Simple Summary
Primary liver cancer is the third most common cause of cancer-related deaths worldwide. Risk factors for primary liver cancer include chronic viral hepatitis B and C infections, alcohol abuse, non-alcoholic fatty liver disease, and obesity. Surgical resection and/or transplantation is the mainstay treatment for candidates with primary liver tumors. However, minimally invasive, image-guided locoregional therapies have become an integral part of liver cancer treatment and management, depending on staging. In this manuscript, the authors provide a comprehensive overview of the antineoplastic mechanisms underpinning locoregional therapies and the current state of the literature on the efficacy of these therapies for primary liver cancer. We also discuss emerging advances in treatment, such as the adjuvant use of immunotherapies and molecular targeting agents with locoregional therapy. This review highlights the emerging technological advancements and image-guided procedures used to treat primary liver cancer.
Abstract
Primary liver cancer is the leading cause of cancer-related deaths worldwide. with incidences predicted to rise over the next several decades. Locoregional therapies, such as radiofrequency or microwave ablation, are described as image-guided percutaneous procedures, which offer either a curative intent for early-stage hepatocellular carcinoma or bridging/downstaging for surgical resection or transplantation. Catheter-driven locoregional therapies, such as transarterial chemoembolization and radioembolization, induce tumor hypoxia, can be palliative, and improve survival for early-to-intermediate hepatocellular carcinoma and unresectable intrahepatic cholangiocarcinoma. Herein, we provide a comprehensive overview of the antineoplastic mechanisms underpinning locoregional therapies, different treatment approaches, and the current state of the literature for the efficacy of locoregional therapies for primary liver cancer. We also discuss emerging advancements, such as the adjuvant use of immunotherapies and molecular targeting agents with locoregional therapy, for the treatment of primary liver cancer.
1. Introduction
Liver cancer constitutes one of the most common causes of malignancy worldwide, and rates for primary liver tumors are steadily rising in the United States [1,2]. The highest reported cases of liver cancer are in Eastern Asia and Middle Africa, and the incidence in men is roughly 2–4 times that of women [1]. Perhaps most alarmingly, liver cancer carries a high risk of mortality, with a 5-year survival rate of 6.5% [1]. Major risk factors have been identified for primary liver cancer, including chronic viral hepatitis B and C infections, alcohol abuse, non-alcoholic fatty liver disease, and obesity. There are two main types of primary liver cancers, including hepatocellular carcinoma and intrahepatic cholangiocarcinoma. In general, surgical resection and/or transplantation is the mainstay treatment for candidates with primary liver tumors. However, locoregional therapies, defined as minimally-invasive, image-guided procedures, have become an integral part of liver cancer treatment and management [3,4,5]. Depending on staging, image-guided locoregional therapies (iLRT), such as ablation (e.g., radiofrequency ablation, microwave ablation, cryoablation), transarterial embolization (TAE) or chemoembolization (TACE), or radioembolization (TARE) can be used as curative, neo-adjunctive, or palliative treatment regimens. The efficacy of these techniques is evaluated by follow-up imaging, via CT or MRI, and the gold standard tool for assessing treatment responses to these techniques is the Response Evaluation Criteria in Solid Tumors (RECIST) [6,7]. It is widely recognized that changes in tumor size, as measured by RECIST, can be used as surrogate endpoints for survival length, meaning that improvements in tumor size often correlate with longer survival times [7]. Within the following manuscript, we provide a brief overview of primary liver cancers and describe the treatment approaches of iLRT, the rationales for treatment, and new emerging evidence for their use.
2. Primary Liver Cancers
2.1. Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide, accounting for 80–90% of primary liver cancer cases [8,9]. The highest incidence rates are seen in sub-Saharan Africa and Southeast Asia, where viral risk factors, such as hepatitis B virus (HBV), are endemic [10]. In developed countries, HCC incidence is rising due to the increasing prevalence of non-alcoholic fatty liver disease, obesity, and diabetes [11]. In the United States, HCC incidence has tripled since the 1980s, accounting for up to 90% of primary liver cancers [12]. The epidemiology of HCC is complex and multifactorial, with risk factors including viral hepatitis, alcohol consumption, metabolic disorders, and exposure to hepatotoxic chemicals [9,10,13].
HCC is a heterogeneous disease with diverse histological subtypes and molecular characteristics. The most common histological subtype is well-differentiated HCC, which accounts for about 30% of cases. Poorly differentiated HCC, also known as hepatoblastoma-like HCC, is a rare, but aggressive, subtype that is associated with worse outcomes [14]. Other histological subtypes also exist, including fibrolamellar, scirrhous, and macrotrabecular-massive subtypes [14,15]. The tumor microenvironment plays a crucial role in HCC development and progression, with chronic inflammation, immune dysregulation, and fibrosis acting as key drivers [14]. Recent advances in molecular profiling technologies have identified new molecular subtypes of HCC, which may have clinical implications for patient stratification and treatment [16].
Prognosis of HCC varies depending on several factors, including tumor stage, liver function, and overall health status. HCC is a deadly disease, with a 5-year survival rate of less than 20% [17]. However, early detection and treatment can significantly improve outcomes. The Barcelona Clinic Liver Cancer (BCLC) staging system is widely used to classify HCC patients into different treatment categories (e.g., surgery, iLRT, and systemic treatment) based on tumor burden, liver function, and performance status (Figure 1) [18]. The classification system was recently updated in 2022, and it has been externally validated and endorsed by the Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) [18]. Patients with early-stage HCC (BCLC stage 0 or A) have better outcomes and are eligible for potentially curative treatments, such as surgical resection, liver transplantation, or ablation therapy. However, most patients present with advanced-stage disease (BCLC stage B or C), so they have limited treatment options. For patients with advanced-stage disease, systemic therapy is the standard of care, but the efficacy of these treatments is modest, and there is an urgent need for new therapeutic options. Patients with end-stage HCC (BCLC stage D) are typically managed with supportive care.
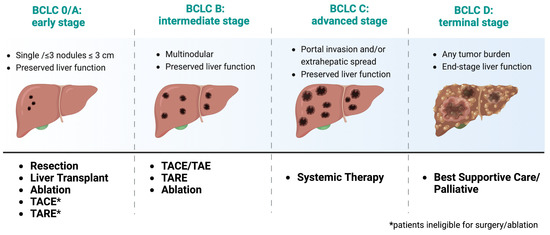
Figure 1.
Treatment recommendations based on recent updates from the 2022 Barcelona Clinic Liver Cancer (BCLC) Guidelines [18]. Adapted from “Barcelona Clinic Liver Cancer (BCLC) Staging and Classification”, by BioRender.com (2023). https://app.biorender.com/biorender-templates, accessed on July 2023.
