What Determines the Class of Immunity an Antigen Induces? A Foundational Question Whose Rational Consideration Has Been Undermined by the Information Overload
Abstract
:Simple Summary
Abstract
1. Prologue
2. Frameworks for Envisaging What Controls the Th1/Th2 Phenotype of a Response
3. The Grounds for the PAMP Model for the Activation of CD4 T Cells
4. The Grounds for the DAMP Model for the Activation of CD4 T Cells
5. The Plausibility/Implausibility of the DAMP/PAMP Models
6. The Quorum Model for Lymphocyte Activation, Including the Activation of CD4 T Cells
7. The General Importance of Immune Class Regulation
8. Variables of Immunization Affecting the Th1/Th2 Phenotype of the Ensuing Response: Salvin’s Findings
8.1. The Generality of Salvin’s Findings
8.2. Immune Deviation and Th Subset Imprinting
9. “Salvin’s Laws” Apply in Diverse Strains of Mice in Their Response to L Major
10. Models and Ideas on What Controls the Th1/Th2 Phenotype of an Immune Response
The DAMP/PAMP-Centric View
11. The Threshold Hypothesis and Its Plausibility
12. How the Threshold Hypothesis Explains the Variables of Immunization Affecting the Th1/Th2 Phenotype of a Response
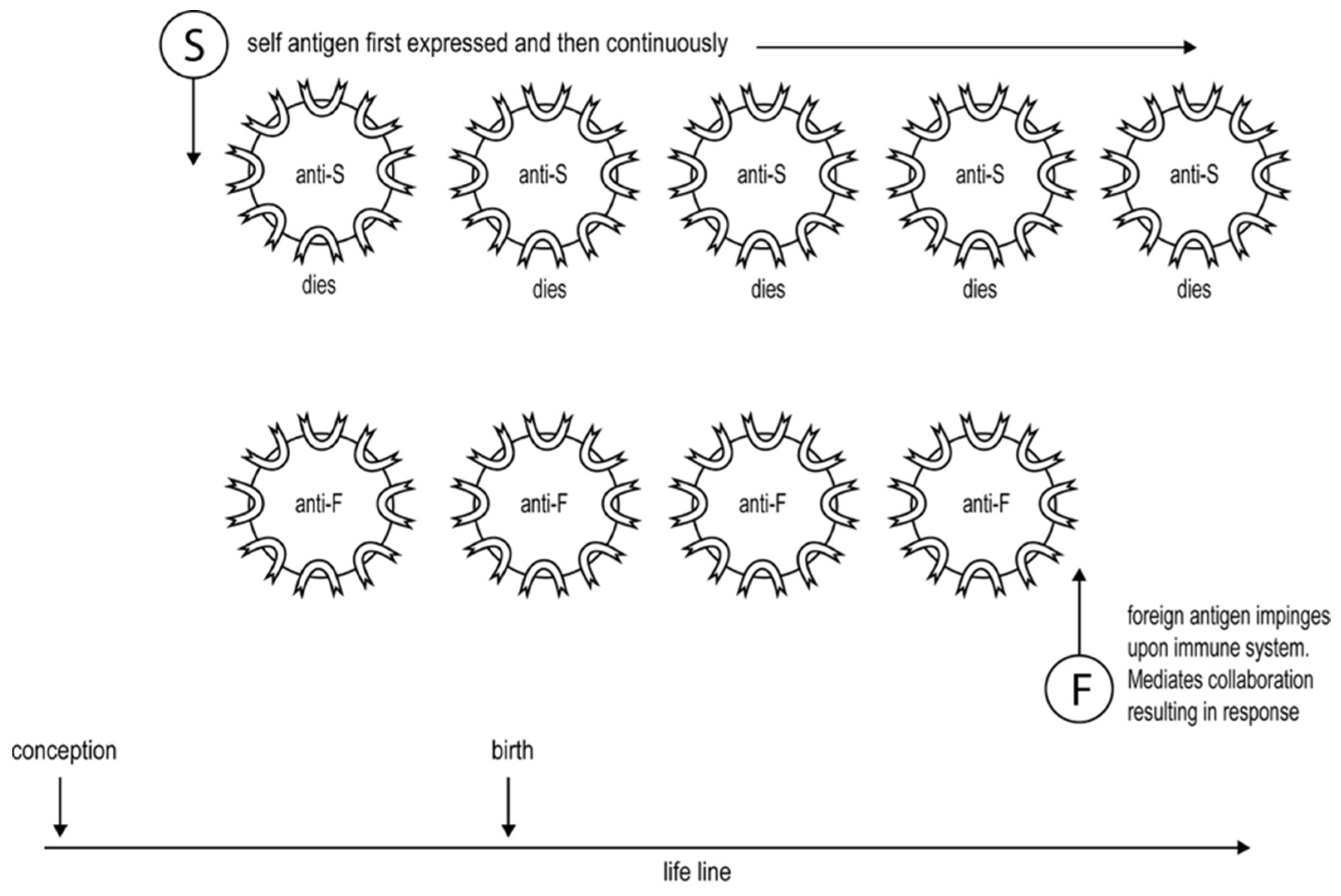
- Pearson and Raffel pointed out in the 1960s that certain antigens were able to induce cell-mediated immunity but not readily detectable antibody. These antigens were either small in size or larger, but being only a slight modification of self. These authors proposed that such antigens are minimally foreign, and it is this characteristic that allows them to be immunogenic only for a cell-mediated response [84]. There will be fewer CD4 T cells specific for such antigens than for more foreign antigens. Even in the presence of amounts of antigen optimal for mediating CD4 T cell interactions, only weak CD4 T cell interactions will take place. Thus, according to the proposed threshold mechanism, Th1 cells will be predominantly generated. I suggested such antigens are susceptible only to cell-mediated attacks, providing a physiological reason underlying this mechanism [14]. This possibility may also explain why cancers are preferentially susceptible to cell-mediated attacks [14,57].
