Regulation of EMT Markers, Extracellular Matrix, and Associated Signalling Pathways by Long Non-Coding RNAs in Glioblastoma Mesenchymal Transition: A Scoping Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
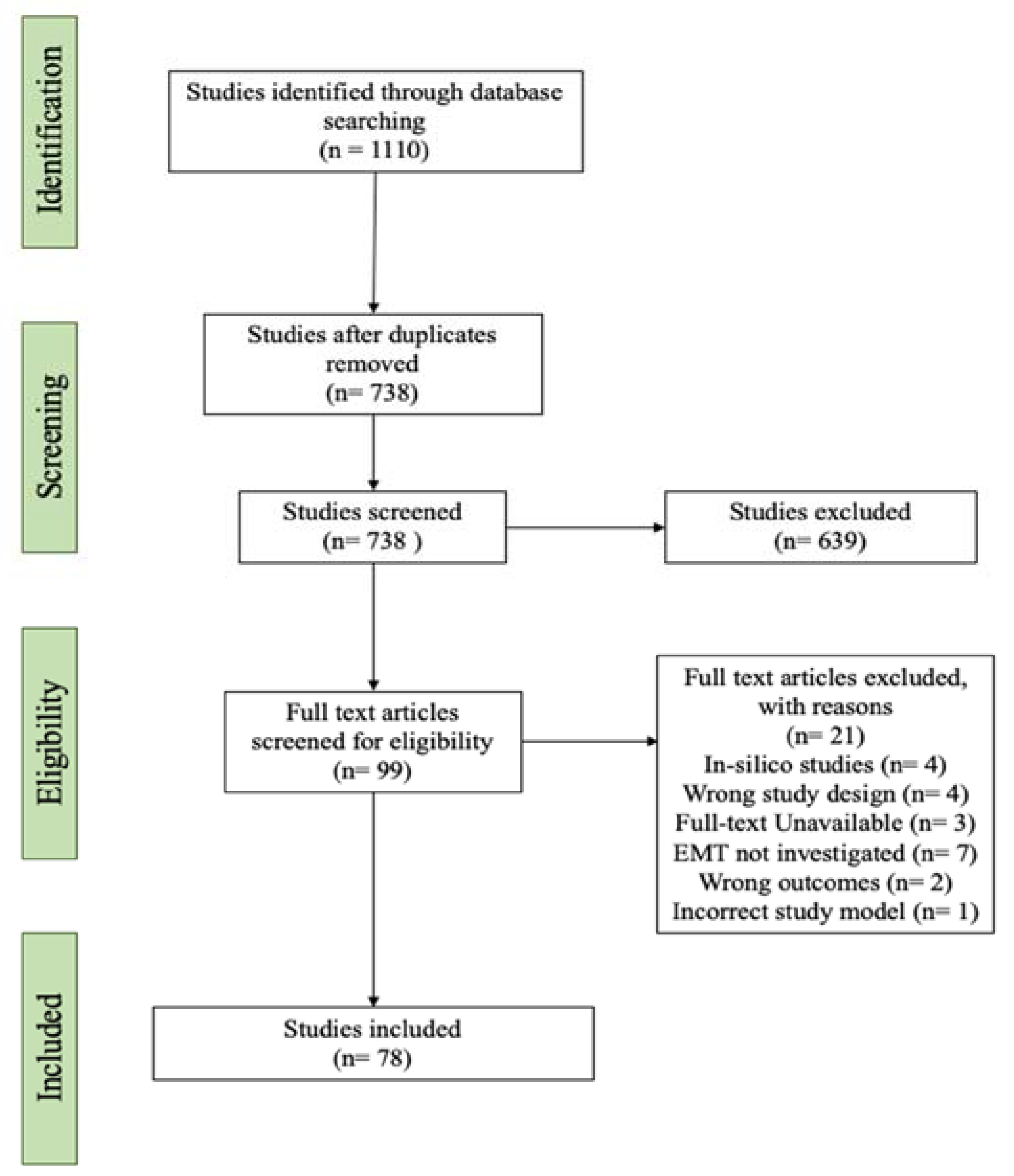
2.1. Literature Search Strategy
2.2. Study Selection
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction and Outcomes
2.5. Validation of lncRNAs via TCGA, and GTEx Datasets
2.6. Gene Set Enrichment Analysis of lncRNAs
3. Results
3.1. Literature Search Results of lncRNAs Regulating GBM MES Transition
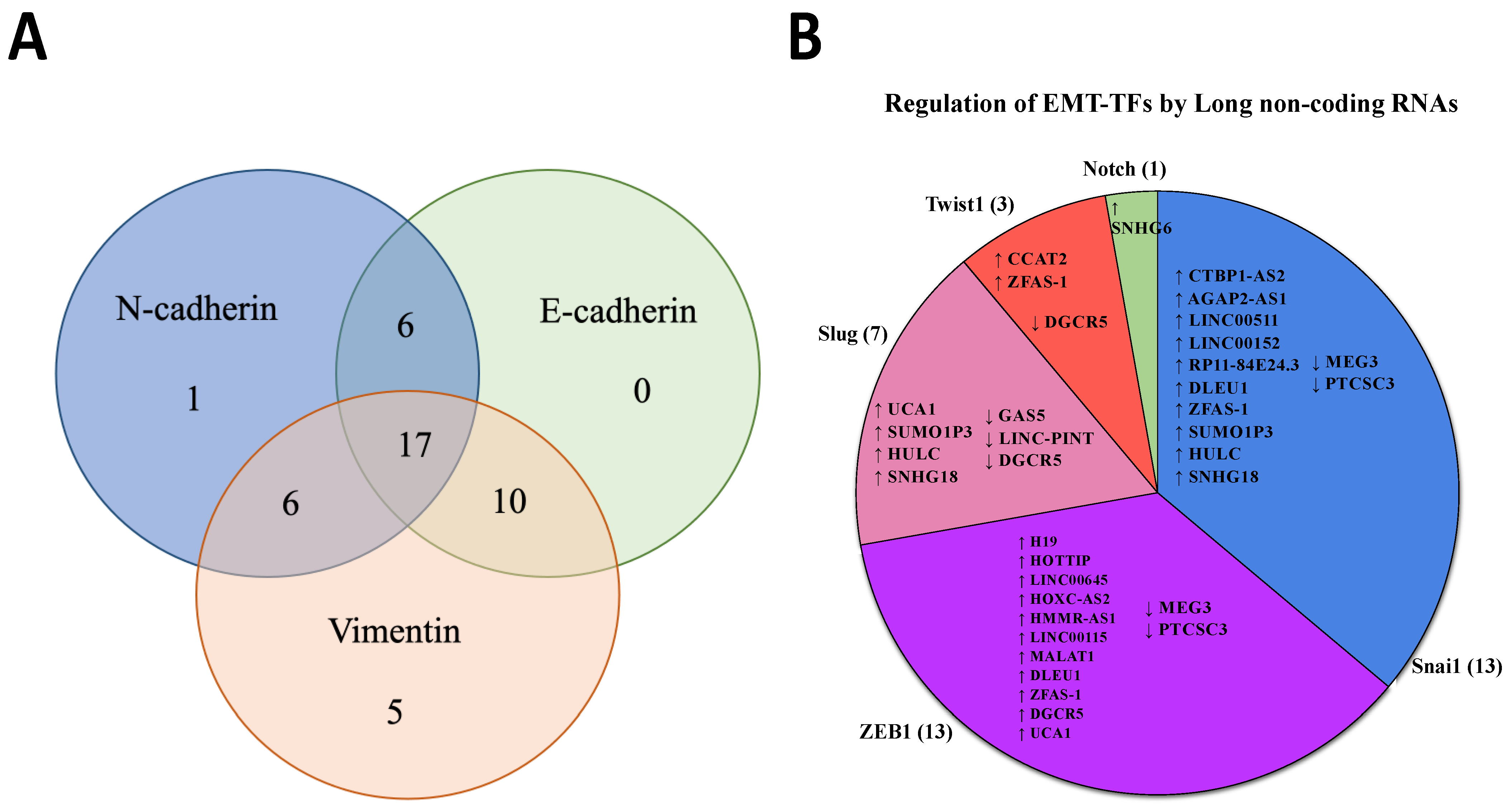
3.2. Dysregulated lncRNAs Affect the Expression of Classical EMT Markers
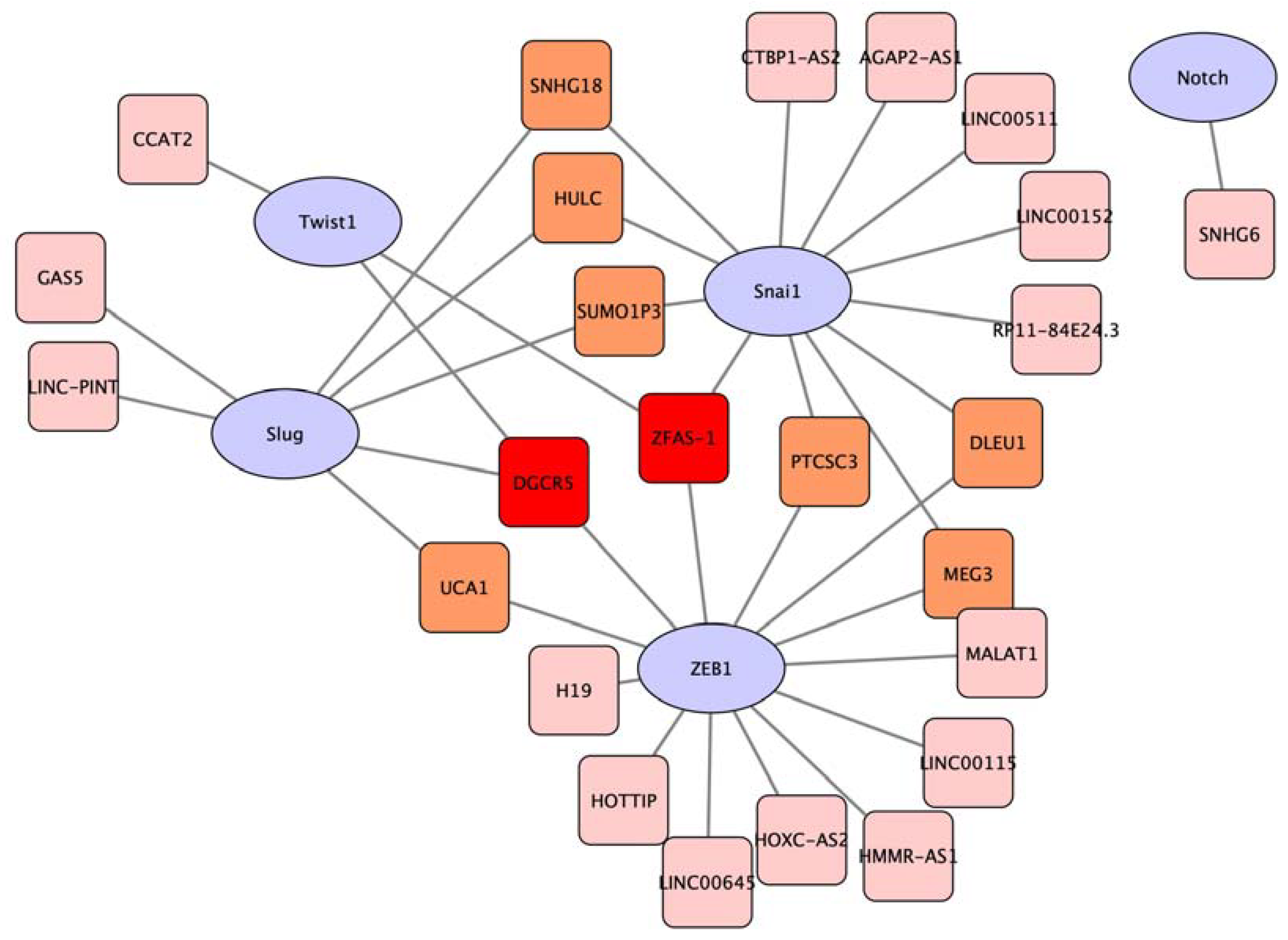
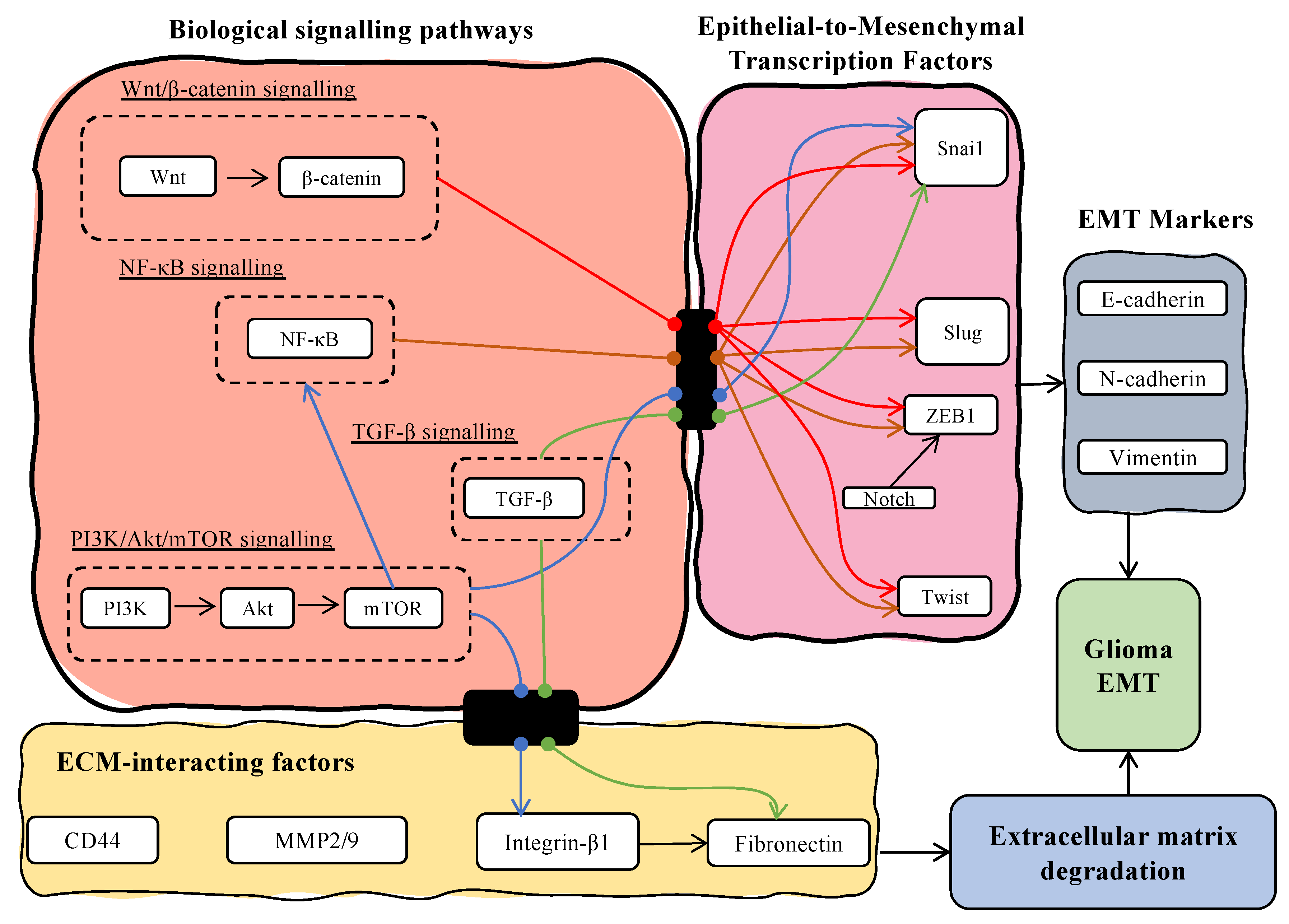
3.3. The Expression of Long Non-Coding RNAs Regulates EMT Transcriptional Factors
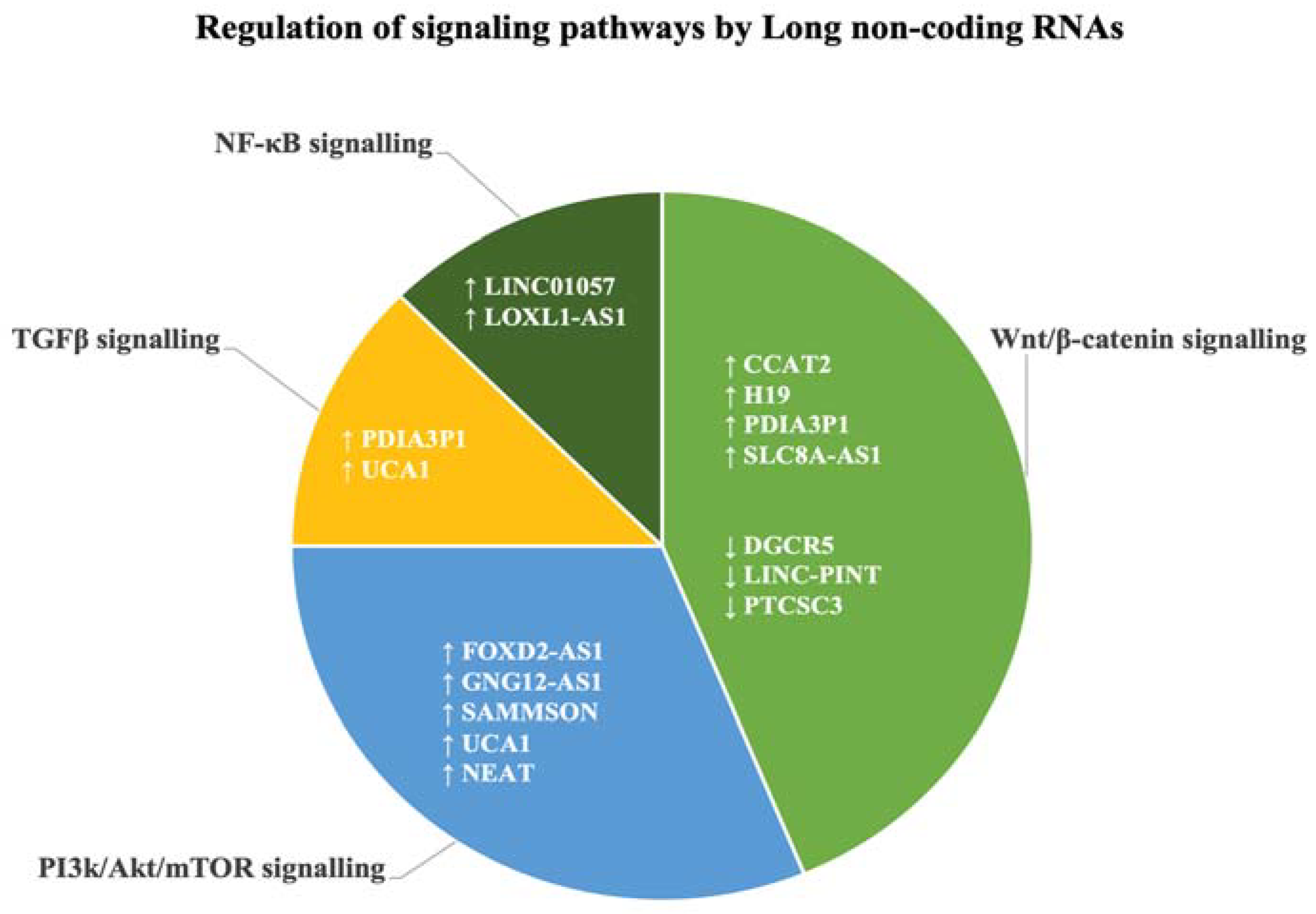
3.4. Long Non-Coding RNAs Target EMT-Associated Biological Signalling Pathways
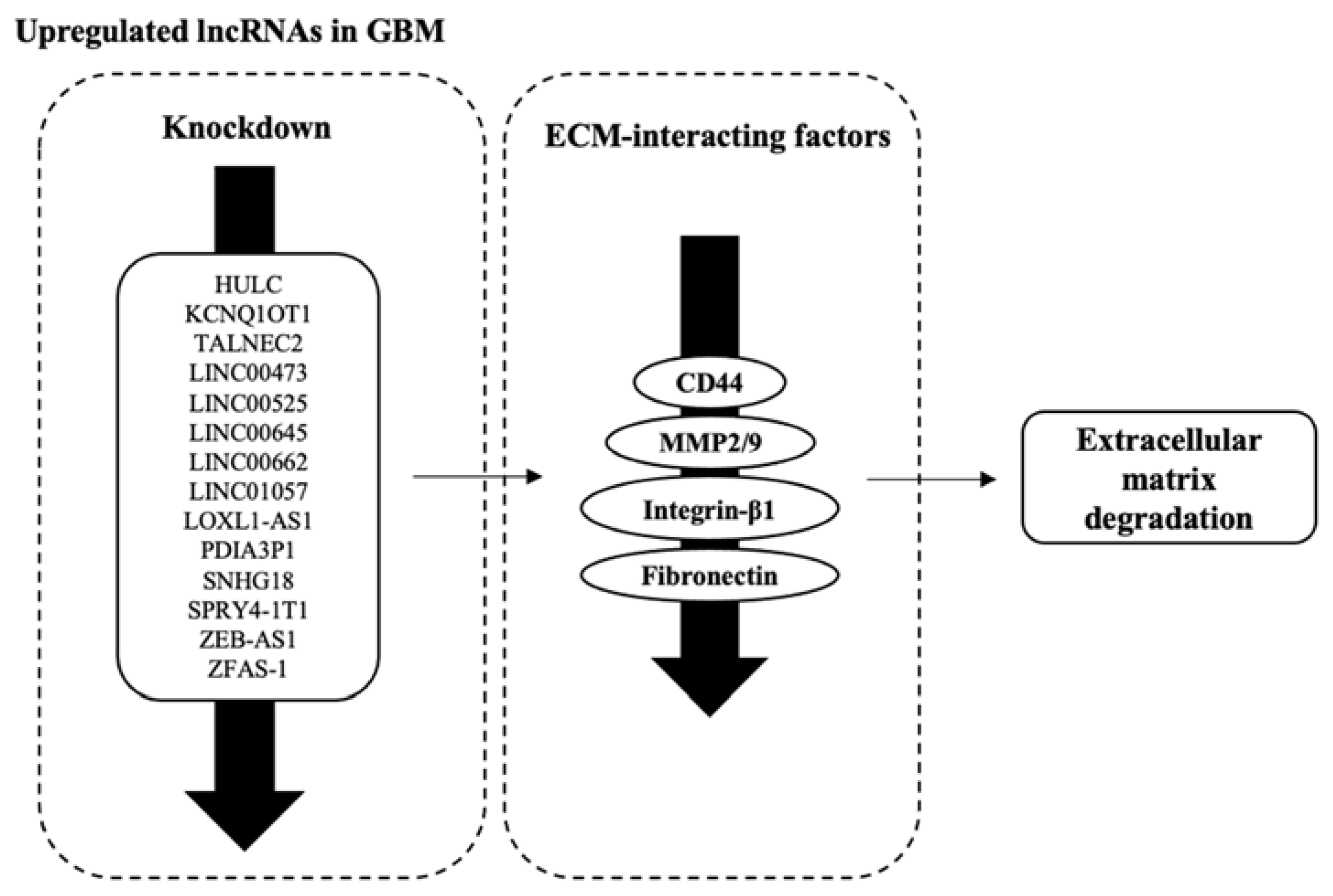
3.5. Factors Facilitating ECM Degradation Are Affected by lncRNAs’ Dysregulation
3.6. Downregulated lncRNAs and Their Potential Tumour-Suppressive Roles in GBM MES Transition
3.7. Long Non-Coding RNAs Are Dysregulated in the Clinical Setting
3.8. Dysregulated lncRNAs Affect Cell Phenotype through Transcriptional and Translational Regulation
4. Discussion
4.1. EMT Markers E- and N-Cadherins Are Commonly Used as Markers of MES Transition in GBM
4.2. Transcription Factors for EMT Play an Important Role in the Overarching Regulation of MES Transition in GBM
4.3. Transcription Factors of EMT Influence Components of Cancer-Associated Signalling Pathways Which Leads to GBM MES Transition
4.4. Long Non-Coding RNAs Influence GBM Microenvironment through Various ECM Components Which Contribute to MES Transition
4.5. Research Is Lacking Outside of Cell Studies to Observe Clinical Relevance of the lncRNAs in GBM MES Transition
4.6. Future Perspective in lncRNA Studies: Moving forward from Cell Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- So, J.S.; Kim, H.; Han, K.S. Mechanisms of Invasion in Glioblastoma: Extracellular Matrix, Ca(2+) Signaling, and Glutamate. Front. Cell Neurosci. 2021, 15, 663092. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zong, H. Developmental origins of brain tumors. Curr. Opin. Neurobiol. 2012, 22, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Vollmann-Zwerenz, A.; Leidgens, V.; Feliciello, G.; Klein, C.A.; Hau, P. Tumor Cell Invasion in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 1932. [Google Scholar] [CrossRef]
- Iwadate, Y. Epithelial-mesenchymal transition in glioblastoma progression. Oncol. Lett. 2016, 11, 1615–1620. [Google Scholar] [CrossRef]
- Roche, J. The Epithelial-to-Mesenchymal Transition in Cancer. Cancers 2018, 10, 52. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef]
- Ansieau, S.; Collin, G.; Hill, L. EMT or EMT-Promoting Transcription Factors, Where to Focus the Light? Front. Oncol. 2014, 4, 353. [Google Scholar] [CrossRef]
- Liu, X.; Yun, F.; Shi, L.; Li, Z.H.; Luo, N.R.; Jia, Y.F. Roles of Signaling Pathways in the Epithelial-Mesenchymal Transition in Cancer. Asian Pac. J. Cancer Prev. 2015, 16, 6201–6206. [Google Scholar] [CrossRef]
- Noh, M.G.; Oh, S.J.; Ahn, E.J.; Kim, Y.J.; Jung, T.Y.; Jung, S.; Kim, K.K.; Lee, J.H.; Lee, K.H.; Moon, K.S. Prognostic significance of E-cadherin and N-cadherin expression in Gliomas. BMC Cancer 2017, 17, 583. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.H.L. Long non-coding RNA in glioblastoma invasion: Angiogenesis and mesenchymal transition via PI3K and Wnt signalling. Asia Pac. J. Mol. Biol. Biotechnol. 2023, 31, 36–52. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Gugnoni, M.; Ciarrocchi, A. Long Noncoding RNA and Epithelial Mesenchymal Transition in Cancer. Int. J. Mol. Sci. 2019, 20, 1924. [Google Scholar] [CrossRef]
- O’Brien, S.J.; Bishop, C.; Hallion, J.; Fiechter, C.; Scheurlen, K.; Paas, M.; Burton, J.; Galandiuk, S. Long non-coding RNA (lncRNA) and epithelial-mesenchymal transition (EMT) in colorectal cancer: A systematic review. Cancer Biol. 2020, 21, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Deng, F.; Qin, Y.; Zhao, Z.; Wu, Z.; Xing, Z.; Ji, A.; Wang, Q.J. Long non-coding RNA regulation of epithelial–mesenchymal transition in cancer metastasis. Cell Death Dis. 2016, 7, e2254. [Google Scholar] [CrossRef]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repečka, K.; McDade, F.; Kamath, A.; Banerjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Vivian, J.; Rao, A.A.; Nothaft, F.A.; Ketchum, C.; Armstrong, J.; Novak, A.; Pfeil, J.; Narkizian, J.; Deran, A.D.; Musselman-Brown, A.; et al. Toil enables reproducible, open source, big biomedical data analyses. Nat. Biotechnol. 2017, 35, 314–316. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.M.; Song, Y.L. Knockdown of long noncoding RNA AB073614 inhibits glioma cell proliferation and migration via affecting epithelial-mesenchymal transition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3997–4002. [Google Scholar] [PubMed]