2.2. Intrahepatic Cholangiocarcinoma
Intrahepatic cholangiocarcinoma (ICCA) is a rare and aggressive cancer arising from the biliary tree within the hepatic parenchymal system [19]. ICCA exhibits traits of cholangiocyte differentiation, and it is likely to originate, mainly, from the epithelial cells that line the bile ducts, known as cholangiocytes [19,20]. Nevertheless, the tumors can also emerge from peribiliary glands and hepatocytes, depending on the location and underlying liver condition. It is the second most common type of primary liver cancer, after HCC carcinoma, accounting for roughly 10–15% of primary liver cancers [21,22]. Reports have also shown progressive increases in the incidence of ICCA worldwide [23,24,25]. However, the epidemiology of ICC remains complex and poorly understood due to its rarity and lack of population-based studies [25].
In the United States, the incidence of cholangiocarcinoma has almost tripled over the past three decades [26]. Similar to HCC, chronic liver disease, including cirrhosis and hepatitis B or C infection, is a significant risk factor for ICCA [19]. Other risk factors include exposure to certain chemicals, such as thorium dioxide and vinyl chloride, as well as inflammatory bowel disease [19,27,28]. There is also a strong association between cholangiocarcinoma and liver fluke parasitic infections within parts of Southeast Asia [19]. Prognosis of cholangiocarcinoma is poor, with a 5 year overall survival rate ranging from 25–31% and a recurrence rate ranging from 40–64% [29,30]. Even worse, ICCA is often beyond the limits of surgical therapy at the time of diagnosis, and the median survival time after treatment with chemoradiotherapy is only 10 months [31]. Surgical resection is the only potentially curative treatment for ICCA, but only a minority of patients are eligible for surgery due to the advanced stage of the disease at the time of diagnosis [32]. Therefore, for unresectable disease, candidates must rely on other non-surgical methods for disease management, such as iLRT or chemotherapy. In the next section, we provide an overview of techniques and current evidence in support of iLRT in the context of primary liver cancer.
3. Image-Guided, Tumor-Directed Locoregional Therapies
3.1. Rationale for Liver Cancer Treatment
The majority of the liver’s blood supply, about 80%, is received from the portal vein, while only 20% comes from the hepatic artery [33,34]. This division of blood supply has been an important framework for directing the locoregional therapy used to treat HCC. Furthermore, iLRTs have become crucial components to HCC management as curative, adjunctive, and palliative treatment options for individuals who do not qualify for surgery (Table 1). On the other hand, ICCA is less vascular than HCC, suggesting iLRT may not play as significant of a role in treating this type of tumor. Nevertheless, numerous studies have demonstrated interventional iLRTs can provide survival benefits in cases of unresectable ICCA [35]. Although these therapies are generally used for palliative purposes for ICCA, they can also help control the disease (Table 1). However, as mentioned above, studying these methods can be difficult due to the rarity of ICCA combined with the small number of eligible patients for each non-curative treatment method.

Table 1.
Image-guided locoregional therapies and their clinical utility.
3.2. Ablation Techniques
Ablative therapies include different procedures, such as percutaneous ethanol injection (PEI), radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation [3,4]. However, the goal of thermal ablation is to use heat extremes to induce tumor death through coagulative necrosis, eliminating undetected cancer microenvironments [37]. The procedure can be performed under moderate sedation or general anesthesia, and it involves the use of a percutaneous probe that navigates to the region of the tumor under CT or MRI guidance. In the context of RFA, the probe delivers frictional high-frequency alternating current to the target tissue, generating heat and, ultimately, the coagulative necrosis of the tumor. Temperatures (50–100 °C) produced by RFA denature proteins, disrupt cellular membranes, and induce thermal coagulation, leading to tumor cell death(Figure 2) [37,38]. After the procedure, patients are usually monitored through multiphasic CT or magnetic resonance imaging (MRI) to evaluate imaging response (Figure 2b). This assessment is typically done 1 month after the procedure. RFA has gained recognition as a well-established therapeutic approach due to its effectiveness, reproducibility, minimal incidence of complications, and widespread accessibility [39]. MVA, which employs electromagnetic energy to induce tumor cell injury, can also be particularly advantageous for liver tumors, due to its enhanced and predictable convection profile, sustained higher intratumoral temperatures, quicker ablation durations, and feasibility of treating multiple lesions, concurrently, using multiple probes [39,40,41].
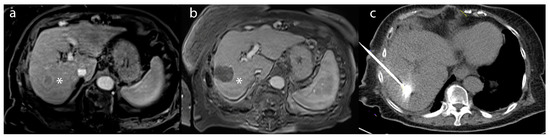
Figure 2.
A 55-year-old male patient with (a) a lesion in segment 6 biopsy–proven as hepatocellular carcinoma (*)—on post-contrast T1-weighted imaging. (b) After microwave ablation, the lesion (*) demonstrated a lack of enhancement compatible with a complete radiographic response on the 1-month follow-up MRI (c) intraprocedural treatment CT of microwave ablation.
In general, thermal ablation is used to induce an adequate margin (usually 5–10 mm) around the tumor. If a sufficient margin around the tumor can be achieved, ablation is considered curative [3,5,42]. The efficacy of complete necrosis, after ablation for a single HCC lesion from 2–3 cm, is approximately 90% [38], but its efficacy decreases with larger or later-stage lesions, where undetected microsatellites are often found. For early-stage HCC, meta-analyses of four randomized control trials (RCT) found no differences in all-cause mortality between surgical intervention and radiofrequency ablation [43]. As with HCC, ablation techniques have proven to be a safe and well-tolerated therapeutic approach for ICCA, specifically, in patients harboring small tumors that have not invaded beyond the confines of the bile duct and surrounding tissue and, therefore, may be considered a potentially curative modality [4,35]. Furthermore, due to the highly aggressive and heterogeneous nature of ICCA, many patients with ICCA are not surgical candidates because advanced disease is common at the time of presentation [28]. Patients with unresectable or recurrent ICCA tumors treated with RFA exhibit 1, 3, and 5-year overall survival rates of 82, 47, and 24%, respectively [44]. It has been reported that RFA provides significant improvement in the median overall survival (OS) rates, which range from 20 to 60 months [4]. This stands in stark contrast to the median OS rates of 3–8 months observed in patients with unresectable ICC who did not receive any form of treatment [4].
