Gradients of Variation in the At-Vessel Mortality Rate between Twelve Species of Sharks and Skates Sampled through a Fishery-Independent Trawl Survey in the Asinara Gulf (NW Mediterranean Sea)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
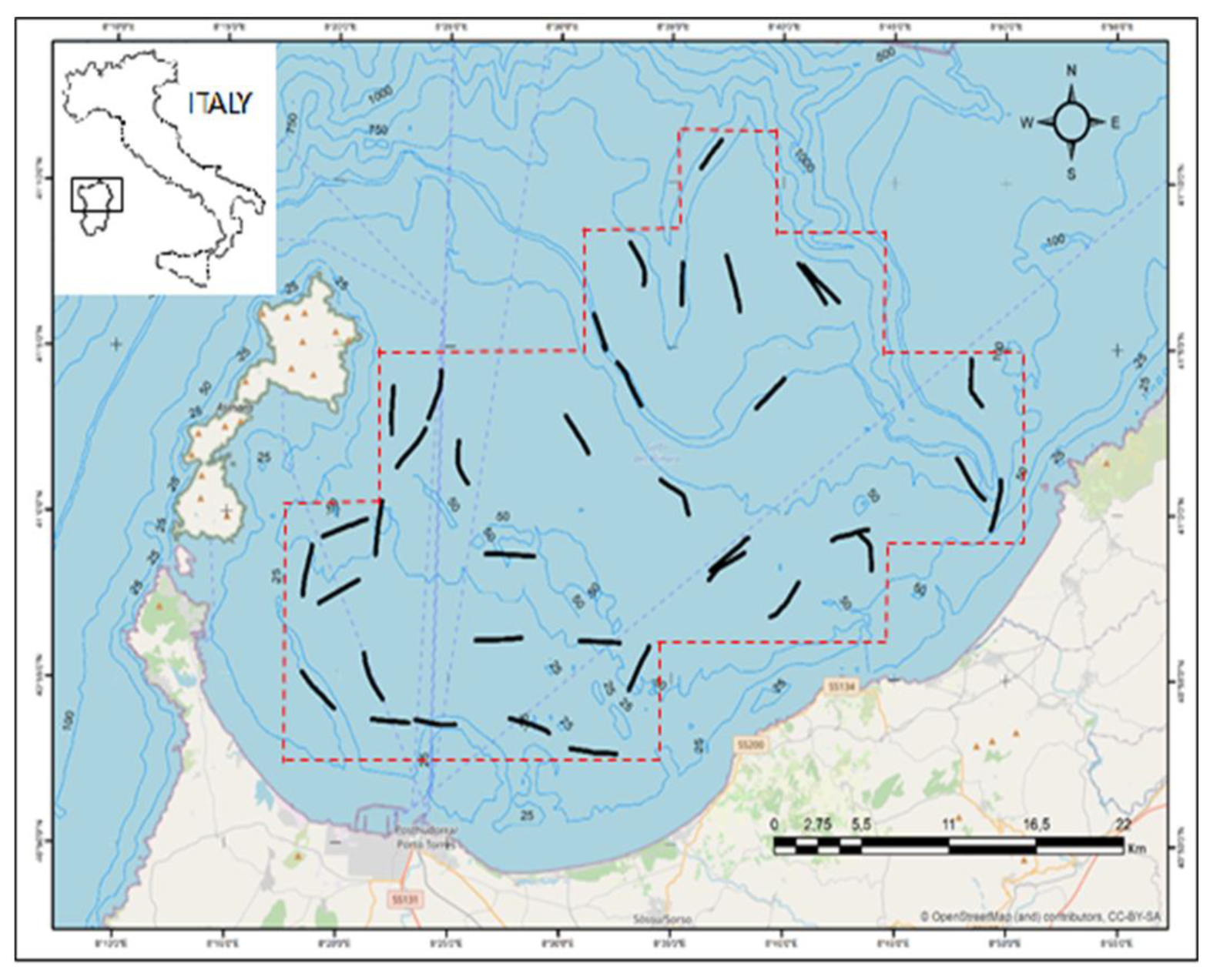
2.1. Sampling Design and On-Board Activities
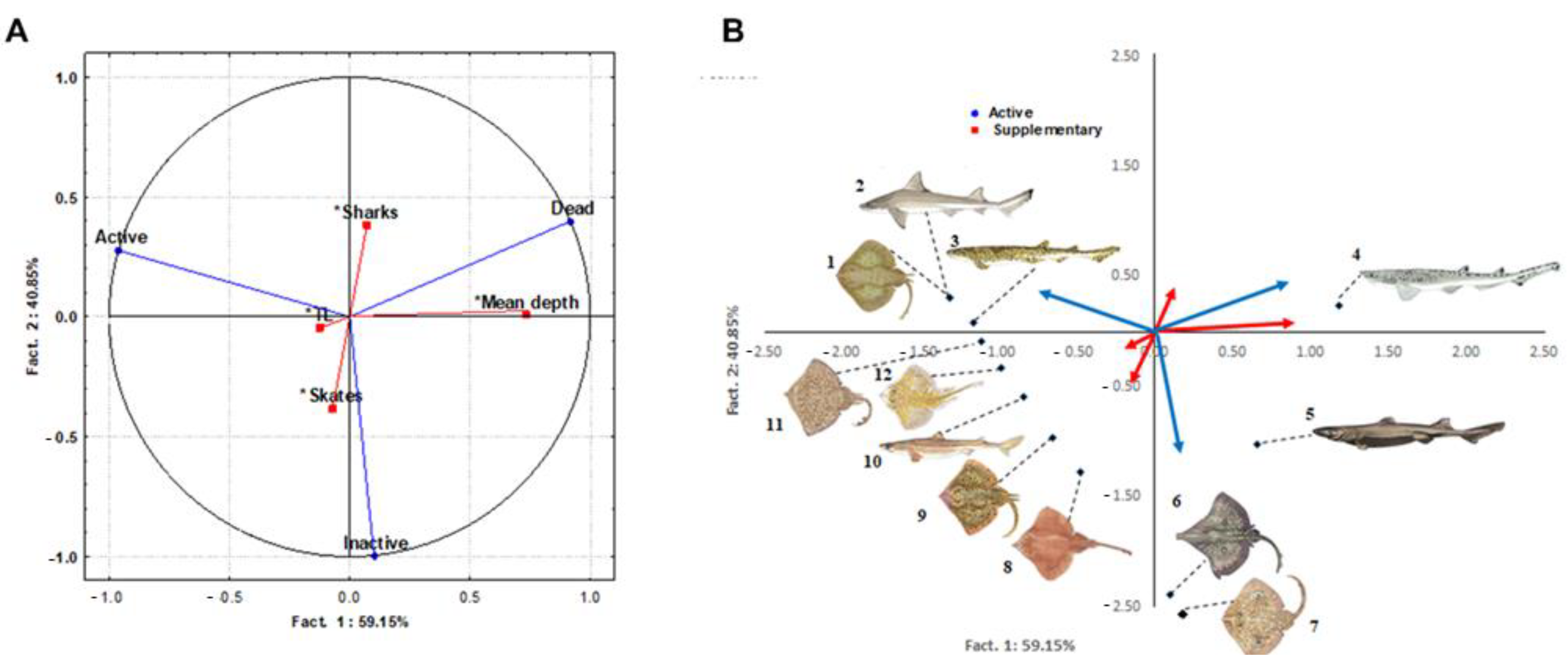
2.2. Data Analysis
3. Results
3.1. General Data
- 1.
