Neuromodulation of Neural Oscillations in Health and Disease
Simple Summary
Abstract
1. Introduction
2. Neural Oscillations in Cognition and Neurological Disorders
2.1. Frequency Spectral Analysis
2.1.1. Healthy Functions
2.1.2. Neurological Diseases
2.2. Cross-Frequency Coupling Analysis
2.2.1. Healthy Functions
2.2.2. Neurological Diseases
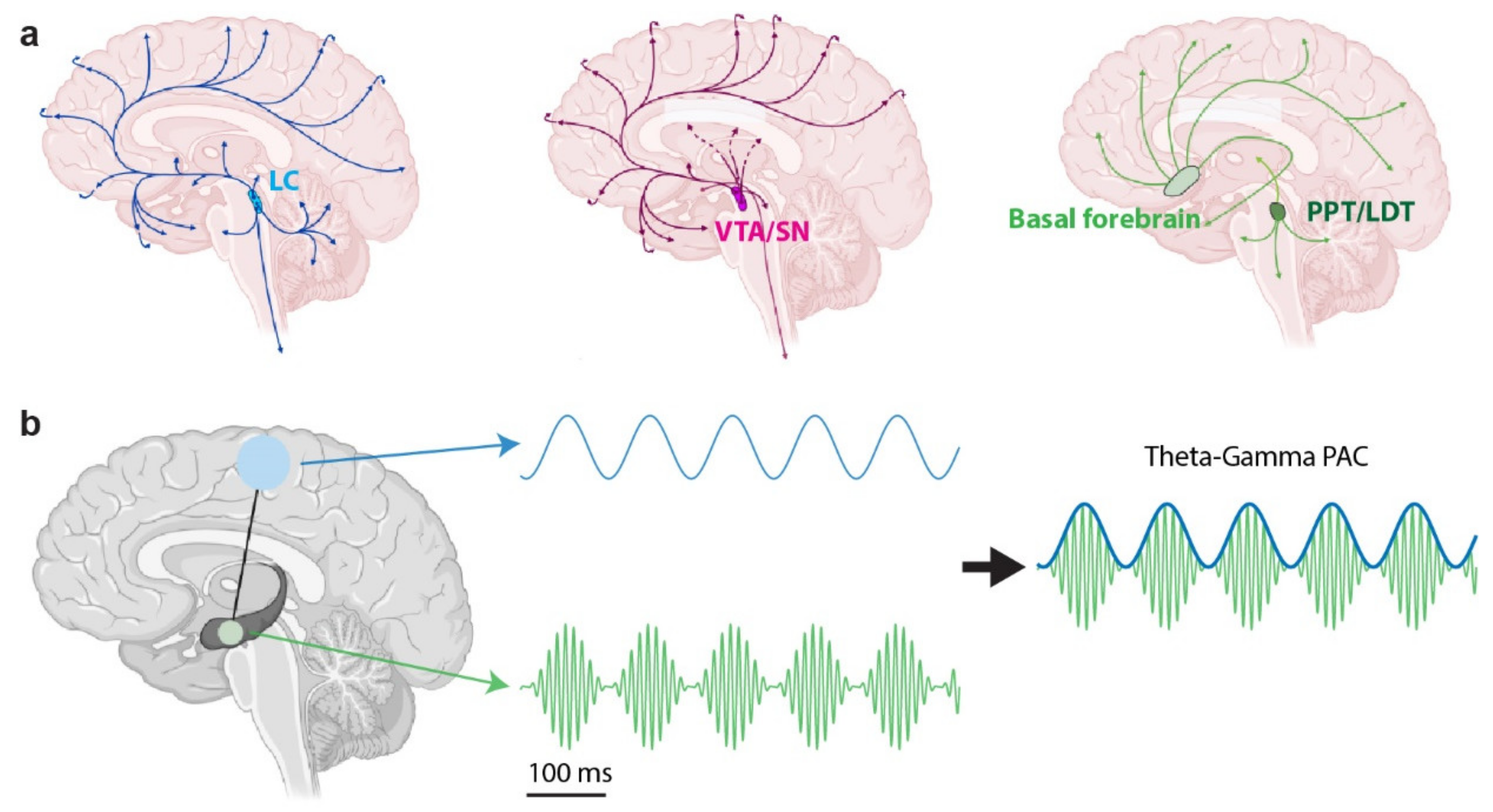
3. The Neuromodulatory System’s Role in Neural Oscillations
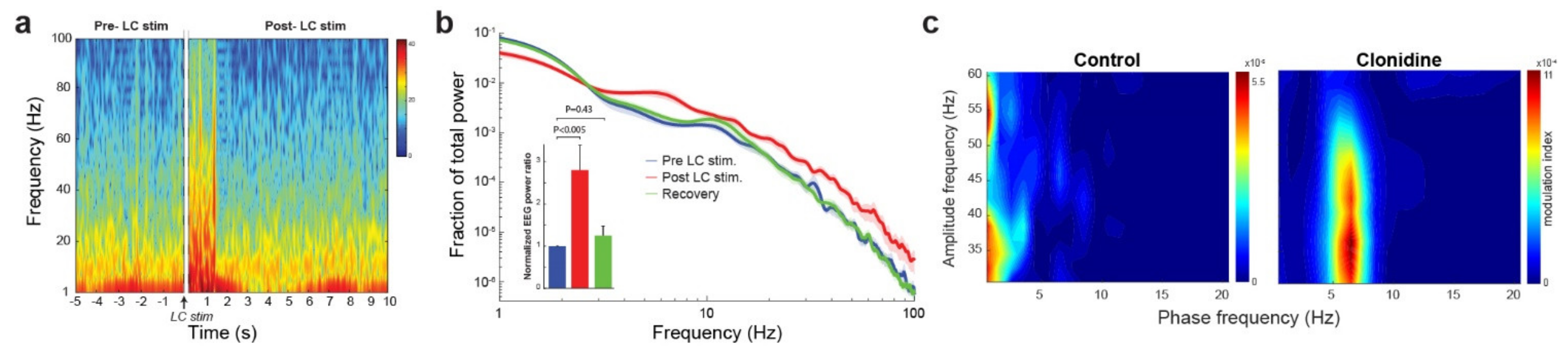
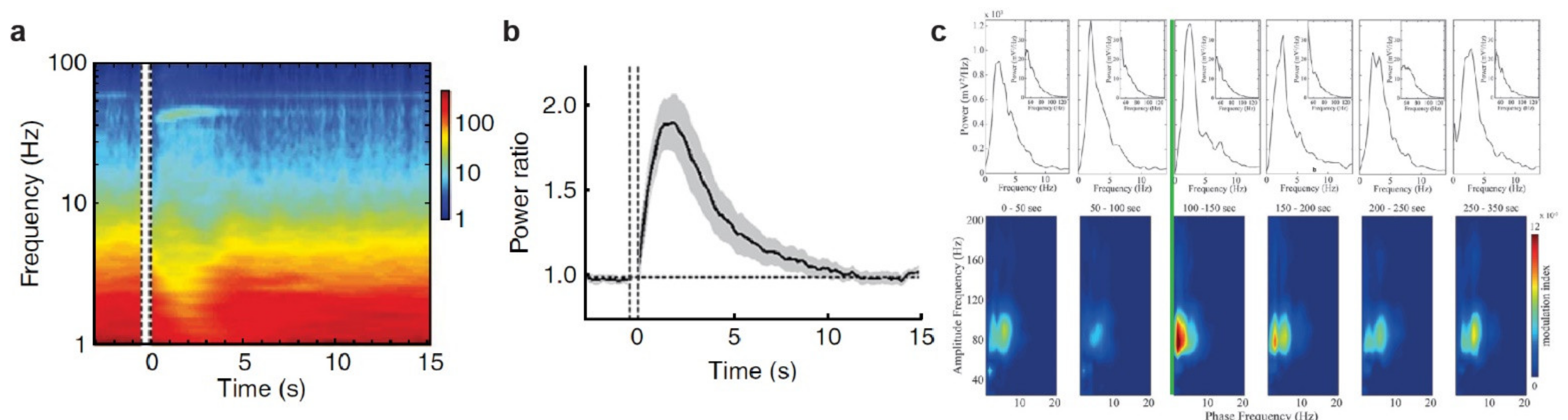
3.1. The Noradrenergic System
3.2. The Cholinergic System
3.3. The Dopaminergic System
4. Pupil-Linked Arousal and Neural Oscillations
5. Future Neuromodulation Technology
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buzsáki, G.; Draguhn, A. Neuronal Oscillations in Cortical Networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J. Cortex and mind: Unifying cognition; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Delis, I.; Ince, R.A.A.; Sajda, P.; Wang, Q. Neural encoding of active multi-sensing enhances perceptual decision-making via a synergistic cross-modal interaction. J. Neurosci. 2022, 42, 2344–2355. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Anastassiou, C.A.; Koch, C. The origin of extracellular fields and currents—EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 2012, 13, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Delis, I.; Dmochowski, J.P.; Sajda, P.; Wang, Q. Correlation of neural activity with behavioral kinematics reveals distinct sensory encoding and evidence accumulation processes during active tactile sensing. NeuroImage 2018, 175, 12–21. [Google Scholar] [CrossRef]
- Michel, C.; Lehmann, D.; Henggeler, B.; Brandeis, D. Localization of the sources of EEG delta, theta, alpha and beta frequency bands using the FFT dipole approximation. Electroencephalogr. Clin. Neurophysiol. 1992, 82, 38–44. [Google Scholar] [CrossRef]
- Friston, K.J. LFP and oscillations-what do they tell us? Curr. Opin. Neurobiol. 2015, 31, 1–6. [Google Scholar] [CrossRef]
- Hyafil, A.; Giraud, A.-L.; Fontolan, L.; Gutkin, B. Neural Cross-Frequency Coupling: Connecting Architectures, Mechanisms, and Functions. Trends Neurosci. 2015, 38, 725–740. [Google Scholar] [CrossRef]
- Salimpour, Y.; Anderson, W.S. Cross-Frequency Coupling Based Neuromodulation for Treating Neurological Disorders. Front. Neurosci. 2019, 13, 125. [Google Scholar] [CrossRef]
- Mormann, F.; Fell, J.; Axmacher, N.; Weber, B.; Lehnertz, K.; Elger, C.E.; Fernández, G. Phase/amplitude reset and theta–gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus 2005, 15, 890–900. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, M.; Wang, L.; Lin, L.; Xu, J. Phase Coupled Firing of Prefrontal Parvalbumin Interneuron with High Frequency Oscillations. Front. Cell. Neurosci. 2020, 14, 610741. [Google Scholar] [CrossRef]
- Womelsdorf, T.; Schoffelen, J.M.; Oostenveld, R.; Singer, W.; Desimone, R.; Engel, A.K.; Fries, P. Modulation of neuronal interactions through neuronal synchronization. Science 2007, 316, 1609–1612. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, J.F.; Cohen, M.X.; Allen, J.J. Prelude to and resolution of an error: EEG phase synchrony reveals cognitive control dynamics during action monitoring. J. Neurosci. 2009, 29, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.; Ludowig, E.; Rosburg, T.; Axmacher, N.; Elger, C.E. Phase-locking within human mediotemporal lobe predicts memory formation. Neuroimage 2008, 43, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Fell, J.; Axmacher, N. The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 2011, 12, 105–118. [Google Scholar] [CrossRef]
- Başar, E. Brain oscillations in neuropsychiatric disease. Dialogues Clin. Neurosci. 2013, 15, 291–300. [Google Scholar] [CrossRef]
- Salimpour, Y.; Mills, K.A.; Hwang, B.Y.; Anderson, W.S. Phase- targeted stimulation modulates phase-amplitude coupling in the motor cortex of the human brain. Brain Stimul. 2022, 15, 152–163. [Google Scholar] [CrossRef]
- Warsi, N.M.; Yan, H.; Wong, S.M.; Yau, I.; Breitbart, S.; Go, C.; Gorodetsky, C.; Fasano, A.; Kalia, S.K.; Rutka, J.T.; et al. Vagus Nerve Stimulation Modulates Phase-Amplitude Coupling in Thalamic Local Field Potentials. Neuromodulation, 2022; in press. [Google Scholar] [CrossRef]
- Rodenkirch, C.; Carmel, J.B.; Wang, Q. Rapid Effects of Vagus Nerve Stimulation on Sensory Processing Through Activation of Neuromodulatory Systems. Front. Neurosci. 2022, 16, 922424. [Google Scholar] [CrossRef]
- Hulsey, D.R.; Riley, J.R.; Loerwald, K.W.; Rennaker, R.L.; Kilgard, M.P.; Hays, S.A. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp. Neurol. 2017, 289, 21–30. [Google Scholar] [CrossRef]
- Chen, L.L.; Madhavan, R.; Rapoport, B.I.; Anderson, W.S. A method for real-time cortical oscillation detection and phase-locked stimulation. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; Volume 2011, pp. 3087–3090. [Google Scholar] [CrossRef]
- Descarries, L.; Watkins, K.C.; Lapierre, Y. Noradrenergic axon terminals in the cerebral cortex of rat. III. Topometric ultrastructural analysis. Brain Res. 1977, 133, 197–222. [Google Scholar] [CrossRef]