3.3. Transarterial Chemoembolization Techniques
The conventional transarterial chemoembolization (cTACE) procedure functions through a distinctive mechanism of action that involves impeding tumor-feeding arteries by injecting chemotherapeutic agents, namely doxorubicin or cisplatin, mixed with the radiopaque contrast agent, lipiodol (Figure 3) [3,42,44,45,46]. The process is intended to limit the supply of nutrients and oxygen to the tumor, thereby causing its necrosis and subsequent shrinkage [46]. This embolic technique works by creating an embolus within the tumor-feeding artery, obstructing the blood flow, and trapping the chemotherapeutic agents within the tumor, leading to a local, sustained release of the chemotherapeutic agents (Figure 3c) [46]. The lipiodol facilitates the visualization of the infused agents under fluoroscopy and CT imaging, thus aiding in the accurate delivery of the embolic agent to the targeted area. The procedure itself takes approximately 1–2 h, and patients are typically monitored overnight before being discharged the following day. Incorporating drug-eluting beads, designated as DEB-TACE, has become increasingly popular in numerous medical centers, as it employs embolic microspheres or beads containing chemotherapy drugs [47]. Among the benefits of DEB-TACE over conventional TACE, it enables a steady and regulated administration of the therapeutic agent, thereby prolonging local exposure to the tumor while minimizing systemic exposure [48,49,50].
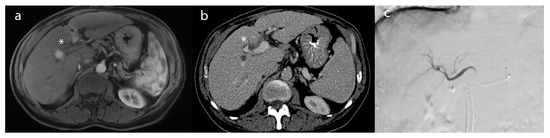
Figure 3.
A 62-year-old female patient with (a) an arterially-enhancing lesion (*) in segment 8, compatible with hepatocellular carcinoma on post-contrast T1-weighted imaging. (b) After TACE, the lesion (*) demonstrated a lack of enhancement, compatible with a complete radiographic response on the 2 month follow-up CT. (c) Intraprocedural angiogram of TACE depicting the embolic distribution of the right lobar artery.
TACE is considered first-line therapy for unresectable liver cancer, namely HCC [18,51,52]. The ideal candidates for TACE are patients who have preserved liver function and present with either multinodular or isolated large tumors larger than 3 cm, without any signs of extrahepatic metastasis, vascular invasion, or cancer-related symptoms, and who are not eligible for percutaneous or surgical interventions [18]. TACE has been shown to provide a survival benefit for HCC, as evidenced by a systematic review of 7 randomized control trials yielding an overall improvement in 2-year survival (OR = 0.53 (0.32–0.89); p = 0.017) [53]. A large retrospective study found median OS to improve by 6 months with the use of TACE vs. supportive care (8 vs 2 months; p ≤ 0.01) [54]. For ICCA, retrospective investigations have shown a statistically significant increase in median survival time for patients receiving TACE, as compared to those who only received supportive treatment (12.2 vs 3.3 months; p < 0.001, respectively) [55]. A recent meta-analysis of 11 studies also confirmed the overall survival benefits of TACE for unresectable ICCA compared to supportive treatment [56].
3.4. Transarterial Radioembolization Techniques
Transarterial radioembolization (TARE), also known as selective internal radiation therapy (SIRT), was developed under similar technical principles to TACE with the addition of utilizing radioactive beads (e.g., microspheres) that are injected into the hepatic artery under fluoroscopic guidance in order to embolize tumor-supplying vessels [57,58,59,60]. The microspheres are loaded with a beta-emitting isotope, such as yttrium-90 (Y-90), which emits high-energy radiation that causes permanent DNA damage, apoptosis, and destroys cancer cells within the hepatic parenchyma [57]. Unique to TARE, in order to achieve successful radioembolization via adequate cytoreduction and free radical formation, a balance of adequate microsphere coverage and normal oxygen tension to targeted cancer cells is essential. Thus, the process requires appropriately sized particles (20–60 mm) [61,62,63].
Indications for TARE overlap with those of TACE for liver tumor treatment. In a recent update from the BCLC guidelines for image-guided locoregional therapy use, radioembolization has been established as a viable treatment modality for very early-stage (BCLC 0) and early-stage (BCLC A) HCC [18]. These new recommendations are largely based on a 2021 study (Local radioEmbolization using Glass Microspheres for the Assessment of Tumor Control with Y-90 or LEGACY) that investigated the efficacy of radioembolization as a treatment option for early-stage HCC [18,64]. TARE was effective for treating patients with a single HCC tumor measuring less than 8 cm and a preserved performance status. The study reported an objective response rate of 88.3% and median overall survival (OS) of 57.9 months [64]. In fact, a small randomized control trial of patients with HCC (BCLC-A or BCLC-B) showed a longer total time to progression for TARE (n = 24; >26 months) compared to cTACE (n = 21; 6.8 months) [65]. Data supporting TARE for ICCA are also promising, albeit mostly retrospective, studies with small sample sizes. Outcomes between TACE and TARE are similar (14.2 vs 13.5 months), with no appreciable differences at 2 years [66].
3.5. Combining Image-Guided Locoregional Modalities
In recent years, an increasing volume of literature has emerged endorsing the practice of integrating various locoregional therapeutic modalities. This approach is intended to produce a synergistic effect, resulting in enhanced treatment efficacy and improved therapeutic responses. There have been several rationales behind the etiology of why combining thermal ablation may be synergistic. For example, obstructing the hepatic artery and ceasing blood flow in the target zone via embolization can increase the lethal thermal coagulation zone by reducing the tissue cooling due to perfusion, which is coined as “heat sink” [67]. Secondly, a larger volume of sublethal hyperthermia is exposed to high concentrations of the chemotherapeutic agent. This hyperthermic exposure leads to increased cellular membrane permeability, improved intratumoral accumulation of chemotherapy, and increased sensitivity of cytotoxic drugs [67,68,69]. The resulting increase in the volume of coagulative necrosis, including the lethal and sublethal hyperthermic zones, leads to the widening of the ablation margin, which ultimately improves local control by destroying microscopic satellite lesions that are adjacent to the central tumor [67,69,70]. A combination therapy that has garnered significant research attention is the integration of RFA and TACE. Several meta-analyses have demonstrated that this dual approach can enhance overall survival rates beyond those achievable by monotherapy, without incurring any discernible changes in associated complication rates [71,72]. To our knowledge, no studies to date have sought to determine the efficacy of multimodality image-guided locoregional therapy approaches on ICCA outcomes. Nevertheless, based on existing evidence, it does appear that multimodal therapies have a possible advantage, in terms of survival, for primary liver cancer.
4. Locoregional and Immunological Therapies
4.1. Immunological Basis of Image-Guided Tumor-Directed Therapies
The liver’s diverse cellular composition, including myeloid cells and lymphocytes, makes its immune microenvironment complex. This microenvironment suppresses anti-tumor activity and is a significant obstacle to treating HCC, which is an immunogenic tumor that develops in an immune-suppressed environment. For example, the liver contains macrophages, called Kupffer Cells, as well as T-regulatory and myeloid-derived suppressor cells [73,74]. In the setting of HCC, immune cell activity can be increased, and it can inhibit T-cell cytotoxicity, as well as immune suppression. Immunodysregulation among certain key cells, such as mature dendritic cells and tumor-associated macrophages has also been identified to correlate with poor prognosis [75,76,77].
