Unveiling the Potential Distribution of the Highly Threatened Madeira Pipistrelle (Pipistrellus maderensis): Do Different Evolutionary Significant Units Exist?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Phylogenetic Analysis
2.3. Occurrence Records
2.4. Environmental Predictors
2.5. Model Development and Analysis of Environmental Matching
3. Results
3.1. Genetic Analysis
3.2. Macaronesian Model and MESS Analysis
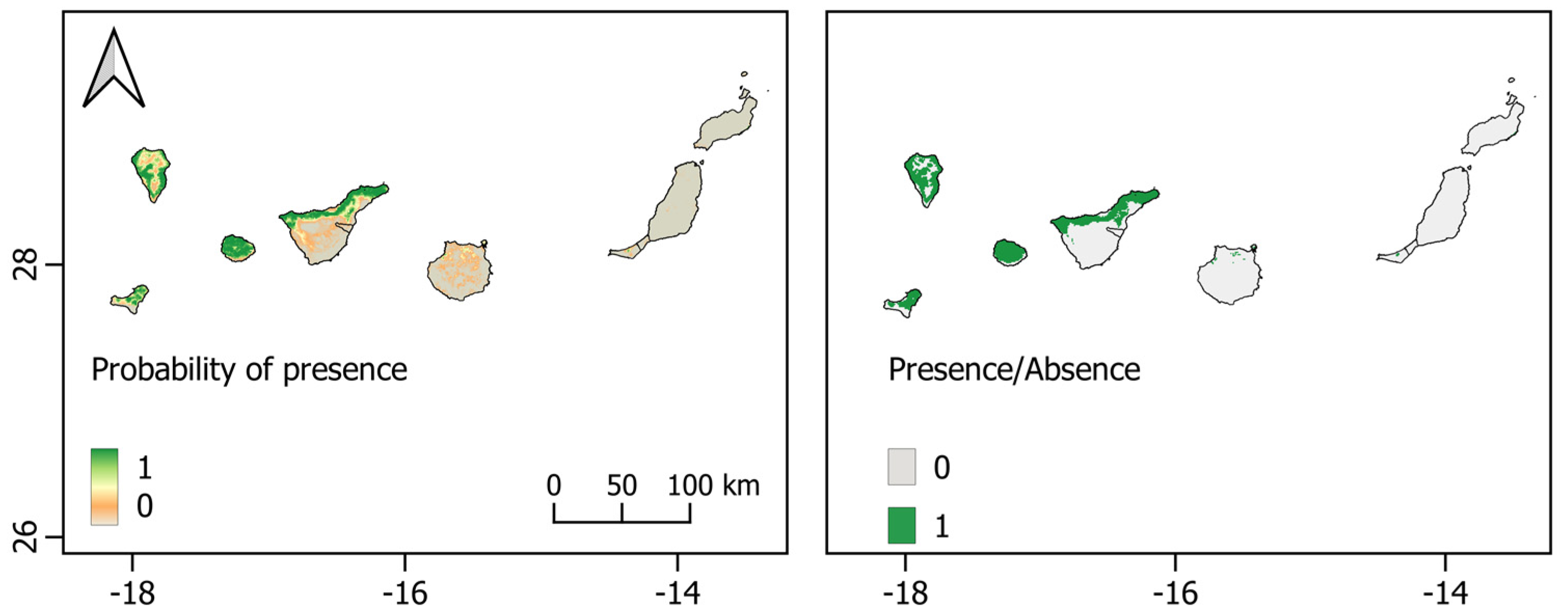
3.3. Archipelago-Specific Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Russell, J.C.; Kueffer, C. Island biodiversity in the Anthropocene. Annu. Rev. Environ. Resour. 2019, 44, 31–60. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; Kreft, H.; Irl, S.D.; Norder, S.; Ah-Peng, C.; Borges, P.A.; Burns, K.C.; de Nascimento, L.; Meyer, J.Y.; Montes, E.; et al. Scientists’ warning—The outstanding biodiversity of islands is in peril. Glob. Ecol. Conserv. 2021, 31, e01847. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R.; Briscoe, D.A.; Ballou, J.D. Introduction to Conservation Genetics; Cambridge University Press: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Connor, E.F.; Courtney, A.C.; Yoder, J.M. Individuals–area relationships: The relationship between animal population density and area. Ecology 2000, 81, 734–748. [Google Scholar] [CrossRef]
- Franks, S.J. Genetics, evolution, and conservation of island plants. J. Plant Biol. 2010, 53, 1–9. [Google Scholar] [CrossRef]
- Heinen, J.H.; van Loon, E.E.; Hansen, D.M.; Kissling, W.D. Extinction-driven changes in frugivore communities on oceanic islands. Ecography 2018, 41, 1245–1255. [Google Scholar] [CrossRef]
- Jones, K.E.; Mickleburgh, S.P.; Sechrest, W.; Walsh, A.L.; Fleming, T.H.; Racey, P.A. Global overview of the conservation of island bats: Importance, challenges and opportunities. In Island Bats: Evolution, Ecology, and Conservation; University of Chicago Press: Chicago, IL, USA, 2009; pp. 496–530. [Google Scholar]
- Wright, N.A.; Steadman, D.W.; Witt, C.C. Predictable evolution toward flightlessness in volant island birds. Proc. Natl. Acad. Sci. USA 2016, 113, 4765–4770. [Google Scholar] [CrossRef]
- Moore, N.W. The diurnal flight of the Azorean bat (Nyctalus azoreum) and the avifauna of the Azores. J. Zool. 1975, 177, 483–486. [Google Scholar] [CrossRef]
- Russo, D.; Maglio, G.; Rainho, A.; Meyer, C.F.; Palmeirim, J.M. Out of the dark: Diurnal activity in the bat Hipposideros ruber on São Tomé island (West Africa). Mamm. Biol. 2011, 76, 701–708. [Google Scholar] [CrossRef]
- Chua, M.A.; Aziz, S.A. Into the light: Atypical diurnal foraging activity of Blyth’s horseshoe bat, Rhinolophus lepidus (Chiroptera: Rhinolophidae) on Tioman Island, Malaysia. Mammalia 2018, 83, 78–83. [Google Scholar] [CrossRef]
- Biscardi, S.; Russo, D.; Casciani, V.; Cesarini, D.; Mei, M.; Boitani, L. Foraging requirements of the endangered long-fingered bat: The influence of micro-habitat structure, water quality and prey type. J. Zool. 2007, 273, 372–381. [Google Scholar] [CrossRef]
- Almenar, D.; Aihartza, J.; Goiti, U.; Salsamendi, E.; Garin, I. Foraging behaviour of the long-fingered bat Myotis capaccinii: Implications for conservation and management. Endanger. Species Res. 2009, 8, 69–78. [Google Scholar] [CrossRef]
- Davy, C.M.; Russo, D.; Fenton, M.B. Use of native woodlands and traditional olive groves by foraging bats on a Mediterranean island: Consequences for conservation. J. Zool. 2007, 273, 397–405. [Google Scholar] [CrossRef]
- Russo, D.; Mucedda, M.; Bello, M.; Biscardi, S.; Pidinchedda, E.; Jones, G. Divergent echolocation call frequencies in insular rhinolophids (Chiroptera): A case of character displacement? J. Biogeogr. 2007, 34, 2129–2138. [Google Scholar] [CrossRef]
