Phenotypic Plasticity in Juvenile Frogs That Experienced Predation Pressure as Tadpoles Does Not Alter Their Locomotory Performance
Abstract
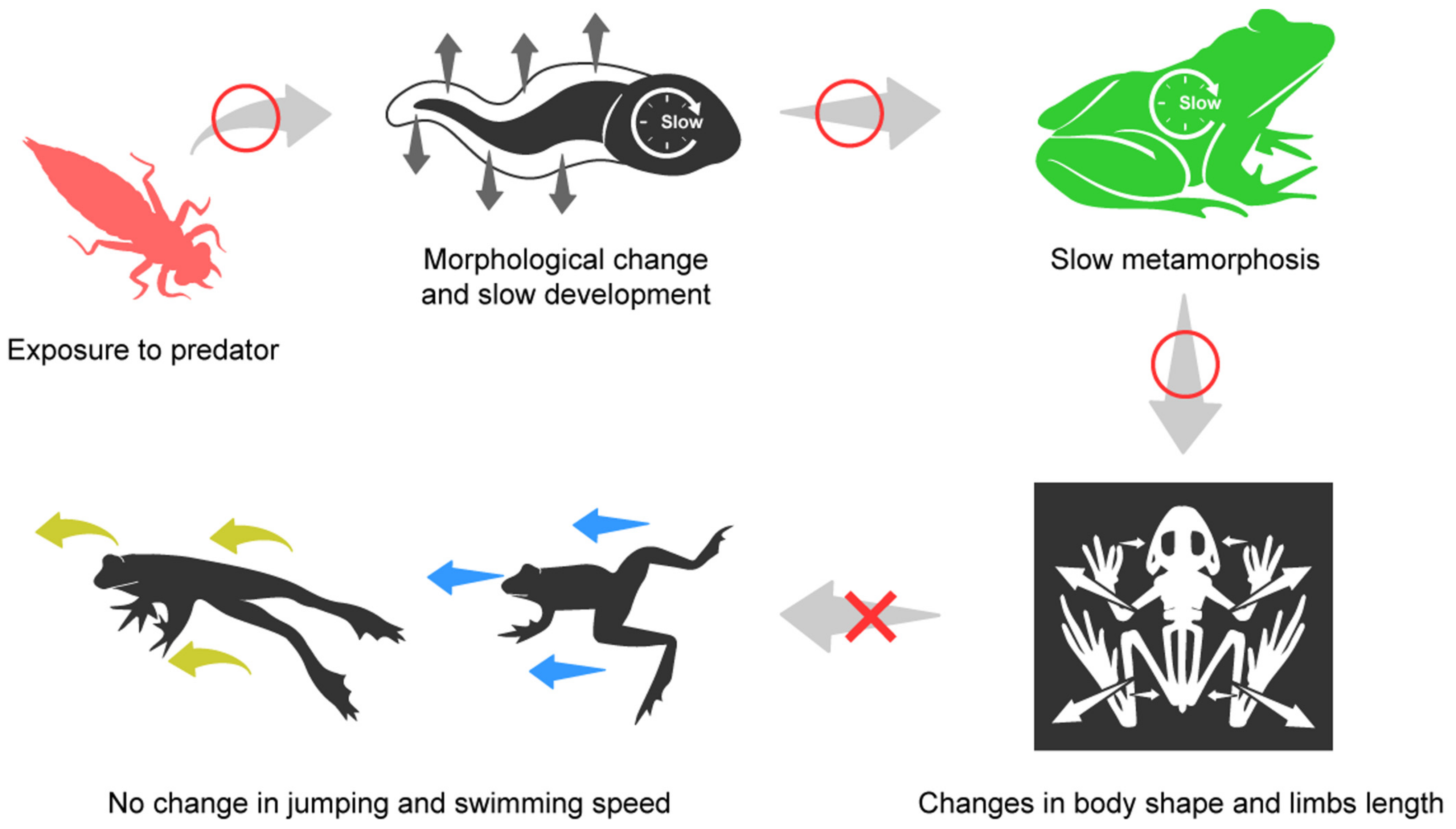
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Collection and Experimental Condition
2.2. Analysis of Growth and Developmental Rate
2.3. Measurement of Jumping and Swimming Speed
2.4. Identification of Morphological Change
2.5. Statistical Analysis
3. Results
3.1. Differences in the Physical Conditions, Development, and Tail Shape of Tadpoles as Caused by Predator Pressure
3.2. Comparison Regarding the Physical Conditions, Metamorphosis Timing, and Skeletal Shape of Juvenile Frogs
3.3. Comparison of Jumping and Swimming Speeds between Predation Group and Control Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wake, D.B.; Koo, M.S. Amphibians. Curr. Biol. 2018, 28, R1237–R1241. [Google Scholar] [CrossRef] [PubMed]
- AmphibiaWeb. Amphibians. Available online: https://amphibiaweb.org/amphibian/amph_index.html (accessed on 15 November 2022).