- Li, X.; Yu, B.; Sun, Q.; Zhang, Y.; Ren, M.; Zhang, X.; Li, A.; Yuan, J.; Madisen, L.; Luo, Q.; et al. Generation of a whole-brain atlas for the cholinergic system and mesoscopic projectome analysis of basal forebrain cholinergic neurons. Proc. Natl. Acad. Sci. USA 2018, 115, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Björklund, A.; Dunnett, S.B. Dopamine neuron systems in the brain: An update. Trends Neurosci. 2007, 30, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Rodenkirch, C.; Liu, Y.; Schriver, B.J.; Wang, Q. Locus coeruleus activation enhances thalamic feature selectivity via norepinephrine regulation of intrathalamic circuit dynamics. Nat. Neurosci. 2019, 22, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Slater, C.; Liu, Y.; Weiss, E.; Yu, K.; Wang, Q. The Neuromodulatory Role of the Noradrenergic and Cholinergic Systems and Their Interplay in Cognitive Functions: A Focused Review. Brain Sci. 2022, 12, 890. [Google Scholar] [CrossRef] [PubMed]
- Foote, S.L.; Bloom, F.E. Activity of Norepinephrine-Containing Locus Coeruleus Neurons in the Unanesthetized Squirrel Monkey. In Catecholamines: Basic and Clinical Frontiers; Usdin, E., Kopin, I.J., Barchas, J., Eds.; Pergamon Press Inc.: Pergamon, Turkey, 1979; pp. 625–627. [Google Scholar]
- Totah, N.K.; Logothetis, N.K.; Eschenko, O. Synchronous spiking associated with prefrontal high γ oscillations evokes a 5-Hz rhythmic modulation of spiking in locus coeruleus. J. Neurophysiol. 2021, 125, 1191–1201. [Google Scholar] [CrossRef]
- Szabadi, E. Functional Organization of the Sympathetic Pathways Controlling the Pupil: Light-Inhibited and Light-Stimulated Pathways. Front. Neurol. 2018, 9, 1069. [Google Scholar] [CrossRef]
- Joshi, S.; Li, Y.; Kalwani, R.M.; Gold, J.I. Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron 2016, 89, 221–234. [Google Scholar] [CrossRef]
- Reimer, J.; Froudarakis, E.; Cadwell, C.R.; Yatsenko, D.; Denfield, G.H.; Tolias, A.S. Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron 2014, 84, 355–362. [Google Scholar] [CrossRef]
- Reimer, J.; McGinley, M.J.; Liu, Y.; Rodenkirch, C.; Wang, Q.; McCormick, D.A.; Tolias, A.S. Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat. Commun. 2016, 7, 13289. [Google Scholar] [CrossRef]
- McGinley, M.J.; David, S.V.; McCormick, D.A. Cortical membrane potential signature of optimal states for sensory signal detection. Neuron 2015, 87, 179–192. [Google Scholar] [CrossRef]
- Liu, Y.; Narasimhan, S.; Schriver, B.J.; Wang, Q. Perceptual Behavior Depends Differently on Pupil-Linked Arousal and Heartbeat Dynamics-Linked Arousal in Rats Performing Tactile Discrimination Tasks. Front. Syst. Neurosci. 2021, 14, 614248. [Google Scholar] [CrossRef] [PubMed]
- Saby, J.N.; Marshall, P.J. The Utility of EEG Band Power Analysis in the Study of Infancy and Early Childhood. Dev. Neuropsychol. 2012, 37, 253–273. [Google Scholar] [CrossRef]
- Stefanics, G.; Hangya, B.; Hernádi, I.; Winkler, I.; Lakatos, P.; Ulbert, I. Phase Entrainment of Human Delta Oscillations Can Mediate the Effects of Expectation on Reaction Speed. J. Neurosci. 2010, 30, 13578–13585. [Google Scholar] [CrossRef]
- Amzica, F.; Steriade, M. Electrophysiological correlates of sleep delta waves. Electroencephalogr. Clin. Neurophysiol. 1998, 107, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Senoussi, M.; Verbeke, P.; Desender, K.; De Loof, E.; Talsma, D.; Verguts, T. Theta oscillations shift towards optimal frequency for cognitive control. Nat. Hum. Behav. 2022, 6, 1000–1013. [Google Scholar] [CrossRef] [PubMed]
- Herweg, N.A.; Solomon, E.A.; Kahana, M.J. Theta Oscillations in Human Memory. Trends Cogn. Sci. 2020, 24, 208–227. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.E.; Jensen, O. The Theta-Gamma Neural Code. Neuron 2013, 77, 1002–1016. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.; Buzsáki, G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizophr. Bull. 2008, 34, 974–980. [Google Scholar] [CrossRef]
- Kolev, V.; Yordanova, J. Analysis of phase-locking is informative for studying event-related EEG activity. Biol. Cybern. 1997, 76, 229–235. [Google Scholar] [CrossRef]
- Başar, E.; Schürmann, M.; Başar-Eroglu, C.; Karakaş, S. Alpha oscillations in brain functioning: An integrative theory. Int. J. Psychophysiol. 1997, 26, 5–29. [Google Scholar] [CrossRef]
- Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Cooper, N.R.; Croft, R.J.; Dominey, S.J.J.; Burgess, A.P.; Gruzelier, J.H. Paradox lost? Exploring the role of alpha oscillations during externally vs. internally directed attention and the implications for idling and inhibition hypotheses. Int. J. Psychophysiol. 2003, 47, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Busch, N.A.; Dubois, J.; VanRullen, R. The phase of ongoing EEG oscillations predicts visual perception. J. Neurosci. 2009, 29, 7869–7876. [Google Scholar] [CrossRef]
- Foxe, J.J.; Snyder, A.C. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Front. Psychol. 2011, 2, 154. [Google Scholar] [CrossRef]
- Pittman-Polletta, B.R.; Quach, A.; Mohammed, A.I.; Romano, M.; Kondabolu, K.; Kopell, N.J.; Han, X.; McCarthy, M.M. Striatal cholinergic receptor activation causes a rapid, selective and state-dependent rise in cortico-striatal β activity. Eur. J. Neurosci. 2018, 48, 2857–2868. [Google Scholar] [CrossRef]
