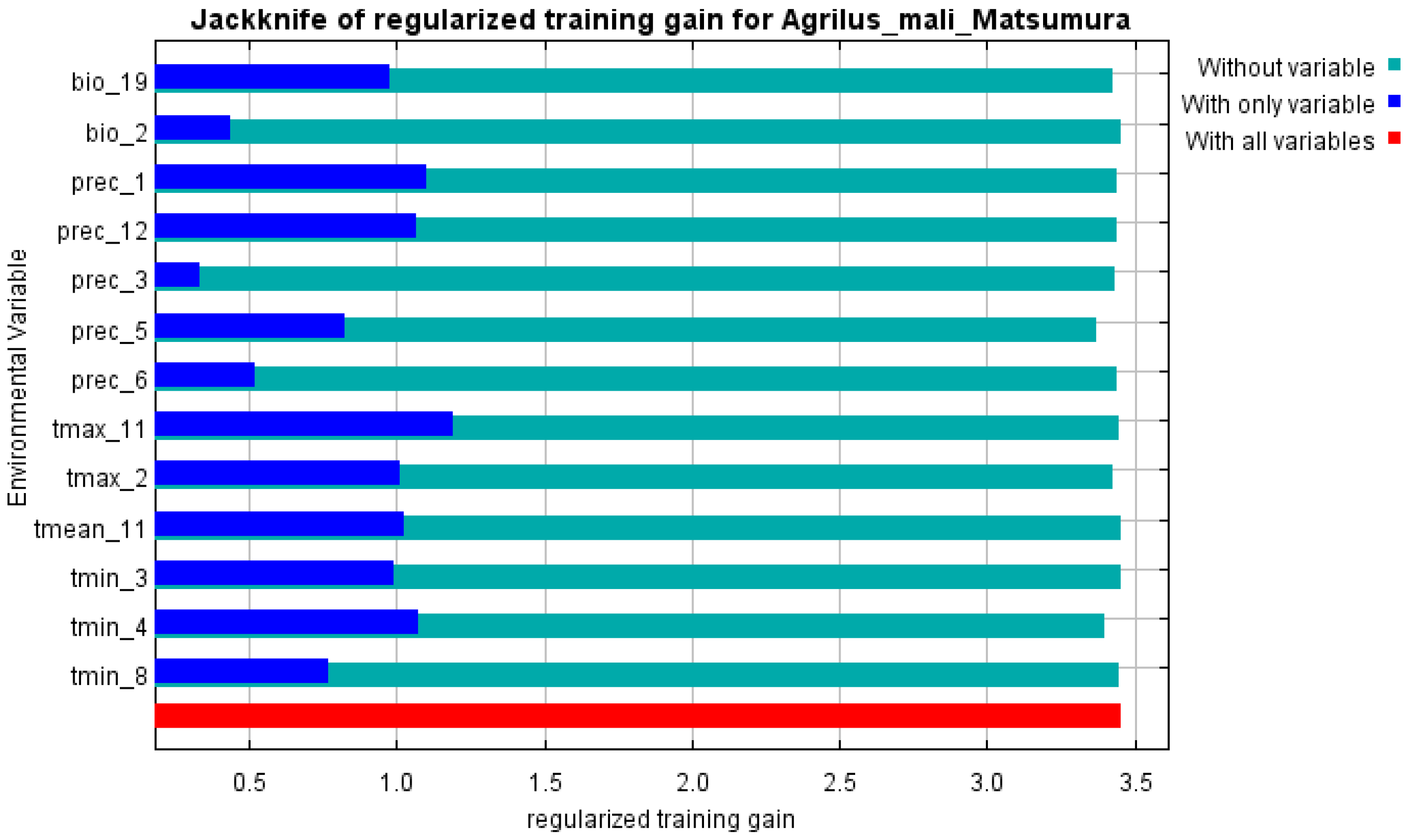
2.2. Environmental Variables
2.3. Modeling Methods and Statistical Analysis
2.4. Calculation of Geometric Center and Displacement
3. Results
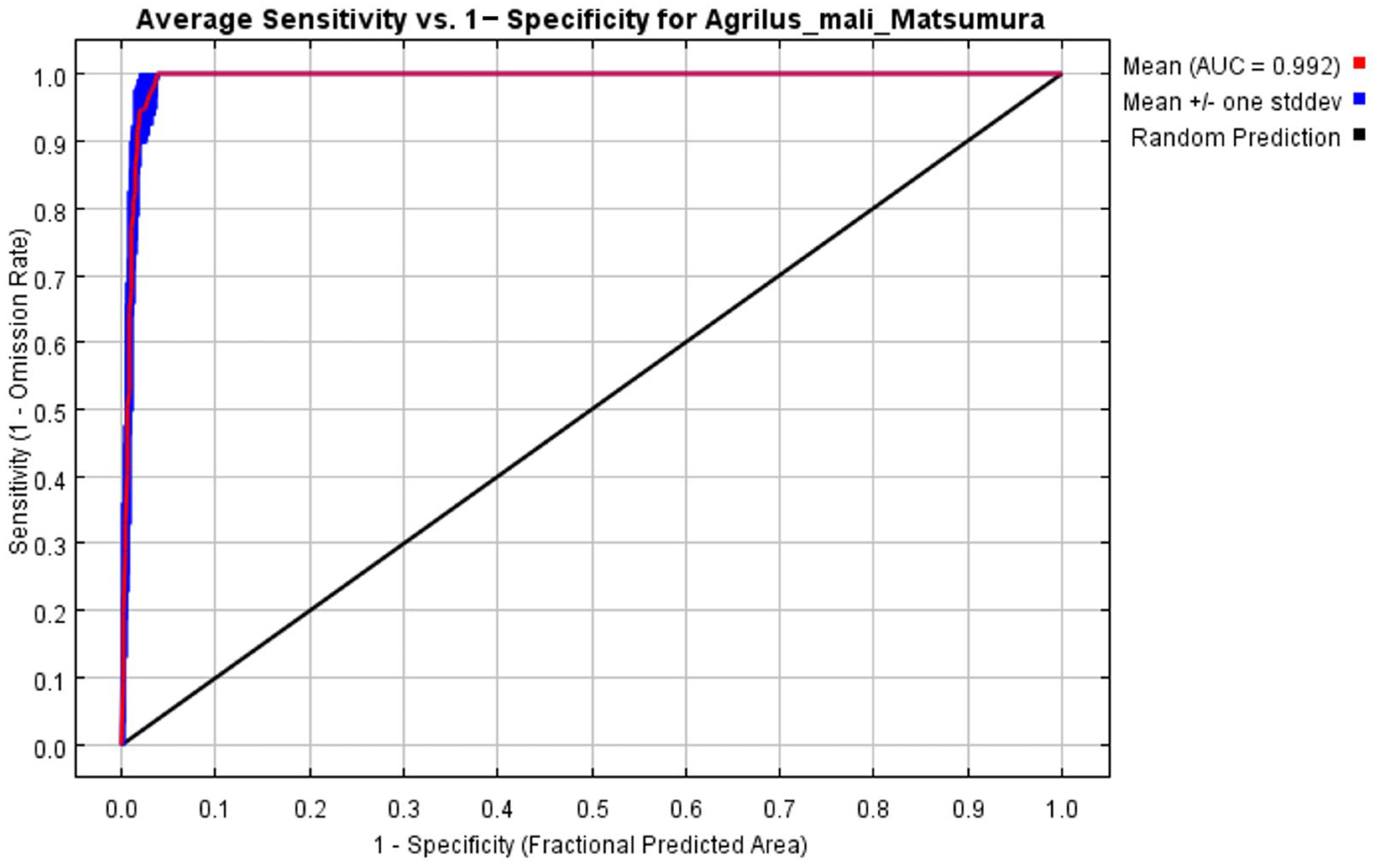
3.1. Evaluation of the MaxEnt Model
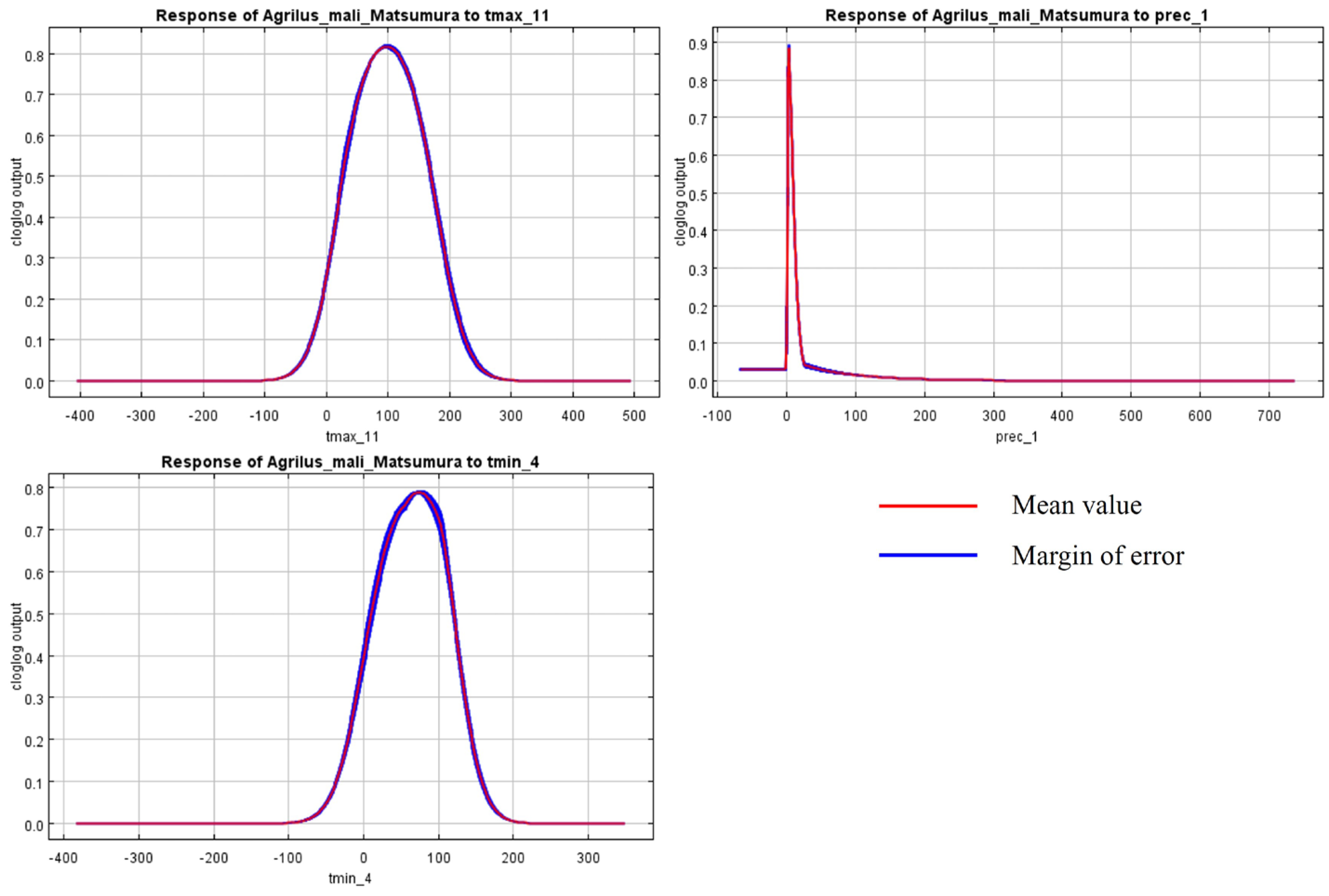
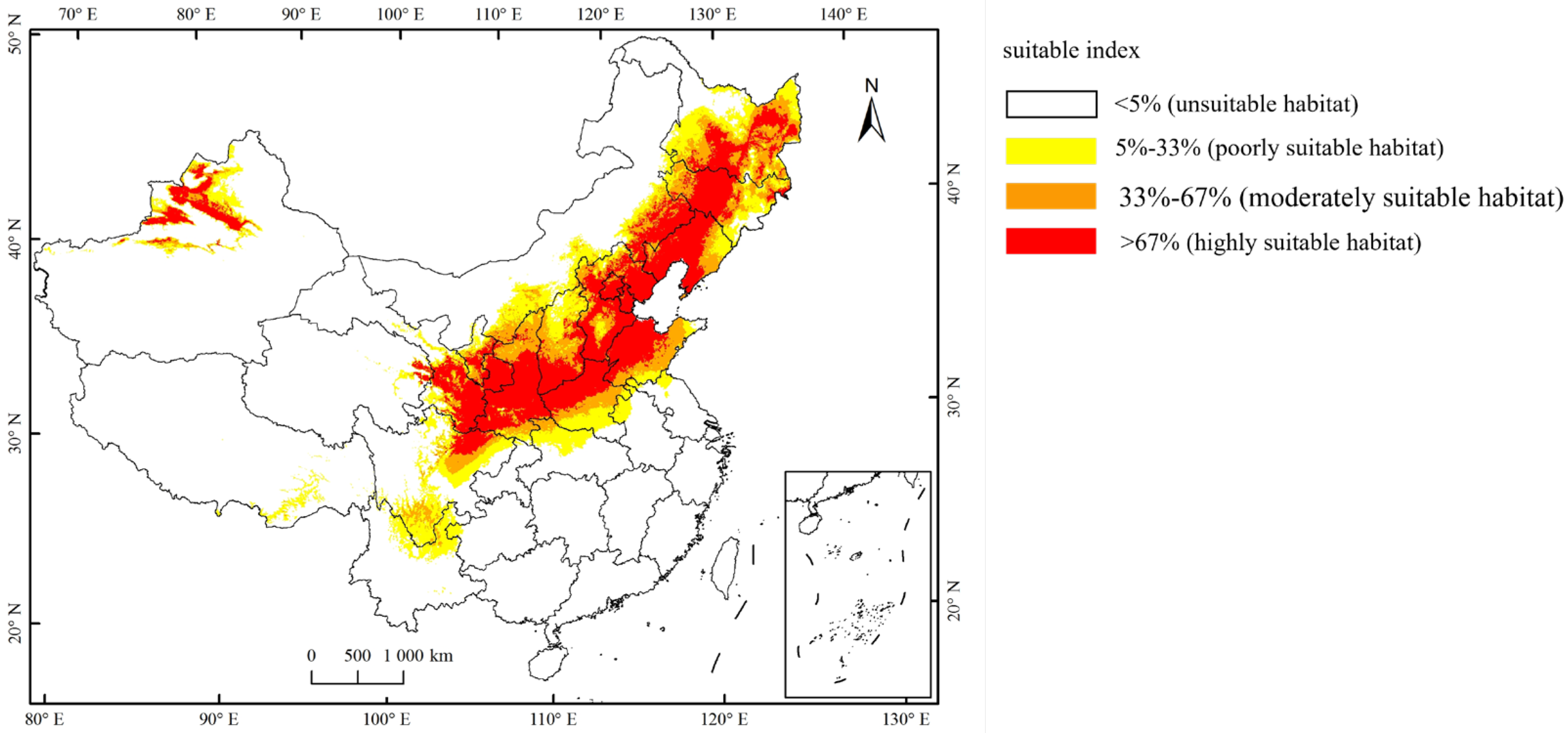
