CpG ODN/Mangiferin Dual Delivery through Calcium Alginate Hydrogels Inhibits Immune-Mediated Osteoclastogenesis and Promotes Alveolar Bone Regeneration in Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
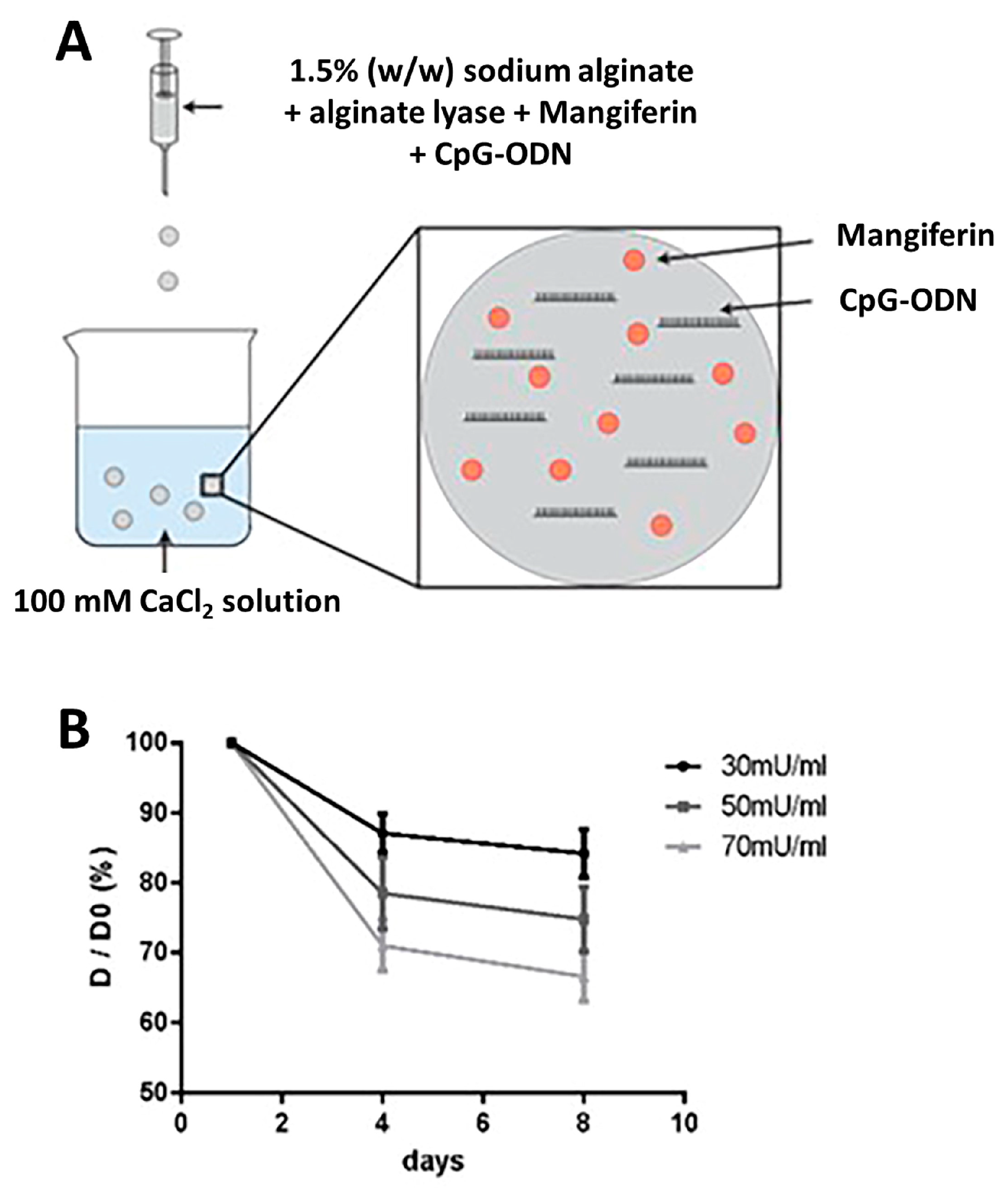
2.1. Fabrication of Controlled Degradable Microbeads
2.2. Hydrogel Microbeads Encapsulated with CpG ODN/MAG
2.3. Bacterial Culture
2.4. Bacterial Live/Dead Cell Detection
2.5. Splenocytes Cell Isolation and Culture
2.6. Co-Culture of Splenocytes/RAW264.7 Cells
2.7. qPCR
2.8. Tartrate-Resistant Acid Phosphatase (TRAP) Staining
2.9. Induction of Alveolar Bone Defects in Mice
2.10. Micro CT
2.11. Histological Analysis
2.12. Statistical Analysis
3. Results
3.1. Determination of the Degradation Rate of CpG ODN/MAG-Containing Microbeads
3.2. Mangiferin Inhibits P. gingivalis Survival
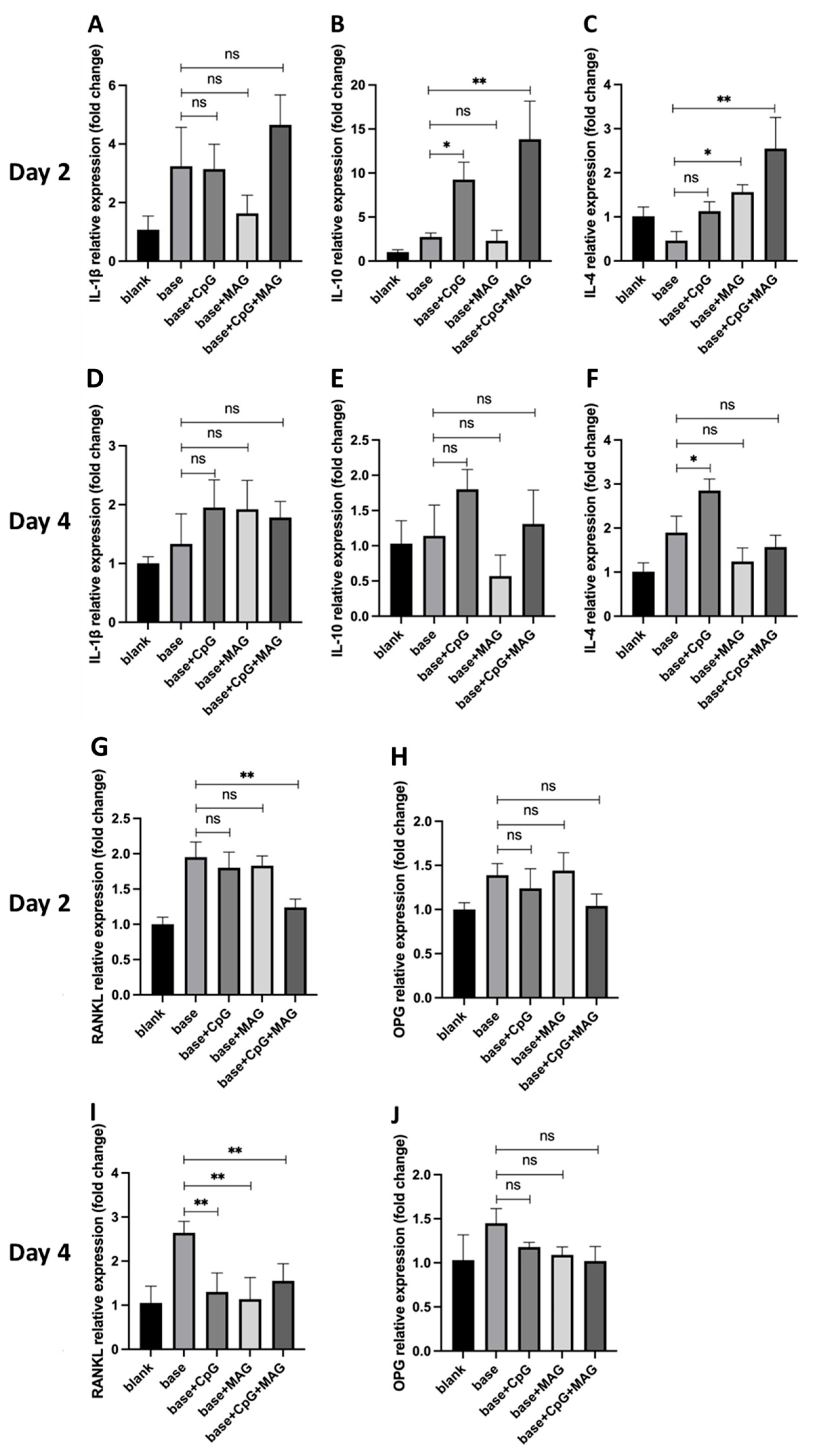
3.3. CpG ODN + MAG Microbeads Modulated the Expressions of Inflammatory Cytokines and RANKL in P. gingivalis-Challenged Splenocytes
3.4. CpG ODN + MAG Microbeads Reduce Osteoclast Activation
3.5. CpG ODN/Mangiferin Microbeads Enhance Alveolar Bone Regeneration in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Drissi, H.; Sanjay, A. The Multifaceted Osteoclast; Far and Beyond Bone Resorption. J. Cell Biochem. 2016, 117, 1753–1756. [Google Scholar] [CrossRef] [PubMed]
- Katsimbri, P. The biology of normal bone remodelling. Eur. J. Cancer Care 2017, 26, e12740. [Google Scholar] [CrossRef]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Y. The Role of the Immune Microenvironment in Bone Regeneration. Int. J. Med. Sci. 2021, 18, 3697–3707. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef]
- Gilbert, L.; He, X.; Farmer, P.; Boden, S.; Kozlowski, M.; Rubin, J.; Nanes, M.S. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology 2000, 141, 3956–3964. [Google Scholar] [CrossRef]
- Cavagis, A.; Takamori, E.; Granjeiro, J.; Oliveira, R.; Ferreira, C.; Peppelenbosch, M.; Zambuzzi, W. TNFα contributes for attenuating both Y397FAK and Y416Src phosphorylations in osteoblasts. Oral Dis. 2014, 20, 780–786. [Google Scholar] [CrossRef]
- Lorenzo, J. Interactions between immune and bone cells: New insights with many remaining questions. J. Clin. Investig. 2000, 106, 749–752. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Solakoglu, Ö.; Heydecke, G.; Amiri, N.; Anitua, E. The use of plasma rich in growth factors (PRGF) in guided tissue regeneration and guided bone regeneration. A review of histological, immunohistochemical, histomorphometrical, radiological and clinical results in humans. Ann. Anat. 2020, 231, 151528. [Google Scholar] [CrossRef]
