Unraveling the Immune Microenvironment in Classic Hodgkin Lymphoma: Prognostic and Therapeutic Implications
Abstract
Simple Summary
Abstract
1. Introduction
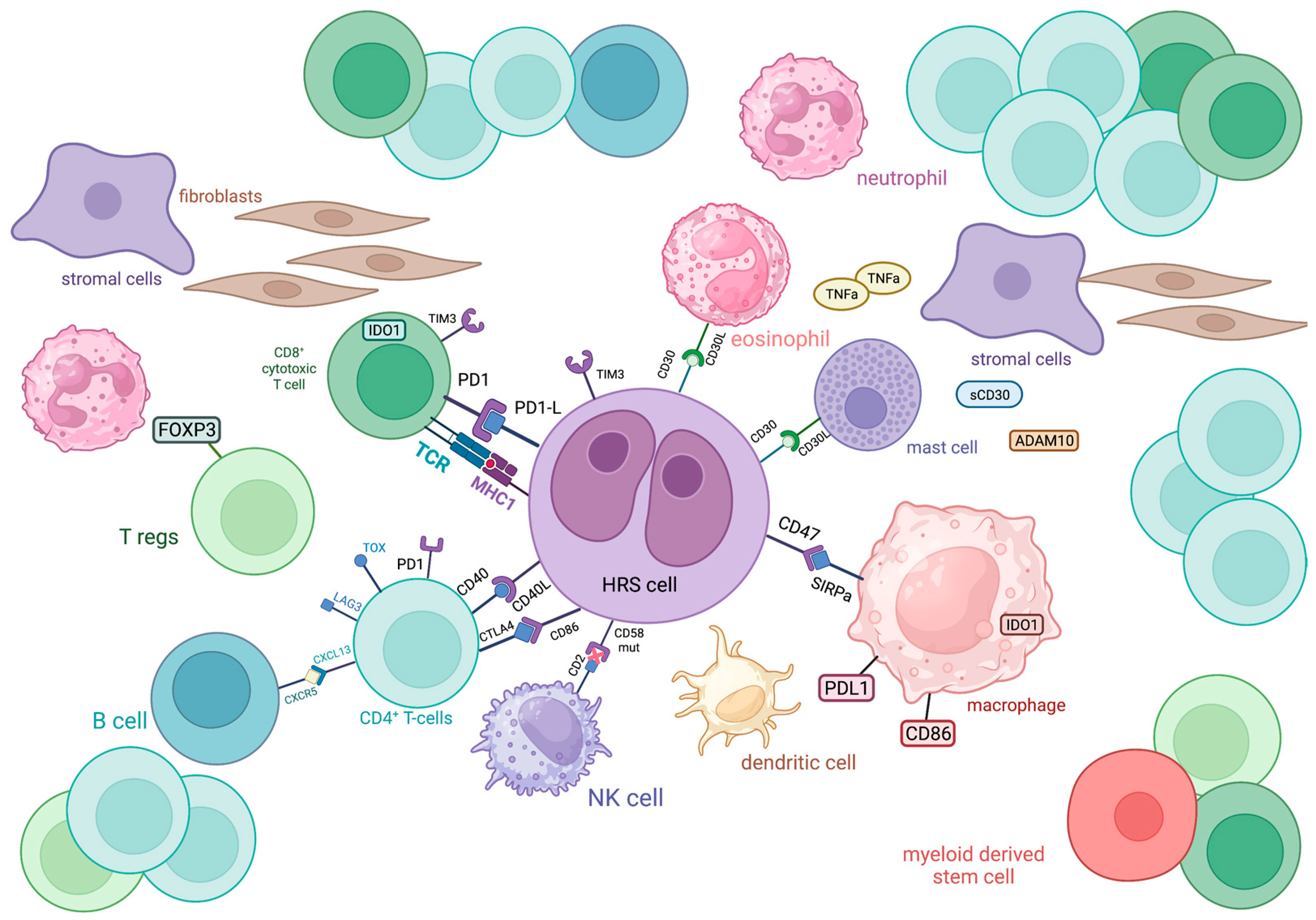
2. Overview of Cellular Components of TME in cHL
2.1. T-Cells
2.1.1. CD4+ T-Cells
2.1.2. CD8+ T-Cells
2.2. B-Cells
2.3. Plasma Cells
2.4. NK-Cells
2.5. Myeloid Cells
2.6. Mast Cells
2.7. Dendritic Cells
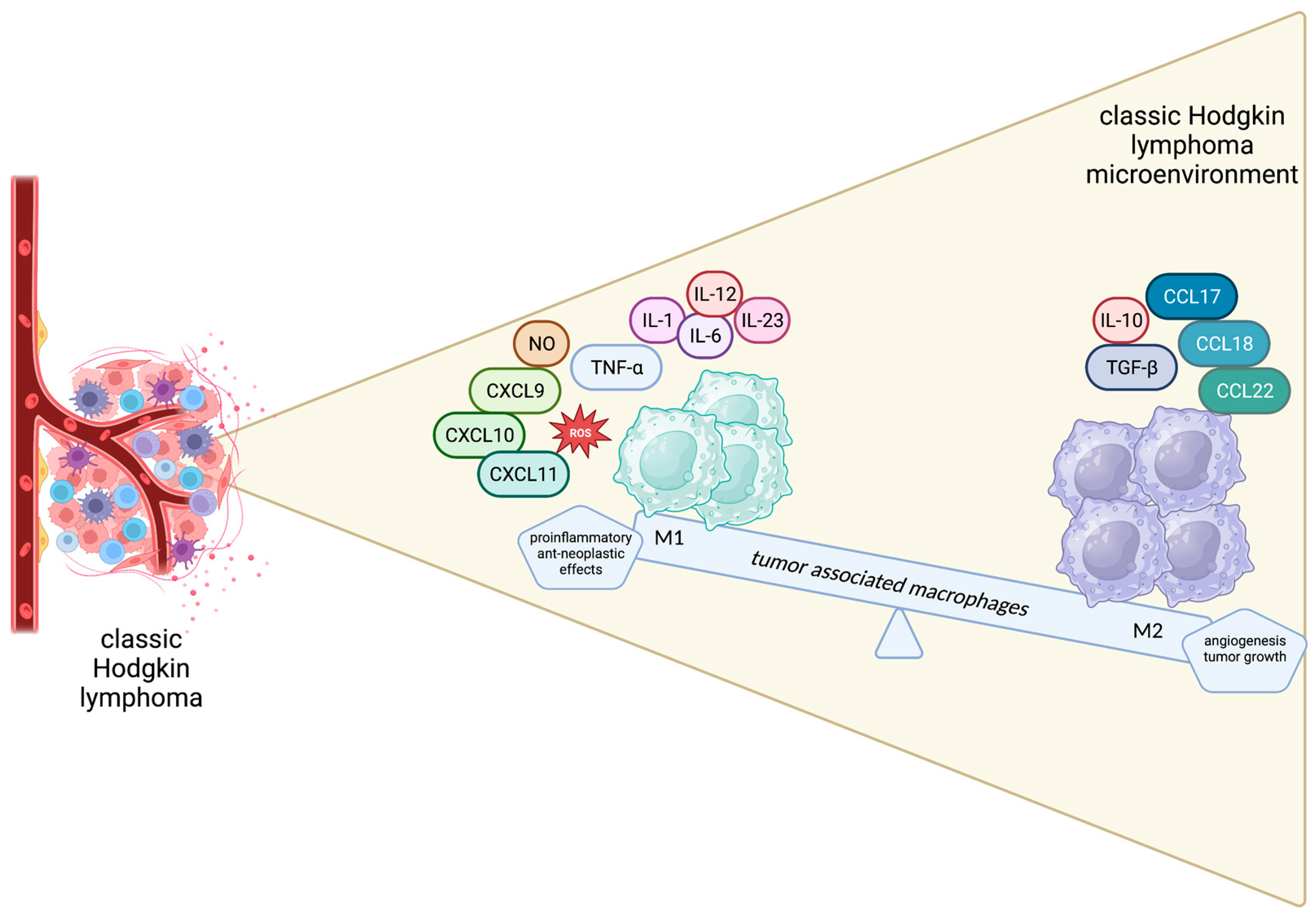
2.8. Tumor-Associated Macrophages
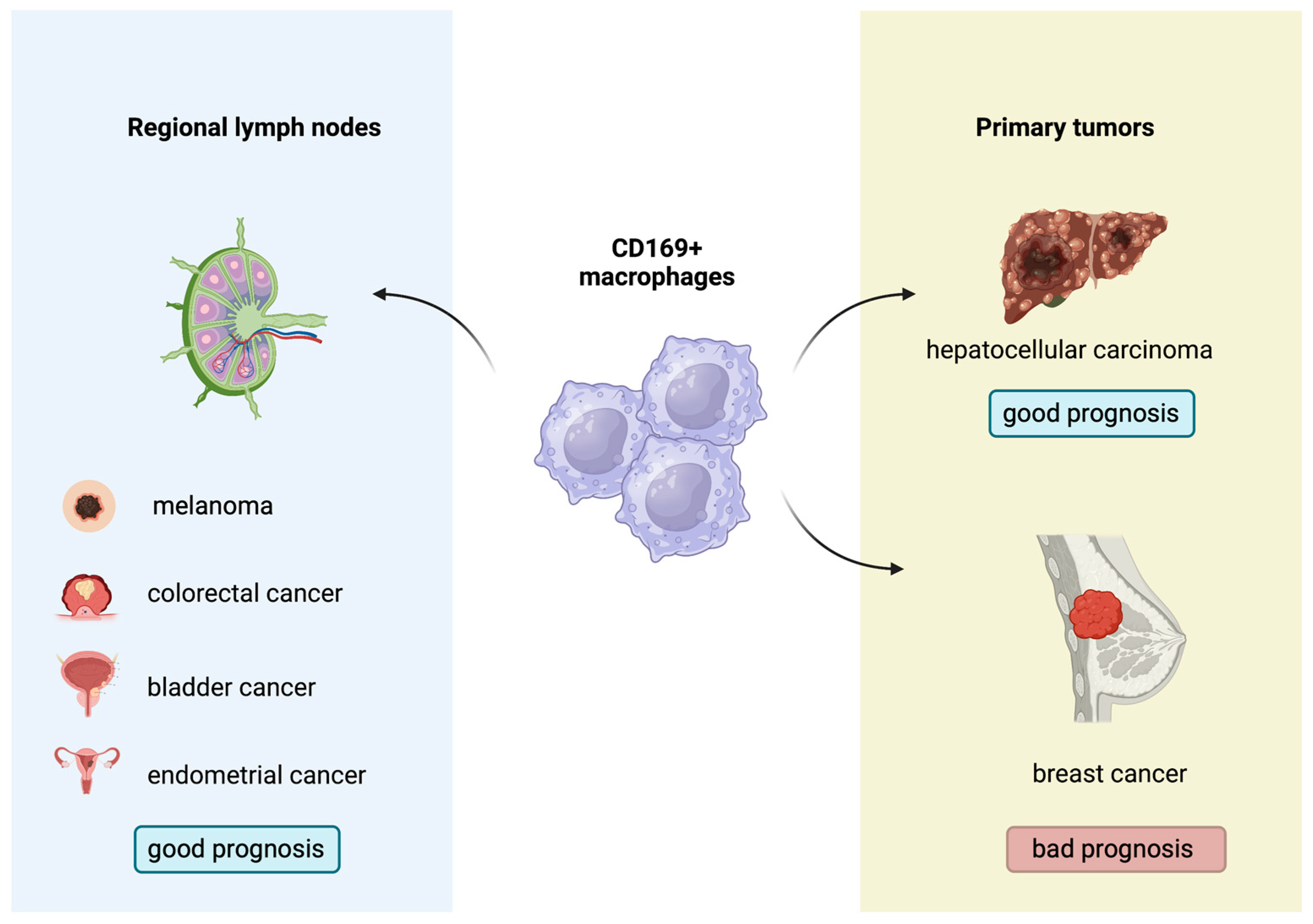
2.9. CD169+ Macrophages: A New Regulator of Antitumor Immunity
3. Immune Evasion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eichenauer, D.A.; Aleman, B.M.P.; André, M.; Federico, M.; Hutchings, M.; Illidge, T.; Engert, A.; Ladetto, M.; ESMO Guidelines Committee. Hodgkin lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv19–iv29. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M. Hodgkin lymphoma: 2023 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2022, 97, 1478–1488. [Google Scholar] [CrossRef]
- Rengstl, B.; Newrzela, S.; Heinrich, T.; Weiser, C.; Thalheimer, F.B.; Schmid, F.; Warner, K.; Hartmann, S.; Schroeder, T.; Küppers, R.; et al. Incomplete cytokinesis and re-fusion of small mononucleated Hodgkin cells lead to giant multinucleated Reed–Sternberg cells. Proc. Natl. Acad. Sci. USA 2013, 110, 20729–20734. [Google Scholar] [CrossRef] [PubMed]
- Weniger, M.A.; Küppers, R. Molecular biology of Hodgkin lymphoma. Leukemia 2021, 35, 968–981. [Google Scholar] [CrossRef]
- Satou, A.; Takahara, T.; Nakamura, S. An Update on the Pathology and Molecular Features of Hodgkin Lymphoma. Cancers 2022, 14, 2647. [Google Scholar] [CrossRef]
- Küppers, R.; Schwering, I.; Bräuninger, A.; Rajewsky, K.; Hansmann, M.-L. Biology of Hodgkin’s lymphoma. Ann. Oncol. 2002, 13 (Suppl. S1), 11–18. [Google Scholar] [CrossRef]
- Bräuninger, A.; Schmitz, R.; Bechtel, D.; Renné, C.; Hansmann, M.-L.; Küppers, R. Molecular biology of Hodgkin’s and Reed/Sternberg cells in Hodgkin’s lymphoma. Int. J. Cancer 2006, 118, 1853–1861. [Google Scholar] [CrossRef]
- Roemer, M.G.M.; Advani, R.H.; Ligon, A.H.; Natkunam, Y.; Redd, R.A.; Homer, H.; Connelly, C.F.; Sun, H.H.; Daadi, S.E.; Freeman, G.J.; et al. PD-L1 and PD-L2 Genetic Alterations Define Classical Hodgkin Lymphoma and Predict Outcome. J. Clin. Oncol. 2016, 34, 2690–2697. [Google Scholar] [CrossRef]
- Skinnider, B.F.; Mak, T.W. The role of cytokines in classical Hodgkin lymphoma. Blood 2002, 99, 4283–4297. [Google Scholar] [CrossRef]
- De Charette, M.; Houot, R. Hide or defend, the two strategies of lymphoma immune evasion: Potential implications for immunotherapy. Haematologica 2018, 103, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Satou, A.; Tsuzuki, T.; Nakamura, S. Hodgkin Lymphoma: Biology and Differential Diagnostic Problem. Diagnostics 2022, 12, 1507. [Google Scholar] [CrossRef]
- Alonso-Álvarez, S.; Vidriales, M.B.; Caballero, M.D.; Blanco, O.; Puig, N.; Martin, A.; Peñarrubia, M.J.; Zato, E.; Galende, J.; Bárez, A.; et al. The number of tumor infiltrating T-cell subsets in lymph nodes from patients with Hodgkin lymphoma is associated with the outcome after first line ABVD therapy. Leuk. Lymphoma 2017, 58, 1144–1152. [Google Scholar] [CrossRef]
- Schreck, S.; Friebel, D.; Buettner, M.; Distel, L.; Grabenbauer, G.; Young, L.S.; Niedobitek, G. Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Hematol. Oncol. 2009, 27, 31–39. [Google Scholar] [CrossRef]
- Walker, J.A.; McKenzie, A.N.J. TH2 cell development and function. Nat. Rev. Immunol. 2018, 18, 121–133. [Google Scholar] [CrossRef]
- Cader, F.Z.; Schackmann, R.C.J.; Hu, X.; Wienand, K.; Redd, R.; Chapuy, B.; Ouyang, J.; Paul, N.; Gjini, E.; Lipschitz, M.; et al. Mass cytometry of Hodgkin lymphoma reveals a CD4+ regulatory T-cell–rich and exhausted T-effector microenvironment. Blood 2018, 132, 825–836. [Google Scholar] [CrossRef]