- Jenkinson, N.; Brown, P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends Neurosci. 2011, 34, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Herrojo Ruiz, M.; Kilavik, B.E.; Lundqvist, M.; Starr, P.A.; Aron, A.R. Beta Oscillations in Working Memory, Executive Control of Movement and Thought, and Sensorimotor Function. J. Neurosci. 2019, 39, 8231–8238. [Google Scholar] [CrossRef]
- Yang, Y.; Gritton, H.; Sarter, M.; Aton, S.J.; Booth, V.; Zochowski, M. Theta-gamma coupling emerges from spatially heterogeneous cholinergic neuromodulation. PLoS Comput. Biol. 2021, 17, e1009235. [Google Scholar] [CrossRef]
- Doesburg, S.; Vinette, S.; Cheung, M.; Pang, E. Theta-Modulated Gamma-Band Synchronization among Activated Regions During a Verb Generation Task. Front. Psychol. 2012, 3, 195. [Google Scholar] [CrossRef]
- Csicsvari, J.; Jamieson, B.; Wise, K.D.; Buzsáki, G. Mechanisms of Gamma Oscillations in the Hippocampus of the Behaving Rat. Neuron 2003, 37, 311–322. [Google Scholar] [CrossRef]
- Crowley, K. Differentiating Pathologic Delta from Healthy Physiologic Delta in Patients with Alzheimer Disease. Sleep 2005, 28, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Hiatt, J.F.; Floyd, T.C.; Katz, P.H.; Feinberg, I. Further Evidence of Abnormal Non-Rapid-Eye-Movement Sleep in Schizophrenia. Arch. Gen. Psychiatry 1985, 42, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Linkenkaer-Hansen, K.; Monto, S.; Rytsälä, H.; Suominen, K.; Isometsä, E.; Kähkönen, S. Breakdown of Long-Range Temporal Correlations in Theta Oscillations in Patients with Major Depressive Disorder. J. Neurosci. 2005, 25, 10131–10137. [Google Scholar] [CrossRef] [PubMed]
- Guo, J. Abnormal modulation of theta oscillations in children with attention-deficit/hyperactivity disorder. NeuroImage Clin. 2020, 27, 102314. [Google Scholar] [CrossRef]
- Osipova, D. Altered generation of spontaneous oscillations in Alzheimer’s disease. NeuroImage 2005, 27, 835–841. [Google Scholar] [CrossRef]
- Deiber, M.-P. Linking alpha oscillations, attention and inhibitory control in adult ADHD with EEG neurofeedback. NeuroImage Clin. 2020, 25, 102145. [Google Scholar] [CrossRef]
- Little, S. The functional role of beta oscillations in Parkinson’s disease. Park. Relat. Disord. 2014, 20, S44–S48. [Google Scholar] [CrossRef] [PubMed]
- Swann, N.C.; de Hemptinne, C.; Miocinovic, S.; Qasim, S.; Wang, S.S.; Ziman, N.; Ostrem, J.L.; San Luciano, M.; Galifianakis, N.B.; Starr, P.A. Gamma Oscillations in the Hyperkinetic State Detected with Chronic Human Brain Recordings in Parkinson’s Disease. J. Neurosci. 2016, 36, 6445–6458. [Google Scholar] [CrossRef]
- Mably, A.J. Gamma oscillations in cognitive disorders. Curr. Opin. Neurobiol. 2018, 52, 182–187. [Google Scholar] [CrossRef]
- Palva, S.; Palva, J.M. New vistas for α-frequency band oscillations. Trends Neurosci. 2007, 30, 150–158. [Google Scholar] [CrossRef]
- Yakubov, B.; Das, S.; Zomorrodi, R.; Blumberger, D.M.; Enticott, P.G.; Kirkovski, M.; Rajji, T.K.; Desarkar, P. Cross-frequency coupling in psychiatric disorders: A systematic review. Neurosci. Biobehav. Rev. 2022, 138, 104690. [Google Scholar] [CrossRef] [PubMed]
- Tort, A.B.; Komorowski, R.W.; Manns, J.R.; Kopell, N.J.; Eichenbaum, H. Theta-gamma coupling increases during the learning of item-context associations. Proc. Natl. Acad. Sci. USA 2009, 106, 20942–20947. [Google Scholar] [CrossRef] [PubMed]
- Nadalin, J.K.; Martinet, L.E.; Blackwood, E.B.; Lo, M.C.; Widge, A.S.; Cash, S.S.; Eden, U.T.; Kramer, M.A. A statistical framework to assess cross-frequency coupling while accounting for confounding analysis effects. ELife 2019, 8, e44287. [Google Scholar] [CrossRef] [PubMed]
- Ceni, A.; Olmi, S.; Torcini, A.; Angulo-Garcia, D. Cross frequency coupling in next generation inhibitory neural mass models. Chaos 2020, 30, 053121. [Google Scholar] [CrossRef]
- Buzsáki, G.; Wang, X.-J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225. [Google Scholar] [CrossRef]
- Jirsa, V.; Müller, V. Cross-frequency coupling in real and virtual brain networks. Front. Comput. Neurosci. 2013, 7, 78. [Google Scholar] [CrossRef]
- Cohen, M.X. Assessing transient cross-frequency coupling in EEG data. J. Neurosci. Methods 2008, 168, 494–499. [Google Scholar] [CrossRef]
- Osipova, D.; Hermes, D.; Jensen, O. Gamma power is phase-locked to posterior alpha activity. PLoS ONE 2008, 3, e3990. [Google Scholar] [CrossRef]
- Andino-Pavlovsky, V.; Souza, A.C.; Scheffer-Teixeira, R.; Tort, A.B.L.; Etchenique, R.; Ribeiro, S. Dopamine Modulates Delta-Gamma Phase-Amplitude Coupling in the Prefrontal Cortex of Behaving Rats. Front. Neural Circuits 2017, 11, 29. [Google Scholar] [CrossRef]
- Cole, S.R.; Voytek, B. Brain Oscillations and the Importance of Waveform Shape. Trends Cogn. Sci. 2017, 21, 137–149. [Google Scholar] [CrossRef]