- Russo, D.; Teixeira, S.; Cistrone, L.; Jesus, J.; Teixeira, D.; Freitas, T.; Jones, G. Social calls are subject to stabilizing selection in insular bats. J. Biogeogr. 2009, 36, 2212–2221. [Google Scholar] [CrossRef]
- Kunz, T.H.; Braun de Torrez, E.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Ann. N. Y. Acad. Sci. 2011, 1223, 1–38. [Google Scholar] [CrossRef]
- Rainey, W.E. The role of flying foxes (Pteropodidae) in oceanic island ecosystems of the Pacific. In Ecology, Evolution and Behaviour of Bats; Racey, P.A., Swift, S., Eds.; Clarendon Press: Oxford, UK, 1995; pp. 47–62. [Google Scholar]
- Tuneu-Corral, C.; Puig-Montserrat, X.; Riba-Bertolín, D.; Russo, D.; Rebelo, H.; Cabeza, M.; López-Baucells, A. Pest suppression by bats and management strategies to favour it: A global review. Biol. Rev. 2023. [Google Scholar] [CrossRef]
- Conenna, I.; Rocha, R.; Russo, D.; Cabeza, M. Insular bats and research effort: A review of global patterns and priorities. Mamm. Rev. 2017, 47, 169–182. [Google Scholar] [CrossRef]
- O’Shea, T.J.; Cryan, P.M.; Hayman, D.T.; Plowright, R.K.; Streicker, D.G. Multiple mortality events in bats: A global review. Mamm. Rev. 2016, 46, 175–190. [Google Scholar] [CrossRef]
- Vincenot, C.E.; Koyama, L.; Russo, D. Near threatened? First report of unsuspected human-driven decline factors in the Ryukyu flying fox (Pteropus dasymallus) in Japan. Mamm. Biol. 2015, 80, 273–277. [Google Scholar] [CrossRef]
- Ancillotto, L.; Fichera, G.; Pidinchedda, E.; Veith, M.; Kiefer, A.; Mucedda, M.; Russo, D. Wildfires, heatwaves and human disturbance threaten insular endemic bats. Biodivers. Conserv. 2021, 30, 4401–4416. [Google Scholar] [CrossRef]
- Bosso, L.; Mucedda, M.; Fichera, G.; Kiefer, A.; Russo, D. A gap analysis for threatened bat populations on Sardinia. Hystrix 2016, 27, 212. [Google Scholar] [CrossRef]
- Martin, T.G.; Nally, S.; Burbidge, A.A.; Arnall, S.; Garnett, S.T.; Hayward, M.W.; Lumsden, L.F.; Menkhorst, P.; McDonald-Madden, E.; Possingham, H.P. Acting fast helps avoid extinction. Conserv. Lett. 2012, 5, 274–280. [Google Scholar] [CrossRef]
- Woinarski, J. A Bat’s End: The Christmas Island Pipistrelle and Extinction in Australia; CSIRO Publishing: Clayton, Australia, 2018. [Google Scholar]
- Mickleburgh, S.; Waylen, K.; Racey, P. Bats as bushmeat: A global review. Oryx 2009, 43, 217–234. [Google Scholar] [CrossRef]
- Aziz, S.A.; Olival, K.J.; Bumrungsri, S.; Richards, G.C.; Racey, P.A. The conflict between pteropodid bats and fruit growers: Species, legislation and mitigation. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; Springer: Cham, Switzerland, 2016; pp. 377–426. [Google Scholar] [CrossRef]
- Vincenot, C.E.; Collazo, A.M.; Russo, D. The Ryukyu flying fox (Pteropus dasymallus)—A review of conservation threats and call for reassessment. Mamm. Biol. 2017, 83, 71–77. [Google Scholar] [CrossRef]
- Vincenot, C.E.; Florens, F.V.; Kingston, T. Can we protect island flying foxes? Science 2017, 355, 1368–1370. [Google Scholar] [CrossRef] [PubMed]
- Oleksy, R.Z.; Ayady, C.L.; Tatayah, V.; Jones, C.; Froidevaux, J.S.; Racey, P.A.; Jones, G. The impact of the Endangered Mauritian flying fox Pteropus niger on commercial fruit farms and the efficacy of mitigation. Oryx 2021, 55, 114–121. [Google Scholar] [CrossRef]
- Cox, P.A.; Elmqvist, T.; Pierson, E.D.; Rainey, W.E. Flying foxes as strong interactors in South Pacific island ecosystems: A conservation hypothesis. Conserv. Biol. 1991, 5, 448–454. [Google Scholar] [CrossRef]