- Greaves, P.; Clear, A.; Owen, A.; Iqbal, S.; Lee, A.; Matthews, J.; Wilson, A.; Calaminici, M.; Gribben, J.G. Defining characteristics of classical Hodgkin lymphoma microenvironment T-helper cells. Blood 2013, 122, 2856–2863. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. Th1/Th2 Cells. Inflamm. Bowel Dis. 1999, 5, 285–294. [Google Scholar] [CrossRef]
- Nishimura, T.; Nakui, M.; Sato, M.; Iwakabe, K.; Kitamura, H.; Sekimoto, M.; Ohta, A.; Koda, T.; Nishimura, S. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother. Pharmacol. 2000, 46, S52–S61. [Google Scholar] [CrossRef]
- Cretney, E.; Kallies, A.; Nutt, S.L. Differentiation and function of Foxp3+ effector regulatory T cells. Trends Immunol. 2013, 34, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Álvaro, T.; Lejeune, M.; Salvadó, M.T.; Bosch, R.; García, J.F.; Jaén, J.; Banham, A.H.; Roncador, G.; Montalbán, C.; Piris, M.A. Outcome in Hodgkin’s Lymphoma Can Be Predicted from the Presence of Accompanying Cytotoxic and Regulatory T Cells. Clin. Cancer Res. 2005, 11, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.; Kalani, M.; Golmoghaddam, H.; Ramzi, M.; Arandi, N. Aberrant peripheral blood CD4+ CD25+ FOXP3+ regulatory T cells/T helper-17 number is associated with the outcome of patients with lymphoma. Cancer Immunol. Immunother. 2020, 69, 1917–1928. [Google Scholar] [CrossRef] [PubMed]
- Ferrarini, I.; Rigo, A.; Visco, C.; Krampera, M.; Vinante, F. The Evolving Knowledge on T and NK Cells in Classic Hodgkin Lymphoma: Insights into Novel Subsets Populating the Immune Microenvironment. Cancers 2020, 12, 3757. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Khattri, R.; Cox, T.; Yasayko, S.-A.; Ramsdell, F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003, 4, 337–342. [Google Scholar] [CrossRef]
- Walker, M.R.; Kasprowicz, D.J.; Gersuk, V.H.; Bénard, A.; Van Landeghen, M.; Buckner, J.H.; Ziegler, S.F. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4+CD25– T cells. J. Clin. Investig. 2003, 112, 1437–1443. [Google Scholar] [CrossRef]
- Yagi, H.; Nomura, T.; Nakamura, K.; Yamazaki, S.; Kitawaki, T.; Hori, S.; Maeda, M.; Onodera, M.; Uchiyama, T.; Fujii, S.; et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int. Immunol. 2004, 16, 1643–1656. [Google Scholar] [CrossRef]
- Pillai, V.; Ortega, S.B.; Wang, C.; Karandikar, N.J. Transient regulatory T-cells: A state attained by all activated human T-cells. Clin. Immunol. 2007, 123, 18–29. [Google Scholar] [CrossRef]
- Ferrarini, I.; Rigo, A.; Zamò, A.; Vinante, F. Classical Hodgkin lymphoma cells may promote an IL-17-enriched microenvironment. Leuk. Lymphoma 2019, 60, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Chong, L.C.; Takata, K.; Milne, K.; Hav, M.; Colombo, A.; Chavez, E.A.; Nissen, M.; Wang, X.; Miyata-Takata, T.; et al. Single-Cell Transcriptome Analysis Reveals Disease-Defining T-cell Subsets in the Tumor Microenvironment of Classic Hodgkin Lymphoma. Cancer Discov. 2020, 10, 406–421. [Google Scholar] [CrossRef]
- Aoki, T.; Chong, L.C.; Takata, K.; Milne, K.; Marshall, A.; Chavez, E.A.; Miyata-Takata, T.; Ben-Neriah, S.; Unrau, D.; Telenius, A.; et al. Single-cell profiling reveals the importance of CXCL13/CXCR5 axis biology in lymphocyte-rich classic Hodgkin lymphoma. Proc. Natl. Acad. Sci. USA 2021, 118, e2105822118. [Google Scholar] [CrossRef]
- Koreishi, A.F.; Saenz, A.J.; Persky, D.O.; Cui, H.; Moskowitz, A.; Moskowitz, C.H.; Teruya-Feldstein, J. The Role of Cytotoxic and Regulatory T cells in Relapsed/Refractory Hodgkin Lymphoma. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 206–211. [Google Scholar] [CrossRef]
- Karihtala, K.; Leivonen, S.-K.; Karjalainen-Lindsberg, M.-L.; Chan, F.C.; Steidl, C.; Pellinen, T.; Leppä, S. Checkpoint protein expression in the tumor microenvironment defines the outcome of classical Hodgkin lymphoma patients. Blood Adv. 2022, 6, 1919–1931. [Google Scholar] [CrossRef]
- Le, K.-S.; Amé-Thomas, P.; Tarte, K.; Gondois-Rey, F.; Granjeaud, S.; Orlanducci, F.; Foucher, E.D.; Broussais, F.; Bouabdallah, R.; Fest, T.; et al. CXCR5 and ICOS expression identifies a CD8 T-cell subset with TFH features in Hodgkin lymphomas. Blood Adv. 2018, 2, 1889–1900. [Google Scholar] [CrossRef]