- Five species of sharks, such as the picked dogfish Squalus acanthias Linnaeus, 1758 (SAC), the smooth-hound shark Mustelus mustelus (Linnaeus, 1758) (MMU), the velvet belly Etmopterus spinax (Linnaeus, 1758) (ESP), the small-spotted catshark Scyliorhinus canicula (Linnaeus, 1758) (SCA), and the black-mouth catshark Galeus melastomus Rafinesque, 1810 (GME) (Figure S1);
- 2.
- Seven species of skates, such as the shagreen ray Leucoraja fullonica (Linnaeus, 1758) (LFU), the sandy ray L. circularis (Couch, 1838) (LCI), the spotted ray Raja montagui Fowler, 1910 (RMO), the blonde ray R. brachyura Lafont, 1873 (RBR), the speckled ray R. polystigma Regan, 1923 (RPO), the brown ray R. miraletus Linnaeus, 1758 (RMI), and the long-nosed skate Dipturus oxyrinchus (Linnaeus, 1758) (DOX) (Figure 2).
| Species | n. | Size Range (cm) | Mean Size (TL or DW) ± SD | Haul Depth Range (m) | Haul Mean Depth (m) ± SD | Mean Absolute CPUE (ind/h) ± SD | Relative CPUE (ind/h) |
|---|---|---|---|---|---|---|---|
| DOX | 20 | 24–62 | 43.25 ± 11.73 | 270–360 | 324.2 ± 18.7 | 13.3 ± 7.7 | 1.14 |
| ESP | 42 | 12–42 | 24.92 ± 7.08 | 550–600 | 583.9 ± 12.1 | 42.0 ± 12.0 | 2.40 |
| GME | 721 | 10–49 | 21.81 ± 6.86 | 133–600 | 407.6 ± 62.1 | 206 ± 281.1 | 41.2 |
| LCI | 2 | 42–47 | 44.5 ± 2.50 | 200–210 | 205.0 | 4.0 | 0.11 |
| LFU | 2 | 30–45 | 37.5 ± 7.50 | 70–200 | 135.0 | 4.0 | 0.11 |
| MMU | 1 | 41 | na | 33–35 | 34.0 | 2.0 | 0.06 |
| RBR | 16 | 24–57 | 38.31 ± 9.31 | 33–360 | 209.4 ± 107.7 | 5.3 ± 2.9 | 0.91 |
| RMI | 35 | 13–41 | 26.57 ± 7.56 | 33–136 | 68.0 ± 12.0 | 7.0 ± 4.2 | 2.00 |
| RMO | 12 | 9–47 | 23.50 ± 14.77 | 42–210 | 159.7 ± 60.6 | 6.0 ± 4.7 | 0.68 |
| RPO | 36 | 15–55 | 21.32 ± 10.36 | 43–350 | 140.8 ± 61.0 | 7.2 ± 9.26 | 2.06 |
| SCA | 714 | 10–50 | 23.53 ± 9.75 | 33–480 | 147.9 ± 89.7 | 54.8 ± 59.4 | 40.8 |
| SAC | 7 | 45–72 | 58.14 ± 10.51 | 270–360 | 300.0 ± 13.2 | 7.0 ± 5.0 | 0.40 |
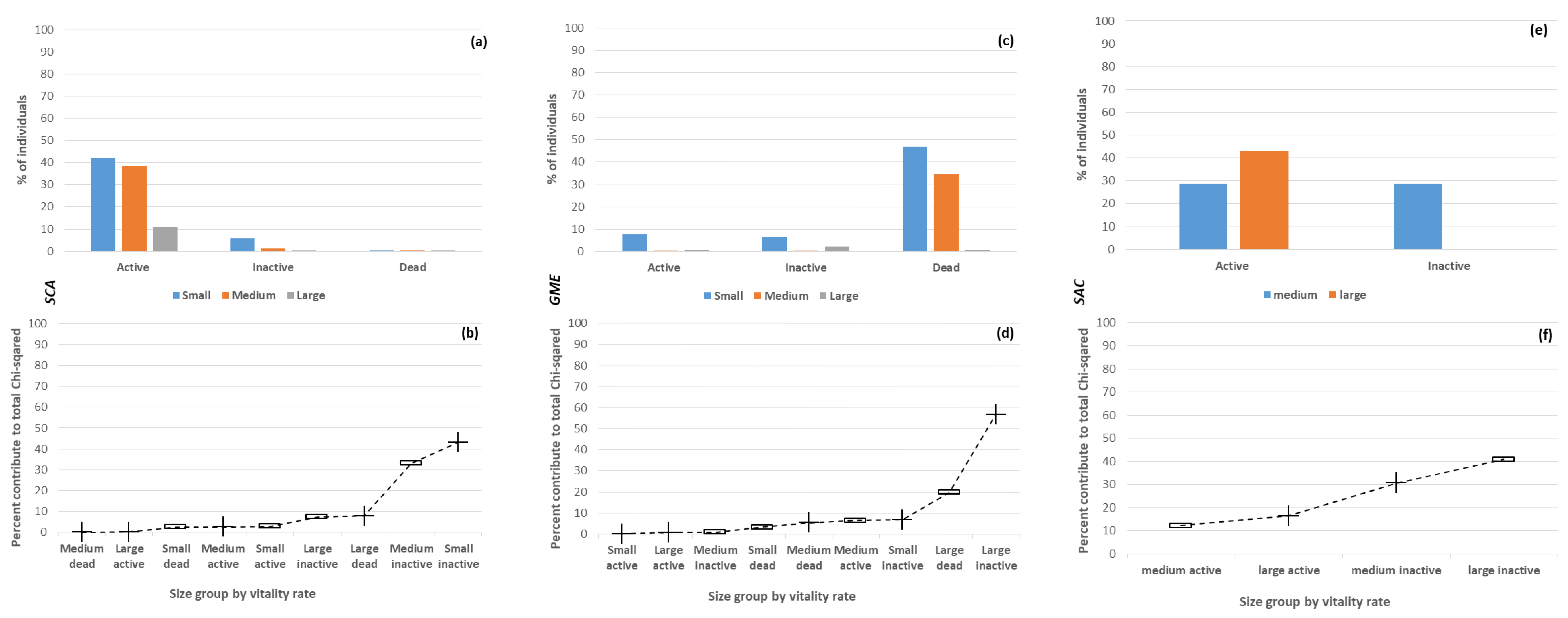
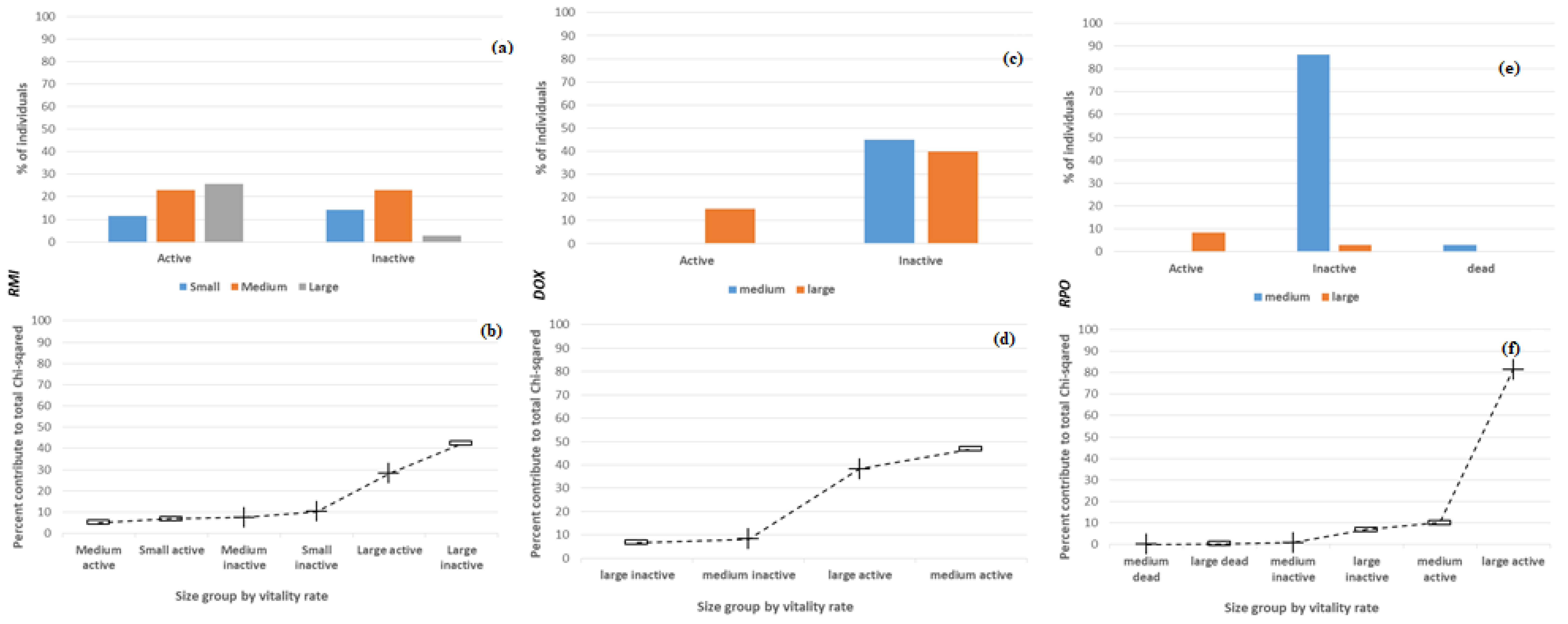
3.2. Intraspecific Variation of the Vitality Rate with Size
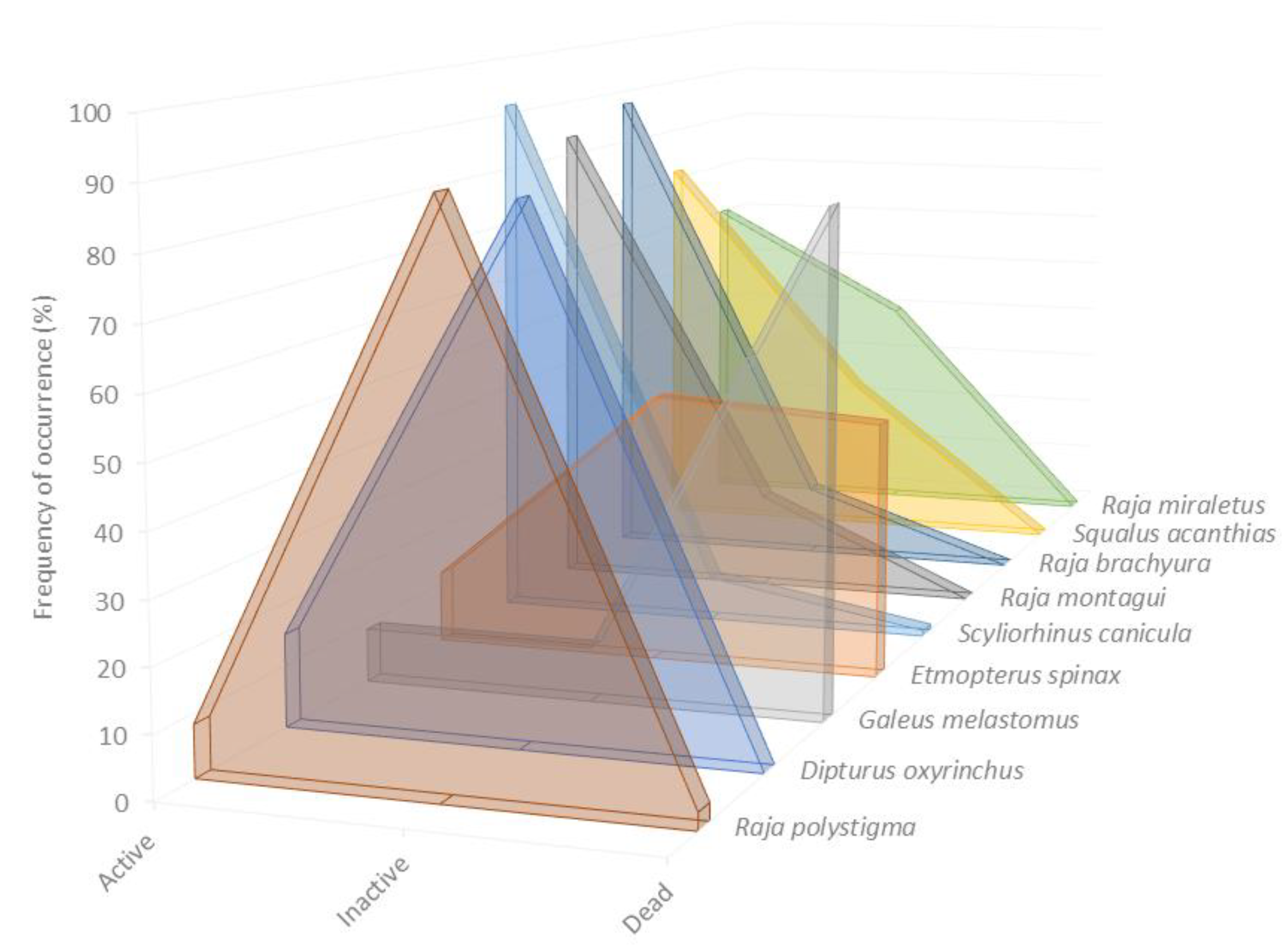
3.3. Interspecific Variation of the Vitality Rate
3.4. Factors Influencing the Vitality Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baum, J.K.; Myers, R.A.; Kehler, D.G.; Worm, B.; Harley, S.J.; Doherty, P.A. Collapse and conservation of shark populations in the Northwest Atlantic. Science 2003, 299, 389–392. [Google Scholar] [CrossRef]
- Myers, R.A.; Baum, J.K.; Shepherd, T.D.; Powers, S.P.; Peterson, C.H. Cascading Effects of the Loss of Apex Predatory Sharks from a Coastal Ocean. Science 2007, 315, 1846–1850. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, F.; Myers, R.A.; Serena, F.; Lotze, H.K. Loss of large predatory sharks from the Mediterranean Sea. Cons. Biol. 2008, 22, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, F.; Worm, B.; Britten, G.; Heithaus, M.R.; Lotze, H.K. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction risk and conservation of the world’s sharks and rays. eLife 2014, 3, e00590. [Google Scholar] [CrossRef] [PubMed]