- Elmqvist, T.; Cox, P.A.; Rainey, W.E.; Pierson, E.D. Restricted pollination on oceanic islands: Pollination of Ceiba pentandra by flying foxes in Samoa. Biotropica 1992, 24, 15–23. [Google Scholar] [CrossRef]
- Meehan, H.J.; McConkey, K.R.; Drake, D.R. Potential disruptions to seed dispersal mutualisms in Tonga, Western Polynesia. J. Biogeogr. 2002, 29, 695–712. [Google Scholar] [CrossRef]
- Fall, P.L.; Drezner, T.D.; Franklin, J. Dispersal ecology of the lowland rain forest in the Vava’u island group, Kingdom of Tonga. N. Z. J. Bot. 2007, 45, 393–417. [Google Scholar] [CrossRef]
- Aziz, S.A.; Clements, G.R.; McConkey, K.R.; Sritongchuay, T.; Pathil, S.; Abu Yazid, M.N.H.; Campos-Arceiz, A.; Forget, P.M.; Bumrungsri, S. Pollination by the locally endangered island flying fox (Pteropus hypomelanus) enhances fruit production of the economically important durian (Durio zibethinus). Ecol. Evol. 2017, 7, 8670–8684. [Google Scholar] [CrossRef] [PubMed]
- Florens, F.B.V.; Baider, C.; Marday, V.; Martin, G.M.N.; Zmanay, Z.; Oleksy, R.; Krivek, G.; Vincenot, C.E.; Strasberg, D.; Kingston, T. Disproportionately large ecological role of a recently mass-culled flying fox in native forests of an oceanic island. J. Nat. Conserv. 2017, 40, 85–93. [Google Scholar] [CrossRef]
- Todd, C.M.; Westcott, D.A.; Martin, J.M.; Rose, K.; McKeown, A.; Hall, J.; Welbergen, J.A. Body-size dependent foraging strategies in the Christmas Island flying-fox: Implications for seed and pollen dispersal within a threatened island ecosystem. Mov. Ecol. 2022, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rogers, H.S.; Donoso, I.; Traveset, A.; Fricke, E.C. Cascading impacts of seed disperser loss on plant communities and ecosystems. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 641–666. [Google Scholar] [CrossRef]
- Juste, J.; Torrent, L.; Méndez-Rodríguez, A.; Howard, K.; García-Mudarra, J.L.; Nogueras, J.; Ibáñez, C. A new Pipistrelle bat from the oceanic Island of Príncipe (Western Central Africa). J. Mammal. 2023, 104, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Dechmann, D.K.N.; Ruczynski, I. Azorean Bat Nyctalus azoreum (Thomas, 1901). In Handbook of the Mammals of Europe. Handbook of the Mammals of Europe; Hackländer, K., Zachos, F.E., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Swift, S.M. Long-eared bats. In Poyser Natural History; T & AD Poyser Ltd.: Hertfordshire, UK, 1998. [Google Scholar]
- Cohen, Y.; Bar-David, S.; Nielsen, M.; Bohmann, K.; Korine, C. An appetite for pests: Synanthropic insectivorous bats exploit cotton pest irruptions and consume various deleterious arthropods. Mol. Ecol. 2020, 29, 1185–1198. [Google Scholar] [CrossRef]
- Salinas-Ramos, V.B.; Ancillotto, L.; Cistrone, L.; Nastasi, C.; Bosso, L.; Smeraldo, S.; Cordero, V.S.; Russo, D. Artificial illumination influences niche segregation in bats. Environ. Pollut. 2021, 284, 117187. [Google Scholar] [CrossRef]
- Georgiakakis, P.; Benda, P. Hanák’s Pipistrelle Pipistrellus hanaki Hulva et Benda, 2004. In Handbook of the Mammals of Europe; Hackländer, K., Zachos, F.E., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Lumsden, L.; Cherry, K. Report on a Preliminary Investigation of the Christmas Island Pipistrelle Pipistrellus Murrayi, in June–July 1994; Arthur Rylah Institute for Environmental Research: Heidelberg, Australia, 1997. [Google Scholar]
- Beeton, B.; Burbidge, A.; Grigg, G.; How, R.; McKenzie, N.; Woinarski, J.C.Z. Final Report Christmas Island Expert Working Group to Minister for the Environment, Heritage and the Arts; DEWHA: Canberra, Australia, 2010. [Google Scholar]