- Canolty, R.T.; Knight, R.T. The functional role of cross-frequency coupling. Trends Cogn. Sci. 2010, 14, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Bandarabadi, M.; Boyce, R.; Gutierrez Herrera, C.; Bassetti, C.L.; Williams, S.; Schindler, K.; Adamantidis, A. Dynamic modulation of theta-gamma coupling during rapid eye movement sleep. Sleep 2019, 42, zsz182. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.L.; Paulsen, O. Hippocampal network oscillations—Recent insights from in vitro experiments. Curr. Opin. Neurobiol. 2015, 31, 40–44. [Google Scholar] [CrossRef]
- Tamura, M.; Spellman, T.J.; Rosen, A.M.; Gogos, J.A.; Gordon, J.A. Hippocampal-prefrontal theta-gamma coupling during performance of a spatial working memory task. Nat. Commun. 2017, 8, 2182. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.X.; Elger, C.E.; Fell, J. Oscillatory activity and phase-amplitude coupling in the human medial frontal cortex during decision making. J. Cogn. Neurosci. 2009, 21, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Watrous, A.J.; Deuker, L.; Fell, J.; Axmacher, N. Phase-amplitude coupling supports phase coding in human ECoG. ELife 2015, 4, e07886. [Google Scholar] [CrossRef] [PubMed]
- Seymour, R.A.; Rippon, G.; Kessler, K. The Detection of Phase Amplitude Coupling during Sensory Processing. Front. Neurosci. 2017, 11, 487. [Google Scholar] [CrossRef]
- Chacko, R.V.; Kim, B.; Jung, S.W.; Daitch, A.L.; Roland, J.L.; Metcalf, N.V.; Corbetta, M.; Shulman, G.L.; Leuthardt, E.C. Distinct phase-amplitude couplings distinguish cognitive processes in human attention. NeuroImage 2018, 175, 111–121. [Google Scholar] [CrossRef]
- Buschman, T.J.; Denovellis, E.L.; Diogo, C.; Bullock, D.; Miller, E.K. Synchronous Oscillatory Neural Ensembles for Rules in the Prefrontal Cortex. Neuron 2012, 76, 838–846. [Google Scholar] [CrossRef]
- Szczepanski, S.M.; Crone, N.E.; Kuperman, R.A.; Auguste, K.I.; Parvizi, J.; Knight, R.T. Dynamic Changes in Phase-Amplitude Coupling Facilitate Spatial Attention Control in Fronto-Parietal Cortex. PLoS Biol. 2014, 12, e1001936. [Google Scholar] [CrossRef]
- Rihs, T.A.; Michel, C.M.; Thut, G. Mechanisms of selective inhibition in visual spatial attention are indexed by α-band EEG synchronization. Eur. J. Neurosci. 2007, 25, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tan, Z.; Xia, W.; Gomes, C.A.; Zhang, X.; Zhou, W.; Liang, S.; Axmacher, N.; Wang, L. Theta oscillations synchronize human medial prefrontal cortex and amygdala during fear learning. Sci. Adv. 2021, 7, eabf4198. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W. Memory processes, brain oscillations and EEG synchronization. Int. J. Psychophysiol. 1996, 24, 61–100. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.; Warden, M.R.; Miller, E.K. Phase-dependent neuronal coding of objects in short-term memory. Proc. Natl. Acad. Sci. USA 2009, 106, 21341–21346. [Google Scholar] [CrossRef] [PubMed]
- De Hemptinne, C.; Ryapolova-Webb, E.S.; Air, E.L.; Garcia, P.A.; Miller, K.J.; Ojemann, J.G.; Ostrem, J.L.; Galifianakis, N.B.; Starr, P.A. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc. Natl. Acad. Sci. USA 2013, 110, 4780–4785. [Google Scholar] [CrossRef]
- Goutagny, R.; Gu, N.; Cavanagh, C.; Jackson, J.; Chabot, J.-G.; Quirion, R.; Krantic, S.; Williams, S. Alterations in hippocampal network oscillations and theta-gamma coupling arise before Aβ overproduction in a mouse model of Alzheimer’s disease. Eur. J. Neurosci. 2013, 37, 1896–1902. [Google Scholar] [CrossRef]
- Mirzayi, P.; Shobeiri, P.; Kalantari, A.; Perry, G.; Rezaei, N. Optogenetics: Implications for Alzheimer’s disease research and therapy. Mol. Brain 2022, 15, 20. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, J.; Kim, B.-N.; Kang, T.; Min, K.J.; Han, D.H.; Lee, Y.S. Theta-phase gamma-amplitude coupling as a neurophysiological marker of attention deficit/hyperactivity disorder in children. Neurosci. Lett. 2015, 603, 25–30. [Google Scholar] [CrossRef]
- Moran, L.V.; Hong, L.E. High vs. low frequency neural oscillations in schizophrenia. Schizophr. Bull. 2011, 37, 659–663. [Google Scholar] [CrossRef]
- Kirihara, K.; Rissling, A.J.; Swerdlow, N.R.; Braff, D.L.; Light, G.A. Hierarchical Organization of Gamma and Theta Oscillatory Dynamics in Schizophrenia. Biol. Psychiatry 2012, 71, 873–880. [Google Scholar] [CrossRef]
- Loizou, L.A. Projections of the nucleus locus coeruleus in the albino rat. Brain Res. 1969, 15, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Russell, G.V. The nucleus locus coeruleus (dorsolateralis tegmenti). Tex. Rep. Biol. Med. 1955, 13, 939–988. [Google Scholar] [PubMed]
- Totah, N.K.; Neves, R.M.; Panzeri, S.; Logothetis, N.K.; Eschenko, O. The Locus Coeruleus Is a Complex and Differentiated Neuromodulatory System. Neuron 2018, 99, 1055–1068.e6. [Google Scholar] [CrossRef]
- Chandler, D.J.; Jensen, P.; McCall, J.G.; Pickering, A.E.; Schwarz, L.A.; Totah, N.K. Redefining Noradrenergic Neuromodulation of Behavior: Impacts of a Modular Locus Coeruleus Architecture. J. Neurosci. 2019, 39, 8239. [Google Scholar] [CrossRef]
- Ross, J.A.; Van Bockstaele, E.J. The Locus Coeruleus- Norepinephrine System in Stress and Arousal: Unraveling Historical, Current, and Future Perspectives. Front. Psychiatry 2020, 11, 601519. [Google Scholar] [CrossRef] [PubMed]
- Sara, S.J.; Bouret, S. Orienting and reorienting: The locus coeruleus mediates cognition through arousal. Neuron 2012, 76, 130–141. [Google Scholar] [CrossRef]