- Sun, Y.; Shen, Y.; Li, X. Knockdown of long non-coding RNA AGAP2-AS1 suppresses the proliferation and metastasis of glioma by targeting microRNA-497-5p. Bioengineered. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.L.; Zhou, H.G.; Liu, J.; Mao, J. Long Noncoding RNA ASB16-AS1 Promotes Proliferation, Migration, and Invasion in Glioma Cells. Biomed Res. Int. 2019, 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, X.S.; Qi, S.J.; Ma, D.Z.; Fan, J.B.; Wang, J.T. Long non-coding RNA BLACAT1 promotes the proliferation and invasion of glioma cells via Wnt/beta-catenin signaling. Exp. Ther. Med. 2019, 17, 4703–4708. [Google Scholar] [CrossRef]
- Cui, B.Z.; Li, B.S.; Liu, Q.; Cui, Y.Q. lncRNA CCAT1 Promotes Glioma Tumorigenesis by Sponging miR-181b. J. Cell. Biochem. 2017, 118, 4548–4557. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Du, T.P.; Song, Y.F.; Gao, Y.; Li, F.Y.; Wu, R.M.; Chen, Y.J.; Li, W.; Zhou, H.; Yang, Y.; et al. Knockdown of Long Noncoding RNA CCAT2 Inhibits Cellular Proliferation, Invasion, and Epithelial-Mesenchymal Transition in Glioma Cells (vol 25, pg 913, 2017). Oncol. Res. 2020, 28, 551–552. [Google Scholar] [CrossRef]
- Li, Y.F.; Zong, J.; Zhao, C. lncRNA CTBP1-AS2 promotes proliferation and migration of glioma by modulating miR-370-3p-Wnt7a-mediated epithelial-mesenchymal transition. Biochem. Cell Biol. 2020, 98, 661–668. [Google Scholar] [CrossRef]
- Yang, J.X.; Sun, Y.; Gao, L.; Meng, Q.; Yang, B.Y. Long non-coding RNA DANCR facilitates glioma malignancy by sponging miR-33a-5p. Neoplasma 2018, 65, 790–798. [Google Scholar] [CrossRef]
- Lv, Q.L.; Wang, L.C.; Li, D.C.; Lin, Q.X.; Shen, X.L.; Liu, H.Y.; Li, M.; Ji, Y.L.; Qin, C.Z.; Chen, S.H. Knockdown lncRNA DLEU1 Inhibits Gliomas Progression and Promotes Temozolomide Chemosensitivity by Regulating Autophagy. Front. Pharmacol. 2020, 11, 15. [Google Scholar] [CrossRef]
- Xu, D.W.; Liu, R.H.; Meng, L.; Zhang, Y.; Lu, G.J.; Ma, P.J. Long non-coding RNA ENST01108 promotes carcinogenesis of glioma by acting as a molecular sponge to modulate miR-489. Biomed. Pharmacother. 2018, 100, 20–28. [Google Scholar] [CrossRef]
- Dong, H.X.; Cao, W.; Xue, J. Long noncoding FOXD2-AS1 is activated by CREB1 and promotes cell proliferation and metastasis in glioma by sponging miR-185 through targeting AKT1. Biochem. Biophys. Res. Commun. 2019, 508, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.Z.; Chang, H.G.; Gao, G.J.; Zhang, B.; Li, X.S.; Jin, B.Z. Long noncoding RNA FOXD2-AS1 promotes glioma malignancy and tumorigenesis via targeting miR-185-5p/CCND2 axis. J. Cell. Biochem. 2019, 120, 9324–9336. [Google Scholar] [CrossRef]
- Zhao, J.; Zeng, X.B.; Zhang, H.Y.; Xiang, J.W.; Liu, Y.S. Long non-coding RNA FOXD2-AS1 promotes cell proliferation, metastasis and EMT in glioma by sponging miR-506-5p. Open Med. 2020, 15, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.J.; Lv, Q.L.; Chen, X.R.; Zhu, X.T.; Liu, S.K.; Li, D.C.; Peng, X.D. Lnc GNG12-AS1 knockdown suppresses glioma progression through the AKT/GSK-3 beta/beta-catenin pathway. Biosci. Rep. 2020, 40, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Ji, X.J.; Wang, H.D. Targeting Long Noncoding RNA HMMR-AS1 Suppresses and Radiosensitizes Glioblastoma. Neoplasia 2018, 20, 456–466. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Wang, W.W.; Liu, G.X.; Xie, S.L.; Li, Q.X.; Li, Y.R.; Lin, Z.Y. Long non-coding RNA HOTTIP promotes hypoxia-induced epithelial-mesenchymal transition of malignant glioma by regulating the miR-101/ZEB1 axis. Biomed. Pharmacother. 2017, 95, 711–720. [Google Scholar] [CrossRef]
- Chen, W.H.; Li, Q.Y.; Zhang, G.L.; Wang, H.; Zhu, Z.H.; Chen, L.K. LncRNA HOXA-AS3 promotes the malignancy of glioblastoma through regulating miR-455-5p/USP3 axis. J. Cell. Mol. Med. 2020, 24, 11755–11767. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Z.Y.; Liu, D.C.; Xie, P. HOXC13-AS-miR-122-5p-SATB1-C-Myc feedback loop promotes migration, invasion and EMT process in glioma. Oncotargets Ther. 2019, 12, 7165–7172. [Google Scholar] [CrossRef]
- Gao, T.H.; Gu, G.Y.; Tian, J.X.; Zhang, R.; Zheng, X.R.; Wang, Y.A.; Pang, Q.; Liu, Q. LncRNA HSP90AA1-IT1 promotes gliomas by targeting miR-8855p-CDK2 pathway. Oncotarget 2017, 8, 75284–75297. [Google Scholar] [CrossRef]
