Distribution and Ecological Risk of Ludwigia peploides in South Korea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Distribution Status and Biological Risk Factors
2.2. Abiotic Risk Factors
3. Results
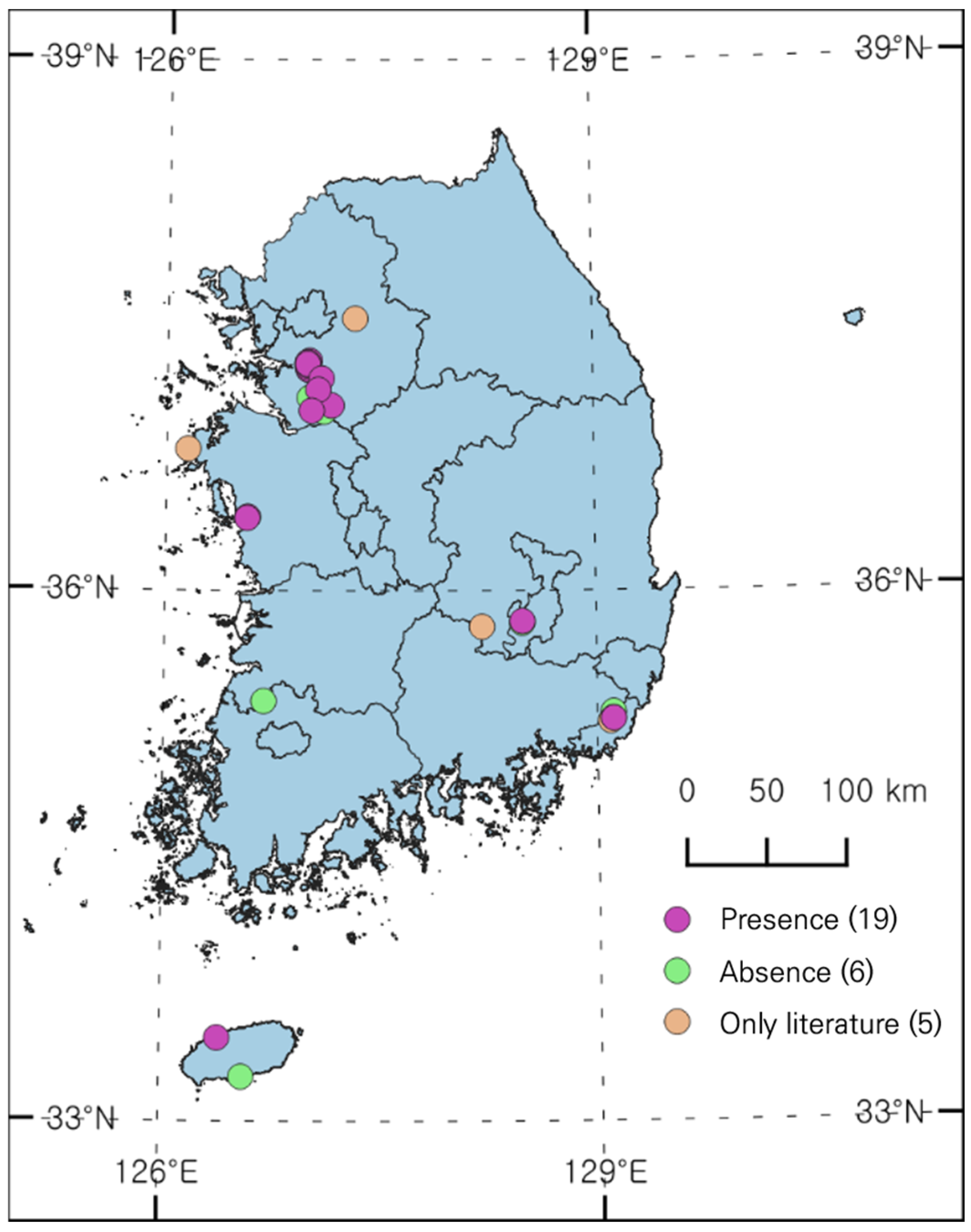
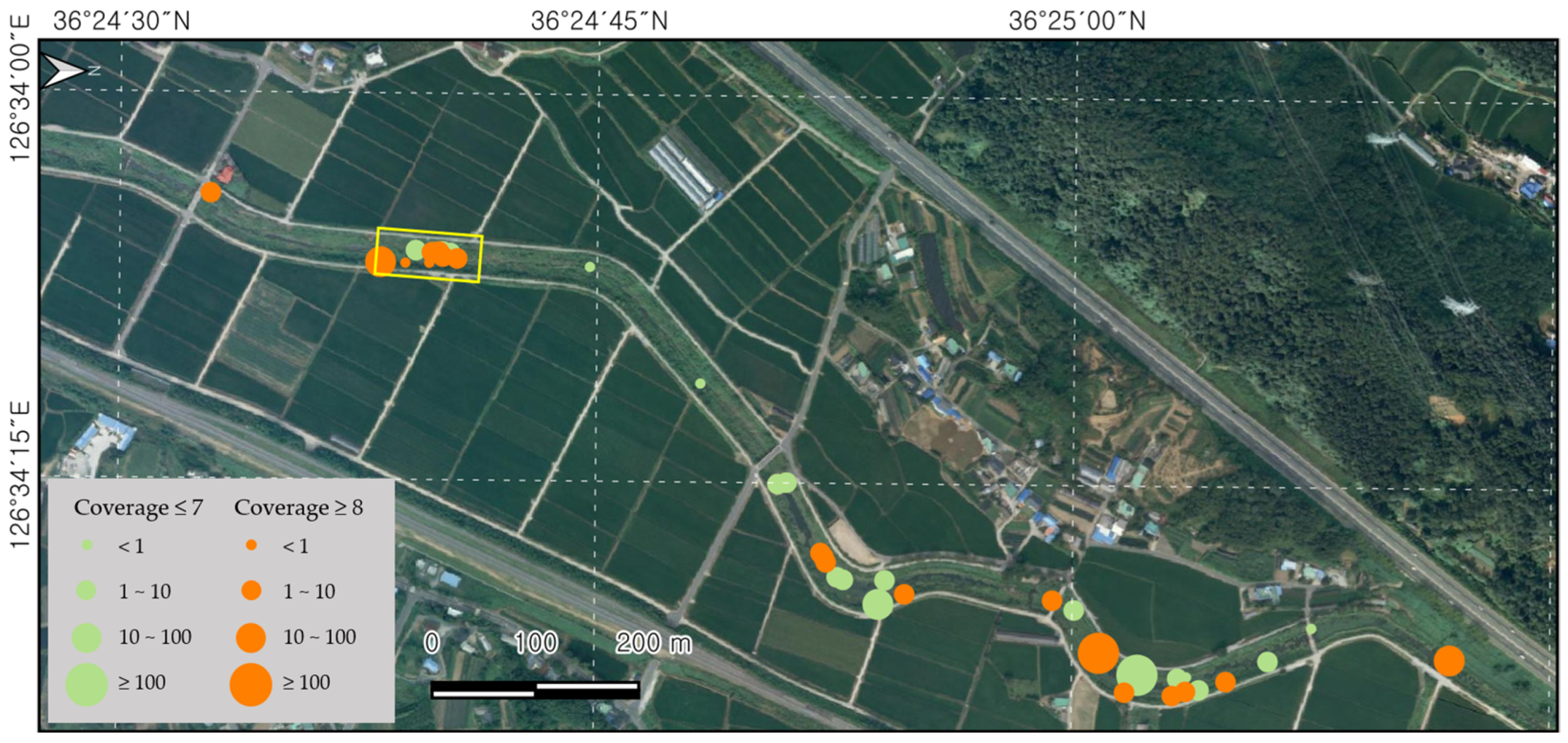
3.1. Distribution Status
3.2. Biological Risk Factors
3.3. Abiotic Risk Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pyšek, P.; Jarošík, V.; Hulme, P.E.; Pergl, J.; Hejda, M.; Schaffner, U.; Vilà, M. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species’ traits and environment. Glob. Chang. Biol. 2012, 18, 1725–1737. [Google Scholar] [CrossRef]
- Vilà, M.; Espinar, J.L.; Hejda, M.; Hulme, P.E.; Jarošík, V.; Maron, J.L.; Pergl, J.; Schaffner, U.; Sun, Y.; Pyšek, P. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol. Lett. 2011, 14, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Mollot, G.; Pantel, J.H.; Romanuk, T.N. The effects of invasive species on the decline in species richness. Adv. Ecol. Res. 2017, 56, 61–83. [Google Scholar]
- Vilà, M.; Basnou, C.; Pyšek, P.; Josefsson, M.; Genovesi, P.; Gollasch, S.; Nentwig, W.; Olenin, S.; Roques, A.; Roy, D. How well do we understand the impacts of alien species on ecosystem services? A pan-European, cross-taxa assessment. Front. Ecol. Environ. 2010, 8, 135–144. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef]
- Convention on Biological Diversity. Decision adopted by the Conference of the Parties to the Convention on Biological Diversity [CBD/COP/DEC/15/4]. Kunming-Montreal Global Biodiversity Framework. 2022. Available online: https://www.cbd.int/doc/decisions/cop-15/cop-15-dec-04-en.pdf (accessed on 28 June 2023).