- Davidson, L.N.K.; Krawchuk, M.A.; Dulvy, N.K. Why have global shark and ray landings declined: Improved management or overfishing? Fish Fish. 2016, 17, 438–458. [Google Scholar] [CrossRef]
- Pacoureau, N.; Rigby, C.L.; Kyne, P.M.; Sherley, R.B.; Winker, H.; Carlson, J.K.; Fordham, S.V.; Barreto, R.; Fernando, D.; Francis, M.P.; et al. Half a century of global decline in oceanic sharks and rays. Nature 2021, 589, 567–571. [Google Scholar] [CrossRef]
- Heppell, S.S.; Crowder, L.B.; Menzel, T.R. Life table analysis of long lived marine species with implications for conservation and management. In Life in the Slow Lane: Ecology and Conservation of Long-Lived Marine Animals; Musick, J.A., Ed.; American Fisheries Society: Grand Rapids, MI, USA, 1999; Volume 23, pp. 137–148. [Google Scholar]
- Musick, J.A.; Burgess, G.; Cailliet, G.; Camhi, M.; Fordham, S. Management of sharks and their relatives. Fisheries 2000, 25, 9–13. [Google Scholar] [CrossRef]
- Espinoza, M.; Araya-Arce, T.; Chaves-Zamora, I.; Chinchilla, I.; Cambra, M. Monitoring elasmobranch assemblages in a data-poor country from the Eastern Tropical Pacific using baited remote underwater video stations. Sci. Rep. 2020, 10, 17175. [Google Scholar] [CrossRef]
- Delaval, A.; Wagner, C.I.; Schwanck, T.; Wood, F.R.; Jones, C.S.; Hoarau, G.; Noble, L.R. Endangered Coastal Elasmobranchs of the North-East Atlantic; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Swift, D.G.; Portnoy, D.S. Identification and Delineation of Essential Habitat for Elasmobranchs in Estuaries on the Texas Coast. Estuar. Coast 2021, 44, 788–800. [Google Scholar] [CrossRef]
- Beerkircher, L.R.; Cortés, E.; Shivji, M. Characteristics of shark bycatch observed on pelagic longlines off the south-eastern United States, 1992–2000. Mar. Fish. Rev. 2002, 64, 40–49. [Google Scholar]
- Gilman, E.; Clarke, S.; Brothers, N.; Alfaroshigueto, J.; Mandelman, J.; Mangel, J.; Petersen, S.; Piovano, S.; Thomson, N.; Dalzell, P. Shark interactions in pelagic longline fisheries. Mar. Pol. 2008, 32, 1–18. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Orbesen, E.S.; Hammerschlag, N.; Serafy, J.E. Vulnerability of oceanic sharks as pelagic longline bycatch. Glob. Ecol. Cons. 2014, 1, 50–59. [Google Scholar] [CrossRef]
- Kroodsma, D.A.; Mayorga, J.; Hochberg, T.; Miller, N.A.; Boerder, K.; Ferretti, F.; Wilson, A.; Bergman, B.; White, T.D.; Block, B.A.; et al. Tracking the global footprint of fisheries. Science 2018, 359, 904–908. [Google Scholar] [CrossRef]
- Queiroz, N.; Humphries, N.E.; Couto, A.; Vedor, M.; da Costa, I.; Sequeira, A.M.M.; Mucientes, G.; Santos, A.M.; Abascal, F.J.; Abercrombie, D.L.; et al. Global spatial risk assessment of sharks under the footprint of fisheries. Nature 2019, 572, 461–466. [Google Scholar] [CrossRef]
- Megalofonou, P.; Damalas, D.; Yannopoulos, C. Composition and abundance of pelagic shark bycatch in the eastern Mediterranean Sea. Cybium 2005, 29, 135–140. [Google Scholar]
- Moro, S.; Jona-Lasinio, G.; Block, B.; Micheli, F.; De Leo, G.; Serena, F.; Bottaro, M.; Scacco, U.; Ferretti, F. Abundance and distribution of the white shark in the Mediterranean Sea. Fish Fish. 2019, 21, 338–349. [Google Scholar] [CrossRef]