- Van Buskirk, J. A comparative test of the adaptive plasticity hypothesis: Relationships between habitat and phenotype in anuran larvae. Am. Nat. 2002, 160, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Richter-Boix, A.; Tejedo, M.; Rezende, E.L. Evolution and plasticity of anuran larval development in response to desiccation. A comparative analysis. Ecol. Evol. 2011, 1, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ruthsatz, K.; Peck, M.A.; Dausmann, K.H.; Sabatino, N.M.; Glos, J. Patterns of temperature induced developmental plasticity in anuran larvae. J. Therm. Biol. 2018, 74, 123–132. [Google Scholar] [CrossRef]
- Relyea, R.A. The lasting effects of adaptive plasticity: Predator-induced tadpoles become long-legged frogs. Ecology 2001, 82, 1947–1955. [Google Scholar] [CrossRef]
- Relyea, R.A. Competitor-induced plasticity in tadpoles: Consequences, cues, and connections to predator-induced plasticity. Ecol. Monogr. 2002, 72, 523–540. [Google Scholar] [CrossRef]
- Relyea, R.A. Fine-tuned phenotypes: Tadpole plasticity under 16 combinations of predators and competitors. Ecology 2004, 85, 172–179. [Google Scholar] [CrossRef]
- DeWitt, T.J.; Sih, A.; Wilson, D.S. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 1998, 13, 77–81. [Google Scholar] [CrossRef]
- Van Buskirk, J.; Anderwald, P.; Lüpold, S.; Reinhardt, L.; Schuler, H. The lure effect, tadpole tail shape, and the target of dragonfly strikes. J. Herpetol. 2003, 37, 420–424. [Google Scholar] [CrossRef]
- Kruger, A.; Morin, P.J. Predators Induce Morphological Changes in Tadpoles of Hyla andersonii. Copeia 2020, 108, 316–325. [Google Scholar] [CrossRef]
- Middlemis Maher, J.; Werner, E.E.; Denver, R.J. Stress hormones mediate predator-induced phenotypic plasticity in amphibian tadpoles. Proc. R. Soc. B Biol. Sci. 2013, 280, 20123075. [Google Scholar] [CrossRef] [PubMed]
- Ramamonjisoa, N.; Rakotonoely, H.; Natuhara, Y. Defense investments and growth responses under different predation risks and gape-limitation predation threats in a tadpole prey. Behav. Ecol. Sociobiol. 2018, 72, 144. [Google Scholar] [CrossRef]
- Zamora-Camacho, F.J.; Cortés-Manzaneque, S.; Aragón, P. Simulated predation pressure in Pelobates cultripes tadpoles modulates morphology at the metamorphic stage. Curr. Zool. 2019, 65, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Relyea, R.A.; Hoverman, J.T. The impact of larval predators and competitors on the morphology and fitness of juvenile treefrogs. Oecologia 2003, 134, 596–604. [Google Scholar] [CrossRef]
- Hagman, M.; Hayes, R.A.; Capon, R.; Shine, R. Alarm cues experienced by cane toad tadpoles affect post-metamorphic morphology and chemical defences. Funct. Ecol. 2009, 23, 126–132. [Google Scholar] [CrossRef]
