Transcriptomic and Metabolomic Studies Reveal That Toll-like Receptor 2 Has a Role in Glucose-Related Metabolism in Unchallenged Zebrafish Larvae (Danio rerio)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Maintenance and Samples Collection
2.2. RNA Isolation, cDNA Synthesis and qPCR
2.3. RNAseq Processing and Analysis
2.4. NMR Sample Preparation
2.5. NMR Measurement and Analysis
2.6. Glucose Measurement
2.7. Statistical Analyses
3. Results
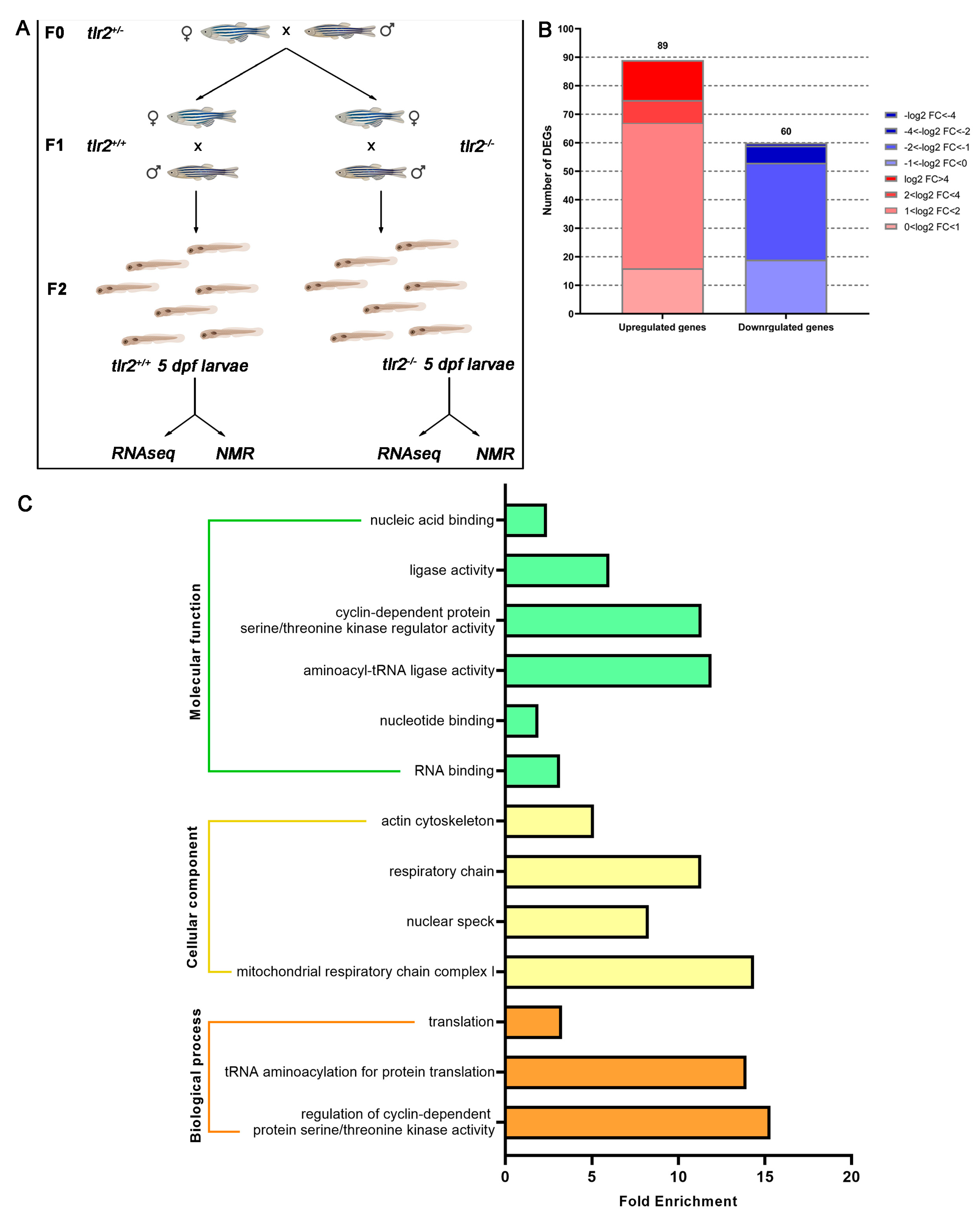
3.1. Transcriptomic Profiling of Tlr2 Mutant Zebrafish
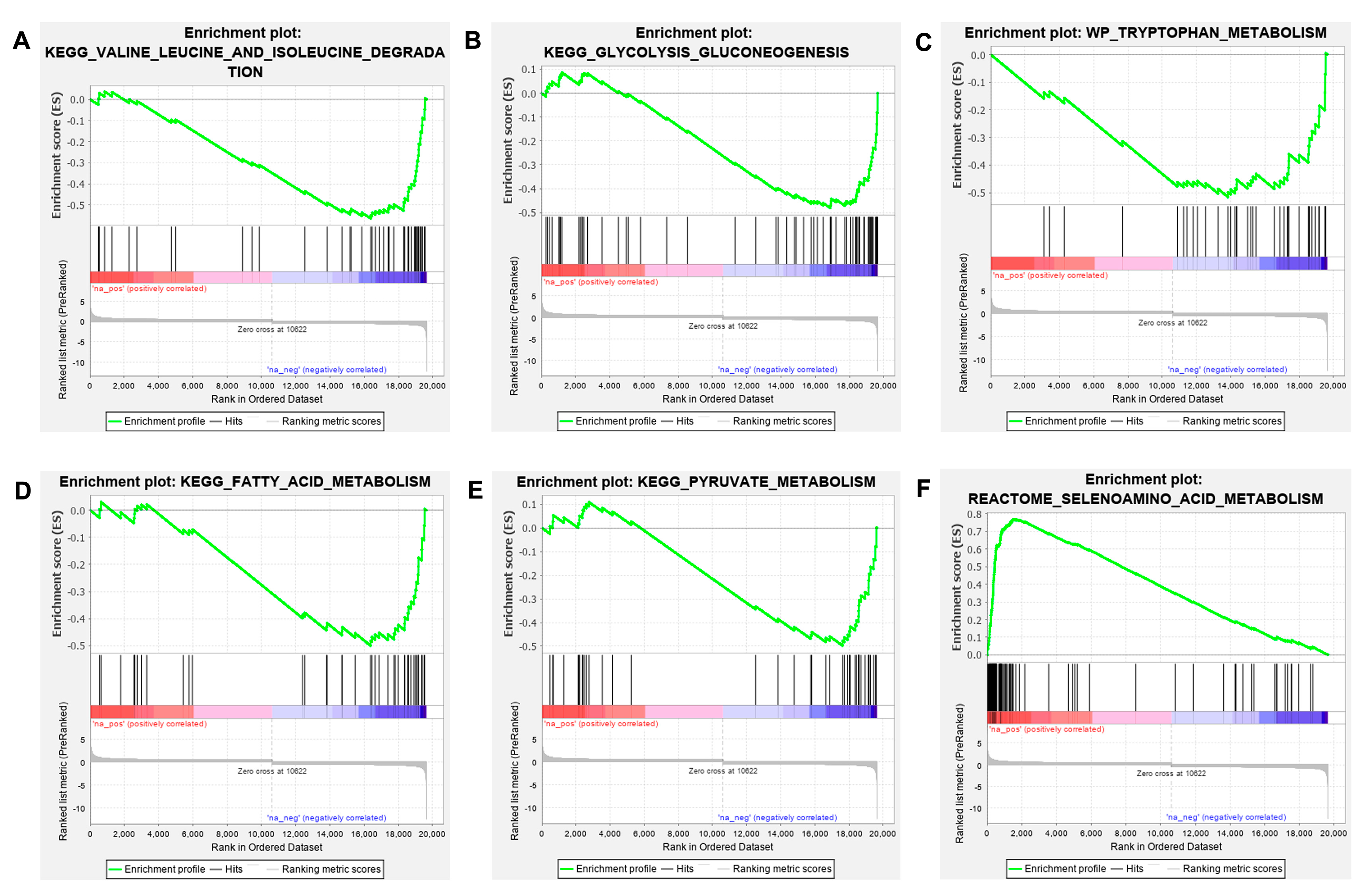
3.2. GSEA Analysis of Tlr2 Mutant Zebrafish
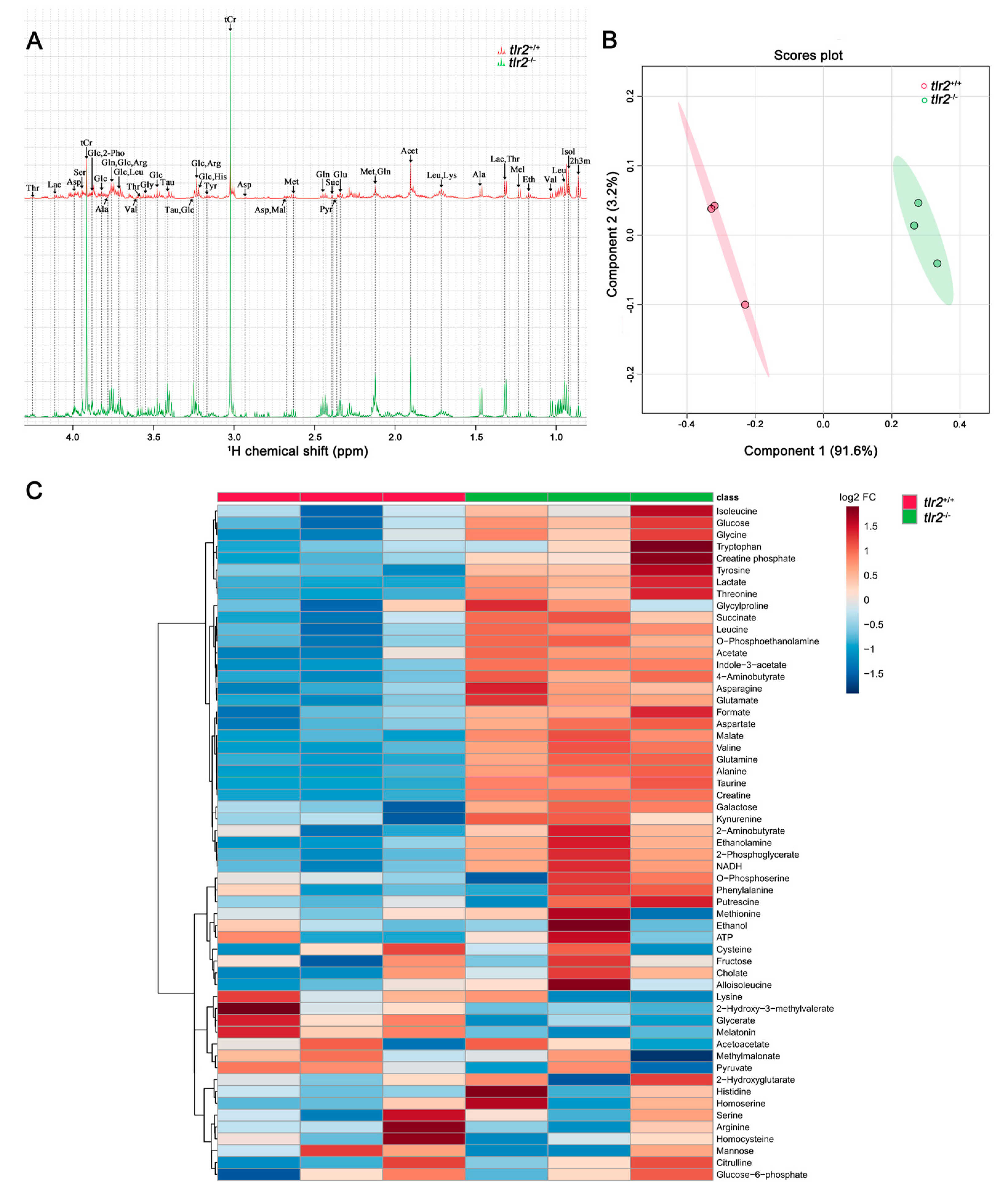
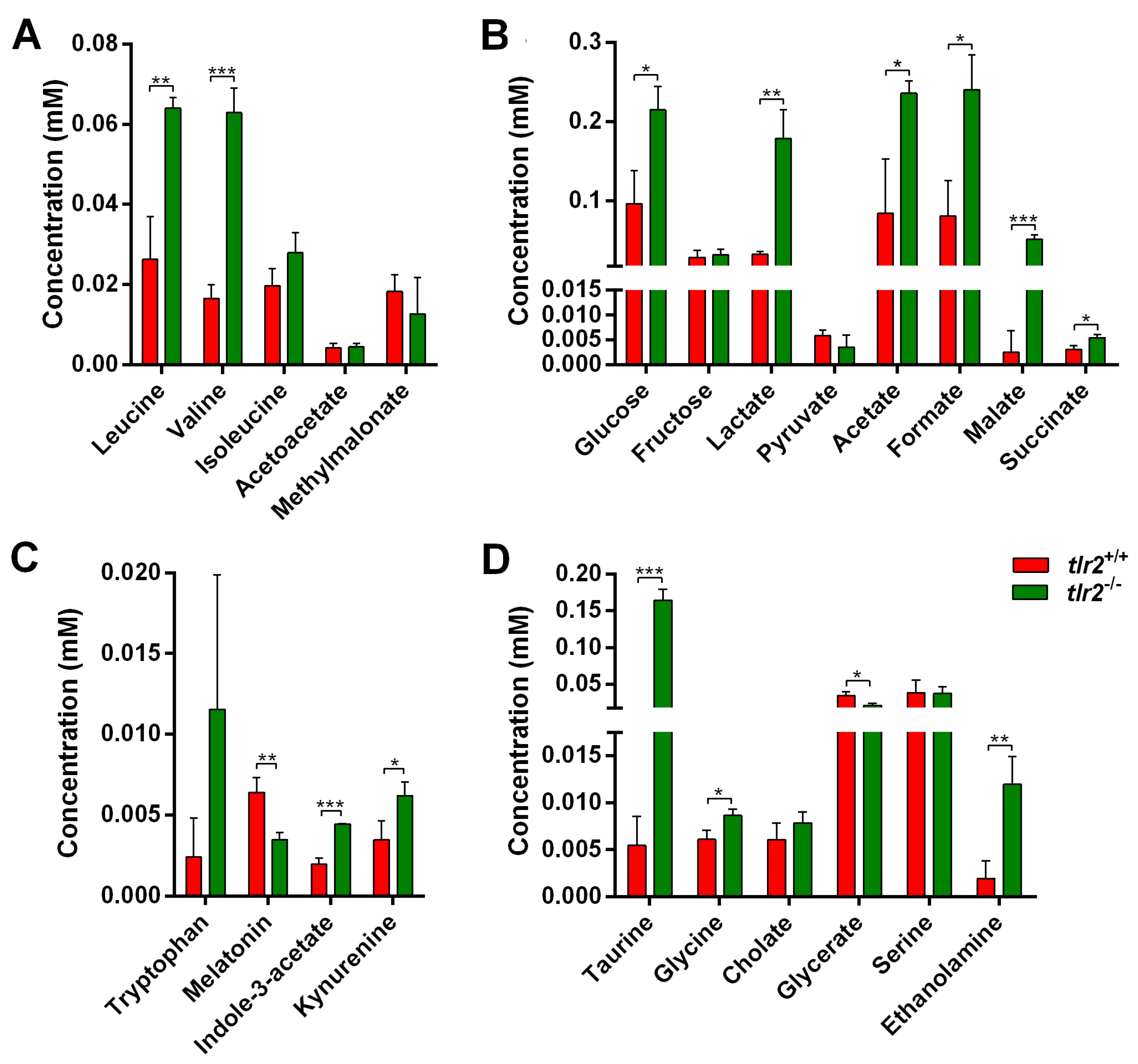
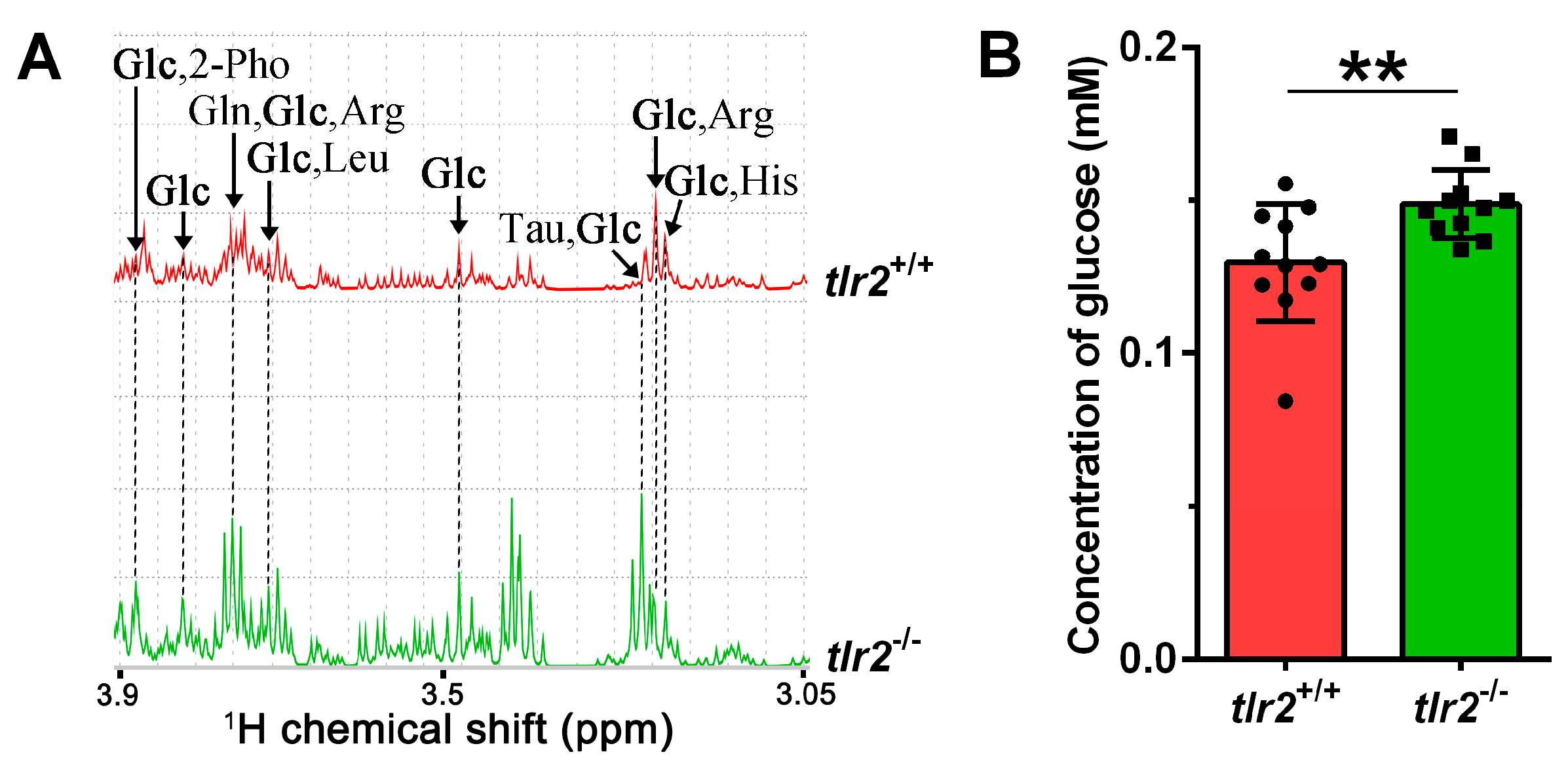
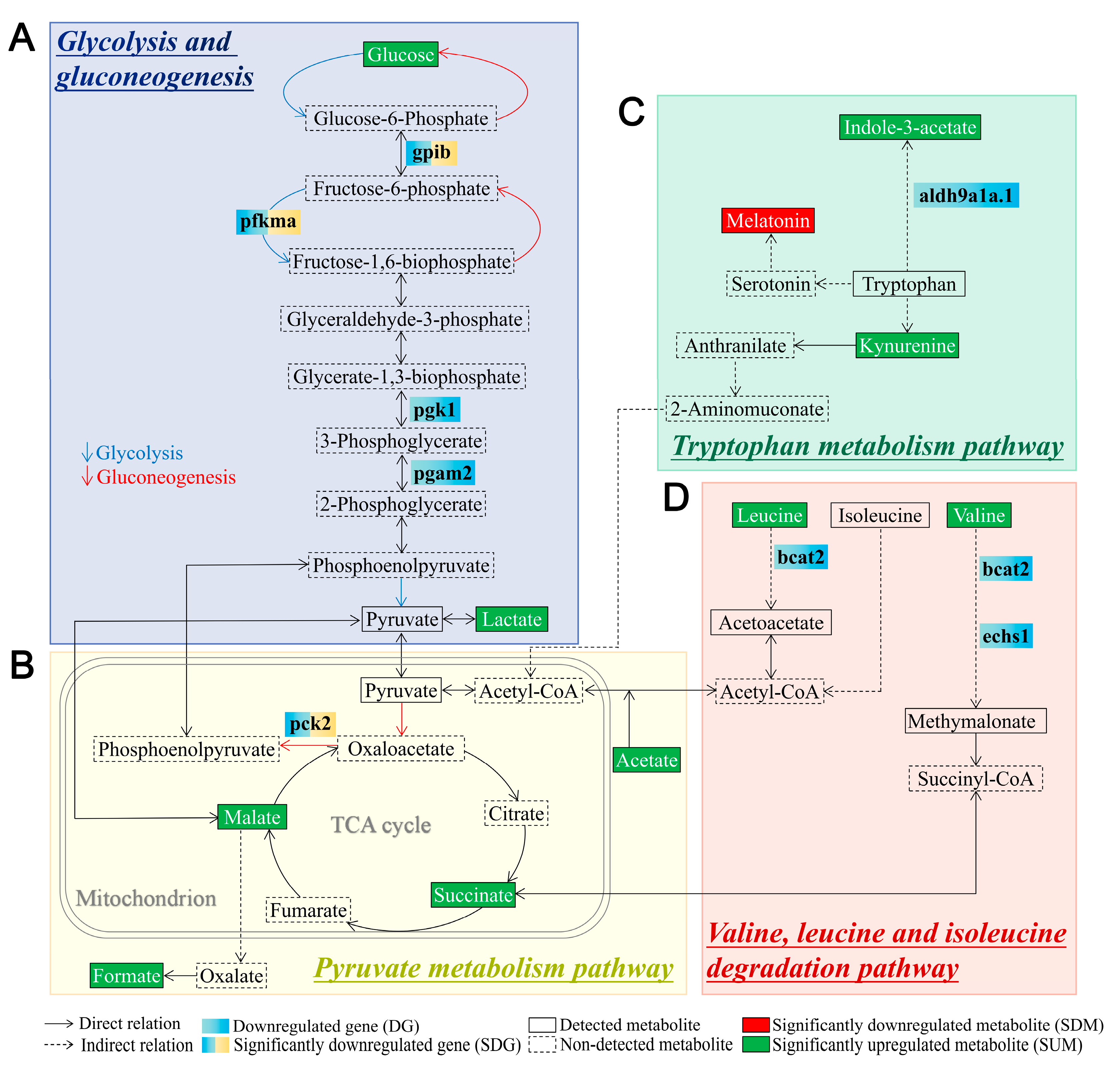
3.3. System Metabolomics Analysis of Tlr2 Mutant Zebrafish
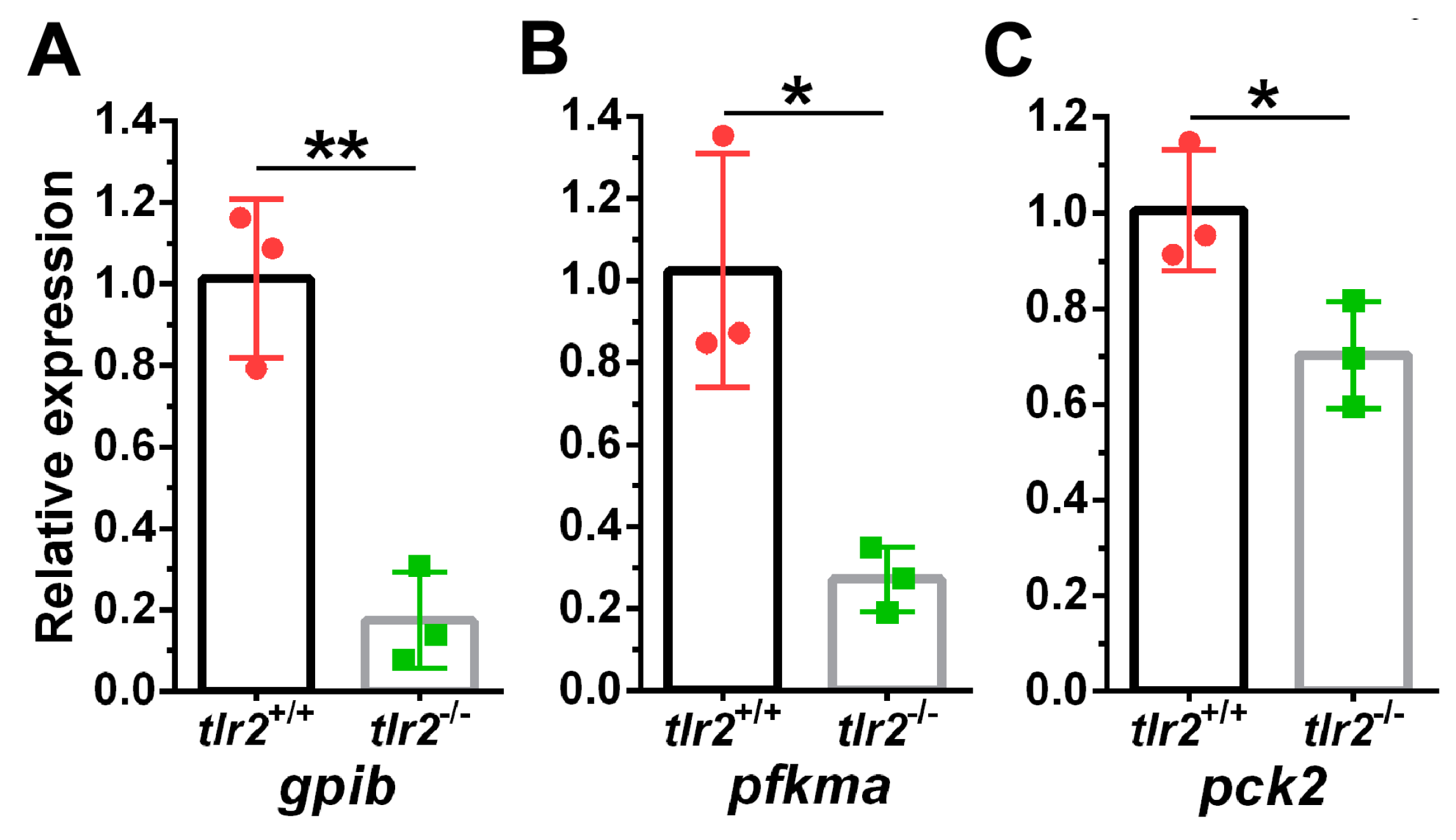
3.4. Glucose Levels and Transcription of Glycolytic Enzymes in tlr2 Mutant Zebrafish
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Lorenzo, A.; Bolli, E.; Tarone, L.; Cavallo, F.; Conti, L. Toll-Like Receptor 2 at the Crossroad between Cancer Cells, the Immune System, and the Microbiota. Int. J. Mol. Sci. 2020, 21, 9418. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Spaink, H.P. The Role of TLR2 in Infectious Diseases Caused by Mycobacteria: From Cell Biology to Therapeutic Target. Biology 2022, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Nascimento, L.; Massari, P.; Wetzler, L.M. The Role of TLR2 in Infection and Immunity. Front. Immunol. 2012, 3, 79. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Sim, J.R.; Yun, C.H.; Han, S.H. Lipoteichoic acids as a major virulence factor causing inflammatory responses via Toll-like receptor 2. Arch. Pharm. Res. 2016, 39, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.E.; Petri, W.A., Jr. TLR2 as a Therapeutic Target in Bacterial Infection. Trends Mol. Med. 2020, 26, 715–717. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 427–439. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Koppenol, W.H.; Bounds, P.L.; Dang, C.V. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 2011, 11, 325–337. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Thompson, C.B. Cellular metabolism and disease: What do metabolic outliers teach us? Cell 2012, 148, 1132–1144. [Google Scholar] [CrossRef]
- Pearce, E.L.; Poffenberger, M.C.; Chang, C.H.; Jones, R.G. Fueling immunity: Insights into metabolism and lymphocyte function. Science 2013, 342, 1242454. [Google Scholar] [CrossRef]
- Chang, C.H.; Curtis, J.D.; Maggi, L.B., Jr.; Faubert, B.; Villarino, A.V.; O’Sullivan, D.; Huang, S.C.; van der Windt, G.J.; Blagih, J.; Qiu, J.; et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013, 153, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.L.; Pearce, E.J. Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Everts, B.; Amiel, E.; Huang, S.C.; Smith, A.M.; Chang, C.H.; Lam, W.Y.; Redmann, V.; Freitas, T.C.; Blagih, J.; van der Windt, G.J.; et al. TLR-driven early glycolytic reprogramming via the kinases TBK1-IKKvarepsilon supports the anabolic demands of dendritic cell activation. Nat. Immunol. 2014, 15, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Tukiman, M.H.; Norazmi, M.N. Immunometabolism of Immune Cells in Mucosal Environment Drives Effector Responses against Mycobacterium tuberculosis. Int. J. Mol. Sci. 2022, 23, 8531. [Google Scholar] [CrossRef] [PubMed]
- Lachmandas, E.; Beigier-Bompadre, M.; Cheng, S.C.; Kumar, V.; van Laarhoven, A.; Wang, X.; Ammerdorffer, A.; Boutens, L.; de Jong, D.; Kanneganti, T.D.; et al. Rewiring cellular metabolism via the AKT/mTOR pathway contributes to host defence against Mycobacterium tuberculosis in human and murine cells. Eur. J. Immunol. 2016, 46, 2574–2586. [Google Scholar] [CrossRef]