- Coelho, R.; Erzini, K.; Bentes, L.; Correia, C.; Lino, P.G.; Monteiro, P.; Ribeiro, J.; Gonçalves, J.M.S. Semi-pelagic longline and trammel net elasmobranch catches in southern Portugal: Catch composition, catch rates, and discards. J. Northwest Atl. Fish. Sci. 2005, 37, 531–537. [Google Scholar] [CrossRef]
- Perez, J.A.A.; Wahrlich, R.A. bycatch assessment of the gillnet monkfish Lophius gastrophysus fishery off southern Brazil. Fish Res. 2005, 72, 81–95. [Google Scholar] [CrossRef]
- Valenzuela, A.; Bustamante, C.; Lamilla, J. Morphological characteristics of five bycatch sharks caught by southern Chilean demersal longline fisheries. Sci. Mar. 2008, 72, 231–237. [Google Scholar]
- Thorpe, T.; Frierson, D. Bycatch mitigation assessment for sharks caught in coastal anchored gillnets. Fish Res. 2009, 98, 102–112. [Google Scholar] [CrossRef]
- Benjamins, S.; Kulka, D.W.; Lawson, J. Recent incidental catch of sharks in gillnet fisheries of Newfoundland and Labrador, Canada. Endanger. Species Res. 2010, 11, 133–146. [Google Scholar] [CrossRef]
- Scacco, U.; Consalvo, I.; Di Muccio, S.; Tunesi, L. On the bycatch of two porbeagle sharks Lamna nasus in the central Adriatic Sea. Mar. Biodivers. Rec. 2012, 5, 1–5. [Google Scholar] [CrossRef]
- McKinnell, S.; Seki, M.P. Shark bycatch in the Japanese high seas squid driftnet fishery in the North Pacific Ocean. Fish Res. 1998, 39, 127–138. [Google Scholar] [CrossRef]
- Tudela, S.; Kai Kai, A.; Maynou, F.; Andalossi, M.E.; Guglielmi, P. Driftnet fishing and biodiversity conservation: The case study of the large-scale Moroccan driftnet fleet operating in the Alboran Sea (SW Mediterranean). Biol. Cons. 2005, 121, 65–78. [Google Scholar] [CrossRef]
- Roff, G.; Brown, C.J.; Priest, M.A.; Mumby, P. Decline of coastal apex shark populations over the past half century. Comm. Biol. 2018, 1, 223. [Google Scholar] [CrossRef]
- Temple, A.J.; Kiszka, J.J.; Stead, S.M.; Wambiji, N.; Brito, A.; Poonian, C.N.S.; Amir, O.A.; Jiddawi, N.; Fennessy, S.T.; Pérez-Jorge, S.; et al. Marine megafauna interactions with small-scale fisheries in the southwestern Indian Ocean: A review of status and challenges for research and management. Rev. Fish Biol. Fish. 2018, 28, 89–115. [Google Scholar] [CrossRef]
- Lloret, J.; Biton-Porsmoguer, S.; Carreño, A.; Di Franco, A.; Sahyoun, R.; Melià, P.; Claudet, J.; Sève, C.; Ligas, A.; Belharet, M.; et al. Recreational and small-scale fisheries may pose a threat to vulnerable species in coastal and offshore waters of the western Mediterranean. ICES J. Mar. Sci. 2019, 77, 2255–2264. [Google Scholar] [CrossRef]
- Smith, H.; Basurto, X. Defining Small-Scale Fisheries and Examining the Role of Science in Shaping Perceptions of Who and What Counts: A Systematic Review. Front. Mar. Sci. 2019, 6, 236. [Google Scholar] [CrossRef]
- FAO. International Standard Statistical Classification of Fishing Gear (ISSCFG, 2016). Coordinating Working Party on Fishery Statistics (CWP). Handbook of Fishery Statistics. 2006. Available online: https://www.fao.org/3/a-bt988e.pdfCouncilregulation (accessed on 1 January 2023).