- Evin, A.; Horáček, I.; Hulva, P. Phenotypic diversification and island evolution of pipistrelle bats (Pipistrellus pipistrellus group) in the Mediterranean region inferred from geometric morphometrics and molecular phylogenetics. J. Biogeogr. 2011, 38, 2091–2105. [Google Scholar] [CrossRef]
- Preble, J.H.; Vincenot, C.E.; Hill, D.A.; Ohte, N. Capturing endangered endemic Okinawan bats with acoustic lures. J. Nat. Conserv. 2021, 64, 126074. [Google Scholar] [CrossRef]
- Ferreira, D.F.; Gibb, R.; López-Baucells, A.; Nunes, N.J.; Jones, K.E.; Rocha, R. Species-specific responses to land-use change in island insectivorous bats. J. Nat. Conserv. 2022, 67, 126177. [Google Scholar] [CrossRef]
- Aguillon, S.; Le Minter, G.; Lebarbenchon, C.; Hoarau, A.O.; Toty, C.; Joffrin, L.; Ramanantsalama, R.V.; Augros, S.; Tortosa, P.; Mavingui, P.; et al. A population in perpetual motion: Highly dynamic roosting behavior of a tropical island endemic bat. Ecol. Evol. 2023, 13, e9814. [Google Scholar] [CrossRef] [PubMed]
- Benda, P.; Hanák, V.; Horáček, I.; Hulva, P.; Lučan, R.; Ruedi, M. Bats (Mammalia: Chiroptera) of the Eastern Mediterranean. Part 5. Bat fauna of Cyprus: Review of records with confirmation of six species new for the island and description of a new subspecies. Acta Soc. Zool. Bohem. 2007, 71, 71–130. [Google Scholar]
- Hulva, P.; Benda, P.; Hanak, V.; Evin, A.; Horacek, I. New mitochondrial lineages within the Pipistrellus pipistrellus complex from Mediterranean Europe. Folia Zool. 2007, 56, 378–388. [Google Scholar]
- Benda, P.; Georgiakakis, P.; Dietz, C.; Hanák, V.; Galanaki, K.; Markantonatou, V.; Chudárková, A.; Hulva, P.; Horáček, I. Bats (Mammalia: Chiroptera) of the Eastern Mediterranean and Middle East. Part 7. The bat fauna of Crete, Greece. Acta Soc. Zool. Bohem. 2008, 72, 105–190. [Google Scholar]
- Bray, T.C.; Mohammed, O.B.; Alagaili, A.N. Phylogenetic and demographic insights into Kuhl’s Pipistrelle, Pipistrellus kuhlii, in the Middle East. PLoS ONE 2013, 8, e57306. [Google Scholar] [CrossRef]
- Andriollo, T.; Naciri, Y.; Ruedi, M. Two mitochondrial barcodes for one biological species: The case of European Kuhl’s pipistrelles (Chiroptera). PLoS ONE 2015, 10, e0134881. [Google Scholar] [CrossRef]
- Barratt, E.M.; Deaville, R.; Burland, T.M.; Bruford, M.W.; Jones, G.; Racey, P.A.; Wayne, R.K. DNA answers the call of pipistrelle bat species. Nature 1997, 387, 138–139. [Google Scholar] [CrossRef]
- Benda, P.; Hulva, P.; Gaisler, J. Systematic status of African populations of Pipistrellus pipistrellus complex (Chiroptera: Vespertilionidae), with a description of a new species from Cyrenaica, Libya. Acta Chiropterologica 2004, 6, 193–217. [Google Scholar] [CrossRef]
- Dietz, C.; von Helversen, O. Illustrated Identification Key to the Bats of Europe; Electronic Publication Version 1.0. Released 15.12.2004; Dietz & von Helversen: Tuebingen, Germany; Erlangen, Germany, 2004. [Google Scholar]
- Rainho, A.; Marques, J.T.; Palmeirim, J.M. Os Morcegos Dos Arquipélagos Dos Açores e Da Madeira: Um Contributo Para a Sua Conservação; Instituto da Conservaçao da Natureza: Lisbon, Portugal, 2002. [Google Scholar]
- Teixeira, S. Os morcegos (Mammalia: Chiroptera) do Arquipélago da Madeira: Identificação Morfológica e Acústica. Um Contributo para a sua Conservação. Tese de Licenciatura, Universidade da Madeira, Funchal, Portugal, 2005; 50p. [Google Scholar]
- Medeiros, F.; Fonseca, A.; Gouveia, C.; Nunes, R.; Vieira, J.; Veiga, M.; Nóia, M.; Fraga, M. Conservação dos Vertebrados Terrestres das Flores e do Corvo. XIII Expedição Científica do Departamento de Biologia—Flores e Corvo 2007. Rel. Com. Dep. Biol. 2008, 35, 49–58. Available online: http://hdl.handle.net/10400.3/713 (accessed on 8 July 2023).