Several pieces of evidence suggest that iLRT not only directly impact tumor cells but also exerts an immune modulation effect, which may clarify their increased effectiveness when used in combination with immunotherapies [76,78]. Mouse models have shown that animals treated with RFA exhibit increased dendritic cell-related antitumor T-cell immune responses and tumor regression [79]. There has also been evidence that, in addition to inducing thermal coagulative necrosis, RFA can increase heat shock protein expression in the surrounding zone and activate concomitant CD4+ and CD8+ T-cell effector responses [80,81]. CD4+ T-cell and cytokine activations have also been observed after MVA treatment [82]. Apart from activating T-cells, ablation can also regulate anti-tumor immunity by inhibiting myeloid-derived suppressor cells, which correlates with improved recurrence-free survival [76,83,84].
Evidence also supports TACE as a treatment for modulating innate and adaptive immunity. Intra-arterial chemoembolization delivery can lead to the release of cellular debris, pro-inflammatory cytokines, and danger-associated molecular patterns. This triggers a priming effect on adaptive immunity [85]. A prospective investigation of 79 patients with HCC found higher levels of T helper cells 1 month after TACE (p = 0.036) [86]. An investigation analyzing the peripheral blood of 114 patients with HCC showed a marked increase in programmed cell death protein 1, which was also associated with improved prognosis [87]. A handful of investigations have also observed changes in immune responses to Y90 radioembolization for HCC and ICCA [88,89,90].
4.2. Combining Image-Guided Therapies with Immunotherapy
The observed immunological changes following iLRT have sparked a burgeoning interest in augmenting the efficacy of locoregional therapy through the implementation of combination regimens involving immunotherapy agents [78]. A majority of these investigations have been done in the context of HCC. Sorefanib, a tyrosine kinase inhibitor, has long been considered a salvage systemic therapy for advanced HCC [18]. Prospective, multi-center investigations have highlighted statistically improved progression-free survival for combined TACE + sorafenib (25.2 months) vs. monotherapy (13.5 months) [91]. The meta-analysis has also supported increased time to progression for combination therapy, but it did not identify differences in overall survival [92]. Several studies have also explored other kinase inhibitors (i.e., brivanib and orantinib) with TACE, but they have failed to meet primary overall survival endpoints [78]. Other immunotherapies include programmed death protein-1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors. Randomized control trials, exploring the role of (neo)adjuvant immunotherapies in concert with RFA, are currently underway [78]. Observational investigations, combining CTLA-4 inhibitors (i.e., Tremelimumab) and TACE for patients with advanced HCC and hepatitis C, have exhibited a resultant reduction in viral load and an increase in intratumoral CD8+ cells from tumor biopsies [93]. A phase 1 clinical trial is underway using Tremelinumab in combination with radiofrequency ablation or TACE [94]. PD-1 inhibitors, such as Lenvatinib, are also being explored for unresectable HCC. A prospective investigation showed combination therapy with TACE at a higher objective response rate (67.9% vs. 29.6%, p < 0.001) and overall survival period (23.9 vs. 15.3 months, p < 0.001) [95]. Although still in its infancy, these efforts to enhance the efficacy of iLRT and enhance anti-tumor immune response display promising results.
5. Discussion and Future Directions
Primary liver cancers are highly aggressive and, often, fatal diseases that affect millions of people around the world. Considering the heterogeneity of liver cancers and the various prognostic factors that must be considered when determining treatment eligibility, image-guided therapies represent distinctive and pioneering modalities for managing HCC and ICCA. Thermal ablation, TACE, and TARE are all effective locoregional therapies for the treatment of HCC and ICCA. Ablation presents a potentially curative therapeutic option for individuals with early-stage HCC who are not eligible for surgical intervention. Ablation (e.g., RFA or MWA) also improves outcomes for patients with unresectable or recurrent ICCA. Other image-guided therapies, such as chemoembolization, offer improved survival benefits for ICCA and early-to-intermediate-stage HCC. TARE is also a viable treatment modality for early-stage HCC and ICCA, as established by recent guidelines. Although further research is required to investigate and refine the utilization of these tools, they offer a promising, minimally-invasive approach for managing and enhancing outcomes in patients with complex or arduous liver diseases. The combination of different locoregional therapies may produce a synergistic effect, resulting in enhanced treatment efficacy and improved therapeutic responses. These therapies also exert an immune modulation effect, making them candidates for combination with immunotherapies.
The identification of specific immune and molecular changes also offers potential for future developments in disease monitoring. For example, the alpha-fetal protein (AFP) has been long considered a prognostic and treatment response serum biomarker, for surgery and locoregional therapy, in patients with HCC [96,97,98]. Serum AFP response to iLRT has been shown to stratify the risk of HCC recurrence following a liver transplant [99]. Currently, the most commonly utilized biomarkers for the detection and monitoring of ICCA include a carbohydrate antigen (CA19-9) and a carcinoembryonic antigen (CEA) [100]. Given that iLRT can induce immune and molecular modulating effects, such as with CD4+, CD8+ T cells, and T regulatory cells, it may offer an additional circulating biomarker to monitor treatment response [101]. For example, increased levels of T helper cells post-TACE are associated with greater OS (p = 0.007) [86]. As such, in combination with imaging, such as dynamic CT and MRI [102], immunological and molecular biomarkers offer new monitoring methods for treatment response [76,101]
6. Conclusions
Primary liver cancers are highly aggressive and often fatal diseases that affect millions of people around the world. Considering the heterogeneity of liver cancers and the various prognostic factors that must be considered when determining treatment eligibility, image-guided therapies represent distinctive and pioneering modalities for managing HCC and ICCA. Ablation presents a potentially curative therapeutic option for individuals with early-stage HCC who are not eligible for surgical intervention. Ablation (e.g., RFA or MWA) also improves outcomes for patients with unresectable or recurrent ICCA. Other image-guided therapies, such as chemoembolization and radioembolization, offer improved survival benefits for ICCA and early-to-intermediate-stage HCC. Although further research is required to investigate and refine the utilization of these tools, they offer a promising, minimally invasive approach for managing and enhancing outcomes in patients with complex or arduous liver diseases.