- Ferretti, M. Inventario Degli Attrezzi da Pesca Nelle Marinerie Italiane, Relazione Commissionata dal Ministero Della Marina Mercantile, Direzione Generale Della Pesca Marittima. 1981. Available online: https://www.unimar.it/wp-content/uploads/2017/04/25.-MAPP_primi_risultati.pdf (accessed on 12 December 2022).
- Scacco, U.; Andaloro, F.; Castriota, L.; Campagnuolo, S.; Vacchi, M. Cartilaginous fishes as a component of trawl discard in the Strait of Sicily. NAFO SCR Doc. 2002, 2, 87. [Google Scholar]
- Stobutzki, I.C.; Miller, M.J.; Heales, D.S.; Brewer, D.T. Sustainability of elasmobranchs caught as bycatch in a tropical prawn (shrimp) trawl fishery. Fish Bull. 2002, 100, 800–821. [Google Scholar]
- Carbonell, A.; Alemany, F.; Merella, P.; Quetglas, A.; Roman, E. The bycatch of sharks in the western Mediterranean (Balearic Islands) trawl fishery. Fish Res. 2003, 61, 7–18. [Google Scholar] [CrossRef]
- Amande, M.J.; Chassot, E.; Chavance, P.; Pianet, R. Silky shark (Carcharhinus falciformis) bycatch in the French tuna purse-seine fishery of the Indian Ocean. IOTC WPEB 2008, 22, 60. [Google Scholar]
- Scacco, U.; Consalvo, I.; Mostarda, E. First documented catch of the giant devil ray Mobula mobular (Chondrichthyes: Mobulidae) in the Adriatic Sea. Mar. Biodivers. Rec. 2009, 2, E93. [Google Scholar] [CrossRef]
- Fortuna, C.M.; Vallini, C.; Filidei, E., Jr.; Ruffino, M.; Consalvo, I.; Di Muccio, S.; Gion, C.; Scacco, U.; Tarulli, E.; Giovanardi, O.; et al. Bycatch of cetaceans and other species of conservation concern during pair trawl fishing operations in the Adriatic Sea (Italy). Chem. Ecol. 2010, 26, 65–76. [Google Scholar] [CrossRef]
- Psomadakis, P.; Dalù, M.; Scacco, U.; Vacchi, M. A rare batoid fish Gymnura altavela (Chondrichthyes, Gymnuridae) captured in the Tyrrhenian Sea. Mar. Biodivers. Rec. 2008, 1, E6. [Google Scholar] [CrossRef]
- Biton Porsmoguer, S.; Lloret, J. Potential impacts of bottom trawling on species of skates (Rajiformes: Rajidae): The case of the Gulf of Cádiz and the Western Mediterranean. Cybium 2020, 44, 255–263. [Google Scholar] [CrossRef]
- Carpentieri, P.; Nastasi, A.; Sessa, M.; Srour, A. (Eds.) Incidental Catch of Vulnerable Species in Mediterranean and Black Sea Fisheries: A Review; GFCM Studies and Reviews No. 101; FAO: Rome, Italy, 2021. [Google Scholar]
- FAO. Catalogue of Fishing Gears Designs; Fishing News Books Ltd.: London, UK, 1972; p. 160. [Google Scholar]
- FAO. Fishery Information, Data Statistics Service and Fishing Technology Service (comps). In Definition and Classification of Fishery Vessel Types; FAO: Rome, Italy, 1985; Volume 267, pp. 1–63. Available online: http://www.fao.org/3/a-bq842e.pdf, (accessed on 1 January 2023).
- Froese, R.; Demirel, N.; Coro, G.; Kleisner, K.M.; Winker, H. Estimating fisheries reference points from catch and resilience. Fish Fish. 2017, 18, 506–526. [Google Scholar] [CrossRef]
- Cortés, E. Demographic analysis as an aid in shark stock assessment and management. Fish Res. 1998, 39, 199–208. [Google Scholar] [CrossRef]
- Cortés, E. Incorporating uncertainty into demographic modelling: Application to shark populations and their conservation. Conserv. Biol 2002, 16, 1048–1062. [Google Scholar] [CrossRef]
- Holling, C.S. Engineering Resilience versus Ecological. In Engineering within Ecological Constraints; Schulze, Ed.; National Academy Press: Washington, DC, USA, 1996; pp. 31–43. [Google Scholar]
- Ellis, J.R.; Mccully, S.R.; Poisson, P.F. A review of capture and post-release mortality of elasmobranchs. J. Fish Biol. 2017, 90, 653–722. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.; Mourier, J.; Rummer, J.L. Blacktip reef sharks (Carcharhinus melanopterus) show high capacity for wound healing and recovery following injury. Conserv. Physiol. 2015, 3, cov062. [Google Scholar] [CrossRef]
- Pianka, E.R. On r- and K-Selection. Am. Nat. 1970, 104, 592–597. [Google Scholar] [CrossRef]
- MacArthur, R.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967; ISBN 978-0-691-08836-5. [Google Scholar]
- Hyatt, M.W.; Anderson, P.A.; O’Donnell, P.M.; Berzins, I.K. Assessment of acid–base derangements among bonnethead (Sphyrna tiburo), bull (Carcharhinus leucas) and lemon (Negaprion brevirostris) sharks from gillnet and longline capture and handling methods. Comp. Biochem. Physiol. 2012, 162, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Marshall, H.; Field, L.; Afiadata, A.; Sepulveda, C.; Skomal, G.; Bernal, D. Hematological indicators of stress in longline-captured sharks. Comp. Biochem. Physiol. 2012, 162, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Skomal, G.B.; Mandelman, J.W. The physiological response to anthropogenic stressors in marine elasmobranch fishes: A review with a focus on the secondary response. Comp. Biochem. Physiol. 2012, 162, 146–155. [Google Scholar] [CrossRef]
- Dapp, D.R.; Huveneers, C.; Walker, T.I.; Drew, M.; Reina, R.D. Moving from measuring to predicting bycatch mortality: Predicting the capture condition of a longline-caught pelagic shark. Front. Mar. Sci. 2016, 2, 126. [Google Scholar] [CrossRef]
- CoNiSMA Contabilità Ambientale Nelle Aree Marine Protette Italiane Area Marina Protetta Isola Dell’asinara Interventi Realizzati a Valere sulle Specifiche Risorse Assegnate per L’implementazione della Rendicontazione Naturalistica (Ecorendiconto). 2014 Report Finale. Available online: https://www.torredelcerrano.it/wp-content/uploads/2016/11/2_Documento-Contabilit%C3%A0_ambientale_FEDERPARCHI-Roma-15-aprile-2014.pdf (accessed on 23 February 2023).