- Yin, T.T.; Wu, J.; Hu, Y.C.; Zhang, M.; He, J. Long non-coding RNA HULC stimulates the epithelial-mesenchymal transition process and vasculogenic mimicry in human glioblastoma. Cancer Med. 2021, 10, 5270–5282. [Google Scholar] [CrossRef]
- Ding, P.F.; Liang, B.; Shou, J.X.; Wang, X.J. lncRNA KCNQ1OT1 promotes proliferation and invasion of glioma cells by targeting the miR-375/YAP pathway. Int. J. Mol. Med. 2020, 46, 1983–1992. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Soufiany, I.; Lyu, X.; Lu, C.; Wei, Y.; Shi, Z.; You, Y. SP1-upregulated LBX2-AS1 promotes the progression of glioma by targeting the miR-491-5p/LIF axis. J. Cancer 2021, 13, 6989–7002. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yu, B.; Li, Y.; Zhang, W.; Alvarez, A.A.; Hu, B.; Cheng, S.Y.; Feng, H. TGF-β-activated lncRNA LINC00115 is a critical regulator of glioma stem-like cell tumorigenicity. EMBO Rep. 2019, 20, e48170. [Google Scholar] [CrossRef] [PubMed]
- Brodie, S.; Lee, H.K.; Jiang, W.; Cazacu, S.; Xiang, C.L.; Poisson, L.M.; Datta, I.; Kalkanis, S.; Ginsberg, D.; Brodie, C. The novel long non-coding RNA TALNEC2, regulates tumor cell growth and the stemness and radiation response of glioma stem cells. Oncotarget 2017, 8, 31798–31814. [Google Scholar] [CrossRef]
- Cai, J.Q.; Zhang, J.W.; Wu, P.F.; Yang, W.T.; Ye, Q.L.; Chen, Q.; Jiang, C.L. Blocking LINC00152 suppresses glioblastoma malignancy by impairing mesenchymal phenotype through the miR-612/AKT2/NF-B pathway. J. Neuro-Oncol. 2018, 140, 225–236. [Google Scholar] [CrossRef]
- Reon, B.J.; Karia, B.T.R.; Kiran, M.; Dutta, A. LINC00152 Promotes Invasion through a 3′-Hairpin Structure and Associates with Prognosis in Glioblastoma. Mol. Cancer Res. 2018, 16, 1470–1482. [Google Scholar] [CrossRef]
- Li, F.; Shen, Z.Z.; Xiao, C.M.; Sha, Q.K. YY1-mediated up-regulation of lncRNA LINC00466 facilitates glioma progression via miR-508/CHEK1. J. Gene Med. 2021, 23, 15. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Wang, G.W.; Xu, L.P.; Yao, Z.Q.; Song, L.J. Long non-coding RNA LINC00473 promotes glioma cells proliferation and invasion by impairing miR-637/CDK6 axis. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3896–3903. [Google Scholar] [CrossRef]
- Du, X.L.; Tu, Y.M.; Liu, S.; Zhao, P.Z.; Bao, Z.Y.; Li, C.; Li, J.H.; Pan, M.H.; Ji, J. LINC00511 contributes to glioblastoma tumorigenesis and epithelial-mesenchymal transition via LINC00511/miR-524-5p/YB1/ZEB1 positive feedback loop. J. Cell. Mol. Med. 2020, 24, 1474–1487. [Google Scholar] [CrossRef]
- Liu, Z.; Tao, B.; Li, L.; Liu, P.; Xia, K.; Zhong, C. LINC00511 knockdown suppresses glioma cell malignant progression through miR-15a-5p/AEBP1 axis. Brain Res. Bull. 2021, 173, 82–96. [Google Scholar] [CrossRef]
- Wan, Y.; Liang, F.; Wei, M.J.; Liu, Y. Long non-coding RNA LINC00525 regulates the proliferation and epithelial to mesenchymal transition of human glioma cells by sponging miR-338-3p. AMB Express 2020, 10, 9. [Google Scholar] [CrossRef]
- Li, C.L.; Zheng, H.S.; Hou, W.L.; Bao, H.B.; Xiong, J.S.; Che, W.L.; Gu, Y.F.; Sun, H.M.; Liang, P. Long non-coding RNA linc00645 promotes TGF-beta-induced epithelial-mesenchymal transition by regulating miR-205-3p-ZEB1 axis in glioma. Cell Death Dis. 2019, 10, 17. [Google Scholar] [CrossRef]
- Geng, Y.B.; Wu, Y.L.; Xu, C.; Li, T.; Zhang, L.W. Long Non-Coding RNA LINC00662 Regulated Proliferation and Migration by Targeting miR-34a-5p/LMAN2L Axis in Glioma. Oncotargets Ther. 2020, 13, 10161–10172. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.D.; Luo, L.Y.; Zhang, J.L.; Zhai, D.F.; Huang, D.Q.; Yin, J.; Zhou, Q.; Zhang, Q.; Zheng, G.P. lncRNA LINC01057 promotes mesenchymal differentiation by activating NF-kappa B signaling in glioblastoma. Cancer Lett. 2021, 498, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Lulli, V.; Buccarelli, M.; Ilari, R.; Castellani, G.; De Dominicis, C.; Di Giamberardino, A.; D’Alessandris, Q.G.; Giannetti, S.; Martini, M.; Stumpo, V.; et al. Mir-370-3p Impairs Glioblastoma Stem-Like Cell Malignancy Regulating a Complex Interplay between HMGA2/HIF1A and the Oncogenic Long Non-Coding RNA (lncRNA) NEAT1. Int. J. Mol. Sci. 2020, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xu, A.; Wu, B.; Wang, M.; Chen, Z. Long noncoding RNA NEAT1 promotes progression of glioma as a ceRNA by sponging miR-185-5p to stimulate DNMT1/mTOR signaling. J. Cell Physiol. 2021, 236, 121–130. [Google Scholar] [CrossRef]
- Pan, T.; Xue, M. LncRNA-NNT-AS1 contributes to the progression of glioma by miR-582-5p/EZH2 axis. Cytotechnology 2021, 73, 473–482. [Google Scholar] [CrossRef]
