Parasites of Sardinella maderensis (Lowe, 1838) (Actinopterygii: Clupeidae) and Their Potential as Biological Tags for Stock Identification along the Coast of West Africa
Abstract
Simple Summary
Abstract
1. Introduction
- It is more appropriate for studies of small delicate species of fish, such as small clupeoids, for which artificial tags can be used with difficulty, or not at all.
- Using parasite as tags is more cost-effective than artificial tagging because fish samples can be obtained from the routine sampling of commercial or research vessel catches without the need for costly dedicated sampling programs.
- The use of parasite tags has an advantage over the use of host genetics because it can often be used to identify subpopulations of fish distinguished by behavioral differences, but between which there is still a considerable amount of gene flow which can render genetic studies inconclusive.
2. Materials and Methods
2.1. Sample Collection
2.2. Morphological Data
2.3. Parasite Collection
2.4. Parasite Preparation and Preservation
2.5. Data Analysis
2.5.1. Morphological Data Analysis
2.5.2. Parasitological Data Analysis
3. Results
3.1. Morphological Data
3.1.1. Comparison between Fish Total Length and Body Weight across Sample Locations (Benin and Ghana)
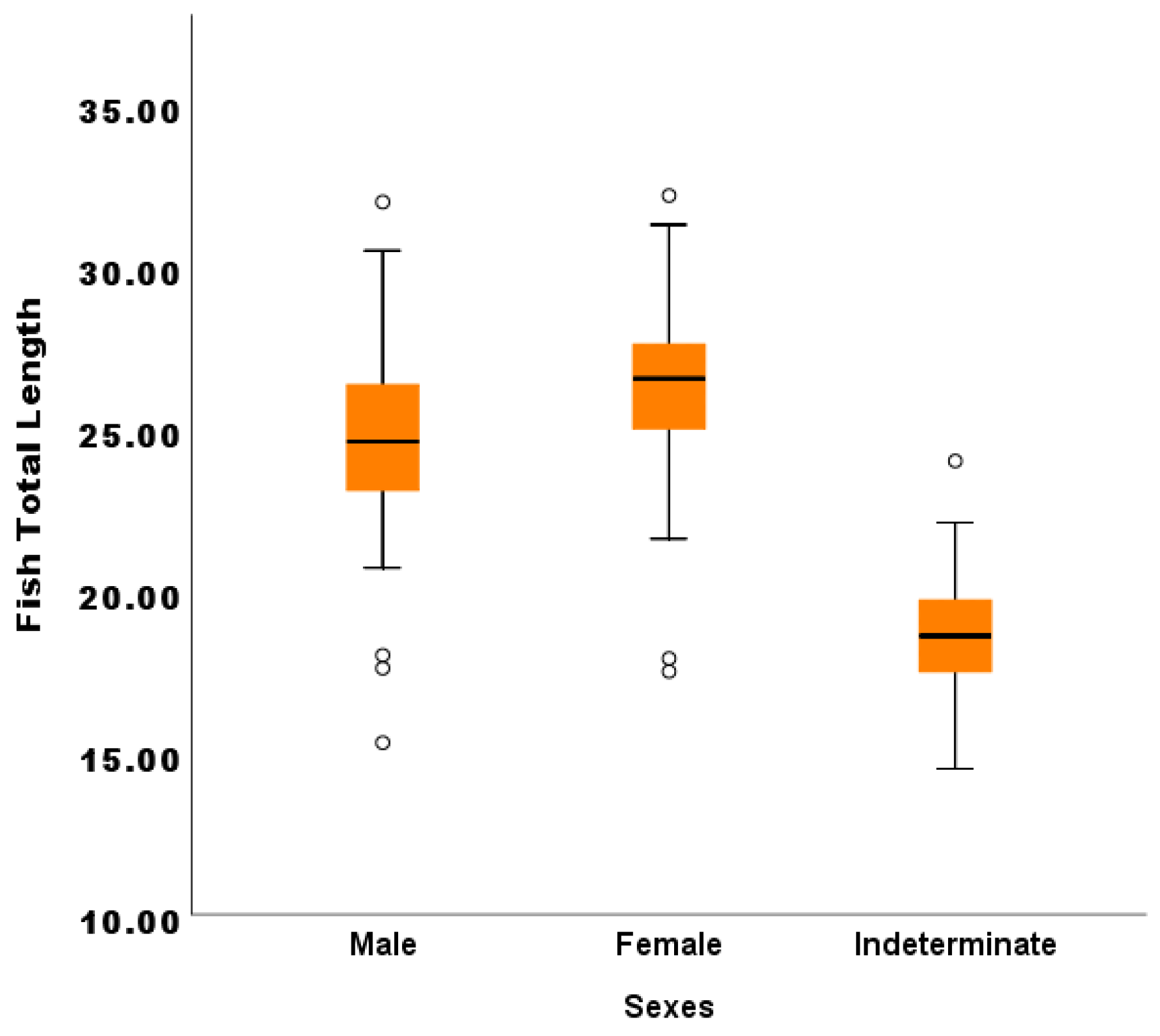
3.1.2. Comparison between the Fish Total Lengths and Body Weight across Sex Categories (Male, Female and Indeterminate Sex)
3.2. Parasite Data
3.2.1. Comparison of Parasite Prevalence and Mean Abundance of Infection across Sampling Locations
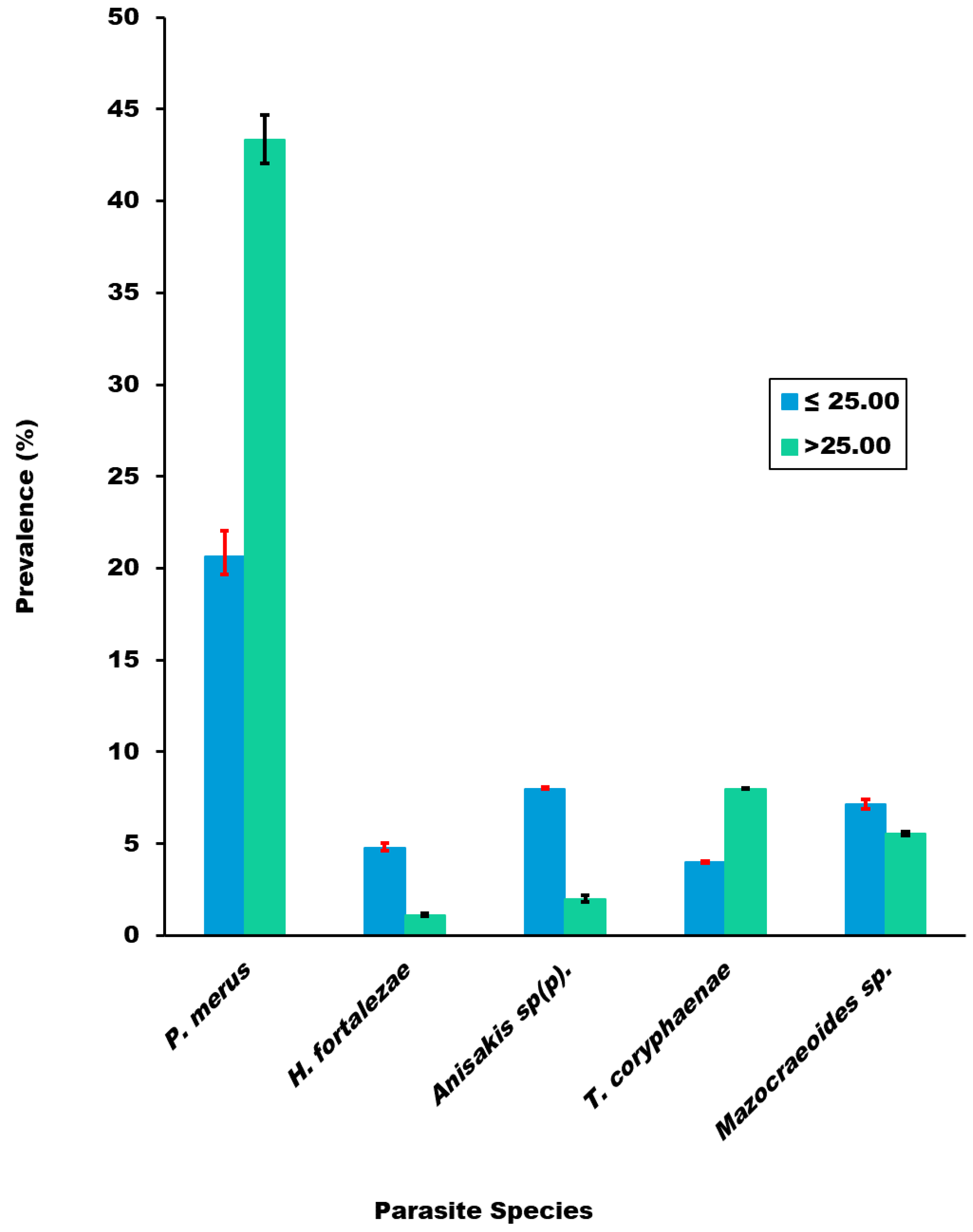
3.2.2. Comparison of Parasite Prevalence and Mean Abundance of Infection across Fish Length Classes
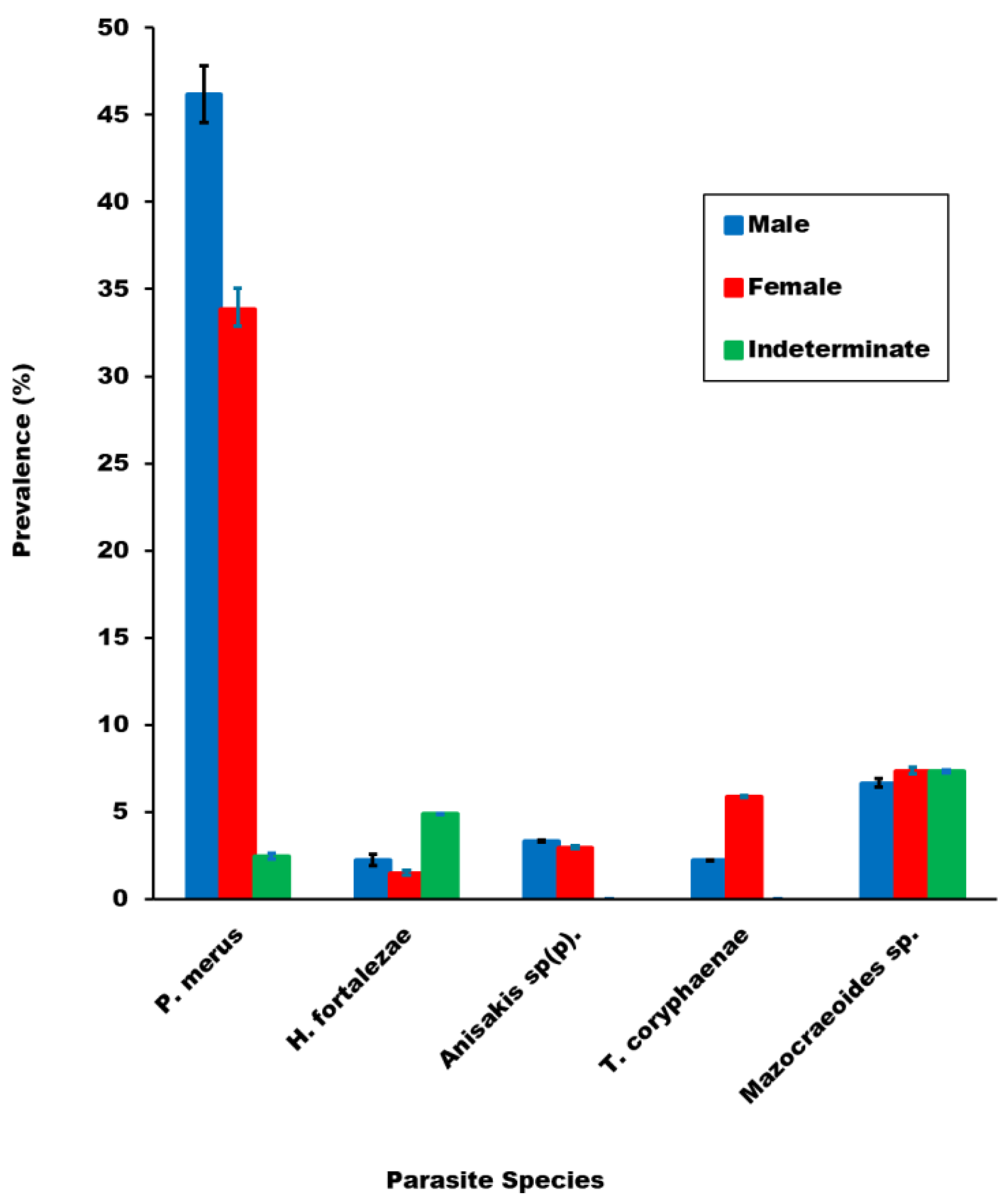
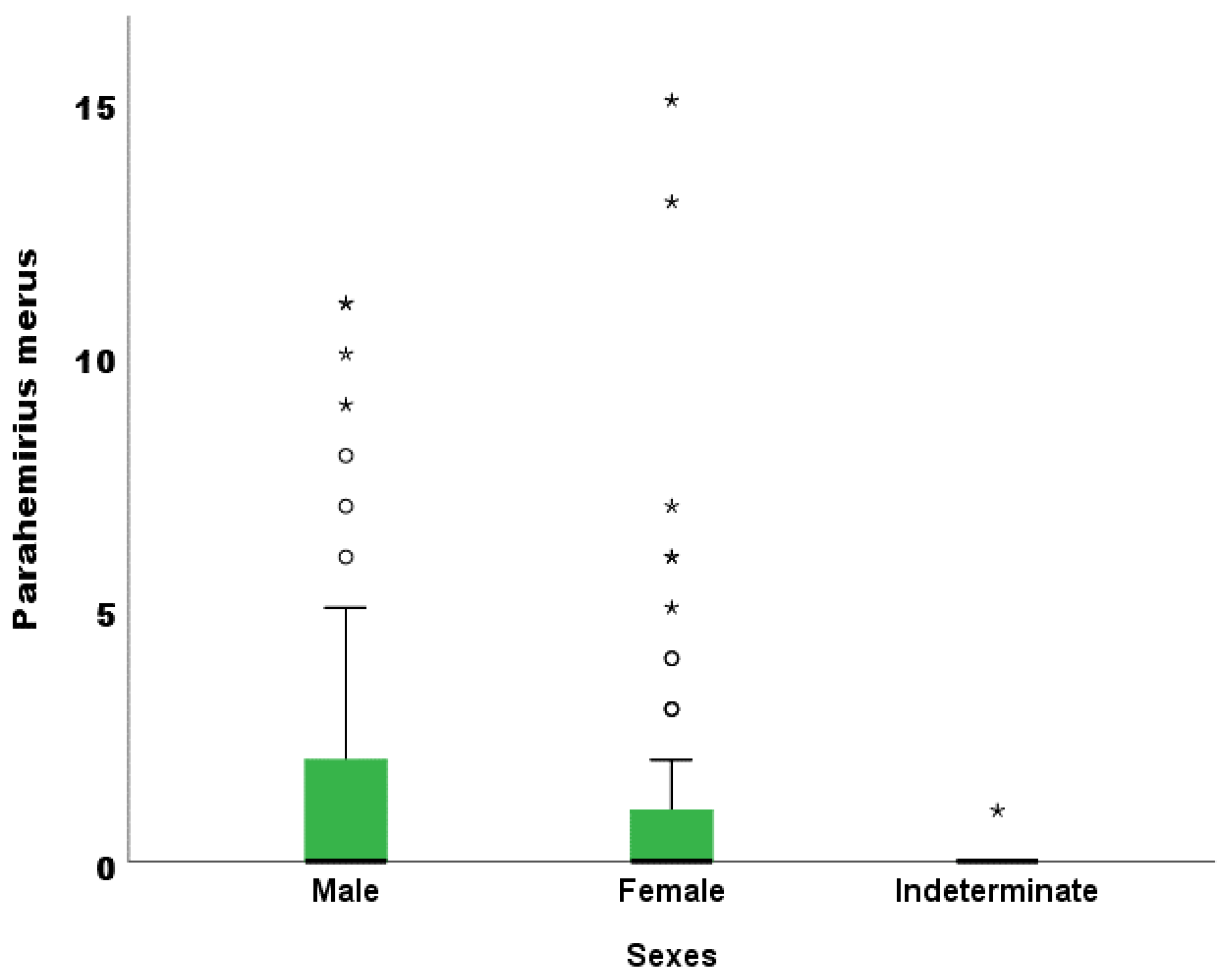
3.2.3. Comparison of Parasite Prevalence and Mean Abundance of Infection across Fish Sex Categories
3.2.4. Relationship between Abundance of Parasites and Fish Length
4. Discussion
4.1. Fish Morphological Data
4.2. Parasitological Data
4.2.1. Relationship between Abundance of Parasite and the Fish Sizes
4.2.2. Parasites Selected as Potential Biological Tags
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reed, C.C. A review of parasite studies of commercially important marine fishes in Sub-Saharan Africa. Parasitology 2015, 142, 109–124. [Google Scholar] [CrossRef]
- Nunoo, F.K.E.; Asiedu, B.; Kombat, E.O. Sardinella and Other Small Pelagics Value and Supply Chain of the Fishery Sector, Ghana; USAID: Narragansett, RI, USA, 2015.