- National Institute of Ecology (NIE). Guidebook for National Inhabitants Survey of Alien Species; National Institute of Ecology: Chungcheongnam-do, Republic of Korea, 2021; 161p, ISBN 979-11-6698-022-0. (In Korean) [Google Scholar]
- Kim, D.E. Management system of invasive alien species threatening biodiversity in Korea and suggestions for the improvement. J. Environ. Impact Assess. 2018, 27, 33–55. [Google Scholar]
- Hussner, A.; Heidbüchel, P.; Coetzee, J.; Gross, E.M. From introduction to nuisance growth: A review of traits of alien aquatic plants which contribute to their invasiveness. Hydrobiologia 2021, 848, 2119–2151. [Google Scholar] [CrossRef]
- Santos, M.J.; Anderson, L.W.; Ustin, S.L. Effects of invasive species on plant communities: An example using submersed aquatic plants at the regional scale. Biol. Invasions 2011, 13, 443–457. [Google Scholar] [CrossRef]
- Hejda, M.; Chytrý, M.; Pergl, J.; Pyšek, P. Native-range habitats of invasive plants: Are they similar to invaded-range habitats and do they differ according to the geographical direction of invasion? Divers. Distrib. 2015, 21, 312–321. [Google Scholar] [CrossRef]
- Bolpagni, R. Towards global dominance of invasive alien plants in freshwater ecosystems: The dawn of the Exocene? Hydrobiologia 2021, 848, 2259–2279. [Google Scholar] [CrossRef]
- Gurnell, A.; Thompson, K.; Goodson, J.; Moggridge, H. Propagule deposition along river margins: Linking hydrology and ecology. J. Ecol. 2008, 96, 553–565. [Google Scholar] [CrossRef]
- Thiébaut, G.; Dutartre, A. Management of invasive aquatic plants in France. In Aquatic Ecosystem Research Trends; Nairne, G.H., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2009; pp. 25–46. ISBN 978-1-60692-772-4. [Google Scholar]
- Thouvenot, L.; Haury, J.; Thiébaut, G. A success story: Water primroses, aquatic plant pests. Aquat. Conserv. Mar. Freshw. Ecosyst. 2013, 23, 790–803. [Google Scholar] [CrossRef]
- Zotos, A.; Sarika, M.; Lucas, E.; Dimopoulos, P. Ludwigia peploides subsp. montevidensis: A New Alien. Taxon. for the Flora of Greece and the Balkans. J. Biol. Res. 2006, 5, 71–78. [Google Scholar]
- Buzjak, S.; Sedlar, Z. Ludwigia peploides (Kunth.) P.H. Raven–floating water primrose, a new species in Croatian Flora from the list of invasive alien species of union concern. Nat. Croat. 2018, 27, 351–356. [Google Scholar] [CrossRef]
- Ruaux, B.; Greulich, S.; Haury, J.; Berton, J.P. Sexual reproduction of two alien invasive Ludwigia (Onagraceae) on the middle Loire River, France. Aquat. Bot. 2009, 90, 143–148. [Google Scholar] [CrossRef]
- Robert, H.; Lafontaine, R.-M.; Beudels-Jamar, R.C.; Delsinne, T. Risk Analysis of the Water Primrose Ludwigia peploides (Kunth) P.H. Raven—Risk Analysis Report of Non-Native Organisms in Belgium; Royal Belgian Institute of Natural Sciences for the Federal Public Service Health, Food Chain Safety and Environment: Brussels, Begium, 2013; 35p. [Google Scholar]
- Kim, H.W.; Son, D.C.; Park, S.H.; Jang, C.S.; Sun, E.M.; Jo, H.Y.; Yun, S.M.; Chang, K.S. Unrecorded alien plant in South Korea: Ludwigia peploides subsp. montevidensis (Spreng.) P.H. Raven. Korean J. Plant Res. 2019, 32, 201–206. [Google Scholar]