- Tan, Z.; Xie, N.; Banerjee, S.; Cui, H.; Fu, M.; Thannickal, V.J.; Liu, G. The monocarboxylate transporter 4 is required for glycolytic reprogramming and inflammatory response in macrophages. J. Biol. Chem. 2015, 290, 46–55. [Google Scholar] [CrossRef]
- Jantsch, J.; Wiese, M.; Schodel, J.; Castiglione, K.; Glasner, J.; Kolbe, S.; Mole, D.; Schleicher, U.; Eckardt, K.U.; Hensel, M.; et al. Toll-like receptor activation and hypoxia use distinct signaling pathways to stabilize hypoxia-inducible factor 1alpha (HIF1A) and result in differential HIF1A-dependent gene expression. J. Leukoc. Biol. 2011, 90, 551–562. [Google Scholar] [CrossRef]
- Obaid, M.; Udden, S.M.N.; Alluri, P.; Mandal, S.S. LncRNA HOTAIR regulates glucose transporter Glut1 expression and glucose uptake in macrophages during inflammation. Sci. Rep. 2021, 11, 232. [Google Scholar] [CrossRef]
- Chatzopoulou, A.; Roy, U.; Meijer, A.H.; Alia, A.; Spaink, H.P.; Schaaf, M.J. Transcriptional and metabolic effects of glucocorticoid receptor alpha and beta signaling in zebrafish. Endocrinology 2015, 156, 1757–1769. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saikia, S.K. Use of Zebrafish as a Model Organism to Study Oxidative Stress: A Review. Zebrafish 2022, 19, 165–176. [Google Scholar] [CrossRef]
- Breuer, M.; Patten, S.A. A Great Catch for Investigating Inborn Errors of Metabolism-Insights Obtained from Zebrafish. Biomolecules 2020, 10, 1352. [Google Scholar] [CrossRef] [PubMed]
- Spaink, H.P.; Cui, C.; Wiweger, M.I.; Jansen, H.J.; Veneman, W.J.; Marin-Juez, R.; de Sonneville, J.; Ordas, A.; Torraca, V.; van der Ent, W.; et al. Robotic injection of zebrafish embryos for high-throughput screening in disease models. Methods 2013, 62, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Veneman, W.J.; Stockhammer, O.W.; de Boer, L.; Zaat, S.A.; Meijer, A.H.; Spaink, H.P. A zebrafish high throughput screening system used for Staphylococcus epidermidis infection marker discovery. BMC Genom. 