Author Contributions
Conceptualization, C.R.C. and M.S.M.; resources, M.S.M.; writing—original draft preparation, C.R.C.; writing—review and editing, M.S.M.; visualization, C.R.C.; supervision, M.S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Augustine, M.M.; Fong, Y. Epidemiology and Risk Factors of Biliary Tract and Primary Liver Tumors. Surg. Oncol. Clin. 2014, 23, 171–188. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.S.; Khandpur, U.; Cloyd, J.M.; Mumtaz, K.; Dowell, J.D. Locoregional Therapy Approaches for Hepatocellular Carcinoma: Recent Advances and Management Strategies. Cancers 2020, 12, 1914. [Google Scholar] [CrossRef]
- Labib, P.L.; Davidson, B.R.; Sharma, R.A.; Pereira, S.P. Locoregional Therapies in Cholangiocarcinoma. Hepatic Oncol. 2017, 4, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Ribes, J.; Díaz, M.; Cléries, R. Primary Liver Cancer: Worldwide Incidence and Trends. Gastroenterology 2004, 127, S5–S16. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.; Makary, M.S.; Beal, E.W. Locoregional Therapy for Intrahepatic Cholangiocarcinoma. Cancers 2023, 15, 2384. [Google Scholar] [CrossRef]
- Llovet, J.M.; Lencioni, R. MRECIST for HCC: Performance and Novel Refinements. J. Hepatol. 2020, 72, 288–306. [Google Scholar] [CrossRef]
- Ko, C.-C.; Yeh, L.-R.; Kuo, Y.-T.; Chen, J.-H. Imaging Biomarkers for Evaluating Tumor Response: RECIST and Beyond. Biomark. Res. 2021, 9, 52. [Google Scholar] [CrossRef]
- Center, M.M.; Jemal, A. International Trends in Liver Cancer Incidence Rates. Cancer Epidemiol Biomark. Prev 2011, 20, 2362–2368. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef]
- Janevska, D.; Chaloska-Ivanova, V.; Janevski, V. Hepatocellular Carcinoma: Risk Factors, Diagnosis and Treatment. Open Access Maced. J. Med. Sci. 2015, 3, 732–736. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Henry, L. Epidemiology of Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma. JHEP Rep. 2021, 3, 100305. [Google Scholar] [CrossRef]
- Flores, Y.N.; Datta, G.D.; Yang, L.; Corona, E.; Devineni, D.; Glenn, B.A.; Bastani, R.; May, F.P. Disparities in Hepatocellular Carcinoma Incidence, Stage, and Survival: A Large Population-Based Study. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1193–1199. [Google Scholar] [CrossRef]
- Balogh, J.; Victor, D.; Asham, E.H.; Burroughs, S.G.; Boktour, M.; Saharia, A.; Li, X.; Ghobrial, R.M.; Monsour, H.P. Hepatocellular Carcinoma: A Review. J. Hepatocell. Carcinoma 2016, 3, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Chidambaranathan-Reghupaty, S.; Fisher, P.B.; Sarkar, D. Hepatocellular Carcinoma (HCC): Epidemiology, Etiology and Molecular Classification. Adv. Cancer Res. 2021, 149, 1–61. [Google Scholar] [CrossRef] [PubMed]
- Schlageter, M.; Terracciano, L.M.; D’Angelo, S.; Sorrentino, P. Histopathology of Hepatocellular Carcinoma. World J. Gastroenterol. 2014, 20, 15955–15964. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Z.; Xu, X. Molecular Subtyping of Hepatocellular Carcinoma: A Step toward Precision Medicine. Cancer Commun. 2020, 40, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Brar, G.; Greten, T.F.; Graubard, B.I.; McNeel, T.S.; Petrick, J.L.; McGlynn, K.A.; Altekruse, S.F. Hepatocellular Carcinoma Survival by Etiology: A SEER-Medicare Database Analysis. Hepatol. Commun. 2020, 4, 1541–1551. [Google Scholar] [CrossRef]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65. [Google Scholar] [CrossRef]
- Halder, R.; Amaraneni, A.; Shroff, R.T. Cholangiocarcinoma: A Review of the Literature and Future Directions in Therapy. Hepatobiliary Surg. Nutr. 2022, 11, 555–566. [Google Scholar] [CrossRef]
- Ustundag, Y.; Bayraktar, Y. Cholangiocarcinoma: A Compact Review of the Literature. World J. Gastroenterol. 2008, 14, 6458–6466. [Google Scholar] [CrossRef] [PubMed]
- Massarweh, N.N.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control 2017, 24, 1073274817729245. [Google Scholar] [CrossRef]
- Patel, T. Worldwide Trends in Mortality from Biliary Tract Malignancies. BMC Cancer 2002, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Robinson, S.; Toledano, M.; Arora, S.; Keegan, T.; Hargreaves, S.; Beck, A.; Khan, S.; Elliott, P.; Thomas, H. Increase in Mortality Rates from Intrahepatic Cholangiocarcinoma in England and Wales 1968–1998. Gut 2001, 48, 816–820. [Google Scholar] [CrossRef]
- Van Dyke, A.L.; Shiels, M.S.; Jones, G.S.; Pfeiffer, R.M.; Petrick, J.L.; Beebe-Dimmer, J.L.; Koshiol, J. Biliary Tract Cancer Incidence and Trends in the United States by Demographic Group, 1999–2013. Cancer 2019, 125, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Zhu, A.X.; Fuchs, C.S.; Brooks, G.A. Forty-Year Trends in Cholangiocarcinoma Incidence in the U.S.: Intrahepatic Disease on the Rise. Oncologist 2016, 21, 594–599. [Google Scholar] [CrossRef]
- Tyson, G.L.; El-Serag, H.B. Risk Factors of Cholangiocarcinoma. Hepatology 2011, 54, 173–184. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next Horizon in Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Shirono, T.; Niizeki, T.; Iwamoto, H.; Shimose, S.; Suzuki, H.; Kawaguchi, T.; Kamachi, N.; Noda, Y.; Okamura, S.; Nakano, M.; et al. Therapeutic Outcomes and Prognostic Factors of Unresectable Intrahepatic Cholangiocarcinoma: A Data Mining Analysis. J. Clin. Med. 2021, 10, 987. [Google Scholar] [CrossRef]
- Mavros, M.N.; Economopoulos, K.P.; Alexiou, V.G.; Pawlik, T.M. Treatment and Prognosis for Patients with Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-Analysis. JAMA Surg. 2014, 149, 565–574. [Google Scholar] [CrossRef]