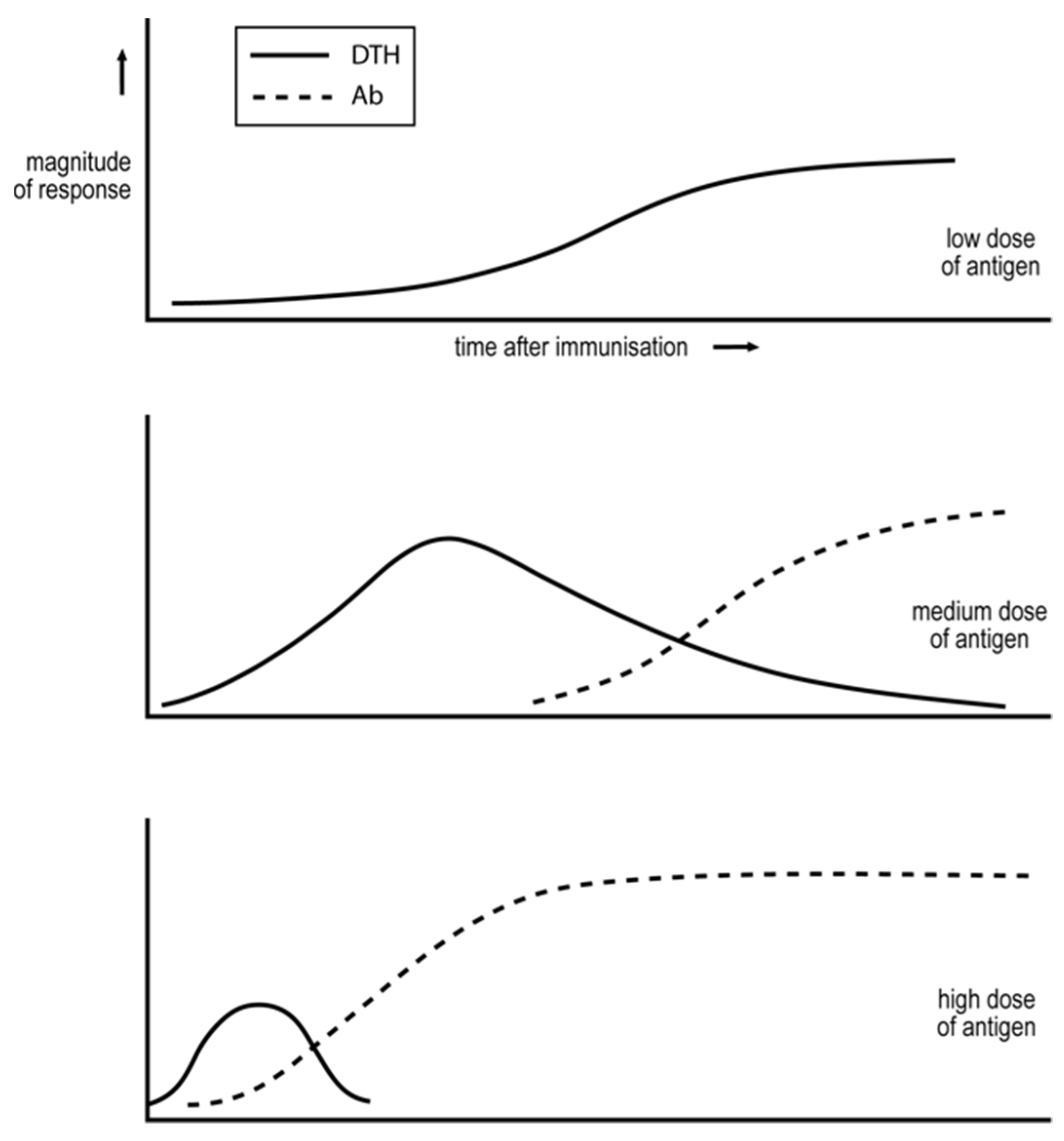
- More foreign antigens, for which there are naturally more CD4 T helper cells, can induce the generation of Th1 or Th2 cells, depending upon the circumstances of immunization. Immunization with low doses of antigen, well below that optimal for mediating CD4 T cell cooperation, will initially only support weak CD4 T cell collaboration and so the generation of Th1 cells, see Figure 2. It is known that foreign antigens cause their corresponding CD4 T cells to multiply. Thus, so long as the level of antigen is sufficiently sustained, the interaction between the CD4 T cells will become stronger with time, thus explaining why the response evolves with time from an exclusive Th1 towards a Th2 phenotype, see Figure 2. Immunizing with higher levels of antigen, more optimal for mediating CD4 T cell collaboration, results in even more rapid responses [7,14].