- Gandhi, M.; Moll, G.; Smith, C.; Dua, U.; Lambley, E.; Ramuz, O.; Gill, D.; Marlton, P.; Seymour, J.F.; Khanna, R. Galectin-1 mediated suppression of Epstein-Barr virus–specific T-cell immunity in classic Hodgkin lymphoma. Blood 2007, 110, 1326–1329. [Google Scholar] [CrossRef]
- Jachimowicz, R.D.; Pieper, L.; Reinke, S.; Gontarewicz, A.; Plütschow, A.; Haverkamp, H.; Frauenfeld, L.; Fend, F.; Overkamp, M.; Jochims, F.; et al. Whole-slide image analysis of the tumor microenvironment identifies low B-cell content as a predictor of adverse outcome in patients with advanced-stage classical Hodgkin lymphoma treated with BEACOPP. Haematologica 2021, 106, 1684–1692. [Google Scholar] [CrossRef]
- Panico, L.; Tenneriello, V.; Ronconi, F.; Lepore, M.; Cantore, N.; Dell’angelo, A.C.; Ferbo, L.; Ferrara, F. High CD20+ background cells predict a favorable outcome in classical Hodgkin lymphoma and antagonize CD68+ macrophages. Leuk. Lymphoma 2015, 56, 1636–1642. [Google Scholar] [CrossRef]
- Calabretta, E.; D’amore, F.; Carlo-Stella, C. Immune and Inflammatory Cells of the Tumor Microenvironment Represent Novel Therapeutic Targets in Classical Hodgkin Lymphoma. Int. J. Mol. Sci. 2019, 20, 5503. [Google Scholar] [CrossRef] [PubMed]
- Tudor, C.S.; Distel, L.V.; Eckhardt, J.; Hartmann, A.; Niedobitek, G.; Buettner, M. B cells in classical Hodgkin lymphoma are important actors rather than bystanders in the local immune reaction. Hum. Pathol. 2013, 44, 2475–2486. [Google Scholar] [CrossRef] [PubMed]
- Gholiha, A.R.; Hollander, P.; Hedstrom, G.; Sundstrom, C.; Molin, D.; Smedby, K.E.; Hjalgrim, H.; Glimelius, I.; Amini, R.; Enblad, G. High tumour plasma cell infiltration reflects an important microenvironmental component in classic Hodgkin lymphoma linked to presence of B-symptoms. Br. J. Haematol. 2019, 184, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.A.; Maurer, M.J.; Cerhan, J.R.; Katzmann, J.A.; Ansell, S.M.; Habermann, T.M.; Macon, W.R.; Weiner, G.J.; Link, B.K.; Witzig, T.E. Elevated serum free light chains are associated with inferior event free and overall survival in Hodgkin lymphoma. Am. J. Hematol. 2011, 86, 998–1000. [Google Scholar] [CrossRef]
- Chiu, J.; Ernst, D.M.; Keating, A. Acquired Natural Killer Cell Dysfunction in the Tumor Microenvironment of Classic Hodgkin Lymphoma. Front. Immunol. 2018, 9, 267. [Google Scholar] [CrossRef]
- Stannard, K.A.; Lemoine, S.; Waterhouse, N.J.; Vari, F.; Chatenoud, L.; Gandhi, M.K.; Martinet, L.; Smyth, M.J.; Guillerey, C. Human peripheral blood DNAM-1neg NK cells are a terminally differentiated subset with limited effector functions. Blood Adv. 2019, 3, 1681–1694. [Google Scholar] [CrossRef]
- Raber, P.; Ochoa, A.C.; Rodríguez, P.C. Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: Mechanisms of T cell suppression and therapeutic perspectives. Immunol. Investig. 2012, 41, 614–634. [Google Scholar] [CrossRef]
- Elliott, L.A.; Doherty, G.A.; Sheahan, K.; Ryan, E.J. Human Tumor-Infiltrating Myeloid Cells: Phenotypic and Functional Diversity. Front. Immunol. 2017, 8, 86. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, C.; Sabattini, E.; Agostinelli, C. Immune Microenvironment Features and Dynamics in Hodgkin Lymphoma. Cancers 2021, 13, 3634. [Google Scholar] [CrossRef]
- Romano, A.; Parrinello, N.L.; Vetro, C.; Forte, S.; Chiarenza, A.; Figuera, A.; Motta, G.; Palumbo, G.A.; Ippolito, M.; Consoli, U.; et al. Circulating myeloid-derived suppressor cells correlate with clinical outcome in Hodgkin Lymphoma patients treated up-front with a risk-adapted strategy. Br. J. Haematol. 2015, 168, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Parrinello, N.L.; Chiarenza, A.; Motta, G.; Tibullo, D.; Giallongo, C.; La Cava, P.; Camiolo, G.; Puglisi, F.; Palumbo, G.A.; et al. Immune off-target effects of Brentuximab Vedotin in relapsed/refractory Hodgkin Lymphoma. Br. J. Haematol. 2019, 185, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Axdorph, U.; Porwit-MacDonald, A.; Grimfors, G.; Björkholm, M. Tissue Eosinophilia in Relation to Immunopathological and Clinical Characteristics in Hodgkin’s Disease. Leuk. Lymphoma 2001, 42, 1055–1065. [Google Scholar] [CrossRef]