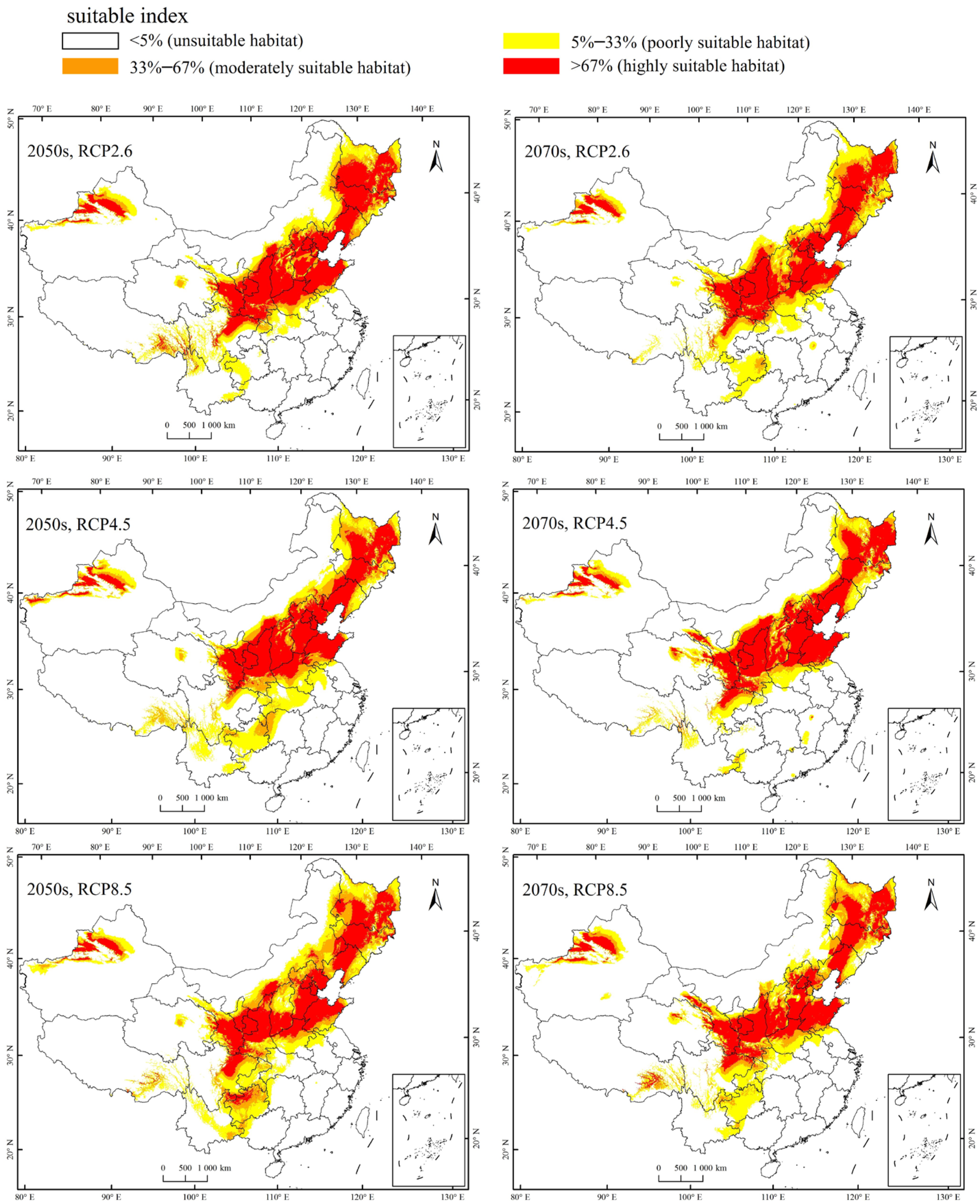
3.2. Spatial Pattern of A. mali Distribution under Global Warming
3.3. Change in the Distribution Area of A. mali under Global Warming Scenarios
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AJB | Apple jewel beetle |
| A. mali | Agrilus mali |
| XUAR | Xinjiang Uygur Autonomous Region |
| IPCC | Intergovernmental Panel on Climate Change |
| GBIF | Global Biodiversity Information Facility |
| CABI | Center for Agriculture and Biosciences International |
| RCPs | Representative CO2 Concentration Pathways |
| CIAT | International Centre for Tropical Agriculture |
| ROC | Receiver operating characteristic |
| AUC | Area under the curve |
References
- Wang, C.; Zhao, J.; Sui, J.; Zhao, F.; Ma, F. First report on Agrilus mali in Xinjiang. Xinjiang Agric. Sci. 1995, 05, 225–226. [Google Scholar]
- EPPO. Agrilus mali-Distribution. EPPO Global Database. 2018. Available online: https://gd.eppo.int/taxon/AGRLMA/distribution (accessed on 10 June 2024).