2013, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Veneman, W.J.; Marin-Juez, R.; de Sonneville, J.; Ordas, A.; Jong-Raadsen, S.; Meijer, A.H.; Spaink, H.P. Establishment and optimization of a high throughput setup to study Staphylococcus epidermidis and Mycobacterium marinum infection as a model for drug discovery. J. Vis. Exp. 2014, 88, e51649. [Google Scholar] [CrossRef]
- Kanwal, Z.; Wiegertjes, G.F.; Veneman, W.J.; Meijer, A.H.; Spaink, H.P. Comparative studies of Toll-like receptor signalling using zebrafish. Dev. Comp Immunol. 2014, 46, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.X.; Marin-Juez, R.; Meijer, A.H.; Spaink, H.P. Common and specific downstream signaling targets controlled by Tlr2 and Tlr5 innate immune signaling in zebrafish. BMC Genom. 2015, 16, 547. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Yang, S.; Shimada, Y.; Munch, M.; Marin-Juez, R.; Meijer, A.H.; Spaink, H.P. Infection and RNA-seq analysis of a zebrafish tlr2 mutant shows a broad function of this toll-like receptor in transcriptional and metabolic control and defense to Mycobacterium marinum infection. BMC Genom. 2019, 20, 878. [Google Scholar] [CrossRef]
- Hu, W.; van Steijn, L.; Li, C.; Verbeek, F.J.; Cao, L.; Merks, R.M.H.; Spaink, H.P. A Novel Function of TLR2 and MyD88 in the Regulation of Leukocyte Cell Migration Behavior During Wounding in Zebrafish Larvae. Front. Cell Dev. Biol. 2021, 9, 624571. [Google Scholar] [CrossRef]
- Hu, W.K.B.; Koch, E.V.; Larmers, G.E.M.; Forn-Cuní, G.; Spaink, H.P. Specificity of the innate immune responses to different classes of non-tuberculous mycobacteria. Front. Immunol. 2023, 13, 1075473. [Google Scholar] [CrossRef]