- Wang, S.B.; Qi, Y.H.; Gao, X.; Qiu, W.; Liu, Q.L.; Guo, X.F.; Qian, M.Y.; Chen, Z.H.; Zhang, Z.P.; Wang, H.Z.; et al. Hypoxia-induced lncRNA PDIA3P1 promotes mesenchymal transition via sponging of miR-124-3p in glioma. Cell Death Dis. 2020, 11, 17. [Google Scholar] [CrossRef]
- Bi, Y.Y.; Ji, J.; Zhou, Y.X. LncRNA-PVT1 indicates a poor prognosis and promotes angiogenesis via activating the HNF1B/EMT axis in glioma. J. Cancer 2021, 12, 5732–5744. [Google Scholar] [CrossRef]
- Chang, L.; Wang, J.; Zhou, F.; Wang, D.; Chen, R.; Zhang, Y.; Zhang, J. LncRNA RP11-84E24.3 drives tumorigenesis and epithelial-to-mesenchymal transition of glioma cells by promoting TFAP2C-mediated activation of SNAI1. J. Neurooncol. 2021, 151, 157–171. [Google Scholar] [CrossRef]
- Ni, H.Z.; Wang, K.; Xie, P.; Zuo, J.D.; Liu, W.G.; Liu, C. LncRNA SAMMSON Knockdown Inhibits the Malignancy of Glioblastoma Cells by Inactivation of the PI3K/Akt Pathway. Cell. Mol. Neurobiol. 2021, 41, 79–90. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Yang, H.; Zhu, X.L.; Zhang, Y.; Lv, K. Knockdown of long non-coding RNA SLC8A1-AS1 attenuates cell invasion and migration in glioma via suppression of Wnt/beta-catenin signaling pathways. Brain Res. Bull. 2021, 176, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.B.; Xu, C.; Wang, Y.; Zhang, L.W. Long non-coding RNA SNHG11 promotes cell proliferation, invasion and migration in glioma by targeting miR-154-5p. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4901–4908. [Google Scholar] [PubMed]
- Zheng, R.; Yao, Q.; Li, X.; Xu, B. Long Noncoding Ribonucleic Acid SNHG18 Promotes Glioma Cell Motility via Disruption of α-Enolase Nucleocytoplasmic Transport. Front. Genet. 2019, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yang, B.Y.; Liu, B.; Yang, J.X.; Sun, Y. Long non-coding RNA SNHG6 promotes glioma tumorigenesis by sponging miR-101-3p. Int. J. Biol. Mrk. 2018, 33, 148–155. [Google Scholar] [CrossRef]
- Nie, J.; Feng, Y.; Wang, H.; Lian, X.Y.; Li, Y.F. Long Non-Coding RNA SNHG6 Supports Glioma Progression Through Upregulation of Notch1, Sox2, and EMT. Front. Cell Dev. Biol. 2021, 9, 7. [Google Scholar] [CrossRef]
- Liu, H.; Lv, Z.; Guo, E. Knockdown of long noncoding RNA SPRY4-IT1 suppresses glioma cell proliferation, metastasis and epithelial-mesenchymal transition. Int. J. Clin. Exp. Pathol. 2015, 8, 9140–9146. [Google Scholar]
- Lou, J.Y.; Luo, J.; Yang, S.C.; Ding, G.F.; Liao, W.; Zhou, R.X.; Qiu, C.Z.; Chen, J.M. Long non-coding RNA SUMO1P3 promotes glioma progression via the Wnt/β-catenin pathway. Eur. Rev. Med. Pharm. Sci. 2020, 24, 9571–9580. [Google Scholar] [CrossRef]
- Chen, H.Y.; Liu, Z.Y.; Chang, H.; Gao, J.B.; Li, Y.J.; Chen, L.H.; Luo, Y.C.; Xu, R.X. Knockdown of long noncoding RNA UCA1 inhibits glioma cell metastasis via reduction of epithelial-mesenchymal transition. Int. J. Clin. Exp. Pathol. 2016, 9, 12621–12626. [Google Scholar]
- Huang, Z.; Zhao, X.Y.; Wu, X.W.; Xiang, L.; Yuan, Y.N.; Zhou, S.; Yu, W.F. LncRNA UCA1 facilitated cell growth and invasion through the miR-206/CLOCK axis in glioma. Cancer Cell Int. 2019, 19, 9. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Zhong, Q.; Wu, J.; Tang, Z. LncRNA UCA1 is necessary for TGF-β-induced epithelial-mesenchymal transition and stemness via acting as a ceRNA for Slug in glioma cells. FEBS Open Bio 2018, 8, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Xiang, W.C.; Shui, S.F.; Han, X.W.; Guo, D.; Yan, L. 11 Long noncoding RNA UCA1 functions as miR-135a sponge to promote the epithelial to mesenchymal transition in glioma. J. Cell. Biochem. 2020, 121, 2447–2457. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Yang, Y.; Guan, J.; Lv, T.; Qu, S.; Fu, Q.; Zhao, H. LncRNA UCA1 sponges miR-204-5p to promote migration, invasion and epithelial-mesenchymal transition of glioma cells via upregulation of ZEB1. Pathol. Res. Pract. 2018, 214, 1474–1481. [Google Scholar] [CrossRef]
- Luo, C.; Quan, Z.; Zhong, B.; Zhang, M.; Zhou, B.; Wang, S.; Luo, X.; Tang, C. LncRNA XIST promotes glioma proliferation and metastasis through miR-133a/SOX4. Exp. Ther. Med. 2020, 19, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.L.; Hu, L.; Chen, S.H.; Sun, B.; Fu, M.L.; Qin, C.Z.; Qu, Q.; Wang, G.H.; He, C.J.; Zhou, H.H. A long noncoding RNA ZEB1-AS1 promotes tumorigenesis and predicts poor prognosis in glioma. Int. J. Mol. Sci. 2016, 17, 1431. [Google Scholar] [CrossRef]
- Gao, K.; Ji, Z.W.; She, K.; Yang, Q.Y.; Shao, L.B. Long non-coding RNA ZFAS1 is an unfavourable prognostic factor and promotes glioma cell progression by activation of the Notch signaling pathway. Biomed. Pharmacother. 2017, 87, 555–560. [Google Scholar] [CrossRef]