- Boda, S.K.; Almoshari, Y.; Wang, H.; Wang, X.; Reinhardt, R.A.; Duan, B.; Wang, D.; Xie, J. Mineralized nanofiber segments coupled with calcium-binding BMP-2 peptides for alveolar bone regeneration. Acta Biomater. 2019, 85, 282–293. [Google Scholar] [CrossRef]
- Schorn, L.; Sproll, C.; Ommerborn, M.; Naujoks, C.; Kübler, N.R.; Depprich, R. Vertical bone regeneration using rhBMP-2 and VEGF. Head Face Med. 2017, 13, 11. [Google Scholar] [CrossRef]
- Pandya, M.; Saxon, M.; Bozanich, J.; Tillberg, C.; Luan, X.; Diekwisch, T.G.H. The Glycoprotein/Cytokine Erythropoietin Promotes Rapid Alveolar Ridge Regeneration In Vivo by Promoting New Bone Extracellular Matrix Deposition in Conjunction with Coupled Angiogenesis/Osteogenesis. Int. J. Mol. Sci. 2021, 22, 2788. [Google Scholar] [CrossRef]
- Lee, J.; Lee, M.S.; Jeoung, D.I.; Kim, Y.M.; Lee, H. Promoter CpG-Site Methylation of the KAI1 Metastasis Suppressor Gene Contributes to Its Epigenetic Repression in Prostate Cancer. Prostate 2017, 77, 350–360. [Google Scholar] [CrossRef]
- Li, H.T.; Chen, Z.G.; Liu, H.; Ye, J.; Zou, X.L.; Wang, Y.H.; Yang, H.L.; Meng, P.; Zhang, T.T. Treatment of allergic rhinitis with CpG oligodeoxynucleotides alleviates the lower airway outcomes of combined allergic rhinitis and asthma syndrome via a mechanism that possibly involves in TSLP. Exp. Lung Res. 2016, 42, 322–333. [Google Scholar] [CrossRef]
- Thapa, S.; Cader, M.S.; Murugananthan, K.; Nagy, E.; Sharif, S.; Czub, M.; Abdul-Careem, M.F. In ovo delivery of CpG DNA reduces avian infectious laryngotracheitis virus induced mortality and morbidity. Viruses 2015, 7, 1832–1852. [Google Scholar] [CrossRef]
- El Kebir, D.; József, L.; Filep, J.G. Neutrophil recognition of bacterial DNA and Toll-like receptor 9-dependent and -independent regulation of neutrophil function. Arch. Immunol. Ther. Exp. 2008, 56, 41–53. [Google Scholar] [CrossRef]
- Sanjuan, M.A.; Rao, N.; Lai, K.T.; Gu, Y.; Sun, S.; Fuchs, A.; Fung-Leung, W.P.; Colonna, M.; Karlsson, L. CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. J. Cell Biol. 2006, 172, 1057–1068. [Google Scholar] [CrossRef]
- Yu, P.; Hu, Y.; Liu, Z.; Kawai, T.; Taubman, M.A.; Li, W.; Han, X. Local Induction of B Cell Interleukin-10 Competency Alleviates Inflammation and Bone Loss in Ligature-Induced Experimental Periodontitis in Mice. Infect. Immun. 2017, 85, e00645-16. [Google Scholar] [CrossRef] [PubMed]