- Cui, Z.; Zhang, Y.; Zhang, X.; Luo, Z.; Zhang, P.; Golec, J.; Poland, T.M.; Zalucki, M.P.; Han, P.; Lu, Z. Life history and mortality factors of Agrilus mali Matsumura (Coleoptera: Buprestidae) in wild apples in Northwestern China. J. Agric. Forest Entomol. 2019, 3, 309–317. [Google Scholar] [CrossRef]
- Cui, X.; Liu, D.; Liu, A. Research progress in integrated management of Agrilus mali. Plant Prot. 2015, 41, 16–23. [Google Scholar]
- Wang, Z. Research on Biological Control of Agrilus mali Matsumura (Coleoptera: Buprestidae) in Stands of Malus sieversii in Xinjiang; Chinese Academy of Forestry: Beijing, China, 2013. [Google Scholar]
- Jun, C.; Long, Z.; Hui, L.; Liang, M.; Zhi, L. Damage of Agrilus mali Matsumura in Wild Apple Forest and Its Assessment. Arid Zone Res. 2018, 35, 1153–1159. [Google Scholar]
- Wang, Z.; Zhang, Y.; Yang, Z.; Wang, X. Determination of larval instars of Agrilus mali matsumura (Coleoptera: Buprestidae). For. Res. 2013, 26, 786–789. [Google Scholar]
- Tohir, A.B.; Bakhtiyor, A.R.; Zhang, D. Characterization of the gut microbiota of invasive Agrilus mali Matsumara (Coleoptera: Buprestidae) using high-throughput sequencing: Uncovering plant cell-wall degrading bacteria. Sci. Rep. 2019, 9, 4923. [Google Scholar] [CrossRef]
- Zhu, H. Agrilus mali made serious damage to wild fruit forest in Gongliu County, Ili City. Plant Prot. 2003, 29, 60. [Google Scholar]
- Volk, G.M.; Henk, A.D.; Richards, C.M.; Forsline, P.L.; Chao, C.T. Malus sieversii: A Diverse Central Asian Apple Species in the USDA-ARS National Plant Germplasm System. HortScience 2013, 48, 1440–1444. [Google Scholar] [CrossRef]
- Zhang, P.; Lv, Z.; Zhang, X.; Zhao, X.; Zhang, Y.; Tanabekova, G.; Bagila, M.; Zhanera, A.; Cui, Z. Age Structure of Malus sieversii Population in Ili of Xinjiang and Kazakhstan. Arid. Zone Res. 2019, 36, 844–853. [Google Scholar]
- Duan, N.; Bai, Y.; Sun, H.; Wang, N.; Ma, Y.; Li, M.; Wang, X.; Jiao, C.; Legall, N.; Mao, L.; et al. Genome re-sequencing reveals the history of apple and supports a two-stage model for fruit enlargement. Nat. Commun. 2017, 8, 249. [Google Scholar] [CrossRef]
- Bozorov, T.A.; Luo, Z.; Li, X.; Zhang, D. Agrilus mali Matsumara (Coleoptera: Buprestidae), a new invasive pest of wild apple in western China: DNA barcoding and life cycle. Ecol. Evol. 2019, 9, 1160–1172. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Wang, Z.; Cao, L.; Duan, J. Recent Outbreaks of the Apple Jewel Beetle (Coleoptera: Buprestidae) in Northwest China: A Potential Risk to Apple Production. In Proceedings of the 25th USDA Interagency Research Forum on Invasive Species, Annapolis, MD, USA, 7–10 January 2014; p. 81. [Google Scholar]
- Xue, S. Occurrence and control of Agrilus mali in Pingliang City. Gansu Agric. Sci. Technol. 2004, 46–47. [Google Scholar]
- Wang, C. Control technology of Agrilus mali in Jianzha County. Qinghai Agro-Technol. Ext. 2006, 39–40. [Google Scholar]
- Zhang, Y.; Liu, B.; Qiu, M.; Liu, Y.; Wu, X.; Xiao, N. Areas Suitable for Growing Apples Moved Northward and Westward in China under the Background of Climate Change: Climatic Degionalization of Apple Based on High-resolution Meteorological Grid Data. Chin. J. Agrometeorol. 2019, 40, 678–691. [Google Scholar]
- Roy, D.B.; Sparks, T.H. Phenology of British butterflies and climate change. Glob. Chang. Biol. 2000, 6, 407–416. [Google Scholar] [CrossRef]
- Guo, K.; Sun, J.; Kang, L. The Responses of Insects to Global Warming. In Recent Advances in Entomological Research; Liu, T.X., Kang, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 118–123. [Google Scholar] [CrossRef]
- Griggs, D.J.; Noguer, M. Climate change 2001: The scientific basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Weather 2002, 57, 267–269. [Google Scholar] [CrossRef]
- Pureswaran, D.S.; Roques, A.; Battisti, A. Forest insects and climate change. Curr. For. Rep. 2018, 4, 35–50. [Google Scholar] [CrossRef]
- Parmesan, C.; Ryrholm, N.; Stefanescu, C.; Hill, J.K.; Thomas, C.D.; Descimon, H.; Huntley, B.; Kaila, L.; Kullberg, J.; Tammaru, T.; et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 1999, 399, 579–583. [Google Scholar] [CrossRef]