- Bouret, S.; Sara, S.J. Locus coeruleus activation modulates firing rate and temporal organization of odour-induced single-cell responses in rat piriform cortex. Eur. J. Neurosci. 2002, 16, 2371–2382. [Google Scholar] [CrossRef]
- Grella, S.L.; Neil, J.M.; Edison, H.T.; Strong, V.D.; Odintsova, I.V.; Walling, S.G.; Martin, G.M.; Marrone, D.F.; Harley, C.W. Locus Coeruleus Phasic, But Not Tonic, Activation Initiates Global Remapping in a Familiar Environment. J. Neurosci. 2019, 39, 445–455. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Rajkowski, J.; Cohen, J. Role of locus coeruleus in attention and behavioral flexibility. Biol. Psychiatry 1999, 46, 1309–1320. [Google Scholar] [CrossRef]
- Hayat, H.; Regev, N.; Matosevich, N.; Sales, A.; Paredes-Rodriguez, E.; Krom, A.J.; Bergman, L.; Li, Y.; Lavigne, M.; Kremer, E.J.; et al. Locus coeruleus norepinephrine activity mediates sensory-evoked awakenings from sleep. Sci. Adv. 2020, 6, eaaz4232. [Google Scholar] [CrossRef]
- Foote, S.L.; Aston-Jones, G.; Bloom, F.E. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc. Natl. Acad. Sci. USA 1980, 77, 3033–3037. [Google Scholar] [CrossRef]
- Liu, Y.; Rodenkirch, C.; Moskowitz, N.; Schriver, B.; Wang, Q. Dynamic Lateralization of Pupil Dilation Evoked by Locus Coeruleus Activation Results from Sympathetic, Not Parasympathetic, Contributions. Cell Rep. 2017, 20, 3099–3112. [Google Scholar] [CrossRef]
- Broncel, A.; Bocian, R.; Kłos-Wojtczak, P.; Konopacki, J. Effects of locus coeruleus activation and inactivation on hippocampal formation theta rhythm in anesthetized rats. Brain Res. Bull. 2020, 162, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Lemon, N.; Aydin-Abidin, S.; Funke, K.; Manahan-Vaughan, D. Locus coeruleus activation facilitates memory encoding and induces hippocampal LTD that depends on beta-adrenergic receptor activation. Cereb. Cortex 2009, 19, 2827–2837. [Google Scholar] [CrossRef] [PubMed]
- Geiller, T.; Sadeh, S.; Rolotti, S.V.; Blockus, H.; Vancura, B.; Negrean, A.; Murray, A.J.; Rozsa, B.; Polleux, F.; Clopath, C.; et al. Local circuit amplification of spatial selectivity in the hippocampus. Nature 2022, 601, 105–109. [Google Scholar] [CrossRef]
- Natsume, K.; Kometani, K. Desynchronization of carbachol-induced theta-like activities by alpha-adrenergic agents in guinea pig hippocampal slices. Neurosci. Res. 1999, 33, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Hajós, M.; Hoffmann, W.E.; Robinson, D.D.; Yu, J.H.; Hajós-Korcsok, E. Norepinephrine but not serotonin reuptake inhibitors enhance theta and gamma activity of the septo-hippocampal system. Neuropsychopharmacology 2003, 28, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Cohen, J.D. Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J. Comp. Neurol. 2005, 493, 99–110. [Google Scholar] [CrossRef]
- Bouret, S.; Sara, S.J. Network reset: A simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005, 28, 574–582. [Google Scholar] [CrossRef]
- McBurney-Lin, J.; Lu, J.; Zuo, Y.; Yang, H. Locus Coeruleus-Norepinephrine Modulation of Sensory Processing and Perception: A Focused Review. Neurosci. Biobehav. Rev. 2019, 105, 190–199. [Google Scholar] [CrossRef]
- Dahl, M.J.; Mather, M.; Werkle-Bergner, M. Noradrenergic modulation of rhythmic neural activity shapes selective attention. Trends Cogn. Sci. 2022, 26, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Durán, E.; Yang, M.; Neves, R.; Logothetis, N.K.; Eschenko, O. Modulation of Prefrontal Cortex Slow Oscillations by Phasic Activation of the Locus Coeruleus. Neuroscience 2021, 453, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Semba, K.; Fibiger, H.C. Organization of central cholinergic systems. Prog. Brain Res. 1989, 79, 37–63. [Google Scholar] [CrossRef] [PubMed]
- Woolf, N.J.; Eckenstein, F.; Butcher, L.L. Cholinergic systems in the rat brain: I. projections to the limbic telencephalon. Brain Res. Bull. 1984, 13, 751–784. [Google Scholar] [CrossRef]
- Kása, P.; Rakonczay, Z.; Gulya, K. The cholinergic system in Alzheimer’s disease. Prog. Neurobiol. 1997, 52, 511–535. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Albin, R.L. The cholinergic system and Parkinson disease. Behav. Brain Res. 2011, 221, 564–573. [Google Scholar] [CrossRef]
- Rye, D.B.; Wainer, B.H.; Mesulam, M.-M.; Mufson, E.J.; Saper, C.B. Cortical projections arising from the basal forebrain: A study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience 1984, 13, 627–643. [Google Scholar] [CrossRef]
- Woolf, N.J. Cholinergic systems in mammalian brain and spinal cord. Prog. Neurobiol. 1991, 37, 475–524. [Google Scholar] [CrossRef]
- Passetti, F.; Dalley, J.W.; O’Connell, M.T.; Everitt, B.J.; Robbins, T.W. Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. Eur. J. Neurosci. 2000, 12, 3051–3058. [Google Scholar] [CrossRef]
- Sarter, M.; Parikh, V.; Howe, W.M. Phasic acetylcholine release and the volume transmission hypothesis: Time to move on. Nat. Rev. Neurosci. 2009, 10, 383–390. [Google Scholar] [CrossRef]