- Fonseca, A.; Gonçalves, V.; Medeiros, F. Estudo da Fauna Chiroptera da Ilha de Santa Maria. XIV Expedição Científica do Departamento de Biologia—Santa Maria 2009. Rel. Com. Dep. Biol. 2009, 36, 59–63. [Google Scholar]
- Teixeira, S.; Jesus, J. Echolocation calls of bats from Madeira Island: Acoustic characterization and implications for surveys. Acta Chiropterologica 2009, 11, 183–190. [Google Scholar] [CrossRef]
- Jesus, J.; Teixeira, S.; Teixeira, D.; Freitas, T.; Russo, D. Vertebrados Terrestres Autóctones dos Arquipélagos da Madeira e Selvagens—Répteis e Mamíferos. Colecção Biodiversidade Madeirense. Avaliação Conservação.; Direcção Regional do Ambiente, SRARN Funchal, Madeira: Madeira, Portugal, 2009; Volume 6, pp. 54–62. [Google Scholar]
- Teixeira, S. The Madeira Pipistrelle Pipistrellus maderensis. In Bat Calls of Britain and Europe: A Guide to Species Identification; Pelagic Publishing: Exeter, UK, 2019; pp. 373–382. [Google Scholar]
- Rocha, R. Madeiran pipistrelle Pipistrellus maderensis (Dobson, 1878). In Handbook of the Mammals of Europe; Hackländer, K., Zachos, F.E., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Trujillo, D. Murciélagos de las Islas Canarias; Ministerio de Agricultura Pesca y Alimentación, ICONA: Madrid, España, 1991. [Google Scholar]
- Pestano, J.; Brown, R.P.; Suárez, N.M.; Fajardo, S. Phylogeography of pipistrelle-like bats within the Canary Islands, based on mtDNA sequences. Mol. Phylogenet. Evol. 2003, 26, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Trujillo, D.; Gonzalez, C. Pipistrellus maderensis (Dobson, 1878) (Chiroptera: Vespertilionidae), una nueva adicion a la fauna de las islas Azores (oceano Atlantico). Vieraea 2011, 39, 215–218. [Google Scholar] [CrossRef]
- Rainho, A. Positive Interactions Drive Bat Distribution in a Remote Oceanic Archipelago (Azores, Portugal). Diversity 2022, 14, 17. [Google Scholar] [CrossRef]
- Carvalho, A.M.G.; Brandão, J.M. Geologia do Arquipélago da Madeira; National Museum of Natural History and Science: Lisboa, Portugal, 1991. [Google Scholar]
- Geldmacher, J.; Hoernle, K. The 72 Ma geochemical evolution of the Madeira hotspot (eastern North Atlantic): Recycling of palaeozoic (≤500 Ma) basaltic and gabbroic crust. Earth Planet. Sci. Lett. 2000, 183, 73–92. [Google Scholar] [CrossRef]
- A List of the Terrestrial Fungi, Flora and Fauna of Madeira and Selvagens Archipelagos; Borges, P.A.V., Abreu, C., Aguiar, A.M.F., Carvalho, P., Jardim, R., Melo, I., Oliveira, P., Sérgio, C., Serrano, A.R.M., Vieira, P., Eds.; Direcção Regional do Ambiente da Madeira: Madeira, Portugal; Universidade dos Açores, Funchal and Angra do Heroísmo: Madeira, Portugal, 2008. [Google Scholar]
- Martín Esquivel, J.L.; Arechavaleta, M.; Borges, P.A.V.; Faria, B. Top 100. Las 100 Especies Amenazadas Prioritarias de Gestión en la Región Europea Biogeográfica de la Macaronesia; Consejería de Medio Ambiente y Ordenación Territorial, Gobierno de Canárias: Canarias, España, 2008. [Google Scholar]
- Silva, L.; Ojeda Land, E.; Rodriguez Luengo, J.L.; Borges, P.A.; Oliveira, P.; Jardim, R. Invasive alien species in Macaronesia. In Invasive Terrestrial Flora & Fauna of Macaronesia. TOP 100 in Azores, Madeira and Canaries; ARENA: Azores, Portugal, 2008; pp. 159–165. [Google Scholar]
- Whittaker, R.J.; Fernández-Palacios, J.M. Island Biogeography: Ecology, Evolution, and Conservation; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Florencio, M.; Patiño, J.; Nogué, S.; Traveset, A.; Borges, P.A.V.; Schaefer, H.; Amorim, I.R.; Arnedo, M.; Ávila, S.P.; Cardoso, P.; et al. Macaronesia as a Fruitful Arena for Ecology, Evolution, and Conservation Biology. Front. Ecol. Evol. 2021, 9, 718169. [Google Scholar] [CrossRef]