- Pascucci, V.; Cossu, A.; De Luca, M.; Santonastaso, A.; Pireddu, L. Accordo di Cooperazione Con L’università Degli Studi di SassarI, Dipartimento di Architettura (DADU) e L’ente Parco Dell’asinara, Amp “Isola Dell’asinara” D.M 22 del 11 Febbraio 2015 “ISOLA DELL’ARSINARA”-Strategia Marina Nelle Aree Marine Protette. Relazione sul Monitoraggio “Habitat a Coralligeno Nell’amp dell’Asinara”; Università di Sassari DADU: Alghero, Italy, 2018. [Google Scholar]
- Bell, J.D.; Harmelin-Vivien, M.L. Fish fauna of French Mediterranean Posidonia oceanica seagrass meadows. II—Feeding habits. Téthys 1983, 11, 1–14. [Google Scholar]
- Google Earth Web. Physical Profiles of Marine Grounds of the Asinara Gulf 2023. Available online: https://earth.google.com/web/@41.13577142,8.74816503,127069.68668974a,0d,35y,0.0257h,0t,0r?utm_source=earth7&utm_campaign=vine&hl=it (accessed on 23 February 2023).
- Telesca, L.; Belluscio, A.; Criscoli, A.; Ardizzone, G.; Apostolaki, E.T.; Fraschetti, S.; Gristina, M.; Knittweis, L.; Martin, C.S.; Pergent, G. Seagrass meadows (Posidonia oceanica) distribution and trajectories of change. Sci. Rep. 2015, 5, 12505. [Google Scholar] [CrossRef]
- Cocito, S.; Ferdeghini, F. Carbonate standing stock and carbonate production of the bryozoan Pentapora fascialis in the north-western Mediterranean. Facies 2001, 45, 25–30. [Google Scholar] [CrossRef]
- Tonin, S. Economic value of marine biodiversity improvement in coralligenous habitats. Ecol. Ind. 2018, 85, 1121–1132. [Google Scholar] [CrossRef]
- Cossu, A.; De Luca, M. Indagine sui fondi duri ai fini della attuazione della direttiva della Strategia Marina—Sardegna Settentrionale. Biol. Mar. Medit. 2016, 23, 178–181. [Google Scholar]
- Cossu, A.; Cressa, L.; Gazale, V.; Ragazzola, F. On The Circalittoral Benthic Communities In The Asinara Marine Park. Biol. Mar. Medit. 2009, 16, 256–257. [Google Scholar]
- Progetto Strategico MARTE+, Sottoprogetto SB, Modelli di Governance e Monitoraggio per la Salvaguardia e Valorizzazione Delle Risorse Ittiche, Componente 4: Monitoraggio delle Risorse Ittiche e Acquacoltura Sostenibile. Risultati del monitoraggio integrativo: La Pesca Artigianale nel Territorio Transfrontaliero: Capacita’, Sforzo di Pesca e Caratterizzazione dei Principali Metiers. Interreg technical report. 2013. Available online: http://www.maritimeit-fr.net/documents/12943000/12967214/MARTE%2B-IT.pdf/6c841325-cb8b-4df0-a544-ad46998bfdb5 (accessed on 23 February 2023).
- Francour, P. Pluriannual analysis of the reserve effect on ichthyofauna in the Scandola natural reserve (Corsica, north-western Mediterranean). Oceanol. Acta 1994, 17, 309–317. [Google Scholar]
- MIPAAF. National Fishery Data Collection Program Italian Work Plan for data Collection in the Fisheries and Aquaculture Sectors 2020–2021; Version 1.0; MIPAAF: Rome, Italy, 2019. [Google Scholar]
- Catalano, B.; Scacco, U.; Vacchi, M. Notes on biodiversity of cartilaginous fishes of the Asinara Marine Reserve. Biol. Mar. Medit. 2003, 10, 655–658. [Google Scholar]
- Catalano, B.; Dalù, M.; Scacco, U.; Vacchi, M. New biological data on Raja brachyura (Chondrichthyes, Rajidae) from around Asinara Island (NW Sardinia, Western Mediterranean). Ital. J. Zool. 2007, 74, 55–61. [Google Scholar] [CrossRef]
- Bertrand, J.A.; Gil de Sola, L.; Papaconstantinou, C.; Relini, G.; Souplet, A. The general specifications of the MEDITS surveys. Sci. Mar. 2002, 66, 9–17. [Google Scholar] [CrossRef]
- Fischer, W.; Bauchot, M.L.; Schneider, M. Fiches FAO D’identification des Espèces Pour Les Besoins de la Pêche. Méditerranée et Mer Noire; Zones de pêche 37 (2) Vertébrés; FAO: Rome, Italy, 1987. [Google Scholar]
- Serena, F. Field identification guide to the sharks and rays of the Mediterranean and Black Sea. In FAO Species Identification Guide for Fishery Purposes; FAO: Rome, Italy, 2005; 97p. [Google Scholar]
- Benoît, H.P.; Hurlbut, T.; .Chassé, J. Assessing the factors influencing discard mortality of demersal fishes using a semi-quantitative indicator of survival potential. Fish Res. 2010, 106, 436–447. [Google Scholar] [CrossRef]
- Revill, A.S.; Dulvy, N.K.; Holst, R. The survival of discarded lesser-spotted dogfish (Scyliorhinus canicula) in the western English Channel beam trawl fishery. Fish Res. 2005, 71, 121–124. [Google Scholar] [CrossRef]
- Benoît, H.P.; Plante, S.; Kroiz, M.; Hurlbut, T. A comparative analysis of marine fish species susceptibilities to discard mortality: Effects of environmental factors, individual traits and phylogeny. ICES J. Mar. Sci. 2013, 70, 99–113. [Google Scholar] [CrossRef]
- Poisson, F.; Séret, B.; Vernet, A.L.; Goujon, M.; Dagorn, L. Collaborative research: Development of a manual on elasmobranch handling and release best practices in tropical tuna purse-seine fisheries. Mar. Pol. 2014, 44, 312–320. [Google Scholar] [CrossRef]
- StatSoft. Statistica (Data Analysis Software System), Version 6; StatSoft Inc.: Tulsa, OK, USA, 2001. [Google Scholar]
- Barone, M.; Mazzoldi, C.; Serena, F. Sharks, Rays and Chimaeras in Mediterranean and Black Seas—Key to Identification; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Frick, L.H.; Reina, R.D.; Walker, T.I. Stress related changes and post-release survival of Port Jackson sharks (Heterodontus portusjacksoni) and gummy sharks (Mustelus antarcticus) following gill-net and longline capture in captivity. J. Exp. Mar. Biol. Ecol. 2010, 385, 29–37. [Google Scholar] [CrossRef]