13. Support for the Threshold Hypothesis and Paradoxes within the Context of DAMP/PAMP-Centric View
14. The Role of Cytokines in Controlling the Th1/Th2 Phenotype of the Response
15. Foundational Ideas and World Health
16. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Barabasi, A. The Science of Science; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Chu, J.S.G.; Evans, J.A. Slowed canonical progress in large fields of science. Proc. Natl. Acad. Sci. USA 2021, 118, e2021636118. [Google Scholar] [CrossRef]
- Park, M.; Leahey, E.; Funk, R.J. Papers and patents are becoming less disruptive with time. Nature 2023, 613, 138. [Google Scholar] [CrossRef] [PubMed]
- Bloom, N.; Jones, C.I.; Van Reenen, J.; Webb, N. Are ideas getting harder to find? Am. Econ. Rev. 2020, 110, 1104. [Google Scholar] [CrossRef]
- Bretscher, P.A. Information overload and resilience in facing foundational issues. Proc. Natl. Acad. Sci. USA 2022, 119, e2120180119. [Google Scholar] [CrossRef] [PubMed]
- Moore, R. Niels Bohr: The Man, His Science, and the World They Changed; Alfred A. Knopf: New York, NY, USA, 1966. [Google Scholar]
- Bretscher, P. On Analyzing How the Th1/Th2 Phenotype of an Immune Response Is Determined: Classical Observations Must Not Be Ignored. Front. Immunol. 2019, 10, 1234. [Google Scholar] [CrossRef] [PubMed]
- Mossman, T.R.; Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989, 7, 145. [Google Scholar] [CrossRef]
- Matzinger, P. Friendly and dangerous signals: Is the tissue in control? Nat. Immunol. 2007, 8, 11. [Google Scholar] [CrossRef]
- Matzinger, P.; Kamala, T. Tissue-based class control: The other side of tolerance. Nat. Rev. Immunol. 2011, 11, 221–230. [Google Scholar] [CrossRef]
- Fearon, D.T.; Locksley, R.M. The instructive role of innate immunity in the acquired immune response. Science 1996, 272, 50–53. [Google Scholar] [CrossRef]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343. [Google Scholar] [CrossRef]
- Janeway, C.A.; Medzhitov, R. Innate Immune Recognition. Ann. Rev. Immunol. 2002, 20, 197. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, P.A. On the control between cell-mediated, IgM and IgG immunity. Cell. Immunol. 1974, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, P.A. On the mechanism determining the TH1/TH2 phenotype of an immune response, and its pertinence to strategies for the prevention, and treatment, of certain infectious diseases. Scand. J. Immunol. 2014, 79, 361. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, P.A. Analyzing some concepts of immune regulation of the last three decades: Fostering greater research resilience despite the information overload. A personal view. Front. Immunol. 2022, 13, 960742. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989, 54 Pt 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Janeway, C.A. Innate Immunity: The Virtues of a Non-Clonal System. Cell 1997, 91, 295. [Google Scholar] [CrossRef] [PubMed]
- Matzinger, P. Tolerance, danger, and the extended family. Ann. Rev. Immunol. 1994, 12, 991. [Google Scholar] [CrossRef]
- Matzinger, P. The danger model: A renewed sense of self. Science 2002, 296, 301. [Google Scholar] [CrossRef]
- Sharpe, A.H. Mechanisms of costimulation. Immunol. Rev. 2009, 229, 5–11. [Google Scholar] [CrossRef]
- Kappler, J.W.; Roehm, N.; Marrack, P. T cell tolerance by clonal elimination in the thymus. Cell 1989, 49, 273. [Google Scholar] [CrossRef]
- Lederberg, J. Genes and antibodies. Science 1959, 129, 1649. [Google Scholar] [CrossRef] [PubMed]