- Lv, Q.L.; Chen, S.H.; Zhang, X.; Sun, B.; Hu, L.; Qu, Q.; Huang, Y.T.; Wang, G.H.; Liu, Y.L.; Zhang, Y.Y.; et al. Upregulation of long noncoding RNA zinc finger antisense 1 enhances epithelial-mesenchymal transition in vitro and predicts poor prognosis in glioma. Tumour Biol. 2017, 39, 1010428317695022. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Zuo, C.; Zhang, K.; Lei, X.; Wang, J.; Yang, Y.; Zhang, J.; Ma, K.; Wang, S.; et al. Long Non-Coding RNA H19 Regulates Glioma Cell Growth and Metastasis via miR-200a-Mediated CDK6 and ZEB1 Expression. Front. Oncol. 2021, 11, 4556. [Google Scholar] [CrossRef]
- Hu, Q.; Yin, J.; Zeng, A.; Jin, X.; Zhang, Z.; Yan, W.; You, Y. H19 Functions as a Competing Endogenous RNA to Regulate EMT by Sponging miR-130a-3p in Glioma. Cell. Physiol. Biochem. 2018, 50, 150–168. [Google Scholar] [CrossRef]
- Jia, L.; Tian, Y.; Chen, Y.; Zhang, G. The silencing of LncRNA-H19 decreases chemoresistance of human glioma cells to temozolomide by suppressing epithelial-mesenchymal transition via the Wnt/β-Catenin pathway. Onco Targets 2018, 11, 313–321. [Google Scholar] [CrossRef]
- Dong, N.; Guo, J.X.; Han, S.; Bao, L.; Diao, Y.; Lin, Z.X. Positive feedback loop of lncRNA HOXC-AS2/miR-876-5p/ZEB1 to regulate EMT in glioma. Oncotargets Ther. 2019, 12, 7601–7609. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, X.; Yan, D.; Li, D.; Guan, F.; Dong, Y.; Wang, H.; Liu, X.; Yang, B. Long Non-Coding RNA MALAT1 Decreases the Sensitivity of Resistant Glioblastoma Cell Lines to Temozolomide. Cell. Physiol. Biochem. 2017, 42, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, L.; Yin, L. Silencing LncRNA LOXL1-AS1 attenuates mesenchymal characteristics of glioblastoma via NF-κB pathway. Biochem. Biophys. Res. Commun. 2018, 500, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, C.; Zhong, C.; Wen, Y.; Ke, S.; Liao, B. Long non-coding RNA CASC2 targeting miR-18a suppresses glioblastoma cell growth, metastasis and EMT in vitro and in vivo. J. Biosci. 2020, 45, 107. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Long, J.; Yang, C.; Gong, B.; Cheng, M.; Wang, Q.; Tang, J. LncRNA DGCR5 plays a tumor-suppressive role in glioma via the miR-21/Smad7 and miR-23a/PTEN axes. Aging 2020, 12, 20285–20307. [Google Scholar] [CrossRef]
- Yang, F.; Huang, Y.L. DGCR5 suppresses the EMT of pediatric primary glioblastoma multiforme cell and serves as a prognostic biomarker. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10024–10034. [Google Scholar] [CrossRef]
- Zhu, X.P.; Pan, S.A.; Chu, Z.; Zhou, Y.X.; Huang, Y.K.; Han, D.Q. LncRNA GAS5 regulates epithelial-mesenchymal transition and viability of glioma cells by targeting microRNA-106b and regulating PTEN expression. Neurosci. Res. 2021, 170, 32–40. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, Z.; Shen, L.; Tang, T.; Yang, M.; Zheng, X. Long Noncoding RNA LINC-PINT Suppresses Cell Proliferation, Invasion, and EMT by Blocking Wnt/β-Catenin Signaling in Glioblastoma. Front. Pharm. 2020, 11, 586653. [Google Scholar] [CrossRef]
- Lei, J.; Zhou, Z. Effect of long intergenic non-coding RNA 00312 on regulating biological behaviors of glioma cells by targeting microRNA-21-3p. Int. J. Clin. Exp. Med. 2021, 14, 852–863. [Google Scholar]
- Fu, Q.; Li, S.S.; Zhou, Q.J.; Yalikun, K.; Yisireyili, D.; Xia, M. Low LINC00599 expression is a poor prognostic factor in glioma. Biosci. Rep. 2019, 39, 9. [Google Scholar] [CrossRef]
- Lu, X.W.; Xu, N.; Zheng, Y.G.; Li, Q.X.; Shi, J.S. Increased expression of long noncoding RNA LINC00961 suppresses glioma metastasis and correlates with favorable prognosis. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4917–4924. [Google Scholar] [PubMed]
- Gong, X.; Huang, M.Y. Tumor-Suppressive Function of lncRNA-MEG3 in Glioma Cells by Regulating miR-6088/SMARCB1 Axis. Biomed Res. Int. 2020, 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.H.; Bian, E.B.; Xu, Y.D.; Ji, X.H.; Tang, F.; Ma, C.C.; Wang, H.L.; Zhao, B. Meg3 Induces EMT and Invasion of Glioma Cells via Autophagy. Oncotargets Ther. 2020, 13, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Ji, R.; Zhan, W. Long noncoding RNA papillary thyroid carcinoma susceptibility candidate 3 (PTCSC3) inhibits proliferation and invasion of glioma cells by suppressing the Wnt/beta-catenin signaling pathway. BMC Neurol. 2017, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, Y.; Zhang, L.; Wang, J.; Wang, W.; Han, X.; Mu, C.; Gao, D. Identification of novel LncRNA targeting Smad2/PKCα signal pathway to negatively regulate malignant progression of glioblastoma. J. Cell Physiol. 2020, 235, 3835–3848. [Google Scholar] [CrossRef]