- Frick, L.H.; Walker, T.I.; Reina, R.D. Trawl capture of Port Jackson sharks, Heterodontus portusjacksoni and gummy sharks, Mustelus antarcticus, in a controlled setting: Effects of tow duration, air exposure and crowding. Fish Res. 2010, 6, 344–350. [Google Scholar] [CrossRef]
- Heard, M.; Van Rijn, J.A.; Reina, R.D.; Huveneers, C. Impacts of crowding, trawl duration and air exposure on the physiology of stingarees (family: Urolophidae). Cons. Physiol. 2014, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chopin, F.S.; Arimoto, T. The condition of fish escaping from fishing gears—A review. Fish Res. 1995, 21, 315–327. [Google Scholar] [CrossRef]
- Davis, M.W. Key principles for understanding fish bycatch discard mortality. Can. J. Fish Aquat. Sci. 2002, 59, 1834–1843. [Google Scholar] [CrossRef]
- Cook, K.V.; Reid, A.J.; Patterson, D.A.; Robinson, K.A.; Chapman, J.M.; Hinch, S.G.; Cooke, S.J. A synthesis to understand responses to capture stressors among fish discarded from commercial fisheries and options for mitigating their severity. Fish Fish. 2019, 20, 25–43. [Google Scholar] [CrossRef]
- Last, P.R.; White, W.T.; de Carvalho, M.R.; Séret, B.; Stehmann, M.F.W.; Naylor, G.J.P. Rays of the World; CSIRO Publishing: Clayton, Australia, 2016. [Google Scholar]
- Jones, E.G.; Tselepides, A.; Bagley, P.M.; Collins, M.A.; Priede, I.G. Bathymetric distribution of some benthic and benthopelagic species attracted to baited cameras and traps in the deep eastern Mediterranean. Mar. Ecol. Prog. Ser. 2003, 251, 75–80. [Google Scholar] [CrossRef]
- Weigmann, S. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. J. Fish Biol. 2016, 88, 837–1037. [Google Scholar] [CrossRef]
- Cognetti, G.; Sará, M. Biologia Marina; Calderini: Bologna, Italy, 1974. [Google Scholar]
- Ghirardelli, E. La Vita Nelle Acque; Utet: Torino, Italy, 1981. [Google Scholar]
- Newton, G.M. The deep-sea environment—Earth’s final frontier. Aust. Mar. Sci. Bull. 1999, 147, 17–21. [Google Scholar]
- Litherland, L.; Collin, S.P.; Fritsches, K.A. Visual optics and ecomorphology of the growing shark eye: A comparison between deep and shallow water species. J. Exp. Biol. 2009, 212, 3583–3594. [Google Scholar] [CrossRef] [PubMed]
- Cicia, A.M.; Schlenker, L.S.; Sulikowski, J.A.; Mandelman, J.W. Seasonal variations in the physiological stress response to discrete bouts of aerial exposure in the little skate, Leucoraja erinacea. Comp. Biochem. Physiol. 2012, 162, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Hoffmayer, E.R.; Hendon, J.M.; Parsons, G.R. Seasonal modulation in the secondary stress response of a carcharhinid shark, Rhizoprionodon terraenovae. Comp. Biochem. Physiol. 2012, 162, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Gurshin, C.W.D.; Szedlmayer, S.T. Short-term survival and movements of Atlantic sharpnose sharks captured by hook-and-line in the north-east Gulf of Mexico. J. Fish. Biol. 2004, 65, 973–986. [Google Scholar] [CrossRef]
- Talwar, B.; Brooks, E.J.; Mandelman, J.W.; Grubbs, R.D. Stress, post-release mortality, and recovery of commonly discarded deep-sea sharks caught on longlines. Mar. Ecol. Progr. Ser. 2017, 582, 147–161. [Google Scholar] [CrossRef]
- Humborstad, O.B.; Ferter, K.; Kryvi, H.; Fjelldal, P.G. Exophthalmia in wild-caught cod (Gadus morhua L.): Development of a secondary barotrauma effect in captivity. J. Fish Dis. 2017, 40, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cabello, C.; Fernández, A.; Olaso, I.; Sánchez, F. Survival of small-spotted catshark (Scyliorhinus canicula) discarded by trawlers in the Cantabrian Sea. J. Mar. Biol. Assoc. UK 2005, 85, 1145–1150. [Google Scholar] [CrossRef]
- Fennessy, S.T. Incidental capture of elasmobranchs by commercial prawn trawlers on the Tugela Bank, Natal, South Africa. S. Afr. J. Mar. Sci 1994, 14, 287–296. [Google Scholar] [CrossRef]
- Enever, R.; Catchpole, T.L.; Ellis, J.R.; Grant, A. The survival of skates (Rajidae) caught by demersal trawlers fishing in UK waters. Fish Res. 2009, 97, 72–76. [Google Scholar] [CrossRef]
- Meyer, W.; Seegers, U. Basics of skin structure and function in elasmobranchs: A review. J. Fish Biol. 2012, 80, 1940–1967. [Google Scholar] [CrossRef]
- Carrier, J.C.; Musick, J.A.; Heithaus, M.R. (Eds.) Biology of Sharks and Their Relatives, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Reid, D.D.; Krogh, M. Assessment of catches from protective shark meshing off NSW beaches between 1950 and 1990. Mar. Fresh Res. 1992, 43, 283–296. [Google Scholar] [CrossRef]
- Moyes, C.D.; Fragoso, N.; Musyl, M.K.; Brill, R.W. Predicting post release survival in large pelagic fish. Trans. Am. Fish Soc. 2006, 135, 1389–1397. [Google Scholar] [CrossRef]
- Coelho, R.; Infante, P.; Santos, M.N. Application of generalized linear models and generalized estimation equations to model at-haulback mortality of blue sharks captured in a pelagic longline fishery in the Atlantic Ocean. Fish Res. 2013, 145, 66–75. [Google Scholar] [CrossRef]
- Follesa, M.C.; Marongiu, M.F.; Zupa, W.; Bellodi, A.; Cau, A.; Cannas, R.; Colloca, F.; Djurovic, M.; Isajlovic, I.; Jadaud, A.; et al. Spatial variability of Chondrichthyes in the northern Mediterranean. Sci. Mar. 2019, 83, 81–100. [Google Scholar] [CrossRef]
- Psomadakis, P.; Giustino, S.; Vacchi, M. Mediterranean fish biodiversity: An updated inventory with focus on the Ligurian and Tyrrhenian seas. Zootaxa 2012, 3263, 1–46. [Google Scholar] [CrossRef]
- Bellodi, A.; Porcu, C.; Cau, A.; Marongiu, M.F.; Melis, R.; Mulas, A.; Pesci, P.; Follesa, M.C.; Cannas, R. Investigation on the genus Squalus in the Sardinian waters (Central-Western Mediterranean) with implications on its management. Med. Mar. Sci. 2018, 19, 256–272. [Google Scholar] [CrossRef]