- 1van Soest, J.J.; Stockhammer, O.W.; Ordas, A.; Bloemberg, G.V.; Spaink, H.P.; Meijer, A.H. Comparison of static immersion and intravenous injection systems for exposure of zebrafish embryos to the natural pathogen Edwardsiella tarda. BMC Immunol. 2011, 12, 58. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Haks, M.C.; van den Eeden, S.J.F.; Ottenhoff, T.H.M.; Harms, A.C.; Hankemeier, T.; Eeza, M.N.H.; Matysik, J.; Alia, A.; Spaink, H.P. Leptin mutation and mycobacterial infection lead non-synergistically to a similar metabolic syndrome. Metabolomics 2022, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Zhu, A.Q.; Ibrahim, J.G.; Love, M.I. Heavy-tailed prior distributions for sequence count data: Removing the noise and preserving large differences. Bioinformatics 2019, 35, 2084–2092. [Google Scholar] [CrossRef]
- Stephens, M. False discovery rates: A new deal. Biostatistics 2017, 18, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Boland, R.; Heemskerk, M.T.; Forn-Cuni, G.; Korbee, C.J.; Walburg, K.V.; Esselink, J.J.; dos Santos, C.C.; de Waal, A.M.; van der Hoeven, D.C.M.; van der Sar, E.; et al. Repurposing Tamoxifen as Potential Host-Directed Therapeutic for Tuberculosis. mBio 2022, e03024-22. [Google Scholar] [CrossRef]
- Zhang, R.; Varela, M.; Forn-Cuni, G.; Torraca, V.; van der Vaart, M.; Meijer, A.H. Deficiency in the autophagy modulator Dram1 exacerbates pyroptotic cell death of Mycobacteria-infected macrophages. Cell Death Dis. 2020, 11, 277. [Google Scholar] [CrossRef]
- Kabli, S.; Spaink, H.P.; De Groot, H.J.M.; Alia, A. In Vivo Metabolite Profile of Adult Zebrafish Brain Obtained by High-Resolution Localized Magnetic Resonance Spectroscopy. J. Magn. Reson. Imaging 2009, 29, 275–281. [Google Scholar] [CrossRef]
- Ding, Y.; Haks, M.C.; Forn-Cuni, G.; He, J.; Nowik, N.; Harms, A.C.; Hankemeier, T.; Eeza, M.N.H.; Matysik, J.; Alia, A.; et al. Metabolomic and transcriptomic profiling of adult mice and larval zebrafish leptin mutants reveal a common pattern of changes in metabolites and signaling pathways. Cell Biosci. 2021, 11, 126. [Google Scholar] [CrossRef]