- Castanho, R.A.; Naranjo Gomez, J.M.; Vulevic, A.; Couto, G. The land-use change dynamics based on the CORINE Data in the period 1990–2018 in the European archipelagos of the Macaronesia Region: Azores, Canary Islands, and Madeira. ISPRS Int. J. Geo-Inf. 2021, 10, 342. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree, ver. 1.3. 1. Institute of Evolutionary Biology, University of Edinburgh, Edinburgh. 2009. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 24 June 2023).
- Jesus, J.; Teixeira, S.; Freitas, T.; Teixeira, D.; Brehm, A. Genetic identity of Pipistrellus maderensis from the Madeira archipelago: A first assessment, and implications for conservation. Hystrix 2013, 24, 177–180. [Google Scholar]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Peer J. 2017, 5, e4095. [Google Scholar] [CrossRef] [PubMed]
- ArcGIS v., 10.8. Available online: http://www.esri.com/software/arcgis (accessed on 24 June 2023).
- Atlantis—Pipistrellus maderensis (Dobson, 1878). Banco de datos de Biodiversidad de Canarias. 2023. Available online: biodiversidadcanarias.es (accessed on 18 May 2023).
- González-Dionis, J.; Castillo Ruiz, C.; Cruzado-Caballero, P.; Cadavid-Melero, E.D.; Crespo, V. First study of the bat fossil record of the mid-Atlantic volcanic islands. Earth Environ. Sci. Trans. R. Soc. Edinb. 2021, 113, 13–27. [Google Scholar] [CrossRef]
- Teixeira, S. Relatório de Progresso Intercalar do Plano de Monitorização dos Descritores Ecológicos do Projecto de Ampliação do Aproveitamento Hidroeléctrico da Calheta; Descritor Quirópteros. Fase de Construção—Campanha de Primavera/Verão; Relatório elaborado para a EEM; Madeira Fauna & Flora: Funchal, Madeira Julho de, Portugal, 2018; 29p. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Graham, C.H.; Master, L.L.; Albert, D.L. The effect of sample size and species characteristics on performance of different species distribution modeling methods. Ecography 2006, 29, 773–785. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jimenez-Valverde, A.; Hortal, J. The uncertain nature of absences and their importance in species distribution modelling. Ecography 2010, 33, 103–114. [Google Scholar] [CrossRef]
- Araújo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The Meaning and Use of the Area under a Receiver Operating Characteristic (ROC) Curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the Accuracy of Species Distribution Models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Di Febbraro, M.; Martinoli, A.; Russo, D.; Preatoni, D.; Bertolino, S. Modelling the Effects of Climate Change on the Risk of Invasion by Alien Squirrels. Hystrix 2016, 27, 1–8. [Google Scholar] [CrossRef]
- Marmion, M.; Parviainen, M.; Luoto, M.; Heikkinen, R.K.; Thuiller, W. Evaluation of Consensus Methods in Predictive Species Distribution Modelling. Divers. Distrib. 2009, 15, 59–69. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Algar, A.C.; Kharouba, H.M.; Young, E.R.; Kerr, J.T. Predicting the future of species diversity: Macroecological theory, climate change, and direct tests of alternative forecasting methods. Ecography 2009, 32, 22–33. [Google Scholar] [CrossRef]
- Dubuis, A.; Pottier, J.; Rion, V.; Pellissier, L.; Theurillat, J.P.; Guisan, A. Predicting spatial patterns of plant species richness: A comparison of direct macroecological and species stacking modelling approaches. Divers. Distrib. 2011, 17, 1122–1131. [Google Scholar] [CrossRef]