- von Wasielewski, R.; Seth, S.; Franklin, J.; Fischer, R.; Hubner, K.; Hansmann, M.L.; Diehl, V.; Georgii, A. Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin’s disease, allowing for known prognostic factors. Blood 2000, 95, 1207–1213. [Google Scholar] [CrossRef]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef]
- Koh, Y.W.; Kang, H.J.; Park, C.; Yoon, D.H.; Kim, S.; Suh, C.; Kim, J.E.; Kim, C.-W.; Huh, J. Prognostic Significance of the Ratio of Absolute Neutrophil Count to Absolute Lymphocyte Count in Classic Hodgkin Lymphoma. Am. J. Clin. Pathol. 2012, 138, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Molin, D.; Fischer, M.; Xiang, Z.; Larsson, U.; Harvima, I.; Venge, P.; Nilsson, K.; Sundström, C.; Enblad, G.; Nilsson, G. Mast cells express functional CD30 ligand and are the predominant CD30L-positive cells in Hodgkin’s disease. Br. J. Haematol. 2001, 114, 616–623. [Google Scholar] [CrossRef]
- Mizuno, H.; Nakayama, T.; Miyata, Y.; Saito, S.; Nishiwaki, S.; Nakao, N.; Takeshita, K.; Naoe, T. Mast cells promote the growth of Hodgkin’s lymphoma cell tumor by modifying the tumor microenvironment that can be perturbed by bortezomib. Leukemia 2012, 26, 2269–2276. [Google Scholar] [CrossRef]
- Molin, D.; Edström, A.; Glimelius, I.; Glimelius, B.; Nilsson, G.; Sundström, C.; Enblad, G. Mast cell infiltration correlates with poor prognosis in Hodgkin’s lymphoma. Br. J. Haematol. 2002, 119, 122–124. [Google Scholar] [CrossRef]
- Keresztes, K.; Szollosi, Z.; Simon, Z.; Tarkanyi, I.; Nemes, Z.; Illes, A. Retrospective analysis of the prognostic role of tissue eosinophil and mast cells in Hodgkin’s lymphoma. Pathol. Oncol. Res. 2007, 13, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Shodell, M.; Kempin, S.; Siegal, F.P. Plasmacytoid dendritic cell and CD4+ T cell deficiencies in untreated Hodgkin disease: Implications for susceptibility to opportunistic infections. Leuk. Lymphoma 2014, 55, 2656–2657. [Google Scholar] [CrossRef]
- Tudor, C.S.; Bruns, H.; Daniel, C.; Distel, L.V.; Hartmann, A.; Gerbitz, A.; Buettner, M.J. Macrophages and Dendritic Cells as Actors in the Immune Reaction of Classical Hodgkin Lymphoma. PLoS ONE 2014, 9, e114345. [Google Scholar] [CrossRef]
- Alavaikko, M.J.; Blanco, G.; Aine, R.; Lehtinen, T.; Fellbaum, C.; Taskinen, P.J.; Sarpola, M.A.; Hansmann, M.-L. Follicular Dendritic Cells Have Prognostic Relevance in Hodgkin’s Disease. Am. J. Clin. Pathol. 1994, 101, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Galati, D.; Zanotta, S.; Corazzelli, G.; Bruzzese, D.; Capobianco, G.; Morelli, E.; Arcamone, M.; De Filippi, R.; Pinto, A. Circulating dendritic cells deficiencies as a new biomarker in classical Hodgkin lymphoma. Br. J. Haematol. 2018, 184, 594–604. [Google Scholar] [CrossRef]
- Hourani, T.; Holden, J.A.; Li, W.; Lenzo, J.C.; Hadjigol, S.; O’brien-Simpson, N.M. Tumor Associated Macrophages: Origin, Recruitment, Phenotypic Diversity, and Targeting. Front. Oncol. 2021, 11, 788365. [Google Scholar] [CrossRef]
- Cencini, E.; Fabbri, A.; Sicuranza, A.; Gozzetti, A.; Bocchia, M. The Role of Tumor-Associated Macrophages in Hematologic Malignancies. Cancers 2021, 13, 3597. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, H.; Yang, C.; He, L.; Zhang, X.; Peng, L.; Zhu, H.; Gao, L. Role and Mechanisms of Tumor-Associated Macrophages in Hematological Malignancies. Front. Oncol. 2022, 12, 933666. [Google Scholar] [CrossRef]
- Carey, C.D.; Gusenleitner, D.; Lipschitz, M.; Roemer, M.G.M.; Stack, E.C.; Gjini, E.; Hu, X.; Redd, R.; Freeman, G.J.; Neuberg, D.; et al. Topological analysis reveals a PD-L1-associated microenvironmental niche for Reed-Sternberg cells in Hodgkin lymphoma. Blood 2017, 130, 2420–2430. [Google Scholar] [CrossRef]
- Patel, S.S.; Weirather, J.L.; Lipschitz, M.; Lako, A.; Chen, P.-H.; Griffin, G.K.; Armand, P.; Shipp, M.A.; Rodig, S.J. The microenvironmental niche in classic Hodgkin lymphoma is enriched for CTLA-4-positive T cells that are PD-1-negative. Blood 2019, 134, 2059–2069. [Google Scholar] [CrossRef]