- Sumiyoshi, T.; Shima, Y.; Okabayashi, T.; Negoro, Y.; Shimada, Y.; Iwata, J.; Matsumoto, M.; Hata, Y.; Noda, Y.; Sui, K.; et al. Chemoradiotherapy for Initially Unresectable Locally Advanced Cholangiocarcinoma. World J. Surg. 2018, 42, 2910–2918. [Google Scholar] [CrossRef]
- Alvaro, D.; Gores, G.J.; Walicki, J.; Hassan, C.; Sapisochin, G.; Komuta, M.; Forner, A.; Valle, J.W.; Laghi, A.; Rizvi, S.I.; et al. EASL-ILCA Clinical Practice Guidelines on the Management of Intrahepatic Cholangiocarcinoma. J. Hepatol. 2023, 79, 181–208. [Google Scholar] [CrossRef]
- Breedis, C.; Young, G. The Blood Supply of Neoplasms in the Liver. Am. J. Pathol. 1954, 30, 969–977. [Google Scholar]
- Yoshida, K.; Matsui, O.; Miyayama, S.; Ibukuro, K.; Yoneda, N.; Inoue, D.; Kozaka, K.; Minami, T.; Koda, W.; Gabata, T. Isolated Arteries Originating from the Intrahepatic Arteries: Anatomy, Function, and Importance in Intervention. J. Vasc. Interv. Radiol. 2018, 29, 531–537.e1. [Google Scholar] [CrossRef] [PubMed]
- Hare, A.E.; Makary, M.S. Locoregional Approaches in Cholangiocarcinoma Treatment. Cancers 2022, 14, 5853. [Google Scholar] [CrossRef]
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.-W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the Diagnosis and Management of Intrahepatic Cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef]
- Ryan, M.J.; Willatt, J.; Majdalany, B.S.; Kielar, A.Z.; Chong, S.; Ruma, J.A.; Pandya, A. Ablation Techniques for Primary and Metastatic Liver Tumors. World J. Hepatol. 2016, 8, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; He, M.; Fu, C.; Feng, K.; Ma, K.; Zhang, L. Radiofrequency Ablation in the Treatment of Hepatocellular Carcinoma. Int. J. Hyperth. 2022, 39, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Palaia, R.; Delrio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; Curley, S.A. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019, 24, e990–e1005. [Google Scholar] [CrossRef] [PubMed]
- Poulou, L.S.; Botsa, E.; Thanou, I.; Ziakas, P.D.; Thanos, L. Percutaneous Microwave Ablation vs Radiofrequency Ablation in the Treatment of Hepatocellular Carcinoma. World J. Hepatol. 2015, 7, 1054–1063. [Google Scholar] [CrossRef]
- Kalra, N.; Gupta, P.; Chawla, Y.; Khandelwal, N. Locoregional Treatment for Hepatocellular Carcinoma: The Best Is yet to Come. World J. Radiol. 2015, 7, 306–318. [Google Scholar] [CrossRef]
- Criss, C.R.; Makary, M.S. Salvage Locoregional Therapies for Recurrent Hepatocellular Carcinoma. World J. Gastroenterol. 2023, 29, 413–424. [Google Scholar] [CrossRef]
- Majumdar, A.; Roccarina, D.; Thorburn, D.; Davidson, B.R.; Tsochatzis, E.; Gurusamy, K.S. Management of People with Early- or Very Early-stage Hepatocellular Carcinoma. Cochrane Database Syst. Rev. 2017, 2017, CD011650. [Google Scholar] [CrossRef]
- Sommer, C.M.; Kauczor, H.U.; Pereira, P.L. Locoregional Therapies of Cholangiocarcinoma. Visc. Med. 2016, 32, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R. Loco-Regional Treatment of Hepatocellular Carcinoma. Hepatology 2010, 52, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.-S.; He, Q.; Wang, M.-Q. Transcatheter Arterial Chemoembolization: History for More than 30 Years. Int. Sch. Res. Not. 2012, 2012, 480650. [Google Scholar] [CrossRef] [PubMed]
- Melchiorre, F.; Patella, F.; Pescatori, L.; Pesapane, F.; Fumarola, E.; Biondetti, P.; Brambillasca, P.; Monaco, C.; Ierardi, A.M.; Franceschelli, G.; et al. DEB-TACE: A Standard Review. Future Oncol. 2018, 14, 2969–2984. [Google Scholar] [CrossRef] [PubMed]
- Song, J.E.; Kim, D.Y. Conventional vs Drug-Eluting Beads Transarterial Chemoembolization for Hepatocellular Carcinoma. World J. Hepatol. 2017, 9, 808–814. [Google Scholar] [CrossRef]
- Li, H.; Wu, F.; Duan, M.; Zhang, G. Drug-Eluting Bead Transarterial Chemoembolization (TACE) vs. Conventional TACE in Treating Hepatocellular Carcinoma Patients with Multiple Conventional TACE Treatments History: A Comparison of Efficacy and Safety. Medicine 2019, 98, e15314. [Google Scholar] [CrossRef]
- Ikeda, M.; Arai, Y.; Inaba, Y.; Tanaka, T.; Sugawara, S.; Kodama, Y.; Aramaki, T.; Anai, H.; Morita, S.; Tsukahara, Y.; et al. Conventional or Drug-Eluting Beads? Randomized Controlled Study of Chemoembolization for Hepatocellular Carcinoma: JIVROSG-1302. Liver Cancer 2022, 11, 440–450. [Google Scholar] [CrossRef]
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Solà, R.; et al. Arterial Embolisation or Chemoembolisation versus Symptomatic Treatment in Patients with Unresectable Hepatocellular Carcinoma: A Randomised Controlled Trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Lo, C.-M.; Ngan, H.; Tso, W.-K.; Liu, C.-L.; Lam, C.-M.; Poon, R.T.-P.; Fan, S.-T.; Wong, J. Randomized Controlled Trial of Transarterial Lipiodol Chemoembolization for Unresectable Hepatocellular Carcinoma. Hepatology 2002, 35, 1164–1171. [Google Scholar] [CrossRef]
- Llovet, J.M.; Bruix, J. Systematic Review of Randomized Trials for Unresectable Hepatocellular Carcinoma: Chemoembolization Improves Survival. Hepatology 2003, 37, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.-Y.; Li, S.-M.; Fan, H.-Y.; Zhang, L.; Zhao, H.-J.; Li, S.-M. Transarterial Chemoembolization Extends Long-Term Survival in Patients with Unresectable Hepatocellular Carcinoma. Medicine 2018, 97, e11872. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, J.H.; Yoon, H.-J.; Lee, I.-S.; Yoon, H.-K.; Kim, K.-P. Transarterial Chemoembolization versus Supportive Therapy in the Palliative Treatment of Unresectable Intrahepatic Cholangiocarcinoma. Clin. Radiol. 2011, 66, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.-R.; Hu, H.-J.; Liu, F.; Regmi, P.; Jin, Y.-W.; Li, F.-Y. The Effect of Trans Arterial Chemoembolization in the Management of Intrahepatic Cholangiocarcinoma. A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2022, 48, 956–966. [Google Scholar] [CrossRef]