- Dandelot, S.; Robles, C.; Pech, N.; Cazaubon, A.; Verlaque, R. Allelopathic potential of two invasive Alien Ludwigia spp. Aquat. Bot. 2008, 88, 311–316. [Google Scholar] [CrossRef]
- Grutters, B.M.C.; Saccomanno, B.; Gross, E.M.; Van de Waal, D.B.; van Donk, E.; Bakker, E.S. Growth strategy, phylogeny and stoichiometry determine the allelopathic potential of native and non-native plants. Oikos 2017, 126, 1770–1779. [Google Scholar] [CrossRef]
- Inderjit; Duke, S.O. Ecophysiological aspects of allelopathy. Planta 2003, 217, 529–539. [Google Scholar]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and exotic plant invasion: From molecules and genes to species interactions. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef]
- Thiébaut, G.; Thouvenot, L.; Rodríguez-Pérez, H. Allelopathic effect of the invasive Ludwigia hexapetala on growth of three macrophyte species. Front. Plant Sci. 2018, 9, 1835. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environment. Genetic and Ecological Risk Assessment of the Invasion of Alien Plants on Native Species; Ministry of Environment: Tokyo, Japan, 2006. [Google Scholar]
- Lambert, E.; Dutartre, A.; Coudreuse, J.; Haury, J. Relationships between the biomass production of invasive Ludwigia species and physical properties of habitats in France. Hydrobiologia 2010, 656, 173–186. [Google Scholar] [CrossRef]
- Zardini, E.M.; Peng, C.I.; Hoch, P.C. Chromosome numbers in Ludwigia sect. Oligospermum and Sect. Oocarpon (Onagraceae). Taxon 1991, 40, 221–230. [Google Scholar] [CrossRef]
- Staunch, A. The Blue Heron Wetland Restoration Project: Eradication of Ludwigia peploides ssp. montevidensis from the Blue Heron Wetlands of Portland, OR. Master’s Thesis, Alexander Staunch, Portland State University, Portland, OR, USA, 2015. Volume 47. [Google Scholar]
- Dandelot, S.; Verlaque, R.; Dutartre, A.; Cazaubon, A. Ecological, dynamic and taxonomic problems due to Ludwigia (Onagraceae) in France. Hydrobiologia 2005, 551, 131–136. [Google Scholar] [CrossRef]
- Dandelot, S. Les Ludwigia spp. du Sud de la France: Historique, Biosyste’Matique et E’Cologie. Ph.D. Thesis, Université Paul Cézanne, Aix-Marseille III, Aix-Marseille, France, 2004; 218p. (In French). [Google Scholar]
- Legrand, C. Pour Contrôler la Prolifération des Jussies (Ludwigia spp.) dans les Zones Humides Méditerranéennes: Guide Technique; Agence Méditerranéenne de L’environnement: Montpellier, France, 2002; 68p. (In French) [Google Scholar]
- EUR-Lex. Regulation (EU) 2016/1141 of the European Parliament and of the Council of 13 July 2016. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02016R1141-20220802&from=EN (accessed on 29 November 2023).
- Invasoras.pt. Nova Legislação Em Vigor Sobre Espécies Exóticas Invasoras. Available online: https://invasoras.pt/pt/nova-legisla%C3%A7%C3%A3o-em-vigor-sobre-esp%C3%A9cies-ex%C3%B3ticas-invasoras (accessed on 29 November 2023).
- GB Non-Native Species Secretariat. Identification Guide: Ludwigia grandiflora (Water Primrose). Available online: https://www.nonnativespecies.org/assets/Uploads/ID_Ludwigia_grandiflora_Water_Primrose-2-v2.pdf (accessed on 29 November 2023).