- Hančić, S.; Gršković, P.; Gašparov, S.; Kolonić, S.O.; Dominis, M.; Korać, P. Macrophage Infiltration Correlates with Genomic Instability in Classic Hodgkin Lymphoma. Biomedicines 2022, 10, 579. [Google Scholar] [CrossRef]
- Werner, L.; Dreyer, J.H.; Hartmann, D.; Barros, M.H.M.; Büttner-Herold, M.; Grittner, U.; Niedobitek, G. Tumor-associated macrophages in classical Hodgkin lymphoma: Hormetic relationship to outcome. Sci. Rep. 2020, 10, 9410. [Google Scholar] [CrossRef] [PubMed]
- Steidl, C.; Lee, T.; Shah, S.P.; Farinha, P.; Han, G.; Nayar, T.; Delaney, A.; Jones, S.J.; Iqbal, J.; Weisenburger, D.D.; et al. Tumor-Associated Macrophages and Survival in Classic Hodgkin’s Lymphoma. N. Engl. J. Med. 2010, 362, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Touati, M.; Delage-Corre, M.; Monteil, J.; Abraham, J.; Moreau, S.; Remenieras, L.; Gourin, M.-P.; Dmytruk, N.; Olivrie, A.; Turlure, P.; et al. CD68-positive tumor-associated macrophages predict unfavorable treatment outcomes in classical Hodgkin lymphoma in correlation with interim fluorodeoxyglucose-positron emission tomography assessment. Leuk. Lymphoma 2015, 56, 332–341. [Google Scholar] [CrossRef]
- Cuccaro, A.; Annunziata, S.; Cupelli, E.; Martini, M.; Calcagni, M.L.; Rufini, V.; Giachelia, M.; Bartolomei, F.; Galli, E.; D’Alò, F.; et al. CD 68+ cell count, early evaluation with PET and plasma TARC levels predict response in Hodgkin lymphoma. Cancer Med. 2016, 5, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Greaves, P.; Clear, A.; Coutinho, R.; Wilson, A.; Matthews, J.; Owen, A.; Shanyinde, M.; Lister, T.A.; Calaminici, M.; Gribben, J.G. Expression of FOXP3, CD68, and CD20 at Diagnosis in the Microenvironment of Classical Hodgkin Lymphoma Is Predictive of Outcome. J. Clin. Oncol. 2013, 31, 256–262. [Google Scholar] [CrossRef]
- Jakovic, L.R.; Mihaljevic, B.S.; Jovanovic, M.D.P.; Bogdanovic, A.D.; Andjelic, B.M.; Bumbasirevic, V.Z. The prognostic relevance of tumor associated macrophages in advanced stage classical Hodgkin lymphoma. Leuk. Lymphoma 2011, 52, 1913–1919. [Google Scholar] [CrossRef]
- Mohamed, O.; El Bastawisy, A.; Allahlobi, N.; Lateif, M.A.; Zekri, A.R.N.; Shaarawy, S.; Korany, Z.; Mohanad, M.; Bahnassy, A.A. The role of CD68+ macrophage in classical Hodgkin lymphoma patients from Egypt. Diagn. Pathol. 2020, 15, 10–13. [Google Scholar] [CrossRef]
- Yoon, D.H.; Koh, Y.W.; Kang, H.J.; Kim, S.; Suh, C.; Huh, J.; Park, C.-S.; Lee, S.-W. CD68 and CD163 as prognostic factors for Korean patients with Hodgkin lymphoma. Eur. J. Haematol. 2012, 88, 292–305. [Google Scholar] [CrossRef]
- Tan, K.L.; Scott, D.W.; Hong, F.; Kahl, B.S.; Fisher, R.I.; Bartlett, N.L.; Advani, R.H.; Buckstein, R.; Rimsza, L.M.; Connors, J.M.; et al. Tumor-associated macrophages predict inferior outcomes in classic Hodgkin lymphoma: A correlative study from the E2496 Intergroup trial. Blood 2012, 120, 3280–3287. [Google Scholar] [CrossRef] [PubMed]
- Kamper, P.; Bendix, K.; Hamilton-Dutoit, S.; Honoré, B.; Nyengaard, J.R.; D’amore, F. Tumor-infiltrating macrophages correlate with adverse prognosis and Epstein-Barr virus status in classical Hodgkin’s lymphoma. Haematologica 2011, 96, 269–276. [Google Scholar] [CrossRef]
- Agur, A.; Amir, G.; Paltiel, O.; Klein, M.; Dann, E.J.; Goldschmidt, H.; Goldschmidt, N. CD68 staining correlates with the size of residual mass but not with survival in classical Hodgkin lymphoma. Leuk. Lymphoma 2015, 56, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Kayal, S.; Mathur, S.; Karak, A.K.; Kumar, L.; Sharma, A.; Bakhshi, S.; Raina, V. CD68 tumor-associated macrophage marker is not prognostic of clinical outcome in classical Hodgkin lymphoma. Leuk. Lymphoma 2014, 55, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.L.; Nguyen, T.T.; Bien-Willner, G.A.; Chen, L.; Foyil, K.V.; Bartlett, N.L.; Duncavage, E.J.; Hassan, A.; Frater, J.L.; Kreisel, F. CD163 Immunohistochemistry Is Superior to CD68 in Predicting Outcome in Classical Hodgkin Lymphoma. Am. J. Clin. Pathol. 2014, 141, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Azambuja, D.; Natkunam, Y.; Biasoli, I.; Lossos, I.; Anderson, M.; Morais, J.; Spector, N. Lack of association of tumor-associated macrophages with clinical outcome in patients with classical Hodgkin’s lymphoma. Ann. Oncol. 2012, 23, 736–742. [Google Scholar] [CrossRef]