- Amponsah, S.K.K.; Ofori-Danson, P.K.; Nunoo, F.K.E.; Ameyaw, G.A. Estimates of population parameters for Sardinella maderensis (Lowe, 1838) in the coastal waters of Ghana. Greener J. Agric. Sci. 2019, 9, 23–31. [Google Scholar] [CrossRef]
- Sossoukpe, E.; Djidohokpin, G.; Fiogbe, E.D. Demographic parameters and exploitation rate of Sardinella maderensis (Pisces: Lowe 1838) in the nearshore waters of Benin (West Africa) and their implication for management and conservation. Int. J. Fish. Aquat. Stud. 2016, 4, 165–171. [Google Scholar]
- MacKenzie, K.; Abaunza, P. Parasites as biological tags. In Stock Identification Methods: Applications in Fishery Science, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; pp. 185–203. [Google Scholar] [CrossRef]
- Timi, J.T.; MacKenzie, K. Parasites in fisheries and mariculture. Parasitology 2015, 142, 901–909. [Google Scholar] [CrossRef]
- Reed, C.; MacKenzie, K.; Van Der Lingen, C.D. Parasites of South African sardines, Sardinops sagax,and an assessment of their potential as biological tags. Bull. Eur. Assoc. Fish Pathol. 2012, 32, 41–48. [Google Scholar]
- Van Der Lingen, C.D.; Weston, L.F.; Ssempa, N.N.; Reed, C.C. Incorporating parasite data in population structure studies of South African sardine Sardinops sagax. Parasitology 2015, 142, 156–167. [Google Scholar] [CrossRef]
- Lazar, N.; Yankson, K.; Blay, J.; Ofori-Danson, P.; Markwei, P.; Agbogah, K.; Bannerman, P.; Sotor, M.; Yamoah, K.; Bilisini, W. Status of The Small Pelagic Stocks in Ghana and Recommendations to Achieve Sustainable Fishing 2017; Scientific and Technical Working Group, USAID/Ghana Sustainable Fisheries Management Project (SFMP), Coastal Resources Center, Graduate School of Oceanography, University of Rhode Island: Kingston, RI, USA, 2018. [Google Scholar]
- Klimpel, S.; Kuhn, T.; Münster, J.; Dörge, D.D.; Klapper, R.; Kochmann, J. Techniques. In Parasites of Marine Fish and Cephalopods: A Practical Guide; Springer International Publishing: Cham, Switzerland, 2019; pp. 77–147. [Google Scholar] [CrossRef]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. Revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for parasitologists—A primer to quantitative parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef]
- Osei, I.K.; Blay, J.; Asare, N. An update of the reproductive biology of sardinellas (Family: Clupeidae) in the coastal waters of Ghana. J. Fish Res. 2021, 5, 1–9. [Google Scholar]
- Wehye, A.; Amponsah, S.; Jueseah, A. Growth, mortality and exploitation of Sardinella maderensis (Lowe, 1838) in the Liberian coastal waters. Fish. Aquac. J. 2017, 8, 2. [Google Scholar] [CrossRef]
- Ogunola, O.S.; Onada, O.A. Preliminary investigation of length–weight relationships and condition factors of two commercially important fish species (mullet, Mugil cephalus (Linnaeus 1758) and sardine, Sardinella maderensis (Lowe 1838)) in Okrika Creeks (Niger-Delta) of Nigeria. Reg. Stud. Mar. Sci. 2017, 13, 54–58. [Google Scholar] [CrossRef]
- Sümer, Ç. Length-Weight relationships of 15 lagoon fish species collected in the Beymelek Lagoon (SW Turkey). Cah. Biol. Mar. 2012, 53, 185–188. [Google Scholar]
- Bray, R.A. A review of the genus Parahemiurus Vaz & Pereira, 1930 (Digenea: Hemiuridae). Syst. Parasitol. 1990, 15, 1–21. [Google Scholar] [CrossRef]
- Fischthal, J.H.; Thomas, J.D. Some hemiurid trematodes of marine fishes from Ghana. Helminthol. Soc. Washingt. 1971, 38, 181–189. [Google Scholar]
- Ndiaye, P.I.; Bakhoum, A.J.S.; Sène, A.; Miquel, J. Ultrastructure of the spermatozoon of Parahemiurus merus (Linton, 1910) (Digenea: Hemiuroidea: Hemiuridae), a parasite of Sardinella aurita (Valenciennes, 1847) and S. maderensis (Lowe, 1838) (Teleostei: Clupeidae) in the Senegalese coast. Zool. Anz. 2013, 252, 572–578. [Google Scholar] [CrossRef]