- Chentoufi, A.A.; Polychronakos, C. Insulin expression levels in the thymus modulate insulin specific autoreactive T-cell tolerance: The mechanism by which the IDDM2 locus may predispose to diabetes. Diabetes 2002, 51, 1383. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, P. An integrated view of immunological tolerance. Scand. J. Immunol. 2022, 95, e13207. [Google Scholar] [CrossRef]
- Ehrlich, P.; Morgenroth, J. Uber Hemolysine: Funfte Mittheilung. In Berliner Klinische Wochenschrift; English translation in The Collected Papers of Paul Ehrlich; Pergamon Press: London, UK; New York, NY, USA, 1901; Volume 1 1956, pp. 246–255. [Google Scholar]
- Burnet, F.M.; Fenner, F. The Production of Antibodies; McMillan and Co.: New York, NY, USA, 1949. [Google Scholar]
- Dresser, D.W.; Mitchison, N.A. The mechanism of immunological paralysis. Adv. Immunol. 1968, 8, 129. [Google Scholar] [PubMed]
- Bretscher, P.; Cohn, M. A Theory of Self-Nonself Discrimination. Science 1970, 169, 1042. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, P. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc. Natl. Acad. Sci. USA 1992, 96, 185. [Google Scholar] [CrossRef]
- Al-Yassin, G.A.; Bretscher, P.A. Does T cell activation require a quorum of lymphocytes. J. Immunol. 2018, 201, 2855. [Google Scholar] [CrossRef]
- Claman, H.N.; Chaperon, E.A.; Triplett, R.F. Thymus, marrow cell combinations-synergism in antibody production. Proc. Soc. Exp. Biol. Med. 1966, 122, 1167. [Google Scholar] [CrossRef]
- Raff, M.C. Role of thymus-derived lymphocytes in the secondary humoral immune response in mice. Nature 1970, 226, 1257. [Google Scholar] [CrossRef]
- Keene, J.A.; Forman, J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J. Exp. Med. 1982, 155, 768–782. [Google Scholar] [CrossRef]
- Metcalf, E.S.; Klinman, N.R. In Vitro tolerance induction of neonatal murine B cells. J. Exp. Med. 1976, 143, 1327–1340. [Google Scholar] [CrossRef] [PubMed]
- Goodnow, C.C.; Crosbie, J.; Jorgensen, H.; Brink, R.A.; Basten, A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature 1989, 342, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Guerder, S.; Matzinger, P. A fail-safe mechanism for maintaining self-tolerance. J. Exp. Med. 1992, 176, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Weigle, W.O. The immune response of Rabbits Tolerant to Bovine Serum Albumin to the Injection of Other Heterologous Serum Albumins. J. Exp. Med. 1961, 114, 111. [Google Scholar] [CrossRef]
- Weigle, W.O. The immune response of BSA tolerant rabbits to injections of BSA following the termination of the tolerant state. J. Immunol. 1964, 92, 791. [Google Scholar] [CrossRef]
- Zabriskie, J.B.; Hsu, K.C.; Seegal, B.C. Heart-reactive antibody associated with rheumatic fever: Characterization and diagnostic significance. Clin. Exp. Immunol. 1970, 7, 147. [Google Scholar] [PubMed]
- Guilherme, L.; Cunha-Neto, E.; Coelho, V.; Snitcowsky, R.; Pomerantzeff, P.M.A.; Assis, R.V.; Pedra, F.; Neumann, J.; Goldberg, A.; Patarroyo, M.E.; et al. Human heart-infiltrating T-cell clones from rheumatic heart disease patients recognize both streptococcal and cardiac proteins. Circulation 1995, 92, 420. [Google Scholar] [CrossRef]
- Lin, R.H.; Mamula, M.J.; A Hardin, J.; A Janeway, C. Induction of autoreactive B cells allows priming of autoreactive T cells. J. Exp. Med. 1991, 173, 1433. [Google Scholar] [CrossRef]
- Sher, A.; Coffman, R.L. Regulation of immunity to parasites by T cells and T cell-derived cytokines. Annu. Rev. Immunol. 1992, 10, 389. [Google Scholar] [CrossRef]