- Zaki, M.A.A.; Wada, N.; Ikeda, J.; Shibayama, H.; Hashimoto, K.; Yamagami, T.; Tatsumi, Y.; Tsukaguchi, M.; Take, H.; Tsudo, M.; et al. Prognostic implication of types of tumor-associated macrophages in Hodgkin lymphoma. Virchows Arch. 2011, 459, 361–366. [Google Scholar] [CrossRef]
- Gusak, A.; Fedorova, L.; Lepik, K.; Volkov, N.; Popova, M.; Moiseev, I.; Mikhailova, N.; Baykov, V.; Kulagin, A. Immunosuppressive Microenvironment and Efficacy of PD-1 Inhibitors in Relapsed/Refractory Classic Hodgkin Lymphoma: Checkpoint Molecules Landscape and Macrophage Populations. Cancers 2021, 13, 5676. [Google Scholar] [CrossRef]
- Guo, B.; Cen, H.; Tan, X.; Ke, Q. Meta-analysis of the prognostic and clinical value of tumor-associated macrophages in adult classical Hodgkin lymphoma. BMC Med. 2016, 14, 159. [Google Scholar] [CrossRef]
- Karihtala, K.; Leivonen, S.-K.; Brück, O.; Karjalainen-Lindsberg, M.-L.; Mustjoki, S.; Pellinen, T.; Leppä, S. Prognostic Impact of Tumor-Associated Macrophages on Survival Is Checkpoint Dependent in Classical Hodgkin Lymphoma. Cancers 2020, 12, 877. [Google Scholar] [CrossRef]
- Locatelli, S.L.; Careddu, G.; Serio, S.; Consonni, F.M.; Maeda, A.; Viswanadha, S.; Vakkalanka, S.; Castagna, L.; Santoro, A.; Allavena, P.; et al. Targeting Cancer Cells and Tumor Microenvironment in Preclinical and Clinical Models of Hodgkin Lymphoma Using the Dual PI3Kδ/γ Inhibitor RP6530. Clin. Cancer Res. 2019, 25, 1098–1112. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, Y.; Qiu, C.-H. Functions of CD169 positive macrophages in human diseases (Review). Biomed. Rep. 2021, 14, 26. [Google Scholar] [CrossRef]
- Grabowska, J.; Lopez-Venegas, M.; Affandi, A.J.; Haan, J.D. CD169+ Macrophages Capture and Dendritic Cells Instruct: The Interplay of the Gatekeeper and the General of the Immune System. Front. Immunol. 2018, 9, 2472. [Google Scholar] [CrossRef] [PubMed]
- Affandi, A.J.; Olesek, K.; Grabowska, J.; Twilhaar, M.K.N.; Rodríguez, E.; Saris, A.; Zwart, E.S.; Nossent, E.J.; Kalay, H.; de Kok, M.; et al. CD169 Defines Activated CD14+ Monocytes with Enhanced CD8+ T Cell Activation Capacity. Front. Immunol. 2021, 12, 697840. [Google Scholar] [CrossRef]
- Asano, K.; Nabeyama, A.; Miyake, Y.; Qiu, C.-H.; Kurita, A.; Tomura, M.; Kanagawa, O.; Fujii, S.-I.; Tanaka, M. CD169-Positive Macrophages Dominate Antitumor Immunity by Crosspresenting Dead Cell-Associated Antigens. Immunity 2011, 34, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Komohara, Y.; Ohnishi, K.; Takeya, M. Possible functions of CD169-positive sinus macrophages in lymph nodes in anti-tumor immune responses. Cancer Sci. 2017, 108, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Ohnishi, K.; Shiota, T.; Motoshima, T.; Sugiyama, Y.; Yatsuda, J.; Kamba, T.; Ishizaka, K.; Komohara, Y. CD 169-positive sinus macrophages in the lymph nodes determine bladder cancer prognosis. Cancer Sci. 2018, 109, 1723–1730. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.-Q.; Jiang, Z.-Z.; Li, L.; Wu, Y.; Zheng, L. CD169 identifies an anti-tumour macrophage subpopulation in human hepatocellular carcinoma. J. Pathol. 2016, 239, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Briem, O.; Källberg, E.; Kimbung, S.; Veerla, S.; Stenström, J.; Hatschek, T.; Hagerling, C.; Hedenfalk, I.; Leandersson, K. CD169+ Macrophages in Primary Breast Tumors Associate with Tertiary Lymphoid Structures, Tregs and a Worse Prognosis for Patients with Advanced Breast Cancer. Cancers 2023, 15, 1262. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, K.; Komohara, Y.; Saito, Y.; Miyamoto, Y.; Watanabe, M.; Baba, H.; Takeya, M. CD169-positive macrophages in regional lymph nodes are associated with a favorable prognosis in patients with colorectal carcinoma. Cancer Sci. 2013, 104, 1237–1244. [Google Scholar] [CrossRef]