- Berry, J.P.; Roy, U.; Jaja-Chimedza, A.; Sanchez, K.; Matysik, J.; Alia, A. High-Resolution Magic Angle Spinning Nuclear Magnetic Resonance of Intact Zebrafish Embryos Detects Metabolic Changes Following Exposure to Teratogenic Polymethoxyalkenes from Algae. Zebrafish 2016, 13, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Roy, U.; Conklin, L.; Schiller, J.; Matysik, J.; Berry, J.P.; Alia, A. Metabolic profiling of zebrafish (Danio rerio) embryos by NMR spectroscopy reveals multifaceted toxicity of beta-methylamino-L-alanine (BMAA). Sci. Rep. 2017, 7, 17305. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Lucas, A.; Hyrossova, P.; Novellasdemunt, L.; Vinals, F.; Perales, J.C. Mitochondrial phosphoenolpyruvate carboxykinase (PEPCK-M) is a pro-survival, endoplasmic reticulum (ER) stress response gene involved in tumor cell adaptation to nutrient availability. J. Biol. Chem 2014, 289, 22090–22102. [Google Scholar] [CrossRef]
- Beale, E.G.; Harvey, B.J.; Forest, C. PCK1 and PCK2 as candidate diabetes and obesity genes. Cell Biochem. Biophys 2007, 48, 89–95. [Google Scholar] [CrossRef]
- Modaressi, S.; Brechtel, K.; Christ, B.; Jungermann, K. Human mitochondrial phosphoenolpyruvate carboxykinase 2 gene. Structure, chromosomal localization and tissue-specific expression. Biochem. J. 1998, 333, 359–366. [Google Scholar] [CrossRef]
- Dong, H.; Feng, Y.; Yang, Y.; Hu, Y.; Jia, Y.; Yang, S.; Zhao, N.; Zhao, R. A Novel Function of Mitochondrial Phosphoenolpyruvate Carboxykinase as a Regulator of Inflammatory Response in Kupffer Cells. Front. Cell Dev. Biol. 2021, 9, 726931. [Google Scholar] [CrossRef]
- Leithner, K.; Hrzenjak, A.; Trotzmuller, M.; Moustafa, T.; Kofeler, H.C.; Wohlkoenig, C.; Stacher, E.; Lindenmann, J.; Harris, A.L.; Olschewski, A.; et al. PCK2 activation mediates an adaptive response to glucose depletion in lung cancer. Oncogene 2015, 34, 1044–1050. [Google Scholar] [CrossRef]