- Mattinson, C.E.; Burmeister, J.J.; Quintero, J.E.; Pomerleau, F.; Huettl, P.; Gerhardt, G.A. Tonic and phasic release of glutamate and acetylcholine neurotransmission in sub-regions of the rat prefrontal cortex using enzyme-based microelectrode arrays. J. Neurosci. Methods 2011, 202, 199–208. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teles-Grilo Ruivo, L.M.; Baker, K.L.; Conway, M.W.; Kinsley, P.J.; Gilmour, G.; Phillips, K.G.; Isaac, J.T.R.; Lowry, J.P.; Mellor, J.R. Coordinated Acetylcholine Release in Prefrontal Cortex and Hippocampus Is Associated with Arousal and Reward on Distinct Timescales. Cell Rep. 2017, 18, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Lustig, C.; Berry, A.S.; Gritton, H.; Howe, W.M.; Parikh, V. What do phasic cholinergic signals do? Neurobiol. Learn. Mem. 2016, 130, 135–141. [Google Scholar] [CrossRef]
- Steriade, M. Acetylcholine systems and rhythmic activities during the waking–sleep cycle. Prog. Brain Res. 2004, 145, 179–196. [Google Scholar] [PubMed]
- Goard, M.; Dan, Y. Basal forebrain activation enhances cortical coding of natural scenes. Nat. Neurosci. 2009, 12, 1444–1449. [Google Scholar] [CrossRef]
- Platt, B.; Riedel, G. The cholinergic system, EEG and sleep. Behav. Brain Res. 2011, 221, 499–504. [Google Scholar] [CrossRef]
- Jones, B.E. Activity, modulation and role of basal forebrain cholinergic neurons innervating the cerebral cortex. Prog. Brain Res. 2004, 145, 157–169. [Google Scholar]
- Cape, E.G.; Manns, I.D.; Alonso, A.; Beaudet, A.; Jones, B.E. Neurotensin-Induced Bursting of Cholinergic Basal Forebrain Neurons Promotes γ and θ Cortical Activity Together with Waking and Paradoxical Sleep. J. Neurosci. 2000, 20, 8452–8461. [Google Scholar] [CrossRef]
- Vinogradova, O.S.; Brazhnik, E.S.; Kitchigina, V.F.; Stafekhina, V.S. Acetylcholine, theta-rhythm and activity of hippocampal neurons in the rabbit—IV. Sensory stimulation. Neuroscience 1993, 53, 993–1007. [Google Scholar] [CrossRef]
- Hasselmo, M.E.; Hay, J.; Ilyn, M.; Gorchetchnikov, A. Neuromodulation, theta rhythm and rat spatial navigation. Neural Netw. 2002, 15, 689–707. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Stoiljkovic, M.; Kelley, C.; Nagy, D.; Leventhal, L.; Hajós, M. Selective activation of α7 nicotinic acetylcholine receptors augments hippocampal oscillations. Neuropharmacology 2016, 110, 102–108. [Google Scholar] [CrossRef]
- Howe, W.M.; Gritton, H.J.; Lusk, N.A.; Roberts, E.A.; Hetrick, V.L.; Berke, J.D.; Sarter, M. Acetylcholine Release in Prefrontal Cortex Promotes Gamma Oscillations and Theta-Gamma Coupling during Cue Detection. J. Neurosci. 2017, 37, 3215–3230. [Google Scholar] [CrossRef] [PubMed]
- McNally, J.M.; Aguilar, D.D.; Katsuki, F.; Radzik, L.K.; Schiffino, F.L.; Uygun, D.S.; McKenna, J.T.; Strecker, R.E.; Deisseroth, K.; Spencer, K.M.; et al. Optogenetic manipulation of an ascending arousal system tunes cortical broadband gamma power and reveals functional deficits relevant to schizophrenia. Mol. Psychiatry 2021, 26, 3461–3475. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Brown, R.E.; Kocsis, B.; Kim, T.; McKenna, J.T.; McNally, J.M.; Han, H.B.; Choi, J.H. Optogenetic stimulation of basal forebrain parvalbumin neurons modulates the cortical topography of auditory steady-state responses. Brain Struct. Funct. 2019, 224, 1505–1518. [Google Scholar] [CrossRef]
- Neymotin, S.A.; Hilscher, M.M.; Moulin, T.C.; Skolnick, Y.; Lazarewicz, M.T.; Lytton, W.W. Ih tunes theta/gamma oscillations and cross-frequency coupling in an in silico CA3 model. PLoS ONE 2013, 8, e76285. [Google Scholar] [CrossRef]
- Rasmusson, D.D.; Clow, K.; Szerb, J.C. Modification of neocortical acetylcholine release and electroencephalogram desynchronization due to brainstem stimulation by drugs applied to the basal forebrain. Neuroscience 1994, 60, 665–677. [Google Scholar] [CrossRef]
- Goldman-Rakic, P.S.; Leranth, C.; Williams, S.M.; Mons, N.; Geffard, M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc. Natl. Acad. Sci. USA 1989, 86, 9015–9019. [Google Scholar] [CrossRef]
- Ott, T.; Nieder, A. Dopamine and Cognitive Control in Prefrontal Cortex. Trends Cogn. Sci. 2019, 23, 213–234. [Google Scholar] [CrossRef]
- Williams, S.M.; Goldman-Rakic, P.S. Widespread origin of the primate mesofrontal dopamine system. Cereb. Cortex 1998, 8, 321–345. [Google Scholar] [CrossRef]
- Dreyer, J.K.; Herrik, K.F.; Berg, R.W.; Hounsgaard, J.D. Influence of Phasic and Tonic Dopamine Release on Receptor Activation. J. Neurosci. 2010, 30, 14273–14283. [Google Scholar] [CrossRef] [PubMed]
- Puig, M.V.; Miller, E.K. The Role of Prefrontal Dopamine D1 Receptors in the Neural Mechanisms of Associative Learning. Neuron 2012, 74, 874–886. [Google Scholar] [CrossRef]
- Sharott, A.; Magill, P.J.; Harnack, D.; Kupsch, A.; Meissner, W.; Brown, P. Dopamine depletion increases the power and coherence of beta-oscillations in the cerebral cortex and subthalamic nucleus of the awake rat. Eur. J. Neurosci. 2005, 21, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Eckart, C.; Fuentemilla, L.; Bauch, E.M.; Bunzeck, N. Dopaminergic stimulation facilitates working memory and differentially affects prefrontal low theta oscillations. NeuroImage 2014, 94, 185–192. [Google Scholar] [CrossRef]