- Bonello, J.; Bonnici, L.; Ferrari, A.; Cariani, A.; Schembri, P. Not all that clear cut: Intraspecific morphological variability in Squalus blainville (Risso, 1827) and implications for identification of the species. J. Mar. Biol. Assoc. UK 2016, 96, 1585–1596. [Google Scholar] [CrossRef]
- Ebert, D.A.; Dando, M.; Fowler, S. Sharks of the World: A Complete Guide. Princeton University Press: Princeton, NJ, USA, 2021; p. 624. [Google Scholar]
- Frodella, N.; Cannas, R.; Velonà, A.; Carbonara, P.; Farrell, E.; Fiorentino, F.; Follesa, M.; Garofalo, G.; Hemida, F.; Mancusi, C.; et al. Population connectivity and phylogeography of the Mediterranean endemic skate Raja polystigma and evidence of its hybridization with the parapatric sibling R. montagui. Mar. Ecol. Prog. Ser. 2016, 554, 99–113. [Google Scholar] [CrossRef]
- Poloczanska, E.S.; Brown, C.J.; Sydeman, W.J.; Kiessling, W.; Schoeman, D.S.; Moore, P.J.; Brander, K.; Bruno, J.F.; Buckley, L.B.; Burrows, M.T.; et al. Global imprint of climate change on marine life. Nat. Clim. Chang. 2013, 3, 919–925. [Google Scholar] [CrossRef]
- Griffiths, S.P.; Brewer, D.T.; Heales, D.S.; Milton, D.A.; Stobutzki, I.C. Validating ecological risk assessments for fisheries: Assessing the impacts of turtle excluder devices on elasmobranch bycatch populations in an Australian trawl fishery. Mar. Fresh Res. 2006, 57, 395–401. [Google Scholar] [CrossRef]
- Isaksen, B.; Valdemarsen, J.; Larsen, R.; Karlsen, L. Reduction of fish bycatch in shrimp trawl using a rigid separator grid in the aft belly. Fish Res. 1992, 13, 335–352. [Google Scholar] [CrossRef]
- Kynoch, R.J.; Fryer, R.J.; Neat, F.C. A simple technical measure to reduce bycatch and discard of skates and sharks in mixed-species bottom-trawl fisheries. ICES J. Mar. Sci. 2015, 72, 1861–1868. [Google Scholar] [CrossRef]
- Zeeberg, J.; Corten, A.; de Graaf, A. Bycatch and release of pelagic megafauna in industrial trawler fisheries off Northwest Africa. Fish Res. 2006, 78, 186–195. [Google Scholar] [CrossRef]

| Level of Vitality after Trawl Capture | Description |
|---|---|
| Dead | No body movement nor any contraction of spiracles, gills, and the mouth |
| Inactive | Weak body movements with some inconstant contractions of spiracles, gills, and the mouth |
| Active | Strong and active body movements with constant contractions of spiracles, gills, and the mouth |
| Species | Species Code | Length Range of Size Groups (cm) | ||
|---|---|---|---|---|
| Small | Medium | Large | ||
| Dipturus oxyrinchus | DOX | na | 24–43 | 44–62 |
| Etmopterus spinax | ESP | 12–22 | 23–32 | 33–42 |
| Galeus melastomus | GME | 10–23 | 24–36 | 37–49 |
| Leucoraja circularis | LCI | na | Na | na |
| Leucoraja fullonica | LFU | na | Na | na |
| Mustelus mustelus | MMU | na | Na | na |
| Raja brachyura | RBR | na | 24–40 | 41–57 |
| Raja miraletus | RMI | 13–22 | 23–32 | 33–41 |
| Raja montagui | RMO | na | 9–28 | 29–47 |
| Raja polystigma | RPO | na | 15–35 | 36–55 |
| Scyliorhinus canicula | SCA | 10–23 | 24–37 | 38–50 |
| Squalus acanthias | SAC | na | 45–58 | 59–72 |
| Species | Χ2 | d. f. | p |
|---|---|---|---|
| DOX | 2.89 | 1 | ** |
| ESP | 4.43 | 4 | ns |
| GME | 150.29 | 4 | *** |
| RBR | 0.00 | 1 | ns |
| RMI | 5.32 | 2 | ** |
| RMO | 1.71 | 1 | ns |
| RPO | 26.19 | 2 | *** |
| SCA | 22.46 | 4 | *** |
| SAC | 2.10 | 1 | * |
| Variables | Dead | Inactive | Active | * TL | * Mean Depth | * Sharks | * Skates |
|---|---|---|---|---|---|---|---|
| Dead | 1.00 | −0.30 | −0.77 | −0.14 | 0.68 | 0.22 | −0.22 |
| Inactive | −0.30 | 1.00 | −0.37 | 0.03 | 0.07 | −0.36 | 0.36 |
| Active | −0.77 | −0.37 | 1.00 | 0.11 | −0.70 | 0.03 | −0.03 |
| * TL | −0.14 | 0.03 | 0.11 | 1.00 | −0.09 | −0.18 | 0.18 |
| * Mean depth | 0.68 | 0.07 | −0.70 | −0.09 | 1.00 | 0.21 | −0.21 |
| * Sharks | 0.22 | −0.36 | 0.03 | −0.18 | 0.21 | 1.00 | −1.00 |
| * Skates | −0.22 | 0.36 | −0.03 | 0.18 | −0.21 | −1.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scacco, U.; Fortibuoni, T.; Baini, M.; Franceschini, G.; Giani, D.; Concato, M.; Panti, C.; Izzi, A.; Angiolillo, M. Gradients of Variation in the At-Vessel Mortality Rate between Twelve Species of Sharks and Skates Sampled through a Fishery-Independent Trawl Survey in the Asinara Gulf (NW Mediterranean Sea). Biology 2023, 12, 363. https://doi.org/10.3390/biology12030363
Scacco U, Fortibuoni T, Baini M, Franceschini G, Giani D, Concato M, Panti C, Izzi A, Angiolillo M. Gradients of Variation in the At-Vessel Mortality Rate between Twelve Species of Sharks and Skates Sampled through a Fishery-Independent Trawl Survey in the Asinara Gulf (NW Mediterranean Sea). Biology. 2023; 12(3):363. https://doi.org/10.3390/biology12030363
Chicago/Turabian StyleScacco, Umberto, Tomaso Fortibuoni, Matteo Baini, Gianluca Franceschini, Dario Giani, Margherita Concato, Cristina Panti, Alessia Izzi, and Michela Angiolillo. 2023. "Gradients of Variation in the At-Vessel Mortality Rate between Twelve Species of Sharks and Skates Sampled through a Fishery-Independent Trawl Survey in the Asinara Gulf (NW Mediterranean Sea)" Biology 12, no. 3: 363. https://doi.org/10.3390/biology12030363
APA StyleScacco, U., Fortibuoni, T., Baini, M., Franceschini, G., Giani, D., Concato, M., Panti, C., Izzi, A., & Angiolillo, M. (2023). Gradients of Variation in the At-Vessel Mortality Rate between Twelve Species of Sharks and Skates Sampled through a Fishery-Independent Trawl Survey in the Asinara Gulf (NW Mediterranean Sea). Biology, 12(3), 363. https://doi.org/10.3390/biology12030363