- Nunes-Alves, C.; Booty, M.G.; Carpenter, S.M.; Jayaraman, P.; Rothchild, A.C.; Behar, S.M. In search of a new paradigm for protective immunity to TB. Nat. Rev. Microbiol. 2014, 12, 289. [Google Scholar] [CrossRef]
- Menon, J.; Hoeppner, V.H.; Judd, A.; Power, C.A.; Bretscher, P.A. A hypothesis for the existence of two types of tuberculosis, reflecting two distinct types of immune failure to control the pathogen, based upon prevalence of mycobacterium-specific IgG subclasses. Scand. J. Immunol. 2018, 87, e12665. [Google Scholar] [CrossRef] [PubMed]
- Okulicz, J.F.; Marconi, V.C.; Landrum, M.L.; Wegner, S.; Weintrob, A.; Ganesan, A.; Hale, B.; Crum-Cianflone, N.; Delmar, J.; Barthel, V.; et al. Clinical outcomes of elite controllers, viremic controllers, and long-term non-progressors in the US Department of Defense HIV natural history study. J. Infect. Dis. 2009, 200, 1714. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.G. Immunological regulation and control of experimental leishmaniasis. Int. Rev. Exp. Parasitol. 1980, 28, 21. [Google Scholar]
- Sher, A.; Gazzinelli, R.T.; Oswald, I.P.; Clerici, M.; Kullberg, M.; Pearce, E.J.; Berzofsky, J.A.; Mosmann, T.R.; James, S.L.; MorseIII, H.; et al. Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol. Rev. 1992, 127, 183. [Google Scholar] [CrossRef] [PubMed]
- Titus, R.G.; Ceredig, R.; Cerottini, J.C.; Louis, J.A. Therapeutic effect of anti-L3T4 monoclonal antibody GK1.5 on cutaneous leishmaniasis in genetically susceptible BALB/c mice. J. Immunol. 1985, 135, 2108. [Google Scholar] [CrossRef]
- Sadick, M.D.; Heinzel, F.P.; Shigekane, V.M.; Fisher, W.L.; Locksley, R.M. Cellular and humoral immunity to Leishmania major in genetically susceptible mice after in vivo depletion of L3T4+ T cells. J. Immunol. 1987, 139, 1303. [Google Scholar] [CrossRef]
- Bretscher, P.A.; Wei, G.; Menon, J.N.; Bielefeldt-Ohmann, H. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science 1992, 257, 539. [Google Scholar] [CrossRef]
- Power, C.A.; Wei, G.; Bretscher, P.A. Mycobacterial dose defines the Th1/Th2 nature of the immune response independently of whether immunization is administered by the intravenous, subcutaneous, or intradermal route. Infect. Immunol. 1998, 66, 5743. [Google Scholar] [CrossRef]
- Kiros, T.G.; A Power, C.; Wei, G.; A Bretscher, P. Immunization of newborn and adult mice with low numbers of BCG leads to Th1 responses, Th1 imprints and enhanced protection upon BCG challenge. Immunotherapy 2010, 2, 25. [Google Scholar] [CrossRef]
- Buddle, B.; de Lisle, G.; Pfeffer, A.; Aldwell, F. Immunological responses and protection against Mycobacterium bovis in calves vaccinated with a low dose of BCG. Vaccine 1995, 13, 1123. [Google Scholar] [CrossRef]
- Ten Dam, H.G. Research on BCG vaccination. Adv. Tuber. Res. 1984, 21, 79. [Google Scholar]
- Boon, T.; Coulie, P.G.; Eynde, B.J.V.D.; Bruggen, P.V.D. Human T cell responses against melanoma. Annu. Rev. Immunol. 2006, 24, 175–208. [Google Scholar] [CrossRef]
- Hamilton, D.H.; Bretscher, P.A. Different immune correlates associated with tumor progression and regression: Implications for prevention and treatment of cancer. Cancer Immunol. Immunother. 2008, 57, 1125. [Google Scholar] [CrossRef]
- Klein, G. Tumor-specific transplantation antigens: G.H.A. Clowes Memorial Lecture. Can Res. 1968, 28, 625. [Google Scholar]
- Hellstrom, K.E.; Hellstrom, I. Immunological defense against cancer. In Immunobiology; Good, R.A., Fisher, D.W., Eds.; Sinauer Associates Inc.: Stamford, Connecticut, 1971; pp. 209–221. [Google Scholar]