- Benchenane, K.; Peyrache, A.; Khamassi, M.; Tierney, P.L.; Gioanni, Y.; Battaglia, F.P.; Wiener, S.I. Coherent Theta Oscillations and Reorganization of Spike Timing in the Hippocampal- Prefrontal Network upon Learning. Neuron 2010, 66, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.E.; Van Dort, C.J.; Kenny, J.D.; Pei, J.; Guidera, J.A.; Vlasov, K.Y.; Lee, J.T.; Boyden, E.S.; Brown, E.N.; Solt, K. Optogenetic activation of dopamine neurons in the ventral tegmental area induces reanimation from general anesthesia. Proc. Natl. Acad. Sci. USA 2016, 113, 12826–12831. [Google Scholar] [CrossRef]
- Mallet, N.; Pogosyan, A.; Sharott, A.; Csicsvari, J.; Bolam, J.P.; Brown, P.; Magill, P.J. Disrupted dopamine transmission and the emergence of exaggerated beta oscillations in subthalamic nucleus and cerebral cortex. J. Neurosci. 2008, 28, 4795–4806. [Google Scholar] [CrossRef]
- Iskhakova, L.; Rappel, P.; Deffains, M.; Fonar, G.; Marmor, O.; Paz, R.; Israel, Z.; Eitan, R.; Bergman, H. Modulation of dopamine tone induces frequency shifts in cortico-basal ganglia beta oscillations. Nat. Commun. 2021, 12, 7026. [Google Scholar] [CrossRef]
- Degos, B.; Deniau, J.M.; Chavez, M.; Maurice, N. Chronic but not acute dopaminergic transmission interruption promotes a progressive increase in cortical beta frequency synchronization: Relationships to vigilance state and akinesia. Cereb. Cortex 2009, 19, 1616–1630. [Google Scholar] [CrossRef]
- Ongini, E.; Caporali, M.G.; Massotti, M. Stimulation of dopamine D-1 receptors by SKF 38393 induces EEG desynchronization and behavioral arousal. Life Sci. 1985, 37, 2327–2333. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, C.; An, L.; Wang, R.; Zhang, T. Effects of Dopamine and Serotonin Systems on Modulating Neural Oscillations in Hippocampus-Prefrontal Cortex Pathway in Rats. Brain Topogr. 2016, 29, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Lohani, S.; Martig, A.K.; Deisseroth, K.; Witten, I.B.; Moghaddam, B. Dopamine Modulation of Prefrontal Cortex Activity Is Manifold and Operates at Multiple Temporal and Spatial Scales. Cell Rep. 2019, 27, 99–114.e116. [Google Scholar] [CrossRef]
- Carter, M.E.; Yizhar, O.; Chikahisa, S.; Nguyen, H.; Adamantidis, A.; Nishino, S.; Deisseroth, K.; de Lecea, L. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat. Neurosci. 2010, 13, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Schriver, B.J.; Perkins, S.M.; Sajda, P.; Wang, Q. Interplay between components of pupil-linked phasic arousal and its role in driving behavioral choice in Go/No-Go perceptual decision-making. Psychophysiology 2020, 57, e13565. [Google Scholar] [CrossRef] [PubMed]
- De Gee, J.W.; Tsetsos, K.; Schwabe, L.; Urai, A.E.; McCormick, D.; McGinley, M.J.; Donner, T.H. Pupil-linked phasic arousal predicts a reduction of choice bias across species and decision domains. eLife 2020, 9, e54014. [Google Scholar] [CrossRef] [PubMed]
- Schriver, B.; Bagdasarov, S.; Wang, Q. Pupil-linked arousal modulates behavior in rats performing a whisker deflection direction discrimination task. J. Neurophysiol. 2018, 120, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Lapborisuth, P.; Koorathota, S.; Wang, Q.; Sajda, P. Integrating neural and ocular attention reorienting signals in virtual reality. J. Neural Eng. 2022, 18, 066052. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, S.; Schriver, B.J.; Wang, Q. Adaptive decision making depends on pupil-linked arousal in rats performing tactile discrimination tasks. bioRxiv 2022. [Google Scholar] [CrossRef]
- Händel, B.; Haarmeier, T. Cross-frequency coupling of brain oscillations indicates the success in visual motion discrimination. NeuroImage 2009, 45, 1040–1046. [Google Scholar] [CrossRef]
- Takahashi, K.; Sobczak, F.; Pais-Roldán, P.; Yu, X. Characterizing pupil dynamics coupling to brain state fluctuation based on lateral hypothalamic activity. bioRxiv 2021. [Google Scholar] [CrossRef]
- Rodenkirch, C.; Schriver, B.; Wang, Q. Brain-Machine Interfaces: Restoring and Establishing Communication Channels. In Neural Engineering: From Advanced Biomaterials to 3D Fabrication Techniques; Zhang, L., Kaplan, D., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Park, M.; Hoang, G.M.; Nguyen, T.; Lee, E.; Jung, H.J.; Choe, Y.; Lee, M.H.; Hwang, J.Y.; Kim, J.G.; Kim, T. Effects of transcranial ultrasound stimulation pulsed at 40 Hz on Aβ plaques and brain rhythms in 5×FAD mice. Transl. Neurodegener. 2021, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Thut, G.; Schyns, P.G.; Gross, J. Entrainment of perceptually relevant brain oscillations by non-invasive rhythmic stimulation of the human brain. Front. Psychol. 2011, 2, 170. [Google Scholar] [CrossRef] [PubMed]
- Bahramisharif, A.; Mazaheri, A.; Levar, N.; Schuurman, P.R.; Figee, M.; Denys, D. Deep Brain Stimulation Diminishes Cross-Frequency Coupling in Obsessive-Compulsive Disorder. Biol. Psychiatry 2016, 80, e57–e58. [Google Scholar] [CrossRef] [PubMed]
- Widge, A.S.; Zorowitz, S.; Link, K.; Miller, E.K.; Deckersbach, T.; Eskandar, E.N.; Dougherty, D.D. Ventral Capsule/Ventral Striatum Deep Brain Stimulation Does Not Consistently Diminish Occipital Cross-Frequency Coupling. Biol. Psychiatry 2016, 80, e59–e60. [Google Scholar] [CrossRef]