- Dar, A.; Faizi, S.; Naqvi, S.; Roome, T.; Zikr-ur-Rehman, S.; Ali, M.; Firdous, S.; Moin, S.T. Analgesic and antioxidant activity of mangiferin and its derivatives: The structure activity relationship. Biol. Pharm. Bull. 2005, 28, 596–600. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.H.; Arshad, M.U.; Sultan, M.T. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 2017, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, Y.; Mano, H.; Nakatani, S.; Shimizu, J.; Kataoka, A.; Ogura, K.; Kimira, Y.; Ebata, M.; Wada, M. Mangiferin positively regulates osteoblast differentiation and suppresses osteoclast differentiation. Mol. Med. Rep. 2017, 16, 1328–1332. [Google Scholar] [CrossRef] [PubMed]
- Kearney, C.J.; Mooney, D.J. Macroscale delivery systems for molecular and cellular payloads. Nat. Mater. 2013, 12, 1004–1017. [Google Scholar] [CrossRef]
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D.; Santra, K. Screening of polysaccharides from tamarind, fenugreek and jackfruit seeds as pharmaceutical excipients. Int. J. Biol. Macromol. 2015, 79, 756–760. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D.; Santra, K. Development of pectinate-ispagula mucilage mucoadhesive beads of metformin HCl by central composite design. Int. J. Biol. Macromol. 2014, 66, 203–211. [Google Scholar] [CrossRef]
- Kim, B.S.; Mooney, D.J. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol. 1998, 16, 224–230. [Google Scholar] [CrossRef]
- Breguet, V.; von Stockar, U.; Marison, I.W. Characterization of alginate lyase activity on liquid, gelled, and complexed states of alginate. Biotechnol. Prog. 2007, 23, 1223–1230. [Google Scholar] [CrossRef]
- Formo, K.; Aarstad, O.A.; Skjåk-Bræk, G.; Strand, B.L. Lyase-catalyzed degradation of alginate in the gelled state: Effect of gelling ions and lyase specificity. Carbohydr. Polym. 2014, 110, 100–106. [Google Scholar] [CrossRef]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. Int. J. Pharm. 2019, 569, 118627. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Eskandari, M.; Ghazali, Z.S.; Ghazali, H.S. Cell encapsulation in alginate-based microgels using droplet microfluidics; a review on gelation methods and applications. Biomed. Phys. Eng. Express 2022, 8, 022001. [Google Scholar] [CrossRef]
- Rajmohan, D.; Bellmer, D. Characterization of Spirulina-Alginate Beads Formed Using Ionic Gelation. Int. J. Food Sci. 2019, 2019, 7101279. [Google Scholar] [CrossRef]