- Salem, R.; Lewandowski, R.J.; Sato, K.T.; Atassi, B.; Ryu, R.K.; Ibrahim, S.; Nemcek, A.A.; Omary, R.A.; Madoff, D.C.; Murthy, R. Technical Aspects of Radioembolization with 90Y Microspheres. Tech. Vasc. Interv. Radiol. 2007, 10, 12–29. [Google Scholar] [CrossRef]
- Tohme, S.; Bou Samra, P.; Kaltenmeier, C.; Chidi, A.P.; Varley, P.R.; Tsung, A. Radioembolization for Hepatocellular Carcinoma: A Nationwide 10-Year Experience. J. Vasc. Interv. Radiol. 2018, 29, 912–919.e2. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, H.-C. Radioembolization for Hepatocellular Carcinoma: What Clinicians Need to Know. J. Liver Cancer 2022, 22, 4–13. [Google Scholar] [CrossRef]
- Sangro, B.; Iñarrairaegui, M.; Bilbao, J.I. Radioembolization for Hepatocellular Carcinoma. J. Hepatol. 2012, 56, 464–473. [Google Scholar] [CrossRef]
- Mosconi, C.; Cappelli, A.; Pettinato, C.; Golfieri, R. Radioembolization with Yttrium-90 Microspheres in Hepatocellular Carcinoma: Role and Perspectives. World J. Hepatol. 2015, 7, 738–752. [Google Scholar] [CrossRef] [PubMed]
- d’Abadie, P.; Hesse, M.; Louppe, A.; Lhommel, R.; Walrand, S.; Jamar, F. Microspheres Used in Liver Radioembolization: From Conception to Clinical Effects. Molecules 2021, 26, 3966. [Google Scholar] [CrossRef]
- Golfieri, R. SIR-Spheres Yttrium-90 Radioembolization for the Treatment of Unresectable Liver Cancers. Hepat. Oncol. 2014, 1, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Gordon, A.C.; Mouli, S.; Hickey, R.; Kallini, J.; Gabr, A.; Mulcahy, M.F.; Baker, T.; Abecassis, M.; Miller, F.H.; et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared with Chemoembolization in Patients with Hepatocellular Carcinoma. Gastroenterology 2016, 151, 1155–1163.e2. [Google Scholar] [CrossRef]
- Mosconi, C.; Solaini, L.; Vara, G.; Brandi, N.; Cappelli, A.; Modestino, F.; Cucchetti, A.; Golfieri, R. Transarterial Chemoembolization and Radioembolization for Unresectable Intrahepatic Cholangiocarcinoma—A Systemic Review and Meta-Analysis. Cardiovasc. Interv. Radiol. 2021, 44, 728–738. [Google Scholar] [CrossRef]
- Higgins, M.C.S.S.; Soulen, M.C. Combining Locoregional Therapies in the Treatment of Hepatocellular Carcinoma. Semin. Interv. Radiol. 2013, 30, 74–81. [Google Scholar] [CrossRef]
- Rossi, S.; Garbagnati, F.; Lencioni, R.; Allgaier, H.P.; Marchianò, A.; Fornari, F.; Quaretti, P.; Tolla, G.D.; Ambrosi, C.; Mazzaferro, V.; et al. Percutaneous Radio-Frequency Thermal Ablation of Nonresectable Hepatocellular Carcinoma after Occlusion of Tumor Blood Supply. Radiology 2000, 217, 119–126. [Google Scholar] [CrossRef]
- Galanakis, N.; Kehagias, E.; Matthaiou, N.; Samonakis, D.; Tsetis, D. Transcatheter Arterial Chemoembolization Combined with Radiofrequency or Microwave Ablation for Hepatocellular Carcinoma: A Review. Hepat. Oncol. 2018, 5, HEP07. [Google Scholar] [CrossRef]
- Kung, J.W.C.; Ng, K.K.C. Role of Locoregional Therapies in the Management of Patients with Hepatocellular Carcinoma. Hepatoma Res. 2022, 8, 17. [Google Scholar] [CrossRef]
- Jiang, C.; Cheng, G.; Liao, M.; Huang, J. Individual or Combined Transcatheter Arterial Chemoembolization and Radiofrequency Ablation for Hepatocellular Carcinoma: A Time-to-Event Meta-Analysis. World J. Surg. Oncol. 2021, 19, 81. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhang, T.; Wang, Z.-G.; Liu, H. Clinical Outcome of Small Hepatocellular Carcinoma after Different Treatments: A Meta-Analysis. World J. Gastroenterol. 2014, 20, 10174–10182. [Google Scholar] [CrossRef] [PubMed]
- Hoechst, B.; Voigtlaender, T.; Ormandy, L.; Gamrekelashvili, J.; Zhao, F.; Wedemeyer, H.; Lehner, F.; Manns, M.P.; Greten, T.F.; Korangy, F. Myeloid Derived Suppressor Cells Inhibit Natural Killer Cells in Patients with Hepatocellular Carcinoma via the NKp30 Receptor. Hepatology 2009, 50, 799–807. [Google Scholar] [CrossRef]
- Ringelhan, M.; Pfister, D.; O’Connor, T.; Pikarsky, E.; Heikenwalder, M. The Immunology of Hepatocellular Carcinoma. Nat. Immunol. 2018, 19, 222–232. [Google Scholar] [CrossRef]
- DeNardo, D.G.; Ruffell, B. Macrophages as Regulators of Tumor Immunity and Immunotherapy. Nat. Rev. Immunol. 2019, 19, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Toom, S.; Avula, A.; Kumar, V.; Rahma, O.E. The Immune Modulation Effect of Locoregional Therapies and Its Potential Synergy with Immunotherapy in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2020, 7, 11–17. [Google Scholar] [CrossRef]
- Zhang, Q.; He, Y.; Luo, N.; Patel, S.J.; Han, Y.; Gao, R.; Modak, M.; Carotta, S.; Haslinger, C.; Kind, D.; et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell 2019, 179, 829–845.e20. [Google Scholar] [CrossRef]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional Therapies in the Era of Molecular and Immune Treatments for Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef]
- Dromi, S.A.; Walsh, M.P.; Herby, S.; Traughber, B.; Xie, J.; Sharma, K.V.; Sekhar, K.P.; Luk, A.; Liewehr, D.J.; Dreher, M.R.; et al. Radiofrequency Ablation Induces Antigen-Presenting Cell Infiltration and Amplification of Weak Tumor-Induced Immunity. Radiology 2009, 251, 58–66. [Google Scholar] [CrossRef]
- Widenmeyer, M.; Shebzukhov, Y.; Haen, S.P.; Schmidt, D.; Clasen, S.; Boss, A.; Kuprash, D.V.; Nedospasov, S.A.; Stenzl, A.; Aebert, H.; et al. Analysis of Tumor Antigen-Specific T Cells and Antibodies in Cancer Patients Treated with Radiofrequency Ablation. Int. J. Cancer 2011, 128, 2653–2662. [Google Scholar] [CrossRef]
- Schueller, G.; Paolini, P.; Friedl, J.; Stift, A.; Dubsky, P.; Bachleitner-Hofmann, T.; Jakesz, R.; Gnant, M. Heat Treatment of Hepatocellular Carcinoma Cells: Increased Levels of Heat Shock Proteins 70 and 90 Correlate with Cellular Necrosis. Anticancer Res. 2001, 21, 295–300. [Google Scholar] [PubMed]