- Régnière, J. Predicting insect continental distributions from species physiology. Unasylva 2009, 60, 37–42. [Google Scholar]
- Lemke, D.; Hulme, P.E.; Brown, J.A.; Tadesse, W. Distribution modelling of Japanese honeysuckle (Lonicera japonica) invasion in the Cumberland Plateau and Mountain Region, USA. For. Ecol. Manag. 2011, 262, 139–149. [Google Scholar] [CrossRef]
- Kumar, S.; Spaulding, S.A.; Stohlgren, T.J.; Hermann, K.A.; Schmidt, T.S.; Bahls, L.L. Potential habitat distribution for the freshwater diatom didymosphenia geminata in the continental us. Front. Ecol. Environ. 2009, 7, 415–420. [Google Scholar] [CrossRef]
- Lamb, J.M.; Ralph, T.M.C.; Goodman, S.M.; Bogdanowicz, W.; Fahr, J.; Gajewska, M.; Bates, P.J.J.; Eger, J.; Benda, P.; Taylor, P.J. Phylogeography and predicted distribution of African-Arabian and Malagasy populations of giant mastiff bats, Otomops spp. (Chiroptera: Molossidae). Acta Chiropterol. 2008, 10, 21–40. [Google Scholar] [CrossRef]
- Sobek-Swant, S.; Kluza, D.A.; Cuddington, K.; Lyons, D.B. Potential distribution of emerald ash borer: What can we learn from ecological niche models using Maxent and GARP? For. Ecol. Manag. 2012, 281, 23–31. [Google Scholar] [CrossRef]
- Baek, S.; Kim, M.J.; Lee, J.H. Current and Future Distribution of Ricania shantungensis (Hemiptera: Ricaniidae) in Korea: Application of Spatial Analysis to Select Relevant Environmental Variables for MaxEnt and CLIMEX Modeling. Forests 2019, 10, 490. [Google Scholar] [CrossRef]
- Yan, X.; Zhang, Y.; Liu, Y.; Xiang, W.; Kang, F. The risk analysis and managing countermeasures of Agrilus mali in Shaanxi province. Plant Quar. 2017, 31, 46–52. [Google Scholar]
- Liu, A.; Alim; Xu, Y.; Zhang, X.; Wang, Y.; Jiao, S.; Du, M.; Kereman, Y. Study on Spatial Distribution Patten of Agrilus mali Matsumura Larva in wild fruit forests Xinjiang. J. Northwest For. Univ. 2007, 22, 92–94. [Google Scholar]
- Bowler, M.G. Species abundance distributions, statistical mechanics and the priors of MaxEnt. Theor. Popul. Biol. 2014, 92, 69–77. [Google Scholar] [CrossRef]
- Cao, X.; Lin, C.; Zhou, Y.; Duan, X. Potential distribution of Magnaporthe grisea in China and the world, predicted by MaxEnt. Plant Prot. 2011, 37, 80–83. [Google Scholar]
- Sun, J.; Zhou, G. Inter-decadal Variability of Winter Wheat Planting Zone in China during 1961 to 2010 Simulated by Maximum Entropy (MaxEnt). Chin. J. Agrometeorol. 2012, 33, 481–487. [Google Scholar]
- Dong, S.; Gao, X. Long-term climate change: Interpretation of IPCC fifth assessment report. Progress. Inquisitiones Mutat. Clim. 2014, 10, 56–59. [Google Scholar]
- Zhang, X.; Yun, G.; Yue, Q.; Sha, F. Implications of the Findings from the Working Group I Contribution to the IPCC Fifth Assessment Report on the UNFCCC Process. Progress. Inquisitiones Mutat. Clim. 2014, 10, 14–19. [Google Scholar] [CrossRef]
- Worthington, T.A.; Zhang, T.; Logue, D.R.; Mittelstet, A.R.; Brewer, S.K. Landscape and flow metrics affecting the distribution of a federally-threatened fish: Improving management, model fit, and model transferability. Ecol. Model. 2016, 342, 1–18. [Google Scholar] [CrossRef]
- Songer, M.; Delion, M.; Biggs, A.; Huang, Q. Modeling impacts of climate change on giant panda habitat. Int. J. Ecol. 2012, 2012, 108752. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Xu, X. Analysis of Suitable Bioclimatic Characteristics of Pseudolarix amabilis by Using MaxEnt Model. Sci. Silvae Sin. 2015, 51, 127–131. [Google Scholar]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Phillips, S.J.; Robert, P.A.; Robert, E.S. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Yue, T.; Yue, T.; Fan, Z.; Chen, C.; Sun, X.; Li, B. Surface modelling of global terrestrial ecosystems under three climate change scenarios. Ecol. Model. 2011, 222, 2342–2361. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, X.; Wang, T.; Zhang, P.; Wang, Z.; Zhang, Y.; Kriticos, D.J.; Zalucki, M.P. Malice at the Gates of Eden: Current and future distribution of Agrilus mali threatening wild and domestic apples. Bull. Entomol. Res. 2022, 112, 745–757. [Google Scholar] [CrossRef] [PubMed]