- North, R.J. The murine anti-tumor response and its therapeautic manipulations. Adv. Immunol. 1984, 35, 89. [Google Scholar] [PubMed]
- Awwad, M.; North, R.J. Cyclophosphamide-induced immunologically mediated regression of a cyclophosphamide-resistant murine tumor: A consequence of eliminating precursor L3T4+ suppressor T-cells. Cancer Res. 1989, 49, 1649. [Google Scholar]
- Gorelik, E. Concomitant tumor immunity and the resistance to a second tumor challenge. Adv. Cancer Res. 1983, 39, 71. [Google Scholar]
- Beyer, M.; Schultze, J.L. Regulatory T cells in cancer. Blood 2006, 108, 804. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Sakaguchi, S. Regulatory T cells in immune surveillance and treatment of cancer. Semin. Cancer Biol. 2006, 16, 115. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The three Es of cancer immunoediting. Annu. Rev. Immunol. 2004, 22, 329. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Gabrilovich, D. Sotomayor EM Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 2007, 25, 267. [Google Scholar] [CrossRef] [PubMed]
- Salvin, S.B. Occurrence of delayed hypersensitivity during the development of Arthus type hypersensitivity. J. Exp. Med. 1958, 107, 109. [Google Scholar] [CrossRef]
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.-P.; Raffatellu, M. Mucosal immunity to pathogenic intestinal bacteria. Nat. Rev. Immunol. 2016, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.; Bretscher, P.A. The Th1/Th2 nature of concurrent immune responses to unrelated antigens can be independent. J. Immunol. 1999, 163, 4842. [Google Scholar] [CrossRef] [PubMed]
- Lagrange, P.H.; Mackaness, G.B.; Miller, T.E. Influence of dose and route of antigen injection on the immunological induction of T cells. J. Exp. Med. 1974, 139, 528. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, M.C.; Pearce, E.J.; Hieny, S.E.; Sher, A.; Berzofsky, J.A. Infection with Schistosoma mansoni alters Th1/Th2 cytokine responses to a non-parasite antigen. J. Immunol. 1992, 148, 3264. [Google Scholar] [CrossRef]
- Bretscher, P. The role of cytokines in determining the Th1/Th2 phenotype of an immune response: Coherence of the T cell response and the Cytokine Implementation Hypothesis. Scand. J. Immunol. 2022, 95, e13110. [Google Scholar] [CrossRef]
- Asherson, G.L.; Stone, S.H. Selective and specific inhibition of 24 hour skin reactions in the guinea-pig: I. Immune deviation: Description of the phenomenon and the effect of splenectomy. Immunology 1965, 9, 205. [Google Scholar]
- Parish, C.R. The relationship between humoral and cell-mediated immunity. Transpl. Rev. 1972, 13, 35. [Google Scholar] [CrossRef]
- Parish, C.R.; Liew, F.Y. Immune response to chemically modified flagellin: III Enhanced cell-mediated immunity during high and low zone antibody tolerance to flagellin. J. Exp. Med. 1992, 135, 298. [Google Scholar] [CrossRef]
- Mitchison, N.A. Immunological paralysis as a dosage phenomenon. In Regulation of the Antibody Response; Cinader, B., Ed.; CC Thomas: Springfield, IL, USA, 1967; pp. 54–67. [Google Scholar]
- Menon, J.N.; Bretscher, P.A. Parasite dose determines the Th1/Th2 nature of the response to Leishmania major independently of infection route and strain of host or parasite. Eur. J. Immunol. 1998, 28, 4020. [Google Scholar] [CrossRef]
- Bretscher, P. The Foundations of Immunology and their Pertinence to Medicine; FriesenPress: Victoria, BC, Canada, 2016. [Google Scholar]