- Derbel, H.; Châari, M.; Neifar, L. Digenean species diversity in teleost fishes from the Gulf of Gabes, Tunisia (Western Mediterranean). Parasite 2012, 19, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Feki, M.; Châari, M.; Neifar, L. Spatial variability of helminth parasites and evidence for stock discrimination in the round sardinella, Sardinella aurita (Valenciennes, 1847), off the coast of Tunisia. J. Helminthol. 2016, 90, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Ramdani, S.; Trilles, J.P.; Ramdane, Z. Parasitic fauna of Sardinella aurita (Valenciennes, 1847) from Algerian coast. Zool. Ecol. 2020, 30, 101–108. [Google Scholar] [CrossRef]
- De Almeida, F.M.; Barquete, V.; Pereira Junior, J. Progenetic metacercariae of Parahemiurus merus (Platyhelminthes, Digenea, Hemiurudae) infecting Parasagitta friderici (Chaetognatha) from southern coast Brazil. Atlântica Rio Gd. 2009, 31, 35–38. [Google Scholar] [CrossRef]
- MacKenzie, K. Parasites as biological tags in population studies of marine fish. Parasitol. Int. 1998, 124, 153–163. [Google Scholar] [CrossRef]
- Andres, M.J.; Peterson, M.S.; Overstreet, R.M. Endohelminth parasites of some midwater and benthopelagic stomiiform fishes from the Northern Gulf of Mexico. Gulf Caribb. Res. 2016, 27, 11–19. [Google Scholar] [CrossRef]
- Fontenelle, G.; Knoff, M.; Felizardo, N.N.; Torres, E.J.L.; da Lopes, L.M.S.; Gomes, D.C.; de Clemente, S.C.S. Larvas Anisakidae e Raphidascarididae parasitos de Selene setapinnis (Mitchill, 1815) no estado do Rio de Janeiro, Brasil. Rev. Bras. Parasitol. Vet. 2015, 24, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Diniz, J.B.; Knoff, M.; da Fonseca, M.C.G.; Gomes, D.C.; de Clemente, S.C.S. Cestode and nematode larvae of hygienic-sanitary importance parasitizing Percophis brasiliensis (Actinopterygii) collected from fish markets of the municipality of Niterói, RJ, Brazil. Food Sci. Technol. 2021, 2061, 1–8. [Google Scholar] [CrossRef]
- Deardorff, T.; Overstreet, R. Larval Hysterothylacium (=thynnascaris) (Nematoda: Anisakidae) from fishes and invertebrates in the Gulf of Mexico. In The Helminthological Society of Washington; The Helminthological Society of Washington: Lawrence, KS, USA, 1981; pp. 113–126. [Google Scholar]
- Odum, B.; Amuzie, C.C. Helminth parasites of Caranx hippos (Crevalle Jack) and Sardinella maderensis (Madeiran Sardinella) from two fishing settlements in Okrika, Rivers State, Nigeria. Niger. J. Parasitol. 2021, 42, 75–80. [Google Scholar] [CrossRef]
- Mattiucci, S.; Cipriani, P.; Webb, S.C.; Paoletti, M.; Marcer, F.; Bellisario, B.; Gibson, D.I.; Nascetti, G. Genetic and morphological approaches distinguish the three sibling species of the Anisakis simplex species complex, with a species designation as Anisakis berlandi n. sp. for A. simplex sp. C (Nematoda: Anisakidae). J. Parasitol. 2014, 100, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W. Anisakis simplex (Rudolphi, 1809, Det. Krabbe, 1878) (Nematoda: Ascaridoidea): Morphology and morphometry of larvae from euphausiids and fish, and a review of the life-history and ecology. J. Helminthol. 1983, 57, 205–224. [Google Scholar] [CrossRef]
- Klimpel, S.; Palm, H.W. Anisakid nematode (Ascaridoidea) life cycles and distribution: Increasing zoonotic potential in the time of climate change? In Progress in Parasitology. Parasitology Research Monographs; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 2, pp. 201–222. [Google Scholar] [CrossRef]