- Smeraldo, S.; Di Febbraro, M.; Bosso, L.; Flaquer, C.; Guixé, D.; Lisón, F.; Meschede, A.; Juste, J.; Prüger, J.; Puig-Montserrat, X.; et al. Ignoring seasonal changes in the ecological niche of non-migratory species may lead to biases in potential distribution models: Lessons from bats. Biodivers. Conserv. 2018, 27, 2425–2441. [Google Scholar] [CrossRef]
- Randin, C.F.; Dirnb¨ock, T.; Dullinger, S.; Zimmermann, N.E.; Zappa, M.; Guisan, A. Are niche-based species distribution models transferable in space? J. Biogeogr. 2006, 33, 1689–1703. [Google Scholar] [CrossRef]
- Williams, J.W.; Jackson, S.T.; Kutzbach, J.E. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl. Acad. Sci. USA 2007, 104, 5738–5742. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, M.C.; Hargrove, W.W. The projection of species distribution models and the problem of non-analog climate. Biodivers. Conserv. 2009, 18, 2255–2261. [Google Scholar] [CrossRef]
- Owens, H.L.; Campbell, L.P.; Dornak, L.L.; Saupe, E.E.; Barve, N.; Sober’on, J.; Peterson, A.T. Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecol. Model. 2013, 263, 10–18. [Google Scholar] [CrossRef]
- Yates, K.L.; Bouchet, P.J.; Caley, M.J.; Mengersen, K.; Randin, C.F.; Parnell, S.; Dormann, C.F. Outstanding challenges in the transferability of ecological models. Trends Ecol. Evol. 2018, 33, 790–802. [Google Scholar] [CrossRef] [PubMed]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- Bouchet, P.J.; Miller, D.L.; Roberts, J.J.; Mannocci, L.; Harris, C.M.; Thomas, L. Dsmextra: Extrapolation assessment tools for density surface models. Methods Ecol. Evol. 2020, 11, 1464–1469. [Google Scholar] [CrossRef]
- Mesgaran, M.B.; Cousens, R.D.; Webber, B.L. Here be dragons: A tool for quantifying novelty due to covariate range and correlation change when projecting species distribution models. Divers. Distrib. 2014, 20, 1147–1159. [Google Scholar] [CrossRef]
- Teixeira, S. Chiroptera. In Listagem dos Fungos, Flora e Fauna Terrestres dos Arquipélagos da Madeira e Selvagens; Borges, P.A.V., Abreu, C., Aguiar, A.M.F., Carvalho, P., Jardim, R., Melo, I., Oliveira, P., Sérgio, C., Serrano, A.R.M., Vieira, P., Eds., Eds.; Direcção Regional do Ambiente da Madeira e Universidade dos Açores: Funchal e Angra do Heroísmo, Portugal, 2008; pp. 366–368. [Google Scholar]
- Pearman, P.B.; D’Amen, M.; Graham, C.H.; Thuiller, W.; Zimmermann, N.E. Within -taxon niche structure: Niche conservatism, divergence and predicted effects of climate change. Ecography 2010, 33, 990–1003. [Google Scholar] [CrossRef]
- D’Amen, M.; Zimmermann, N.E.; Pearman, P.B. Conservation of phylogeographic lineages under climate change. Glob. Ecol. Biogeogr. 2013, 22, 93–104. [Google Scholar] [CrossRef]
- Lecocq, T.; Harpke, A.; Rasmont, P.; Schweiger, O. Integrating intraspecific differentiation in species distribution models: Consequences on projections of current and future climatically suitable areas of species. Divers. Distrib. 2019, 25, 1088–1100. [Google Scholar] [CrossRef]






| Archipelago | Island | Locality | Code/ Haplotype | Accession Number | Source |
|---|---|---|---|---|---|
| Canary | |||||
| El Hierro | Frontera | PM#06 | AJ426615 | [69] | |
| Guarazoca | PM#08 | AJ426617 | [69] | ||
| La Gomera | Los Acevinos | PM#06 | AJ426615 | [69] | |
| Agulo | PM#06 | AJ426615 | [69] | ||
| Alajero | PM#06 | AJ426615 | [69] | ||
| Tamargada | PM#05 | AJ426614 | [69] | ||
| Juego de Bolas | PM#07 | AJ426616 | [69] | ||
| Vallehermoso | PM#06 | AJ426615 | [69] | ||
| La Palma | Mirca | PM#09 | AJ426618 | [69] | |
| Mirca | PM#11 | AJ426632 | [69] | ||
| El Paso | PM#10 | n.a. | [69] | ||