- Aguirre Calvo, T.; Santagapita, P. Physicochemical Characterization of Alginate Beads Containing Sugars and Biopolymers. J. Qual. Reliab. Eng. 2016, 2016, 9184039. [Google Scholar] [CrossRef]
- Qiao, P.; Wang, J.; Xie, Q.; Li, F.; Dong, L.; Xu, T. Injectable calcium phosphate-alginate-chitosan microencapsulated MC3T3-E1 cell paste for bone tissue engineering in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4633–4639. [Google Scholar] [CrossRef]
- Nishimura, K.; Shindo, S.; Movila, A.; Kayal, R.; Abdullah, A.; Savitri, I.J.; Ikeda, A.; Yamaguchi, T.; Howait, M.; Al-Dharrab, A.; et al. TRAP-positive osteoclast precursors mediate ROS/NO-dependent bactericidal activity via TLR4. Free Radic. Biol. Med. 2016, 97, 330–341. [Google Scholar] [CrossRef]
- Mazzinelli, E.; Favuzzi, I.; Arcovito, A.; Castagnola, R.; Fratocchi, G.; Mordente, A.; Nocca, G. Oral Mucosa Models to Evaluate Drug Permeability. Pharmaceutics 2023, 15, 1559. [Google Scholar] [CrossRef]
- Bai, L.; Tao, G.; Feng, M.; Xie, Y.; Cai, S.; Peng, S.; Xiao, J. Hydrogel Drug Delivery Systems for Bone Regeneration. Pharmaceutics 2023, 15, 1334. [Google Scholar] [CrossRef]
- Amato, M.; Santonocito, S.; Polizzi, A.; Tartaglia, G.M.; Ronsivalle, V.; Viglianisi, G.; Grippaudo, C.; Isola, G. Local Delivery and Controlled Release Drugs Systems: A New Approach for the Clinical Treatment of Periodontitis Therapy. Pharmaceutics 2023, 15, 1312. [Google Scholar] [CrossRef]
- Sarkar, N.; Maity, A. Chapter 12—Modified alginates in drug delivery. In Tailor-Made Polysaccharides in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 291–325. [Google Scholar]
- Abourehab, M.A.S.; Rajendran, R.R.; Singh, A.; Pramanik, S.; Shrivastav, P.; Ansari, M.J.; Manne, R.; Amaral, L.S.; Deepak, A. Alginate as a Promising Biopolymer in Drug Delivery and Wound Healing: A Review of the State-of-the-Art. Int. J. Mol. Sci. 2022, 23, 9035. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, A.; Dar, A.H.; Pandey, V.K.; Shams, R.; Khan, S.; Panesar, P.S.; Kennedy, J.F.; Fayaz, U.; Khan, S.A. Recent insights into polysaccharide-based hydrogels and their potential applications in food sector: A review. Int. J. Biol. Macromol. 2022, 213, 987–1006. [Google Scholar] [CrossRef] [PubMed]