- Crespi, E.J.; Warne, R.W. Environmental conditions experienced during the tadpole stage alter post-metamorphic glucocorticoid response to stress in an amphibian. Integr. Comp. Biol. 2013, 53, 989–1001. [Google Scholar] [CrossRef]
- Márquez-García, M.; Correa-Solis, M.; Sallaberry, M.; Méndez, M. Effects of pond drying on morphological and life-history traits in the anuran Rhinella spinulosa (Anura: Bufonidae). Evol. Ecol. Res. 2009, 11, 803–815. [Google Scholar]
- Martins, F.M.; Oom, M.d.M.; Rebelo, R.; Rosa, G.M. Differential effects of dietary protein on early life-history and morphological traits in natterjack toad (Epidalea calamita) tadpoles reared in captivity. Zoo Biol. 2013, 32, 457–462. [Google Scholar] [CrossRef]
- Patar, A.; Giri, A.; Boro, F.; Bhuyan, K.; Singha, U.; Giri, S. Cadmium pollution and amphibians–Studies in tadpoles of Rana limnocharis. Chemosphere 2016, 144, 1043–1049. [Google Scholar] [CrossRef]
- Smith, D.C. Factors controlling tadpole populations of the chorus frog (Pseudacris triseriata) on Isle Royale, Michigan. Ecology 1983, 64, 501–510. [Google Scholar] [CrossRef]
- Berven, K.A.; Gill, D.E. Interpreting geographic variation in life-history traits. Am. Zool. 1983, 23, 85–97. [Google Scholar] [CrossRef]
- Newman, R.A. Adaptive plasticity in development of Scaphiopus couchii tadpoles in desert ponds. Evolution 1988, 42, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Semlitsch, R.D.; Harris, R.N.; Wilbur, H.M. Paedomorphosis in Ambystoma talpoideum: Maintenance of population variation and alternative life-history pathways. Evolution 1990, 44, 1604–1613. [Google Scholar] [PubMed]
- Gomez-Mestre, I.; Saccoccio, V.L.; Iijima, T.; Collins, E.; Rosenthal, G.G.; Warkentin, K.M. The shape of things to come: Linking developmental plasticity to post-metamorphic morphology in anurans. J. Evol. Biol. 2010, 23, 1364–1373. [Google Scholar] [CrossRef]
- Buskirk, J.V.; Saxer, G. Delayed costs of an induced defense in tadpoles? Morphology, hopping, and development rate at metamorphosis. Evolution 2001, 55, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Nauwelaerts, S.; Ramsay, J.; Aerts, P. Morphological correlates of aquatic and terrestrial locomotion in a semi-aquatic frog, Rana esculenta: No evidence for a design conflict. J. Anat. 2007, 210, 304–317. [Google Scholar] [CrossRef]
- Zug, G.R. Anuran locomotion: Structure and function. I. Preliminary observations on relation between jumping and osteometrics of appendicular and postaxial skeleton. Copeia 1972, 1972, 613–624. [Google Scholar] [CrossRef]
- Emerson, S.B. Allometry and jumping in frogs: Helping the twain to meet. Evolution 1978, 32, 551–564. [Google Scholar] [CrossRef]
- AmphibiaWeb. Pelophylax nigromaculatus: Dark-Spotted Frog. Available online: https://amphibiaweb.org/species/5109 (accessed on 15 November 2022).