| Los Sauces | PM#10 | n.a. | [69] | ||
| Tenerife | Cueva Fea de Arico | PM#01 | AJ426610 | [69] | |
| Cueva Fea de Arico | PM#03 | AJ426612 | [69] | ||
| Cueva Fea de Arico | PM#04 | AJ426613 | [69] | ||
| La Orotava | PM#01 | AJ426610 | [69] | ||
| Almáciga | PM#02 | AJ426611 | [69] | ||
| La Esperanza | PM#03 | AJ426612 | [69] | ||
| Madeira | |||||
| Madeira | Sta. Porto Moniz | PmSanta1 | KC520774 | [83] | |
| Sta. Porto Moniz | PmSanta2 | KC520773 | [83] | ||
| Sta. Porto Moniz | PmSanta3 | KC520770 | [83] | ||
| Chão da Ribeira | Pm2Chao | KC520771 | [83] | ||
| Chão da Ribeira | Pm3Chao | KC520772 | [83] |
| Archipelago | Island | Number of Records | Source |
|---|---|---|---|
| Azores | |||
| Corvo | 1 | [60] | |
| 2 | [71] | ||
| Flores | 6 | [60] | |
| 8 | [71] | ||
| 1 | [62] | ||
| Graciosa | 1 | [71] | |
| Pico | 2 | [71] | |
| Santa Maria | 9 | [60] | |
| 15 | [63] | ||
| 14 | [70] | ||
| São Jorge | 5 | [71] | |
| Canary | |||
| El Hierro | 3 | [68] | |
| 5 | [69] | ||
| 3 | [86] | ||
| La Gomera | 5 | [68] | |
| 6 | [69] | ||
| 16 | [86] | ||
| La Palma | 5 | [68] | |
| 3 | [69] | ||
| 14 | [86] | ||
| Tenerife | 5 | [68] | |
| 4 | [69] | ||
| 1 | [87] | ||
| 21 | [86] | ||
| Madeira | |||
| Madeira | 386 | [61] | |
| 65 | [64] | ||
| 1 | [88] | ||
| Porto Santo | 3 | [60] | |
| 3 | [61] | ||
| Deserta Grande | 1 | Silva (pers. obs.) |
| Model | AUC (sd) | TSS (sd) |
|---|---|---|
| GBM | 0.96 (0.02) | 0.85 (0.05) |
| GLM | 0.94 (0.02) | 0.80 (0.04) |
| RF | 0.97 (0.01) | 0.84 (0.05) |
| Variable | Univariate Extrapolation (%) | Combinatorial Extrapolation (%) |
|---|---|---|
| BIO2 | 53 | 0 |
| BIO12 | 36 | 0 |
| Artificial illumination | 0.6 | 0 |
| BIO6 | 0.4 | 0 |
| Euclidean distance from mixed agricultural lands | 0.05 | 2.4 |
| Euclidean distance from hydrographical elements | 0.03 | 0.26 |
| Model | AUC (sd) | TSS (sd) |
|---|---|---|
| Azores | 0.87 (0.08) | 0.72 (0.15) |
| Canary Islands | 0.91 (0.06) | 0.77 (0.13) |
| Madeira | 0.88 (0.04) | 0.71 (0.08) |
| Azores Archipelago | Canary Archipelago | Madeira Archipelago |
|---|---|---|
| BIO3 | BIO2 | BIO2 |
| BIO6 | BIO12 | BIO12 |
| Artificial illumination | Euclidean distance from mixed agricultural lands | Artificial illumination |
| Euclidean distance from mixed agricultural lands | BIO6 | |
| Euclidean distance from hydrographical elements | Euclidean distance from mixed agricultural lands | |
| Euclidean distance from hydrographical elements |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, S.; Smeraldo, S.; Russo, D. Unveiling the Potential Distribution of the Highly Threatened Madeira Pipistrelle (Pipistrellus maderensis): Do Different Evolutionary Significant Units Exist? Biology 2023, 12, 998. https://doi.org/10.3390/biology12070998
Teixeira S, Smeraldo S, Russo D. Unveiling the Potential Distribution of the Highly Threatened Madeira Pipistrelle (Pipistrellus maderensis): Do Different Evolutionary Significant Units Exist? Biology. 2023; 12(7):998. https://doi.org/10.3390/biology12070998
Chicago/Turabian StyleTeixeira, Sérgio, Sonia Smeraldo, and Danilo Russo. 2023. "Unveiling the Potential Distribution of the Highly Threatened Madeira Pipistrelle (Pipistrellus maderensis): Do Different Evolutionary Significant Units Exist?" Biology 12, no. 7: 998. https://doi.org/10.3390/biology12070998
APA StyleTeixeira, S., Smeraldo, S., & Russo, D. (2023). Unveiling the Potential Distribution of the Highly Threatened Madeira Pipistrelle (Pipistrellus maderensis): Do Different Evolutionary Significant Units Exist? Biology, 12(7), 998. https://doi.org/10.3390/biology12070998