- Leslie, S.K.; Cohen, D.J.; Sedlaczek, J.; Pinsker, E.J.; Boyan, B.D.; Schwartz, Z. Controlled release of rat adipose-derived stem cells from alginate microbeads. Biomaterials 2013, 34, 8172–8184. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.T.; Stilhano, R.S.; Silva, E.A. Enzymatically degradable alginate hydrogel systems to deliver endothelial progenitor cells for potential revasculature applications. Biomaterials 2018, 179, 109–121. [Google Scholar] [CrossRef]
- Klinman, D.M.; Klaschik, S.; Sato, T.; Tross, D. CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases. Adv. Drug Deliv. Rev. 2009, 61, 248–255. [Google Scholar] [CrossRef]
- Jin, Y.; Zhuang, Y.; Dong, X.; Liu, M. Development of CpG oligodeoxynucleotide TLR9 agonists in anti-cancer therapy. Expert. Rev. Anticancer Ther. 2021, 21, 841–851. [Google Scholar] [CrossRef]
- Jacquet, A. Nucleic acid vaccines and CpG oligodeoxynucleotides for allergen immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 569–575. [Google Scholar] [CrossRef]
- Klier, J.; May, A.; Fuchs, S.; Schillinger, U.; Plank, C.; Winter, G.; Gehlen, H.; Coester, C. Immunostimulation of bronchoalveolar lavage cells from recurrent airway obstruction-affected horses by different CpG-classes bound to gelatin nanoparticles. Vet. Immunol. Immunopathol. 2011, 144, 79–87. [Google Scholar] [CrossRef]
- Demento, S.L.; Bonafé, N.; Cui, W.; Kaech, S.M.; Caplan, M.J.; Fikrig, E.; Ledizet, M.; Fahmy, T.M. TLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitis. J. Immunol. 2010, 185, 2989–2997. [Google Scholar] [CrossRef]
- Heikenwalder, M.; Polymenidou, M.; Junt, T.; Sigurdson, C.; Wagner, H.; Akira, S.; Zinkernagel, R.; Aguzzi, A. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat. Med. 2004, 10, 187–192. [Google Scholar] [CrossRef]
- Montamat, G.; Leonard, C.; Poli, A.; Klimek, L.; Ollert, M. CpG Adjuvant in Allergen-Specific Immunotherapy: Finding the Sweet Spot for the Induction of Immune Tolerance. Front. Immunol. 2021, 12, 590054. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Lin, J.; Hu, Y.; Zhao, Q.; Kawai, T.; Taubman, M.A.; Han, X. B10 Cells Alleviate Periodontal Bone Loss in Experimental Periodontitis. Infect. Immun. 2017, 85, e00335-17. [Google Scholar] [CrossRef]
- Ono, T.; Takayanagi, H. Osteoimmunology in Bone Fracture Healing. Curr. Osteoporos Rep. 2017, 15, 367–375. [Google Scholar] [CrossRef]
- Gungor, B.; Yagci, F.C.; Tincer, G.; Bayyurt, B.; Alpdundar, E.; Yildiz, S.; Ozcan, M.; Gursel, I.; Gursel, M. CpG ODN nanorings induce IFNα from plasmacytoid dendritic cells and demonstrate potent vaccine adjuvant activity. Sci. Transl. Med. 2014, 6, 235ra261. [Google Scholar] [CrossRef]