- Du, L.; Tang, J.-H.; Huang, G.-H.; Xiang, Y.; Lv, S.-Q. The progression of epithelial-mesenchymal transformation in gliomas. Chin. Neurosurg. J. 2017, 3, 23. [Google Scholar] [CrossRef]
- Vesuna, F.; van Diest, P.; Chen, J.H.; Raman, V. Twist is a transcriptional repressor of E-cadherin gene expression in breast cancer. Biochem. Biophys. Res. Commun. 2008, 367, 235–241. [Google Scholar] [CrossRef]
- Chandra, A.; Jahangiri, A.; Chen, W.; Nguyen, A.T.; Yagnik, G.; Pereira, M.P.; Jain, S.; Garcia, J.H.; Shah, S.S.; Wadhwa, H.; et al. Clonal ZEB1-Driven Mesenchymal Transition Promotes Targetable Oncologic Antiangiogenic Therapy Resistance. Cancer Res. 2020, 80, 1498–1511. [Google Scholar] [CrossRef]
- Sánchez-Tilló, E.; Liu, Y.; de Barrios, O.; Siles, L.; Fanlo, L.; Cuatrecasas, M.; Darling, D.S.; Dean, D.C.; Castells, A.; Postigo, A. EMT-activating transcription factors in cancer: Beyond EMT and tumor invasiveness. Cell Mol. Life Sci. 2012, 69, 3429–3456. [Google Scholar] [CrossRef]
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a Master Regulator of Morphogenesis, Plays an Essential Role in Tumor Metastasis. Cell 2004, 117, 927–939. [Google Scholar] [CrossRef]
- Crespo, S.; Kind, M.; Arcaro, A. The role of the PI3K/AKT/mTOR pathway in brain tumor metastasis. J. Cancer Metastasis Treat. 2016, 2, 80–89. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Yue, J.; Hanley, S.J.B.; Kobayashi, N.; Todo, Y.; Watari, H. Long Non-coding RNA NEAT1: A Novel Target for Diagnosis and Therapy in Human Tumors. Front. Genet. 2018, 9, 471. [Google Scholar] [CrossRef] [PubMed]
- Scheau, C.; Badarau, I.A.; Costache, R.; Caruntu, C.; Mihai, G.L.; Didilescu, A.C.; Constantin, C.; Neagu, M. The Role of Matrix Metalloproteinases in the Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma. Anal. Cell. Pathol. 2019, 2019, 9423907. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, X.; Sun, S.; Zhang, X.; Yang, W.; Zhang, J.; Zhang, X.; Jiang, Z. Increased expression of MMP-2 and MMP-9 indicates poor prognosis in glioma recurrence. Biomed. Pharmacother. 2019, 118, 109369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, L.; Li, A.; Han, X. The roles of ZEB1 in tumorigenic progression and epigenetic modifications. Biomed. Pharmacother. 2019, 110, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Zhang, L.; Bao, Y.; Li, B.; He, C.; Gao, M.; Feng, X.; Xu, W.; Zhang, X.; Wang, S. Epithelial-mesenchymal transition, a novel target of sulforaphane via COX-2/MMP2, 9/Snail, ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J. Nutr. Biochem. 2013, 24, 1062–1069. [Google Scholar] [CrossRef]
- Oh, S.J.; Ahn, E.J.; Kim, O.; Kim, D.; Jung, T.Y.; Jung, S.; Lee, J.H.; Kim, K.K.; Kim, H.; Kim, E.H.; et al. The Role Played by SLUG, an Epithelial-Mesenchymal Transition Factor, in Invasion and Therapeutic Resistance of Malignant Glioma. Cell. Mol. Neurobiol. 2019, 39, 769–782. [Google Scholar] [CrossRef]
- Siebzehnrubl, F.A.; Silver, D.J.; Tugertimur, B.; Deleyrolle, L.P.; Siebzehnrubl, D.; Sarkisian, M.R.; Devers, K.G.; Yachnis, A.T.; Kupper, M.D.; Neal, D.; et al. The ZEB1 pathway links glioblastoma initiation, invasion and chemoresistance. EMBO Mol. Med. 2013, 5, 1196–1212. [Google Scholar] [CrossRef]
- Lai, S.W.; Huang, B.R.; Liu, Y.S.; Lin, H.Y.; Chen, C.C.; Tsai, C.F.; Lu, D.Y.; Lin, C. Differential Characterization of Temozolomide-Resistant Human Glioma Cells. Int. J. Mol. Sci. 2018, 19, 127. [Google Scholar] [CrossRef]
- Wu, X.; Yang, L.; Wang, J.; Hao, Y.; Wang, C.; Lu, Z. The Involvement of Long Non-Coding RNAs in Glioma: From Early Detection to Immunotherapy. Front. Immunol. 2022, 13, 2229. [Google Scholar] [CrossRef]
- Phon, B.W.S.; Kamarudin, M.N.A.; Bhuvanendran, S.; Radhakrishnan, A.K. Transitioning pre-clinical glioblastoma models to clinical settings with biomarkers identified in 3D cell-based models: A systematic scoping review. Biomed. Pharmacother. 2022, 145, 112396. [Google Scholar] [CrossRef] [PubMed]
| lncRNA | miRNA Interactions | Knockdown or Overexpression Studies | Effect on EMT Markers Expression | References |
|---|---|---|---|---|
| AB073614 | NA | Knockdown | ↑ E-cadherin ↓ Vimentin | [21] |
| AGAP2-AS1 | mi-497-5p | Knockdown | ↑ E-cadherin ↓ N-cadherin | [22] |
| ASB16-AS1 | NA | Knockdown | ↑ E-cadherin ↓ Vimentin, N-cadherin | [23] |
| BLACAT1 | NA | Knockdown | ↑ E-cadherin ↓ Vimentin//N-cadherin | [24] |
| CCAT1 | miR-181b | Knockdown | ↑ E-cadherin ↓ Vimentin | [25] |
| CCAT2 | NA | Knockdown | ↑ E-cadherin ↓ Vimentin//N-cadherin | [26] |
| CTBP1-AS2 | miR-370-3p | Knockdown | ↑ E-cadherin ↓ Vimentin//N-cadherin | [27] |