- Stanley, A.; Sroufe, J. Mazocraeoides olentangiensis, n. sp., a monogenetic trematode parasitic on the gills of the gizzard shad, Dorosoma cepedianum (Le Sueur). J. Parasitol. 1958, 44, 643–646. [Google Scholar] [CrossRef]
- Sailaja, B.; Shameem, U.; Madhavi, R. Four species of Mazocraeoides (Price, 1936) (Monogenea: Mazocraeidae), including two new species from clupeiform fishes off Visakhapatnam coast, Bay of Bengal. Zootaxa 2019, 4608, 233–246. [Google Scholar] [CrossRef]
- Beverley-Burton, M. Monogenea and Turbellaria. Guide to parasites fishes Canada. Part I. Can. Spec. Publ. Fish. Aquat. Sci. 1984, 74, 5–209. [Google Scholar]
- Caira, J.N.; Jensen, K. A digest of elasmobranch tapeworms. J. Parasitol. 2014, 100, 373–391. [Google Scholar] [CrossRef]
- Palm, H.W.; Waeschenbach, A.; Littlewood, D.T.J. Genetic diversity in the trypanorhynch cestode Tentacularia coryphaenae (Bosc, 1797): Evidence for a cosmopolitan distribution and low host specificity in the teleost intermediate host. Parasitol. Res. 2007, 101, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Nunkoo, M.A.I.; Reed, C.C.; Kerwath, S.E. Community ecology of the metazoan parasites of snoek Thyrsites atun (Euphrasen, 1791) (Perciformes: Gempylidae) off South Africa. African J. Mar. Sci. 2016, 38, 363–371. [Google Scholar] [CrossRef]
- Nunkoo, I.; Weston, M.J.; Reed, C.C.; Der Lingen, C.D.V.; Kerwath, S. First account of the metazoan parasite fauna of oilfish Ruvettus pretiosus Cocco, 1829 (Perciformes: Gempylidae) in South African waters. African Zool. 2017, 52, 237–241. [Google Scholar] [CrossRef]
- Santos, M.J.; Saraiva, A.; Cruz, C.; Eiras, J.C.; Hermida, M.; Ventura, C.; Soares, J.P. Use of parasites as biological tags in stock identification of the black scabbardfish, Aphanopus carbo (Lowe, 1839) (Osteichthyes: Trichiuridae) from Portuguese waters. Sci. Mar. 2009, 73, 55–62. [Google Scholar] [CrossRef]
- Hendricks, J.P. The parasite assemblage of Scomber japonicus (Houttyun, 1782) off South Africa. Master’s Thesis, Faculty of Science, Department of Biological Sciences, University of Cape Town, Cape Town, South Africa, 2019. [Google Scholar]
- Tavares, L.E.; Luque, J.L.; Bicudo, A.J. Community ecology of metazoan parasites of the anchovy Anchoa tricolor (Osteichthyes: Engraulidae) from the coastal zone of the State of Rio de Janeiro, Brazil. Braz. J. Biol. 2005, 65, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Soares, I.A.p.; Vieira, F.M.a.; Luque, J.L. Parasite community of Pagrus pagrus (Sparidae) from Rio de Janeiro, Brazil: Evidence of temporal stability. Rev. Bras. Parasitol. Vet. 2014, 23, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.; Paschoal, F. Community ecology of the metazoan parasites of the atlantic thread herring, Opisthonema oglinum (Lesueur, 1818) (Actinopterygii: Clupeidae) from the Sepetiba Bay, Rio de Janeiro, Brazil. Brazilian J. Biol. 2021, 81, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Ichalal, K.; Ramdane, Z.; Ider, D.; Kacher, M.; Iguerouada, M.; Trilles, J.P.; Courcot, L.; Amara, R. Nematodes parasitizing Trachurus trachurus (L.) and Boops boops (L.) from Algeria. Parasitol. Res. 2015, 114, 4059–4068. [Google Scholar] [CrossRef] [PubMed]
- González, T.; Acuña, E. Influence of host size and sex on the endohelminth infracommunities of the red rockfish Sebastes capensis off Northern Chile. J. Parasitol. 2000, 86, 854–857. [Google Scholar] [CrossRef]
- Diatta, Y.; Bonaventure, L.; Tidiane, C. Étude du régime alimentaire de Sardinella aurita (Valenciennes, 1847) et de S. maderensis (Lowe, 1841) de la côte occidentale de l’Afrique. Bull. L’institut Natl. Des Sci. Technol. La Mer 2016, 43, 21–37. [Google Scholar]