- Park, J.K.; Chung, K.W.; Kim, J.Y.; Do, Y. Population structure and morphological pattern of the black-spotted pond frog (Pelophylax nigromaculatus) inhabiting watershed areas of the Geum River in South Korea. Sustainability 2022, 14, 16530. [Google Scholar] [CrossRef]
- Lillywhite, H.B.; Shine, R.; Jacobson, E.; DeNardo, D.F.; Gordon, M.S.; Navas, C.A.; Wang, T.; Seymour, R.S.; Storey, K.B.; Heatwole, H.; et al. Anesthesia and Euthanasia of Amphibians and Reptiles Used in Scientific Research: Should Hypothermia and Freezing Be Prohibited? Bioscience 2017, 67, 53–61. [Google Scholar] [CrossRef]
- Gong, J.; Lan, H.; Fang, S.-G.; Wan, Q.-H. Development and characterization of 13 polymorphic microsatellite DNA markers for the pond green frog (Rana nigromaculata). J. Genet. 2010, 89, e7–e10. [Google Scholar] [CrossRef]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.A. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Rome, L.C. Jumping performance of frogs (Rana pipiens) as a function of muscle temperature. J. Exp. Biol. 1984, 108, 429–439. [Google Scholar] [CrossRef]
- Rohlf, F.J. TpsDig, Version 2.1.1; Department of Ecology and Evolution, State University of New York: Stony Brook, NY, USA; Available online: http://en.freedownloadmanager.org/Windows-PC/tpsDig2.html (accessed on 16 January 2022).
- Catford, J.A.; Wilson, J.R.; Pyšek, P.; Hulme, P.E.; Duncan, R.P. Addressing context dependence in ecology. Trends Ecol. Evol. 2022, 37, 158–170. [Google Scholar] [CrossRef]
- Relyea, R.A. Predators come and predators go: The reversibility of predator-induced traits. Ecology 2003, 84, 1840–1848. [Google Scholar] [CrossRef]
- Buskirk, J.; Relyea, R.A. Selection for phenotypic plasticity in Rana sylvatica tadpoles. Biol. J. Linn. Soc. 1998, 65, 301–328. [Google Scholar] [CrossRef]
- Buskirk, J.; McCollum, S.A. Plasticity and selection explain variation in tadpole phenotype between ponds with different predator composition. Oikos 1999, 85, 31–39. [Google Scholar] [CrossRef]
- Teplitsky, C.; Plénet, S.; Léna, J.P.; Mermet, N.; Malet, E.; Joly, P. Escape behaviour and ultimate causes of specific induced defences in an anuran tadpole. J. Evol. Biol. 2005, 18, 180–190. [Google Scholar] [CrossRef]
- McIntyre, P.B.; Baldwin, S.; Flecker, A.S. Effects of behavioral and morphological plasticity on risk of predation in a Neotropical tadpole. Oecologia 2004, 141, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Arribas, R.; Touchon, J.C.; Gomez-Mestre, I. Predation and competition differentially affect the interactions and trophic niches of a Neotropical amphibian guild. Front. Ecol. Evol. 2018, 6, 28. [Google Scholar] [CrossRef]
- Lardner, B. Morphological and life history responses to predators in larvae of seven anurans. Oikos 2000, 88, 169–180. [Google Scholar] [CrossRef]
- Nicieza, A.G. Interacting effects of predation risk and food availability on larval anuran behaviour and development. Oecologia 2000, 123, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Mogali, S. Predatory cues influence the behavioral responses and metamorphic traits of Polypedates maculatus (Anura: Rhacophoridae). Asian Herpetol. Res. 2018, 9, 188–194. [Google Scholar]
- Bonett, R.M.; Hoopfer, E.D.; Denver, R.J. Molecular mechanisms of corticosteroid synergy with thyroid hormone during tadpole metamorphosis. Gen. Comp. Endocrinol. 2010, 168, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Sachs, L.M.; Buchholz, D.R. Insufficiency of thyroid hormone in frog metamorphosis and the role of glucocorticoids. Front. Endocrinol. 2019, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Phuge, S.; Tapkir, S.; Bhand, V.; Kour, G.; Pandit, R. Comparative analysis of anti-predator behaviour and life history traits of the tadpoles exposed to predation risk and corticosterone. Proc. Zool Soc. 2020, 70, 220–226. [Google Scholar] [CrossRef]