- Qu, S.; Wang, W.; Li, D.; Li, S.; Zhang, L.; Fu, Y.; Zhang, N. Mangiferin inhibits mastitis induced by LPS via suppressing NF-ĸB and NLRP3 signaling pathways. Int. Immunopharmacol. 2017, 43, 85–90. [Google Scholar] [CrossRef]
- Chan, G.; Mooney, D.J. Ca(2+) released from calcium alginate gels can promote inflammatory responses in vitro and in vivo. Acta Biomater. 2013, 9, 9281–9291. [Google Scholar] [CrossRef]
- Feng, M.; Wei, S.; Zhang, S.; Yang, Y. Anti-Inflammation and Anti-Pyroptosis Activities of Mangiferin via Suppressing NF-κB/NLRP3/GSDMD Signaling Cascades. Int. J. Mol. Sci. 2022, 23, 124. [Google Scholar] [CrossRef]
- Wang, X.; Yuwen, T.; Yanqin, T. Mangiferin Inhibits Inflammation and Cell Proliferation, and Activates Proapoptotic Events via NF-κB Inhibition in DMBA-Induced Mammary Carcinogenesis in Rats. J. Environ. Pathol. Toxicol. Oncol. 2021, 40, 1–9. [Google Scholar] [CrossRef]
- Li, H.; Wang, Q.; Ding, Y.; Bao, C.; Li, W. Mangiferin ameliorates Porphyromonas gingivalis-induced experimental periodontitis by inhibiting phosphorylation of nuclear factor-κB and Janus kinase 1-signal transducer and activator of transcription signaling pathways. J. Periodontal. Res. 2017, 52, 1–7. [Google Scholar] [CrossRef]
- Ang, E.; Liu, Q.; Qi, M.; Liu, H.G.; Yang, X.; Chen, H.; Zheng, M.H.; Xu, J. Mangiferin attenuates osteoclastogenesis, bone resorption, and RANKL-induced activation of NF-κB and ERK. J. Cell Biochem. 2011, 112, 89–97. [Google Scholar] [CrossRef]
- Gu, Y.; Zhang, L.; Bai, Y. Mangiferin promotes the osteogenic differentiation of human periodontal ligament stem cells via TGF-β/SMAD2 signaling. Mol. Med. Rep. 2022, 26, 266. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.; Koduru, J.R.; Rautray, T.R. Mangiferin-Enriched Mn-Hydroxyapatite Coupled with β-TCP Scaffolds Simultaneously Exhibit Osteogenicity and Anti-Bacterial Efficacy. Materials 2023, 16, 2206. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liao, H.; Bao, C.; Xiao, Y.; Wang, Q. Preparation and Evaluations of Mangiferin-Loaded PLGA Scaffolds for Alveolar Bone Repair Treatment Under the Diabetic Condition. AAPS PharmSciTech 2017, 18, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, C.; Fu, L.; Gong, X.; Dou, C.; Cao, Z.; Quan, H.; Li, J.; Kang, F.; Dai, J.; et al. Mangiferin enhances endochondral ossification-based bone repair in massive bone defect by inducing autophagy through activating AMP-activated protein kinase signaling pathway. Faseb J. 2018, 32, 4573–4584. [Google Scholar] [CrossRef]