- Zhang, H.; Hou, X.; Cai, H.; Zhuang, X. Effects of Microwave Ablation on T-Cell Subsets and Cytokines of Patients with Hepatocellular Carcinoma. Minim. Invasive Ther. Allied Technol. 2017, 26, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fu, X.; Li, T.; Yan, H. The Prognostic Value of Myeloid Derived Suppressor Cell Level in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. PLoS ONE 2019, 14, e0225327. [Google Scholar] [CrossRef]
- Iwata, T.; Kondo, Y.; Kimura, O.; Morosawa, T.; Fujisaka, Y.; Umetsu, T.; Kogure, T.; Inoue, J.; Nakagome, Y.; Shimosegawa, T. PD-L1+MDSCs Are Increased in HCC Patients and Induced by Soluble Factor in the Tumor Microenvironment. Sci. Rep. 2016, 6, 39296. [Google Scholar] [CrossRef]
- Pinato, D.J.; Murray, S.M.; Forner, A.; Kaneko, T.; Fessas, P.; Toniutto, P.; Mínguez, B.; Cacciato, V.; Avellini, C.; Diaz, A.; et al. Trans-Arterial Chemoembolization as a Loco-Regional Inducer of Immunogenic Cell Death in Hepatocellular Carcinoma: Implications for Immunotherapy. J. Immunother. Cancer 2021, 9, e003311. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, B.; Huang, Z.-L.; Shi, M.; Yu, X.-J.; Zheng, L.; Li, S.; Li, L. Increased Circulating Th17 Cells after Transarterial Chemoembolization Correlate with Improved Survival in Stage III Hepatocellular Carcinoma: A Prospective Study. PLoS ONE 2013, 8, e60444. [Google Scholar] [CrossRef]
- Guo, J.; Wang, S.; Han, Y.; Jia, Z.; Wang, R. Effects of Transarterial Chemoembolization on the Immunological Function of Patients with Hepatocellular Carcinoma. Oncol. Lett. 2021, 22, 554. [Google Scholar] [CrossRef]
- Greten, T.F.; Mauda-Havakuk, M.; Heinrich, B.; Korangy, F.; Wood, B.J. Combined Locoregional-Immunotherapy for Liver Cancer. J. Hepatol. 2019, 70, 999–1007. [Google Scholar] [CrossRef]
- Chew, V.; Lee, Y.H.; Pan, L.; Nasir, N.J.M.; Lim, C.J.; Chua, C.; Lai, L.; Hazirah, S.N.; Lim, T.K.H.; Goh, B.K.P.; et al. Immune Activation Underlies a Sustained Clinical Response to Yttrium-90 Radioembolisation in Hepatocellular Carcinoma. Gut 2019, 68, 335–346. [Google Scholar] [CrossRef]
- Haag, F.; Manikkam, A.; Kraft, D.; Bär, C.; Wilke, V.; Nowak, A.J.; Bertrand, J.; Omari, J.; Pech, M.; Gylstorff, S.; et al. Selective Internal Radiotherapy Changes the Immune Profiles of Extracellular Vesicles and Their Immune Origin in Patients with Inoperable Cholangiocarcinoma. Cells 2022, 11, 2309. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Ikeda, M.; Torimura, T.; Tanabe, N.; Aikata, H.; Izumi, N.; Yamasaki, T.; Nojiri, S.; Hino, K.; et al. Randomised, Multicentre Prospective Trial of Transarterial Chemoembolisation (TACE) plus Sorafenib as Compared with TACE Alone in Patients with Hepatocellular Carcinoma: TACTICS Trial. Gut 2020, 69, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, W.; Wang, M.; Hu, J.; Wang, E.; Zhao, Y.; Liu, L. Transarterial Chemoembolization plus Sorafenib for the Management of Unresectable Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. BMC Gastroenterol. 2018, 18, 138. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in Combination with Ablation in Patients with Advanced Hepatocellular Carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Cadamuro, M.; Fabris, L.; Zhang, X.; Strazzabosco, M. Tumor Microenvironment and Immunology of Cholangiocarcinoma. Hepatoma Res. 2022, 8, 11. [Google Scholar] [CrossRef]
- Qu, S.; Zhang, X.; Wu, Y.; Meng, Y.; Pan, H.; Fang, Q.; Hu, L.; Zhang, J.; Wang, R.; Wei, L.; et al. Efficacy and Safety of TACE Combined with Lenvatinib Plus PD-1 Inhibitors Compared with TACE Alone for Unresectable Hepatocellular Carcinoma Patients: A Prospective Cohort Study. Front. Oncol. 2022, 12, 874473. [Google Scholar] [CrossRef]
- Muscari, F.; Maulat, C. Preoperative Alpha-Fetoprotein (AFP) in Hepatocellular Carcinoma (HCC): Is This 50-Year Biomarker Still up-to-Date? Transl. Gastroenterol. Hepatol. 2020, 5, 46. [Google Scholar] [CrossRef]
- Lee, J.S.; Chon, Y.E.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.-H.; Kang, W.; Choi, M.S.; Gwak, G.-Y.; et al. Prognostic Value of Alpha-Fetoprotein in Patients Who Achieve a Complete Response to Transarterial Chemoembolization for Hepatocellular Carcinoma. Yonsei Med. J. 2021, 62, 12. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, Y.; Jia, J.; Chen, H.; Bai, W.; Yang, M.; Yin, Z.; He, C.; Zhang, L.; Guo, W.; et al. The Prognostic Value of Alpha-Fetoprotein Response for Advanced-Stage Hepatocellular Carcinoma Treated with Sorafenib Combined with Transarterial Chemoembolization. Sci. Rep. 2016, 6, 19851. [Google Scholar] [CrossRef]
- Chen, I.-H.; Hsu, C.-C.; Yong, C.-C.; Cheng, Y.-F.; Wang, C.-C.; Lin, C.-C.; Chen, C.-L. AFP Response to Locoregional Therapy Can Stratify the Risk of Tumor Recurrence in HCC Patients after Living Donor Liver Transplantation. Cancers 2023, 15, 1551. [Google Scholar] [CrossRef]
- Pavicevic, S.; Reichelt, S.; Uluk, D.; Lurje, I.; Engelmann, C.; Modest, D.P.; Pelzer, U.; Krenzien, F.; Raschzok, N.; Benzing, C.; et al. Prognostic and Predictive Molecular Markers in Cholangiocarcinoma. Cancers 2022, 14, 1026. [Google Scholar] [CrossRef]
- Tampaki, M.; Doumba, P.P.; Deutsch, M.; Koskinas, J. Circulating Biomarkers of Hepatocellular Carcinoma Response after Locoregional Treatments: New Insights. World J. Hepatol. 2015, 7, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Criss, C.; Nagar, A.M.; Makary, M.S. Hepatocellular Carcinoma: State of the Art Diagnostic Imaging. World J. Radiol. 2023, 15, 56–68. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).