- Grewell, B.J.; Thomason, M.J.S.; Netherland, M.D. Establishing Research and Management Priorities for Invasive Water Primroses (Ludwigia spp.); U.S. Army Corps of Engineers Engineer Research and Development Center: Vicksburg, MS, USA, 2016; 53p. [Google Scholar]
- Ministry for Primary Industries Manatū Ahu Matua. Handling Unwanted Organisms. Available online: https://www.mpi.govt.nz/biosecurity/how-to-find-report-and-prevent-pests-and-diseases/handling-unwanted-organisms (accessed on 29 November 2023).
- EPPO (European and Mediterranean Plant Protection Organization). Ludwigia grandiflora and L. peploides Onagraceae—Water Primroses. Available online: https://gd.eppo.int/taxon/LUDPE/documents (accessed on 28 June 2023).
- Kim, Y.O.; Lee, E.J. Comparison of phenolic compounds and the effects of invasive and native species in East Asia: Support for the novel weapons hypothesis. Ecol. Res. 2011, 26, 87–94. [Google Scholar] [CrossRef]


| Region | Part | TFC (mg/g) | TPC (mg/g) | Caffeic Acid (mg/kg) | Myricitrin (mg/kg) | p-Coumaric Acid (mg/kg) | Prunin (mg/kg) |
|---|---|---|---|---|---|---|---|
| Boryeong | Leaf | 9.56 ± 0.02 | 48.29 ± 0.68 | 219.72 ± 1.34 | - | - | 457.60 ± 3.80 |
| Root | 2.18 ± 0.02 | 7.06 ± 0.11 | 27.65 ± 1.80 | - | 17.73 ± 0.24 | - | |
| Osan | Leaf | 7.77 ± 0.10 | 27.96 ± 0.08 | 142.14 ± 0.42 | - | - | 499.81 ± 1.30 |
| Root | 0.78 ± 0.04 | 0.43 ± 0.02 | - | - | 15.94 ± 0.06 | - | |
| Suwon | Leaf | 7.88 ± 0.01 | 42.09 ± 0.33 | 147.94 ± 1.13 | 8681.84 ± 26.13 | - | 585.90 ± 2.57 |
| Root | 1.27 ± 0.04 | 0.41 ± 0.01 | 19.58 ± 0.03 | - | 14.84 ± 0.25 | - |
| Parameter (mg/L) | Osan | Boryeong | Busan | Dague | Anseong | Average |
|---|---|---|---|---|---|---|
| TOC | 8.3 | 2.7 | 2.2 | 6.1 | 3.9 | 4.64 |
| TP | 0.879 | 0.059 | 0.27 | 0.653 | 0.077 | 0.3876 |
| COD | 10.5 | 4.4 | 5.8 | 9 | 13.3 | 8.6 |
| BOD | 3.3 | 1.5 | 2.1 | 3 | 2.6 | 2.5 |
| SS | 5 | 3.2 | 67 | 27.8 | 9.2 | 22.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, A.; Lee, S.I.; Choi, D.; Kim, Y.; Lee, Y.H.; Hong, S.H. Distribution and Ecological Risk of Ludwigia peploides in South Korea. Biology 2024, 13, 768. https://doi.org/10.3390/biology13100768
Jo A, Lee SI, Choi D, Kim Y, Lee YH, Hong SH. Distribution and Ecological Risk of Ludwigia peploides in South Korea. Biology. 2024; 13(10):768. https://doi.org/10.3390/biology13100768
Chicago/Turabian StyleJo, Aram, Soo In Lee, Donghui Choi, Youngha Kim, Yong Ho Lee, and Sun Hee Hong. 2024. "Distribution and Ecological Risk of Ludwigia peploides in South Korea" Biology 13, no. 10: 768. https://doi.org/10.3390/biology13100768
APA StyleJo, A., Lee, S. I., Choi, D., Kim, Y., Lee, Y. H., & Hong, S. H. (2024). Distribution and Ecological Risk of Ludwigia peploides in South Korea. Biology, 13(10), 768. https://doi.org/10.3390/biology13100768