- Kamimura, H.A.; Wang, S.; Chen, H.; Wang, Q.; Aurup, C.; Acosta, C.; Carneiro, A.A.; Konofagou, E.E. Focused ultrasound neuromodulation of cortical and subcortical brain structures using 1.9 MHz. Med. Phys. 2016, 43, 5730. [Google Scholar] [CrossRef] [PubMed]
- Downs, M.E.; Lee, S.A.; Yang, G.; Kim, S.; Wang, Q.; Konofagou, E.E. Non-invasive peripheral nerve stimulation via focused ultrasound in vivo. Phys. Med. Biol. 2018, 63, 035011. [Google Scholar] [CrossRef] [PubMed]
- Rodenkirch, C.; Wang, Q. Rapid and transient enhancement of thalamic information transmission induced by vagus nerve stimulation. J. Neural Eng. 2020, 17, 026027. [Google Scholar] [CrossRef]
- Legon, W.; Sato, T.F.; Opitz, A.; Mueller, J.; Barbour, A.; Williams, A.; Tyler, W.J. Transcranial focused ultrasound modulates the activity of primary somatosensory cortex in humans. Nat. Neurosci. 2014, 17, 322–329. [Google Scholar] [CrossRef]
- McIntyre, C.C.; Grill, W.M. Extracellular Stimulation of Central Neurons: Influence of Stimulus Waveform and Frequency on Neuronal Output. J. Neurophysiol. 2002, 88, 1592–1604. [Google Scholar] [CrossRef]
- Wang, Q.; Millard, D.C.; Zheng, H.J.V.; Stanley, G.B. Voltage-sensitive dye imaging reveals improved topographic activation of cortex in response to manipulation of thalamic microstimulation parameters. J. Neural Eng. 2012, 9, 026008. [Google Scholar] [CrossRef]
- Bari, B.A.; Ollerenshaw, D.R.; Millard, D.C.; Wang, Q.; Stanley, G.B. Behavioral and electrophysiological effects of cortical microstimulation parameters. PLoS ONE 2013, 8, e82170. [Google Scholar] [CrossRef] [PubMed]
- Millard, D.C.; Wang, Q.; Gollnick, C.A.; Stanley, G.B. System identification of the nonlinear dynamics in the thalamocortical circuit in response to patterned thalamic microstimulation in vivo. J. Neural Eng. 2013, 10, 066011. [Google Scholar] [CrossRef]
- Mickle, A.D.; Gereau, R.W.I. A bright future? Optogenetics in the periphery for pain research and therapy. Pain 2018, 159, S65–S73. [Google Scholar] [CrossRef] [PubMed]
- Boggio, P.S.; Rigonatti, S.P.; Ribeiro, R.B.; Myczkowski, M.L.; Nitsche, M.A.; Pascual-Leone, A.; Fregni, F. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int. J. Neuropsychopharmacol. 2008, 11, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Zangen, A.; Moshe, H.; Martinez, D.; Barnea-Ygael, N.; Vapnik, T.; Bystritsky, A.; Duffy, W.; Toder, D.; Casuto, L.; Grosz, M.L.; et al. Repetitive transcranial magnetic stimulation for smoking cessation: A pivotal multicenter double-blind randomized controlled trial. World Psychiatry 2021, 20, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Sadleir, R.; Vannorsdall, T.; Schretlen, D.; Gordon, B. Target Optimization in Transcranial Direct Current Stimulation. Front. Psychiatry 2012, 3, 90. [Google Scholar] [CrossRef]
- Khadka, N.; Truong, D.Q.; Bikson, M. Principles of Within Electrode Current Steering1. J. Med. Devices 2015, 9, 020947. [Google Scholar] [CrossRef]
- Slater, C.; Wang, Q. Alzheimer’s disease: An evolving understanding of noradrenergic involvement and the promising future of electroceutical therapies. Clin. Transl. Med. 2021, 11, e397. [Google Scholar] [CrossRef]
- Sharon, O.; Fahoum, F.; Nir, Y. Transcutaneous Vagus Nerve Stimulation in Humans Induces Pupil Dilation and Attenuates Alpha Oscillations. J.Neurosci. Off. J. Soc. Neurosci. 2021, 41, 320–330. [Google Scholar] [CrossRef]
- Turi, Z.; Mittner, M.; Lehr, A.; Bürger, H.; Antal, A.; Paulus, W. θ-γ Cross-Frequency Transcranial Alternating Current Stimulation over the Trough Impairs Cognitive Control. eNeuro 2020, 7, ENEURO.0126-0120.2020. [Google Scholar] [CrossRef]
- Polanía, R.; Nitsche, M.A.; Korman, C.; Batsikadze, G.; Paulus, W. The Importance of Timing in Segregated Theta Phase-Coupling for Cognitive Performance. Curr. Biol. 2012, 22, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Riddle, J.; McFerren, A.; Frohlich, F. Causal role of cross-frequency coupling in distinct components of cognitive control. Prog. Neurobiol. 2021, 202, 102033. [Google Scholar] [CrossRef] [PubMed]
- Yazdan-Shahmorad, A.; Silversmith, D.B.; Sabes, P.N. Novel techniques for large-scale manipulations of cortical networks in non-human primates. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; Volume 2018, pp. 5479–5482. [Google Scholar] [CrossRef]
- Widge, A.S.; Miller, E.K. Targeting Cognition and Networks Through Neural Oscillations: Next-Generation Clinical Brain Stimulation. JAMA Psychiatry 2019, 76, 671–672. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, E.; Kann, M.; Wang, Q. Neuromodulation of Neural Oscillations in Health and Disease. Biology 2023, 12, 371. https://doi.org/10.3390/biology12030371
Weiss E, Kann M, Wang Q. Neuromodulation of Neural Oscillations in Health and Disease. Biology. 2023; 12(3):371. https://doi.org/10.3390/biology12030371
Chicago/Turabian StyleWeiss, Evan, Michael Kann, and Qi Wang. 2023. "Neuromodulation of Neural Oscillations in Health and Disease" Biology 12, no. 3: 371. https://doi.org/10.3390/biology12030371
APA StyleWeiss, E., Kann, M., & Wang, Q. (2023). Neuromodulation of Neural Oscillations in Health and Disease. Biology, 12(3), 371. https://doi.org/10.3390/biology12030371