- Saito, Y.; Ohnishi, K.; Miyashita, A.; Nakahara, S.; Fujiwara, Y.; Horlad, H.; Motoshima, T.; Fukushima, S.; Jinnin, M.; Ihn, H.; et al. Prognostic Significance of CD169+ Lymph Node Sinus Macrophages in Patients with Malignant Melanoma. Cancer Immunol. Res. 2015, 3, 1356–1363. [Google Scholar] [CrossRef]
- Ohnishi, K.; Yamaguchi, M.; Erdenebaatar, C.; Saito, F.; Tashiro, H.; Katabuchi, H.; Takeya, M.; Komohara, Y. Prognostic significance of CD 169-positive lymph node sinus macrophages in patients with endometrial carcinoma. Cancer Sci. 2016, 107, 846–852. [Google Scholar] [CrossRef]
- Marmey, B.; Boix, C.; Barbaroux, J.-B.; Dieu-Nosjean, M.-C.; Diebold, J.; Audouin, J.; Fridman, W.-H.; Mueller, C.G.; Molina, T.J. CD14 and CD169 expression in human lymph nodes and spleen: Specific expansion of CD14+CD169− monocyte-derived cells in diffuse large B-cell lymphomas. Hum. Pathol. 2006, 37, 68–77. [Google Scholar] [CrossRef]
- Uccini, S.; Al-Jadiry, M.F.; Pepe, G.; Scarpino, S.; Al-Hadad, S.A.; Ruco, L. PD-L1 expression in pediatric Epstein-Barr virus positive classic Hodgkin lymphoma is not associated with 9p24.1 amplification. Pediatr. Blood Cancer 2019, 66, e27757. [Google Scholar] [CrossRef]
- Green, M.R.; Rodig, S.; Juszczynski, P.; Ouyang, J.; Sinha, P.; O’Donnell, E.; Neuberg, D.; Shipp, M.A. Constitutive AP-1 Activity and EBV Infection Induce PD-L1 in Hodgkin Lymphomas and Posttransplant Lymphoproliferative Disorders: Implications for Targeted Therapy. Clin. Cancer Res. 2012, 18, 1611–1618. [Google Scholar] [CrossRef]
- Liu, W.R.; Shipp, M.A. Signaling pathways and immune evasion mechanisms in classical Hodgkin lymphoma. Blood 2017, 130, 2265–2270. [Google Scholar] [CrossRef]
- Kawashima, M.; Higuchi, H.; Kotani, A. Significance of trogocytosis and exosome-mediated transport in establishing and maintaining the tumor microenvironment in lymphoid malignancies. J. Clin. Exp. Hematop. 2021, 61, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Roemer, M.G.; Redd, R.A.; Cader, F.Z.; Pak, C.J.; Abdelrahman, S.; Ouyang, J.; Sasse, S.; Younes, A.; Fanale, M.; Santoro, A.; et al. Major Histocompatibility Complex Class II and Programmed Death Ligand 1 Expression Predict Outcome After Programmed Death 1 Blockade in Classic Hodgkin Lymphoma. J. Clin. Oncol. 2018, 36, 942–950. [Google Scholar] [CrossRef]
- Menéndez, V.; Solórzano, J.L.; Fernández, S.; Montalbán, C.; García, J.F. The Hodgkin Lymphoma Immune Microenvironment: Turning Bad News into Good. Cancers 2022, 14, 1360. [Google Scholar] [CrossRef]
- Hatic, H.; Sampat, D.; Goyal, G. Immune checkpoint inhibitors in lymphoma: Challenges and opportunities. Ann. Transl. Med. 2021, 9, 1037. [Google Scholar] [CrossRef] [PubMed]
- Vardhana, S.; Younes, A. The immune microenvironment in Hodgkin lymphoma: T cells, B cells, and immune checkpoints. Haematologica 2016, 101, 794–802. [Google Scholar] [CrossRef]
- El Halabi, L.; Adam, J.; Gravelle, P.; Marty, V.; Danu, A.; Lazarovici, J.; Ribrag, V.; Bosq, J.; Camara-Clayette, V.; Laurent, C.; et al. Expression of the Immune Checkpoint Regulators LAG-3 and TIM-3 in Classical Hodgkin Lymphoma. Clin. Lymphoma Myeloma Leuk. 2020, 21, 257–266.e3. [Google Scholar] [CrossRef]
- Liu, Y.; Abdul Razak, F.R.; Terpstra, M.; Chan, F.C.; Saber, A.; Nijland, M.; Van Imhoff, G.; Visser, L.; Gascoyne, R.; Steidl, C.; et al. The mutational landscape of Hodgkin lymphoma cell lines determined by whole-exome sequencing. Leukemia 2014, 28, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Schneider, S.; Zühlke-Jenisch, R.; Klapper, W.; Sundström, C.; Hartmann, S.; Hansmann, M.-L.; Siebert, R.; Küppers, R.; Giefing, M. Alterations of the CD58 gene in classical Hodgkin lymphoma. Genes, Chromosom. Cancer 2015, 54, 638–645. [Google Scholar] [CrossRef]