- Kulkarni, P.; Gramapurohit, N. Effect of corticosterone on larval growth, antipredator behaviour and metamorphosis of Hylarana indica. Gen. Comp. Endocrinol. 2017, 251, 21–29. [Google Scholar] [CrossRef]
- Joshi, A.; Wadekar, N.; Gramapurohit, N. Does corticosterone mediate predator-induced responses of larval Hylarana indica? Gen. Comp. Endocrinol. 2017, 251, 30–37. [Google Scholar] [CrossRef]
- Chivers, D.P.; Kiesecker, J.M.; Marco, A.; Wildy, E.L.; Blaustein, A.R. Shifts in life history as a response to predation in western toads (Bufo boreas). J. Chem. Ecol. 1999, 25, 2455–2463. [Google Scholar] [CrossRef]
- Walsh, P.; Downie, J.; Monaghan, P. Predation-induced plasticity in metamorphic duration in Xenopus laevis. Funct. Ecol. 2008, 22, 699–705. [Google Scholar] [CrossRef]
- Xu, W.; Li, Y.Y.; Lou, Q.Q.; Chen, X.R.; Qin, Z.F.; Wie, W.J. Low concentrations of dihydrotestosterone induce female-to-male sex reversal in the frog Pelophylax nigromaculatus. Environ. Toxicol. Chem. 2015, 34, 2370–2377. [Google Scholar] [CrossRef]
- Liu, P.-Y.; Meng, T.; Li, Y.-Y.; Cai, M.; Li, X.-H.; Chen, J.; Qin, Z.-F. Tetrabromoethylcyclohexane affects gonadal differentiation and development in the frog Pelophylax nigromaculatus. Aquat. Toxicol. 2017, 192, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Qin, Z.; Wang, H.; Li, J. A screening assay for thyroid hormone signaling disruption based on thyroid hormone-response gene expression analysis in the frog Pelophylax nigromaculatus. J. Environ. Sci. 2015, 34, 143–154. [Google Scholar] [CrossRef]
- Chen, J.; Meng, T.; Li, Y.; Gao, K.; Qin, Z. Effects of triclosan on gonadal differentiation and development in the frog Pelophylax nigromaculatus. J. Environ. Sci. 2018, 64, 157–165. [Google Scholar] [CrossRef]
- Emerson, S.B. Heterochrony and frogs: The relationship of a life history trait to morphological form. Am. Nat. 1986, 127, 167–183. [Google Scholar] [CrossRef]
- Tejedo, M.; Semlitsch, R.D.; Hotz, H. Covariation of morphology and jumping performance in newly metamorphosed water frogs: Effects of larval growth history. Copeia 2000, 2000, 448–458. [Google Scholar] [CrossRef]
- Emerson, S.B. Jumping and leaping. In Functional Vertebrate Morphology; Hildebrand, M., Bramble, D.M., Liem, K.F., Wake, D.B., Eds.; Harvard University Press: Cambridge, MA, USA, 2013; pp. 198–211. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Do, Y. Phenotypic Plasticity in Juvenile Frogs That Experienced Predation Pressure as Tadpoles Does Not Alter Their Locomotory Performance. Biology 2023, 12, 341. https://doi.org/10.3390/biology12030341
Park J, Do Y. Phenotypic Plasticity in Juvenile Frogs That Experienced Predation Pressure as Tadpoles Does Not Alter Their Locomotory Performance. Biology. 2023; 12(3):341. https://doi.org/10.3390/biology12030341
Chicago/Turabian StylePark, Junkyu, and Yuno Do. 2023. "Phenotypic Plasticity in Juvenile Frogs That Experienced Predation Pressure as Tadpoles Does Not Alter Their Locomotory Performance" Biology 12, no. 3: 341. https://doi.org/10.3390/biology12030341
APA StylePark, J., & Do, Y. (2023). Phenotypic Plasticity in Juvenile Frogs That Experienced Predation Pressure as Tadpoles Does Not Alter Their Locomotory Performance. Biology, 12(3), 341. https://doi.org/10.3390/biology12030341