- Hall, C.J.; Boyle, R.H.; Sun, X.Y.; Wicker, S.M.; Misa, J.P.; Krissansen, G.W.; Print, C.G.; Crosier, K.E.; Crosier, P.S. Epidermal cells help coordinate leukocyte migration during inflammation through fatty acid-fuelled matrix metalloproteinase production. Nat. Commun. 2014, 5, 3880. [Google Scholar] [CrossRef]
- Pagan, A.J.; Lee, L.J.; Edwards-Hicks, J.; Moens, C.B.; Tobin, D.M.; Busch-Nentwich, E.M.; Pearce, E.L.; Ramakrishnan, L. mTOR-regulated mitochondrial metabolism limits mycobacterium-induced cytotoxicity. Cell 2022, 185, 3720–3738. [Google Scholar] [CrossRef]
- Pagan, A.J.; Lee, L.J.; Edwards-Hicks, J.; Moens, C.B.; Tobin, D.M.; Pearce, E.L.; Busch-Nentwich, E.; Ramakrishnan, L. mTOR-associated Mitochondrial Energy Metabolism Limits Mycobacterium ESX-1-induced Cytotoxicity. J. Immunol. 2022, 208, 163.32. [Google Scholar] [CrossRef]
- Massey, W.; Brown, J.M. The Gut Microbial Endocrine Organ in Type 2 Diabetes. Endocrinology 2021, 162, bqaa235. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Zhang, L.; Yang, L.; Chu, H. The critical role of gut microbiota in obesity. Front. Endocrinol. 2022, 13, 1025706. [Google Scholar] [CrossRef]
- Sankararaman, S.; Noriega, K.; Velayuthan, S.; Sferra, T.; Martindale, R. Gut Microbiome and Its Impact on Obesity and Obesity-Related Disorders. Curr. Gastroenterol. Rep. 2022, 25, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, Z.; Xu, C.; Kan, S.; Chen, D. Disturbances of the Gut Microbiota and Microbiota-Derived Metabolites in Inflammatory Bowel Disease. Nutrients 2022, 14, 5140. [Google Scholar] [CrossRef]
- Yiu, J.H.; Dorweiler, B.; Woo, C.W. Interaction between gut microbiota and toll-like receptor: From immunity to metabolism. J. Mol. Med. 2017, 95, 13–20. [Google Scholar] [CrossRef]