- Casagrande, N.; Borghese, C.; Aldinucci, D. Current and Emerging Approaches to Study Microenvironmental Interactions and Drug Activity in Classical Hodgkin Lymphoma. Cancers 2022, 14, 2427. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.-Y.; Yun, J.Y.; Jeon, Y.K.; Kim, S.H.; Park, G.; Huh, J.R.; Oh, S.; Kim, J.E. Indoleamine 2,3-dioxygenase (IDO) is frequently expressed in stromal cells of Hodgkin lymphoma and is associated with adverse clinical features: A retrospective cohort study. BMC Cancer 2014, 14, 335. [Google Scholar] [CrossRef]
- Kim, M.-S.; Park, T.I.; Son, S.-A.; Lee, H.W. Immunohistochemical Features of Indoleamine 2,3-Dioxygenase (IDO) in Various Types of Lymphoma: A Single Center Experience. Diagnostics 2020, 10, 275. [Google Scholar] [CrossRef]
- Masaki, A.; Ishida, T.; Maeda, Y.; Ito, A.; Suzuki, S.; Narita, T.; Kinoshita, S.; Takino, H.; Yoshida, T.; Shiori, K.; et al. Clinical significance of tryptophan catabolism in Hodgkin lymphoma. Cancer Sci. 2018, 109, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.A.; Christie, L.E.; Munro, L.R.; Culligan, D.J.; Johnston, P.W.; Barker, R.N.; Vickers, M.A. Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood 2004, 103, 1755–1762. [Google Scholar] [CrossRef]
- Jaiswal, S.; Jamieson, C.H.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; van Rooijen, N.; Weissman, I.L. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef]
- López-Pereira, B.; Fernández-Velasco, A.A.; Fernández-Vega, I.; Corte-Torres, D.; Quirós, C.; Villegas, J.A.; Palomo, P.; González, S.; González, A.P.; Payer, A.; et al. Expression of CD47 antigen in Reed–Sternberg cells as a new potential biomarker for classical Hodgkin lymphoma. Clin. Transl. Oncol. 2020, 22, 782–785. [Google Scholar] [CrossRef]
- Gholiha, A.R.; Hollander, P.; Löf, L.; Glimelius, I.; Hedstrom, G.; Molin, D.; Hjalgrim, H.; Smedby, K.E.; Hashemi, J.; Amini, R.; et al. Checkpoint CD47 expression in classical Hodgkin lymphoma. Br. J. Haematol. 2022, 197, 580–589. [Google Scholar] [CrossRef]
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D.; Van Rooijen, N.; Weissman, I.L. CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells. Cell 2009, 138, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Aviles-Salas, A.; Orozco-Morales, M.; Hernández-Pedro, N.; Cardona, A.F.; Cabrera-Miranda, L.; Barrios-Bernal, P.; Soca-Chafre, G.; Cruz-Rico, G.; Peña-Torres, M.D.L.; et al. Association between CD47 expression, clinical characteristics and prognosis in patients with advanced non-small cell lung cancer. Cancer Med. 2020, 9, 2390–2402. [Google Scholar] [CrossRef]
- Chew, V.; Toh, H.C.; Abastado, J.-P. Immune Microenvironment in Tumor Progression: Characteristics and Challenges for Therapy. J. Oncol. 2012, 2012, 608406. [Google Scholar] [CrossRef]
- Santisteban-Espejo, A.; Bernal-Florindo, I.; Perez-Requena, J.; Atienza-Cuevas, L.; Maira-Gonzalez, N.; Garcia-Rojo, M. Whole-slide image analysis identifies a high content of Hodgkin Reed-Sternberg cells and a low content of T lymphocytes in tumor microenvironment as predictors of adverse outcome in patients with classic Hodgkin lymphoma treated with ABVD. Front. Oncol. 2022, 12, 1000762. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgoulis, V.; Papoudou-Bai, A.; Makis, A.; Kanavaros, P.; Hatzimichael, E. Unraveling the Immune Microenvironment in Classic Hodgkin Lymphoma: Prognostic and Therapeutic Implications. Biology 2023, 12, 862. https://doi.org/10.3390/biology12060862
Georgoulis V, Papoudou-Bai A, Makis A, Kanavaros P, Hatzimichael E. Unraveling the Immune Microenvironment in Classic Hodgkin Lymphoma: Prognostic and Therapeutic Implications. Biology. 2023; 12(6):862. https://doi.org/10.3390/biology12060862
Chicago/Turabian StyleGeorgoulis, Vasileios, Alexandra Papoudou-Bai, Alexandros Makis, Panagiotis Kanavaros, and Eleftheria Hatzimichael. 2023. "Unraveling the Immune Microenvironment in Classic Hodgkin Lymphoma: Prognostic and Therapeutic Implications" Biology 12, no. 6: 862. https://doi.org/10.3390/biology12060862
APA StyleGeorgoulis, V., Papoudou-Bai, A., Makis, A., Kanavaros, P., & Hatzimichael, E. (2023). Unraveling the Immune Microenvironment in Classic Hodgkin Lymphoma: Prognostic and Therapeutic Implications. Biology, 12(6), 862. https://doi.org/10.3390/biology12060862