| DANCR | miR-33a-5p | Knockdown | ↑ E-cadherin ↓ Vimentin | [28] |
| DLEU1 | NA | Knockdown | ↓ N-cadherin | [29] |
| ENST01108 | miR-489 | Overexpression | ↑ Vimentin ↓ E-cadherin | [30] |
| FOXD2-AS1 | miR-506-5p, miR-185 | Knockdown | ↑ E-cadherin ↓ Vimentin//N-cadherin | [31,32,33] |
| GNG12-AS1 | NA | Knockdown | ↓ Vimentin | [34] |
| HMMR-AS1 | NA | Both | Knockdown↓ Vimentin | [35] |
| HOTTIP | miR-101 | Knockdown | ↑ Vimentin ↓ E-cadherin | [36] |
| HOXA-AS3 | miR-455-5p | Knockdown | ↑ E-cadherin ↓ Vimentin//N-cadherin | [37] |
| HOXC13-AS | miR-122-5p | Knockdown | ↓ Vimentin//N-cadherin | [38] |
| HSP90AA1-IT1 | miR-885-5p | Knockdown | ↓ Vimentin//N-cadherin | [39] |
| HULC | NA | Both | Knockdown ↑ E-cadherin ↓ Vimentin//N-cadherin Overexpression ↑ N-cadherin//Vimentin ↓ E-cadherin | [40] |
| KCNQ1OT1 | miR-375 | Knockdown | ↑ E-cadherin ↓ Vimentin//N-cadherin | [41] |
| LBX2-AS1 | miR-491-5p | Knockdown | ↑ E-cadherin ↓ Vimentin//N-cadherin | [42] |
| LINC00115 | miR-200s | Knockdown | ↑ E-cadherin ↓ Vimentin | [43] |
| LINC00152 | miR-612 and miR-107 | Both | Overexpression ↑ N-cadherin//Vimentin ↓ E-cadherin | [44,45,46] |
| LINC00466 | miR-598 | Knockdown | ↓ Vimentin//N-cadherin | [47] |
| LINC00473 | miR-637 | Knockdown | ↑ E-cadherin ↓ N-cadherin | [48] |
| LINC00511 | miR-524-5p miR-15a-5p | Knockdown | ↑ E-cadherin ↓ Vimentin//N-cadherin | [49,50] |
| LINC00525 | miR-338-3p | Knockdown | ↑ E-cadherin ↓ Vimentin//Fibronectin | [51] |
| LINC00645 | miR-205-3p | Both | Overexpression ↑ Vimentin | [52] |
| LINC00662 | miR-34a-5p | Knockdown | ↓ Vimentin | [53] |
| LINC01057 | NA | Knockdown | ↓ Vimentin | [54] |
| NEAT1 | Mir-370-3p, miR-185-5p | Both | Knockdown ↑ E-cadherin ↓ Vimentin | [55,56] |
| NNT-AS1 | miR-582-5p | Knockdown | ↑ E-cadherin ↓ N-cadherin | [57] |
| PDIA3P1 | miR-124-3p | Both | Knockdown ↓ N-cadherin//Vimentin Overexpression ↑ Vimentin | [58] |
| PVT1 | miR-1207-3p | Knockdown | ↓ N-cadherin//Vimentin | [59] |
| RP11-84E24.3 | NA | Both | Overexpression ↑ N-cadherin//Vimentin ↓ E-cadherin | [60] |
| SAMMSON | NA | Knockdown | ↑ E-cadherin ↓ N-cadherin | [61] |
| SLC8A1-AS1 | NA | Knockdown | ↑ E-cadherin ↓ N-cadherin//Vimentin | [62] |
| SNHG11 | miR-154-5p | Knockdown | ↓ N-cadherin//Vimentin | [63] |
| SNHG18 | NA | Knockdown | ↑ N-cadherin//Vimentin ↓ E-cadherin | [64] |
| SNHG6 | miR-101-3p | Knockdown | ↑ E-cadherin ↓ Vimentin | [65,66] |
| SPRY4-IT1 | NA | Knockdown | ↑ E-cadherin ↓ Vimentin | [67] |
| SUMO1P3 | NA | Both | Knockdown ↑ E-cadherin ↓ N-cadherin//Vimentin | [68] |
| UCA1 | miR-1, miR-203a, miR-204-5p, miR-135a, miR-206 | Both | Knockdown ↑ E-cadherin ↓ N-cadherin//Vimentin Overexpression ↑ N-cadherin//Vimentin ↓ E-cadherin | [69,70,71,72,73] |
| XIST | miR-133a | Knockdown | ↑ E-cadherin ↓ N-cadherin//Vimentin | [74] |
| ZEB1-AS1 | NA | Knockdown | ↑ E-cadherin ↓ N-cadherin | [75] |
| ZFAS-1 | NA | Knockdown | ↑ E-cadherin ↓ N-cadherin | [76,77] |
| lncRNA | miRNA Interactions | Knockdown or overexpression Studies | Effect of lncRNA Expression on EMT-TFs | References |
|---|---|---|---|---|
| AGAP2-AS1 | mi-497-5p | Knockdown | ↓ Snai1 | [22] |
| CCAT2 | NA | Knockdown | ↓ Twist//Snail//β-catenin | [26] |
| CTBP1-AS2 | miR-370-3p | Knockdown | ↓ Snai1 | [27] |
| DLEU1 | NA | Knockdown | ↓ ZEB1//Snai1 | [29] |
| H19 | miR-130a-3p, miR-200a | Both | Overexpression: ↑ ZEB1 | [78,79,80] |
| HMMR-AS1 | NA | Both | Knockdown: ↓ ZEB1 | [35] |
| HOTTIP | miR-101 | Knockdown | HOTTIP indirectly upregulates ZEB1 | [36] |
| HOXC-AS2 | miR-876-5p | Knockdown | ↑ ZEB1, lncRNA also positively regulated by ZEB1 feedback loop | [81] |
| HULC | NA | Both | Overexpression: ↑ Slug//Snai1 | [40] |
| LINC00115 | miR-200s | Knockdown | ↓ ZEB1 | [43] |
| LINC00152 | miR-612 and miR-107 | Both | Overexpression: ↑ Snai1 | [44,45,46] |
| LINC00511 | miR-524-5p miR-15a-5p | Knockdown | ↓ Snai1 | [49,50] |
| LINC00645 | miR-205-3p | Both | Knockdown: ↓ ZEB1 | [52] |
| MALAT1 | NA | Both | Overexpression: ↑ ZEB1 | [82] |
| RP11-84E24.3 | NA | Both | Knockdown: ↓ Snai1 | [60] |
| SNHG18 | NA | Overexpression | ↑ Snai1//Slug | [64] |
| SNHG6 | miR-101-3p | Knockdown | ↓ Notch1 | [65,66] |
| SUMO1P3 | NA | Both | Knockdown: ↓ Slug//Snai1 | [68] |
| UCA1 | miR-1, miR-203a, miR-204-5p, miR-135a, miR-206 | Both | Knockdown: ↓ Slug1//ZEB1 | [69,70,71,72,73] |
| ZFAS-1 | NA | Knockdown | ↓ Snai1//ZEB1//Twist | [76,77] |
| lncRNA | Knockdown or Overexpression Studies | Effect of lncRNA Expression | References |
|---|---|---|---|
| CCAT2 | Knockdown | ↓ β-catenin | [25] |
| FOXD2-AS1 | Knockdown | ↓ AKT1 | [31,32,33] |