- Battisti, A.; Stastny, M.; Buffo, E.; Larsson, S. A rapid altitudinal range expansion in the pine processionary moth produced by the 2003 climatic anomaly. Glob. Chang. Biol. 2006, 12, 662–671. [Google Scholar] [CrossRef]
- Jepsen, J.U.; Hagen, S.B.; Ims, R.A.; Yoccoz, N.G. Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: Evidence of a recent outbreak range expansion. J. Anim. Ecol. 2008, 77, 257–264. [Google Scholar] [CrossRef]
- Trân, J.K.; Ylioja, T.; Billings, R.F.; Régnière, J.; Ayres, M.P. Impact of minimum winter temperatures on the population dynamics of dendroctonus frontalis. Ecol. Appl. 2007, 17, 882–899. [Google Scholar] [CrossRef] [PubMed]
- Shaanxi Provincial Bureau of Statistics; Shaanxi Survey Team of National Bureau of Statistics. Shaanxi Statistical Yearbook; China Statistics Press: Beijing, China, 2019; Volume 12–28. [Google Scholar]
- Koch, F.H.; Yemshanov, D.; Magarey, R.D.; Smith, W.D. Dispersal of Invasive Forest Insects via Recreational Firewood: A Quantitative Analysis. J. Econ. Entomol. 2012, 105, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Meurisse, N.; Rassati, D.; Hurley, B.P.; Brockerhoff, E.G.; Haack, R.A. Common pathways by which non-native forest insects move internationally and domestically. J. Pest Sci. 2019, 92, 13–27. [Google Scholar] [CrossRef]
- Qin, D.H.; Stocker, T. Highlights of the IPCC Working Group I Fifth Assessment Report. Progress. Inquisitiones Mutat. Clim. 2014, 10, 1–6. [Google Scholar]
- Jepsen, J.U.; Lauri, K.; Hagen, S.B.; Schott, T.; Ole, P.L.V.; Nilssen, A.C.; Ims, R.A. Rapid northwards expansion of a forest insect pest attributed to spring phenology matching with sub-Arctic birch. Glob. Chang. Biol. 2010, 17, 2071–2083. [Google Scholar] [CrossRef]
- Ye, G.; You, L.; Lu, C.; Lin, W.; Luo, M.; Tan, F. Global Climate Change and Adaptive Management of Forest Ecosystem. World For. Res. 2015, 28, 1–6. [Google Scholar]
- Wasielewski, O.; Wojciechowicz, T.; Giejdasz, K.; Krishnan, N. Influence of methoprene and temperature on diapause termination in adult females of the over-wintering solitary bee, Osmia rufa L. J. Insect Physiol. 2011, 57, 1682–1688. [Google Scholar] [CrossRef]
- Schebeck, M.; Hansen, E.M.; Schopf, A.; Ragland, G.J.; Bentz, B.J.J.P.E. Diapause and overwintering of two spruce bark beetle species. Physiol. Entomol. 2017, 42, 200–210. [Google Scholar] [CrossRef]
- Sgolastra, F.; William, P.K.; James, S.B.; Theresa, L.P.-S.; Stefano, M.; Jordi, B. The long summer: Pre-wintering temperatures affect metabolic expenditure and winter survival in a solitary bee. J. Insect Physiol. 2011, 57, 1651–1659. [Google Scholar] [CrossRef]
- Xing, F.; Huang, S.; Luo, L.; Liu, Y.; Zhang, L. Diapause termination, post-diapause development and reproduction in the beet webworm, Loxostege sticticalis (Lepidoptera: Pyralidae). J. Insect Physiol. 2010, 56, 1325–1331. [Google Scholar] [CrossRef]
- Irwin, J.T.; Richard, E.L. Mild winter temperatures reduce survival and potential fecundity of the goldenrod gall fly, Eurosta solidaginis (Diptera: Tephritidae). J. Insect Physiol. 2000, 46, 655–661. [Google Scholar] [CrossRef]
- Lecoq, M. Desert Locust Threat to Agricultural Development and Food Security and FAO/International Role in Its Control. In Proceedings of the Symposium on “Desert Locust Control”* at the Eighth Arab Congress of Plant Protection, El-Beida, Libya, 12–16 October 2003; pp. 188–193. [Google Scholar]
- Nielsen, D.G.; Muilenburg, V.L.; Herms, D.A. Interspecific Variation in Resistance of Asian, European, and North American Birches (Betula spp.) to Bronze Birch Borer (Coleoptera: Buprestidae). Environ. Entomol. 2011, 40, 648–653. [Google Scholar] [CrossRef]

| Bioclimatic Characteristic | Abbreviation | Time | Abbreviation |
|---|---|---|---|
| Annual mean temperature | bio1 | ||
| Mean diurnal range (mean max. temp–mean min. temp) | bio2 | Monthly | bio2 * |
| Isothermality (bio2/bio7) (×100) | bio3 | ||
| Temperature seasonality (standard deviation ×100) | bio4 | ||
| Max. temperature of warmest month | bio5 | ||
| Min. temperature of coldest month | bio6 | ||