- Khokhani, P.; Rahmani, N.R.; Kok, A.; Öner, F.C.; Alblas, J.; Weinans, H.; Kruyt, M.C.; Croes, M. Use of Therapeutic Pathogen Recognition Receptor Ligands for Osteo-Immunomodulation. Materials 2021, 14, 1119. [Google Scholar] [CrossRef]
- Fomenko, E.V.; Chi, Y. Mangiferin modulation of metabolism and metabolic syndrome. Biofactors 2016, 42, 492–503. [Google Scholar] [CrossRef]
- Aswal, S.; Kumar, A.; Chauhan, A.; Semwal, R.B.; Kumar, A.; Semwal, D.K. A Molecular Approach on the Protective Effects of Mangiferin Against Diabetes and Diabetes-related Complications. Curr. Diabetes Rev. 2020, 16, 690–698. [Google Scholar] [CrossRef]



| Genes | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | CCCCAGCAAGGACACTGAGCAA | GTGGGTGCAGCGAACTTTATTGATG |
| IL-10 | ATTTGAATTCCCTGGGTGAGAAG | CACAGGGGAGAAATCGATGACA |
| IL-4 | GGTGCGCCATGCACGGAGATG | TGCGAAGCACCTTGGAAGCCC |
| OPG | AGCAGGAGTGCAACCGCACC | TTCCAGCTTGCACCACGCCG |
| RANKL | GGGGGCCGTGCAGAAGGAAC | CTCAGGCTTGCCTCGCTGGG |
| TNF-α | CAACGCCCTCCTGGCCAACG | TCGGGGCAGCCTTGTCCCTT |
| IL-1β | ATGCCTTCCCCAGGGCATGT | CTGAGCGACCTGTCTTGGCCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Y.; Hu, Y.; Huang, S.; Ruiz, S.; Kawai, T.; Bai, Y.; Han, X. CpG ODN/Mangiferin Dual Delivery through Calcium Alginate Hydrogels Inhibits Immune-Mediated Osteoclastogenesis and Promotes Alveolar Bone Regeneration in Mice. Biology 2023, 12, 976. https://doi.org/10.3390/biology12070976
Gu Y, Hu Y, Huang S, Ruiz S, Kawai T, Bai Y, Han X. CpG ODN/Mangiferin Dual Delivery through Calcium Alginate Hydrogels Inhibits Immune-Mediated Osteoclastogenesis and Promotes Alveolar Bone Regeneration in Mice. Biology. 2023; 12(7):976. https://doi.org/10.3390/biology12070976
Chicago/Turabian StyleGu, Yingzhi, Yang Hu, Shengyuan Huang, Sunniva Ruiz, Toshihisa Kawai, Yuxing Bai, and Xiaozhe Han. 2023. "CpG ODN/Mangiferin Dual Delivery through Calcium Alginate Hydrogels Inhibits Immune-Mediated Osteoclastogenesis and Promotes Alveolar Bone Regeneration in Mice" Biology 12, no. 7: 976. https://doi.org/10.3390/biology12070976
APA StyleGu, Y., Hu, Y., Huang, S., Ruiz, S., Kawai, T., Bai, Y., & Han, X. (2023). CpG ODN/Mangiferin Dual Delivery through Calcium Alginate Hydrogels Inhibits Immune-Mediated Osteoclastogenesis and Promotes Alveolar Bone Regeneration in Mice. Biology, 12(7), 976. https://doi.org/10.3390/biology12070976