- Hsieh, C.S.; Macatonia, S.E.; Tripp, C.S.; Wolf, S.F.; O’Garra, A.; Murphy, K.M. Development of TH1 CD4+ T cells through IL- 12 produced by Listeria-induced macrophages. Science 1993, 260, 547. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.L.; Weinberg, A.D.; English, M.; Huston, G. IL-4 directs the development of Th2-like helper effectors. J. Immunol. 1990, 145, 3796. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Fitch, F.W. Anti-proliferative effect of IFN-gamma in immune regulation. I IFN-gamma inhibits the proliferation of Th2 but not Th1 murine helper T cell clones. J. Immunol. 1988, 140, 4245. [Google Scholar] [CrossRef]
- Sadick, M.D.; Heinzel, F.P.; Holaday, B.J.; Coffman, R.L.; Locksley, R.M. Cure of murine leishmaniasis with anti-IL-4 monoclonal antibody. J. Exp. Med. 1990, 171, 115. [Google Scholar] [CrossRef]
- Belosevic, M.I.O.D.R.A.G.; Finbloom, D.S.; Van Der Meide, P.H.; Slayter, M.V.; Nacy, C.A. Administration of monoclonal anti-IFN-gamma antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J. Immunol. 1989, 143, 266. [Google Scholar] [CrossRef]
- Pearson, M.N.; Raffel, S. Macrophage-digested antigen as inducer of delayed hypersensitivity. J. Exp. Med. 1971, 133, 494. [Google Scholar] [CrossRef]
- Bretscher, P. Rediscovering the Immune System as an Integrated Organ; Friesen Press: Altona, Canada, 2016. [Google Scholar]
- Rudulier, C.D.; McKinstry, K.K.; Al-Yassin, G.A.; Kroeger, D.R.; Bretscher, P.A. The Number of Responding CD4 T Cells and the Dose of Antigen Conjointly Determine the Th1/Th2 Phenotype by Modulating B7/CD28 Interactions. J. Immunol. 2014, 192, 5140. [Google Scholar] [CrossRef]
- Kelso, A. Th1 and Th2 subsets: Paradigms lost? Immunol. Today 1995, 16, 374. [Google Scholar] [CrossRef]
- Bretscher, P.A.; Al-Yassin, G. Can interruption/withdrawl of anti-retroviral therapy provide personalized immunotherapy against HIV-1? Scand. J. Immunol. 2020, 92, e12934. [Google Scholar] [CrossRef]
- Bretscher, P.A. Facing the Increased Prevalence of Antibiotic-Resistant, M. tuberculosis: Exploring the Feasibility of Realizing Koch’s Aspiration of Immunotherapy of Tuberculosis. Antibiotics 2022, 11, 371. [Google Scholar] [CrossRef] [PubMed]
- Bretscher, P. The Problem of Host and Pathogen Genetic Variability for Developing Strategies of Universally Efficacious Vaccination against and Personalised Immunotherapy of Tuberculosis: Potential Solutions? Int. J. Mol. Sci. 2023, 24, 1887. [Google Scholar] [CrossRef] [PubMed]
- Savilahti, E.M.; Savilahti, E. Immunol Development of natural tolerance and induced desensitization in cow’s milk allergy. Pediatr. Allergy 2013, 24, 114–121. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bretscher, P. What Determines the Class of Immunity an Antigen Induces? A Foundational Question Whose Rational Consideration Has Been Undermined by the Information Overload. Biology 2023, 12, 1253. https://doi.org/10.3390/biology12091253
Bretscher P. What Determines the Class of Immunity an Antigen Induces? A Foundational Question Whose Rational Consideration Has Been Undermined by the Information Overload. Biology. 2023; 12(9):1253. https://doi.org/10.3390/biology12091253
Chicago/Turabian StyleBretscher, Peter. 2023. "What Determines the Class of Immunity an Antigen Induces? A Foundational Question Whose Rational Consideration Has Been Undermined by the Information Overload" Biology 12, no. 9: 1253. https://doi.org/10.3390/biology12091253
APA StyleBretscher, P. (2023). What Determines the Class of Immunity an Antigen Induces? A Foundational Question Whose Rational Consideration Has Been Undermined by the Information Overload. Biology, 12(9), 1253. https://doi.org/10.3390/biology12091253