- Koch, B.E.V.; Yang, S.X.; Lamers, G.; Stougaard, J.; Spaink, H.P. Intestinal microbiome adjusts the innate immune setpoint during colonization through negative regulation of MyD88. Nat. Commun. 2019, 9, 4099. [Google Scholar] [CrossRef]
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018, 23, 1099–1111. [Google Scholar] [CrossRef]
| No. | ENSEMBL ID | Gene Symbol | Gene Name | Log2 Fold Change | S Value |
|---|---|---|---|---|---|
| 1 | ENSDARG00000103826 | gpib | glucose-6-phosphate isomerase b | −1.57 | 3.24 × 10−5 |
| 2 | ENSDARG00000014179 | pfkma | phosphofructokinase, muscle a | −1.05 | 0.00363 |
| 3 | ENSDARG00000020956 | pck2 | phosphoenolpyruvate carboxykinase 2 | −1.80 | 0.00239 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Liu, L.; Forn-Cuní, G.; Ding, Y.; Alia, A.; Spaink, H.P. Transcriptomic and Metabolomic Studies Reveal That Toll-like Receptor 2 Has a Role in Glucose-Related Metabolism in Unchallenged Zebrafish Larvae (Danio rerio). Biology 2023, 12, 323. https://doi.org/10.3390/biology12020323
Hu W, Liu L, Forn-Cuní G, Ding Y, Alia A, Spaink HP. Transcriptomic and Metabolomic Studies Reveal That Toll-like Receptor 2 Has a Role in Glucose-Related Metabolism in Unchallenged Zebrafish Larvae (Danio rerio). Biology. 2023; 12(2):323. https://doi.org/10.3390/biology12020323
Chicago/Turabian StyleHu, Wanbin, Li Liu, Gabriel Forn-Cuní, Yi Ding, Alia Alia, and Herman P. Spaink. 2023. "Transcriptomic and Metabolomic Studies Reveal That Toll-like Receptor 2 Has a Role in Glucose-Related Metabolism in Unchallenged Zebrafish Larvae (Danio rerio)" Biology 12, no. 2: 323. https://doi.org/10.3390/biology12020323
APA StyleHu, W., Liu, L., Forn-Cuní, G., Ding, Y., Alia, A., & Spaink, H. P. (2023). Transcriptomic and Metabolomic Studies Reveal That Toll-like Receptor 2 Has a Role in Glucose-Related Metabolism in Unchallenged Zebrafish Larvae (Danio rerio). Biology, 12(2), 323. https://doi.org/10.3390/biology12020323