| Temperature annual range (bio5-bio6) | bio7 | ||
| Mean temperature of wettest quarter | bio8 | ||
| Mean temperature of driest quarter | bio9 | ||
| Mean temperature of warmest quarter | bio10 | ||
| Mean temperature of coldest quarter | bio11 | ||
| Annual precipitation | bio12 | ||
| Precipitation of wettest month | bio13 | ||
| Precipitation of driest month | bio14 | ||
| Precipitation seasonality (coefficient of variation) | bio15 | ||
| Precipitation of wettest quarter | bio16 | ||
| Precipitation of driest quarter | bio17 | ||
| Precipitation of warmest quarter | bio18 | ||
| Precipitation of coldest quarter | bio19 | Yearly | bio19 * |
| Minimum temperature | tmin | March, April, and August | tmin3 *, tmin4 * tmin8 * |
| Maximum temperature | tmax | February and November | tamx2 *, tamx11 * |
| Average temperature | tavg | ||
| Precipitation | prec | January, March, May, June, and December | prec1 *, prec3 *, prec5 *, prec6 * prec12 * |
| Mean temperature of month | tmean | November | tmean11 * |
| Total number of variables | 23 | 13 |
| AUC Value | Evaluation Criterion (to Describe Reality) |
|---|---|
| <0.5 | Fails |
| 0.5 ≤ AUC < 0.6 | Fails |
| 0.6 ≤ AUC < 0.7 | Poor |
| 0.7 ≤ AUC < 0.8 | Moderate |
| 0.8 ≤ AUC < 0.9 | Good |
| ≥0.9 | Excellent |
| Period | RCP2.6 SRES-RCP2.6 | RCP4.5 SRES-RCP4.5 | RCP8.5 SRES-RCP8.5 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Displacement (km) | Direction | Angle (°) | Displacement (km) | Direction | Angle (°) | Displacement (km) | Direction | Angle (°) | |
| From the present until the 2050s | 27.19 | Northwest | 156.31 | 37.49 | Northwest | 160.50 | 33.64 | Southwest | 204.74 |
| From the 2050s to the 2070s | 42.81 | Northeast | 7.20 | 24.91 | Northeast | 37.11 | 38.90 | Northwest | 127.20 |
| From the present until the 2070s | 23.97 | Northeast | 42.84 | 31.59 | Northwest | 119.32 | 56.65 | Northwest | 162.63 |
| Period | RCP2.6 SRES-RCP2.6 | RCP4.5 SRES-RCP4.5 | RCP8.5 SRES-RCP8.5 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Displacement (km) | Direction | Angle (°) | Displacement (km) | Direction | Angle (°) | Displacement (km) | Direction | Angle (°) | |
| From the present until the 2050s | 112.44 | Northeast | 23.00 | 125.21 | Northeast | 34.68 | 68.68 | Northeast | 23.78 |
| From the 2050s to the 2070s | 33.14 | Northeast | 43.40 | 46.67 | Northeast | 5.77 | 21.13 | Northwest | 141.99 |
| From the present until the 2070s | 143.96 | Northeast | 27.60 | 167.59 | Northeast | 26.95 | 61.57 | Northeast | 41.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, H.; Jiamahate, A.; Yang, H.; Cao, L.; Dang, Y.; Lu, Z.; Yang, Z.; Bozorov, T.A.; Wang, X. Potential Ecological Distribution of the Beetle Agrilus mali Matsumura (Coleoptera: Buprestidae) in China under Three Climate Change Scenarios, with Consequences for Commercial and Wild Apple Forests. Biology 2024, 13, 803. https://doi.org/10.3390/biology13100803
Zhang Y, Yang H, Jiamahate A, Yang H, Cao L, Dang Y, Lu Z, Yang Z, Bozorov TA, Wang X. Potential Ecological Distribution of the Beetle Agrilus mali Matsumura (Coleoptera: Buprestidae) in China under Three Climate Change Scenarios, with Consequences for Commercial and Wild Apple Forests. Biology. 2024; 13(10):803. https://doi.org/10.3390/biology13100803
Chicago/Turabian StyleZhang, Yanlong, Hua Yang, Aerguli Jiamahate, Honglan Yang, Liangming Cao, Yingqiao Dang, Zhaozhi Lu, Zhongqi Yang, Tohir A. Bozorov, and Xiaoyi Wang. 2024. "Potential Ecological Distribution of the Beetle Agrilus mali Matsumura (Coleoptera: Buprestidae) in China under Three Climate Change Scenarios, with Consequences for Commercial and Wild Apple Forests" Biology 13, no. 10: 803. https://doi.org/10.3390/biology13100803
APA StyleZhang, Y., Yang, H., Jiamahate, A., Yang, H., Cao, L., Dang, Y., Lu, Z., Yang, Z., Bozorov, T. A., & Wang, X. (2024). Potential Ecological Distribution of the Beetle Agrilus mali Matsumura (Coleoptera: Buprestidae) in China under Three Climate Change Scenarios, with Consequences for Commercial and Wild Apple Forests. Biology, 13(10), 803. https://doi.org/10.3390/biology13100803