- Baali, A.; Ouazzani, K.C.; Touhami, F.; El-Achi, A.; Amenzoui, K. Diet composition of round sardinella (Sardinella aurita) and flat sardinella (Sardinella maderensis) in the South of Atlantic Moroccan coast. Egypt. J. Aquat. Biol. Fish. 2020, 24, 73–91. [Google Scholar] [CrossRef]
- MacKenzie, K. Parasites as biological tags in population studies of marine organisms: An update. Parasitology 2002, 124, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Lester, R.J.G.; Barnes, A.; Habib, G. Parasites of skipjack tuna, Katsuwonus pelamis: Fishery implications. Fish. Bull. 1985, 83, 343–356. [Google Scholar]
- Jakob, E.; Palm, H.W. Parasites of commercially important fish species from the Southern Java coast, Indonesia, including the distribution pattern of trypanorhynch cestodes. Verb. Ges. Ichthyol. 2006, 5, 165–191. [Google Scholar]



| Sampling | N | Sex | TL Range (cm) | BW Range (g) | ||
|---|---|---|---|---|---|---|
| Locations | M | I | F | (Mean ± SD) | (Mean ± SD) | |
| Benin | 100 | 50 | 3 | 47 | 14.5–32.20 | 28.00–287.00 |
| (17.70 ± 2.97) | (144.95 ± 47.25) | |||||
| Ghana | 100 | 41 | 38 | 21 | 16.00–32.00 | 38.46–258.92 |
| (16.00 ± 3.98) | (120.53 ± 57.41) | |||||
| Parasites | SI | Benin (Cotonou) | Ghana (Elmina) | ||
|---|---|---|---|---|---|
| (n = 100) | (n = 100) | ||||
| P (%) | MA ± SD | P (%) | MA ± SD | ||
| (95% CI) | (95% CI) | ||||
| Digenea | |||||
| P. merus | S | 45 | 1.63 ± 2.76 | 21 | 0.75 ± 2.08 |
| (1.19–2.3) | (0.43–1.25) | ||||
| Nematode | |||||
| H. fortalezae * | S | 1 | 0.06 ± 0.60 | 4 | 0.3 ± 2.28 |
| (0–0.18) | (0.03–1.3) | ||||
| Anisakis sp(p). | S/L | 5 | 0.14 | 0 | 0 |
| (0.03–0.34) | |||||
| Cestode | |||||
| T. coryphaenae * | V | 6 | 0.07 (0.02–0.13) | 0 | 0 |
| Monogenea | |||||
| Mazocraeoides sp.* | G | 3 | 0.08 ± 0.55 | 11 | 0.3 ± 1.19 |
| (0.01–0.27) | (0.13–0.67) | ||||
| Parasites | Prevalence (%) | Mean Abundance | ||||
|---|---|---|---|---|---|---|
| Benin | Ghana | p-Value | Benin | Ghana | p-Value | |
| Digenea | ||||||
| P. merus | 45 | 21 | <0.05 * | 1.63 | 0.75 | <0.05 * |
| Nematoda | ||||||
| H. fortalezae | 1 | 4 | >0.05 | 0.06 | 0.3 | - |
| Monogenea | ||||||
| Mazocraeoides sp. | 3 | 11 | <0.05 * | 0.08 | 0.3 | >0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogbon, A.M.; Afoakwah, R.; Mireku, K.K.; Tossavi, N.D.; MacKenzie, K. Parasites of Sardinella maderensis (Lowe, 1838) (Actinopterygii: Clupeidae) and Their Potential as Biological Tags for Stock Identification along the Coast of West Africa. Biology 2023, 12, 389. https://doi.org/10.3390/biology12030389
Ogbon AM, Afoakwah R, Mireku KK, Tossavi ND, MacKenzie K. Parasites of Sardinella maderensis (Lowe, 1838) (Actinopterygii: Clupeidae) and Their Potential as Biological Tags for Stock Identification along the Coast of West Africa. Biology. 2023; 12(3):389. https://doi.org/10.3390/biology12030389
Chicago/Turabian StyleOgbon, Abdou Matinou, Richmond Afoakwah, Kwadwo Kesse Mireku, Nounagnon Darius Tossavi, and Ken MacKenzie. 2023. "Parasites of Sardinella maderensis (Lowe, 1838) (Actinopterygii: Clupeidae) and Their Potential as Biological Tags for Stock Identification along the Coast of West Africa" Biology 12, no. 3: 389. https://doi.org/10.3390/biology12030389
APA StyleOgbon, A. M., Afoakwah, R., Mireku, K. K., Tossavi, N. D., & MacKenzie, K. (2023). Parasites of Sardinella maderensis (Lowe, 1838) (Actinopterygii: Clupeidae) and Their Potential as Biological Tags for Stock Identification along the Coast of West Africa. Biology, 12(3), 389. https://doi.org/10.3390/biology12030389