| GNG12-AS1 | Knockdown | ↓ P-AKT | [34] |
| H19 | Both | LncRNA affects Wnt/β-catenin pathway | [78,79,80] |
| LINC01057 | Both | LncRNA activates NF-κB | [54] |
| LOXL1-AS1 | Knockdown | LncRNA activates NF-κB indirectly through RelB repression | [83] |
| NEAT1 | Both | Overexpression: ↓ DNMT1 DNMT1 affects mTOR signalling | [55,56] |
| PDIA3P1 | Both | Knockdown: ↓ β-catenin//TGF-β Overexpression: ↑ TGF-β RELA expression directly proportional to NF-κB activity | [58] |
| SAMMSON | Knockdown | Knockdown inactivated PI3K/Akt pathway | [61] |
| SLC8A1-AS1 | Knockdown | SLC8A1-AS1 knockdown impairs Wnt/β-catenin signalling | [62] |
| UCA1 | Both | Knockdown: ↓ p-AKT LncRNA involves in TGF-β signalling | [69,70,71,72,73] |
| lncRNA | Knockdown or Overexpression Studies | Effect of lncRNA Expression | References |
|---|---|---|---|
| HULC | Both | Overexpression: ↑ MMP2//MMP9 | [40] |
| KCNQ1OT1 | Knockdown | ↓ MMP9 | [41] |
| TALNEC2 | Knockdown | ↓ Fibronectin | [44] |
| LINC00473 | Knockdown | ↓ MMP9//CDK6 | [48] |
| LINC00525 | Knockdown | ↓ Fibronectin | [51] |
| LINC00645 | Both | Overexpression: ↑ Fibronectin | [52] |
| LINC00662 | Knockdown | ↓ Fibronectin | [53] |
| LINC01057 | Both | ↓ CD44 | [54] |
| LOXL1-AS1 | Knockdown | ↓ CD44 | [83] |
| PDIA3P1 | Both | Knockdown: ↓ CD44 | [58] |
| SLC8A1-AS1 | Knockdown | ↑ Claudin | [62] |
| SNHG18 | Overexpression | ↑ MMP2//MMP9 | [64] |
| SPRY4-IT1 | Knockdown | ↓ Fibronectin | [67] |
| ZEB1-AS1 | Knockdown | ↓ MMP2//MMP9//Integrin-β1 | [75] |
| ZFAS-1 | Knockdown | ↓ MMP2//MMP9//Integrin-β1 | [76,77] |
| lncRNA | miRNA Interactor | Knockdown or Overexpression Studies | EMT Suppressed/Activated | References |
|---|---|---|---|---|
| CASC2 | miR-18a | Knockdown | ↑ Vimentin//N-cadherin ↓ E-cadherin | [84] |
| DGCR5 | miR-21 and miR-23a | Overexpression | ↑ E-cadherin//ZO-1//β-catenin ↓ Vimentin//Snai2//Twist//ZEB1//Fibronectin | [85,86] |
| GAS5 | miR-106b | Overexpression | ↑ E-cadherin ↓ Slug//Vimentin GAS5 regulates PTEN through miR-106b | [87] |
| LINC-PINT | NA | Overexpression | ↓ N-cadherin//Vimentin//Slug LINC-PINT also suppresses Wnt/β-catenin signalling | [88] |
| LINC00312 | miR-21-3p | Overexpression | ↑ E-cadherin ↓ Vimentin//N-cadherin//MMP2//MMP9 | [89] |
| LINC00599 | NA | Overexpression | ↑ E-cadherin ↓ Vimentin | [90] |
| LINC00961 | NA | Overexpression | ↑ E-cadherin ↓ Vimentin//N-cadherin | [91] |
| MEG3 | miR-6088 | Both | Knockdown: ↑ E-cadherin ↓ Vimentin//N-cadherin//Snai1 Overexpression: ↑ ZEB1//ZEB2 Regulates SMARCB1 through miR-6088 Also reported to have both oncogenic and tumour-suppressive effects in two separate studies | [92,93] |
| PTCSC3 | NA | Overexpression | ↑ E-cadherin ↓ Fibronectin//Snai1//ZEB1 Overexpression also inhibits Wnt/β-catenin pathway | [94] |
| TCONS_00020456 | NA | Knockdown | ↑ N-cadherin//Vimentin ↓ E-cadherin | [95] |
| lncRNA | Fold Change |
|---|---|
| HOXC13-AS | 68.26 |
| HOXA-AS3 | 50.15 |
| H19 | 31.32 |
| HOXC-AS2 | 26.98 |
| HOTTIP | 11.36 |
| SLC8A1-AS1 | 9.88 |
| AGAP2-AS1 | 9.49 |
| LBX2-AS1 | 6.07 |
| LINC00511 | 5.44 |
| DLEU1 | 4.57 |
| lncRNA | Fold Change |
|---|---|
| MEG3 | 0.11 |
| DGCR5 | 0.12 |
| MALAT1 | 0.30 |
| XIST | 0.33 |
| BLACAT1 | 0.34 |
| KCNQ1OT1 | 0.36 |
| SAMMSON | 0.46 |
| LINC-PINT | 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, D.H.L.; Phon, B.W.S.; Sivalingam, M.; Radhakrishnan, A.K.; Kamarudin, M.N.A. Regulation of EMT Markers, Extracellular Matrix, and Associated Signalling Pathways by Long Non-Coding RNAs in Glioblastoma Mesenchymal Transition: A Scoping Review. Biology 2023, 12, 818. https://doi.org/10.3390/biology12060818
Leung DHL, Phon BWS, Sivalingam M, Radhakrishnan AK, Kamarudin MNA. Regulation of EMT Markers, Extracellular Matrix, and Associated Signalling Pathways by Long Non-Coding RNAs in Glioblastoma Mesenchymal Transition: A Scoping Review. Biology. 2023; 12(6):818. https://doi.org/10.3390/biology12060818
Chicago/Turabian StyleLeung, Dexter Hoi Long, Brandon Wee Siang Phon, Mageswary Sivalingam, Ammu Kutty Radhakrishnan, and Muhamad Noor Alfarizal Kamarudin. 2023. "Regulation of EMT Markers, Extracellular Matrix, and Associated Signalling Pathways by Long Non-Coding RNAs in Glioblastoma Mesenchymal Transition: A Scoping Review" Biology 12, no. 6: 818. https://doi.org/10.3390/biology12060818
APA StyleLeung, D. H. L., Phon, B. W. S., Sivalingam, M., Radhakrishnan, A. K., & Kamarudin, M. N. A. (2023). Regulation of EMT Markers, Extracellular Matrix, and Associated Signalling Pathways by Long Non-Coding RNAs in Glioblastoma Mesenchymal Transition: A Scoping Review. Biology, 12(6), 818. https://doi.org/10.3390/biology12060818







