Unsaturated Fatty Acids and Their Immunomodulatory Properties
Abstract
Simple Summary
Abstract
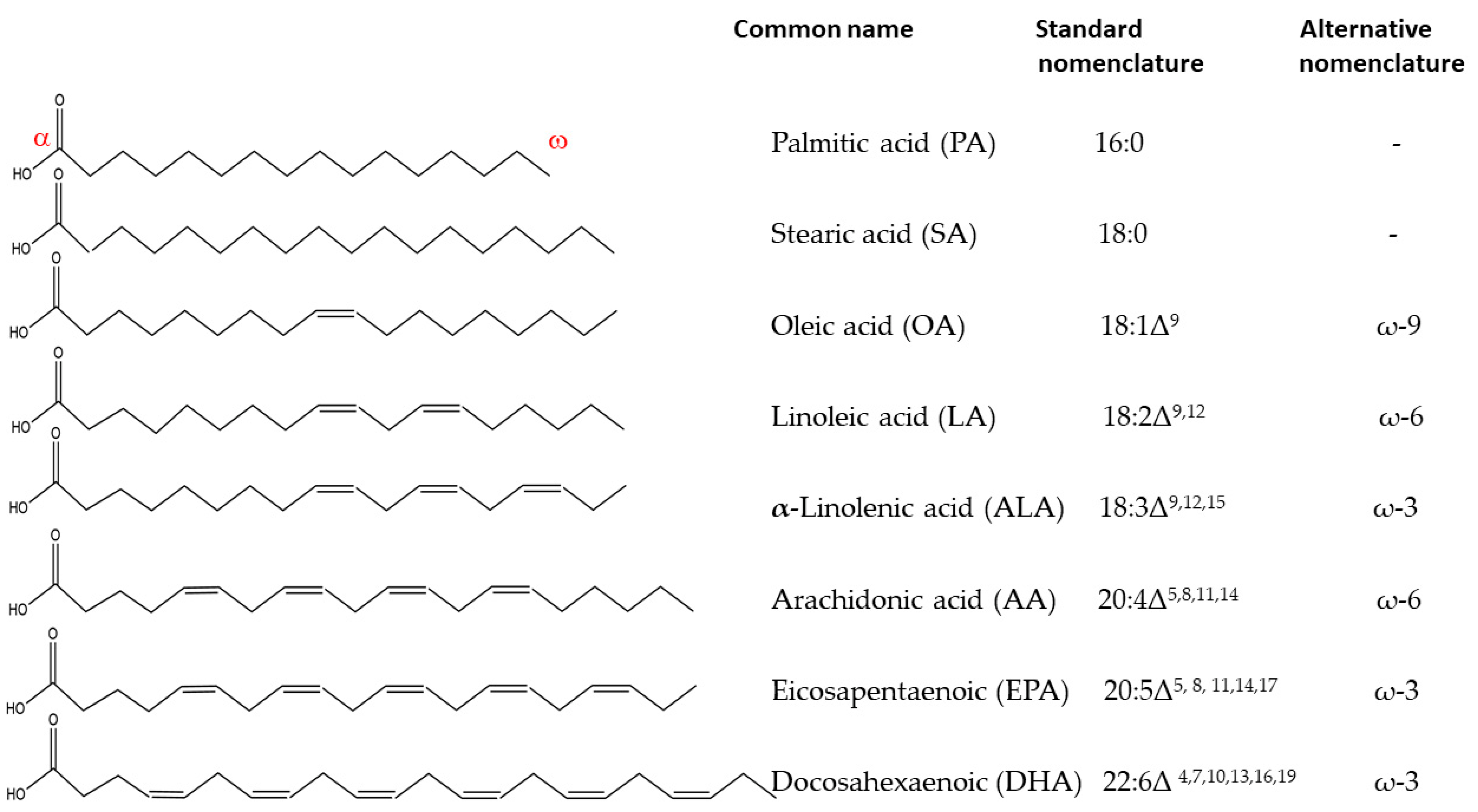
1. Fatty Acids in Living Systems
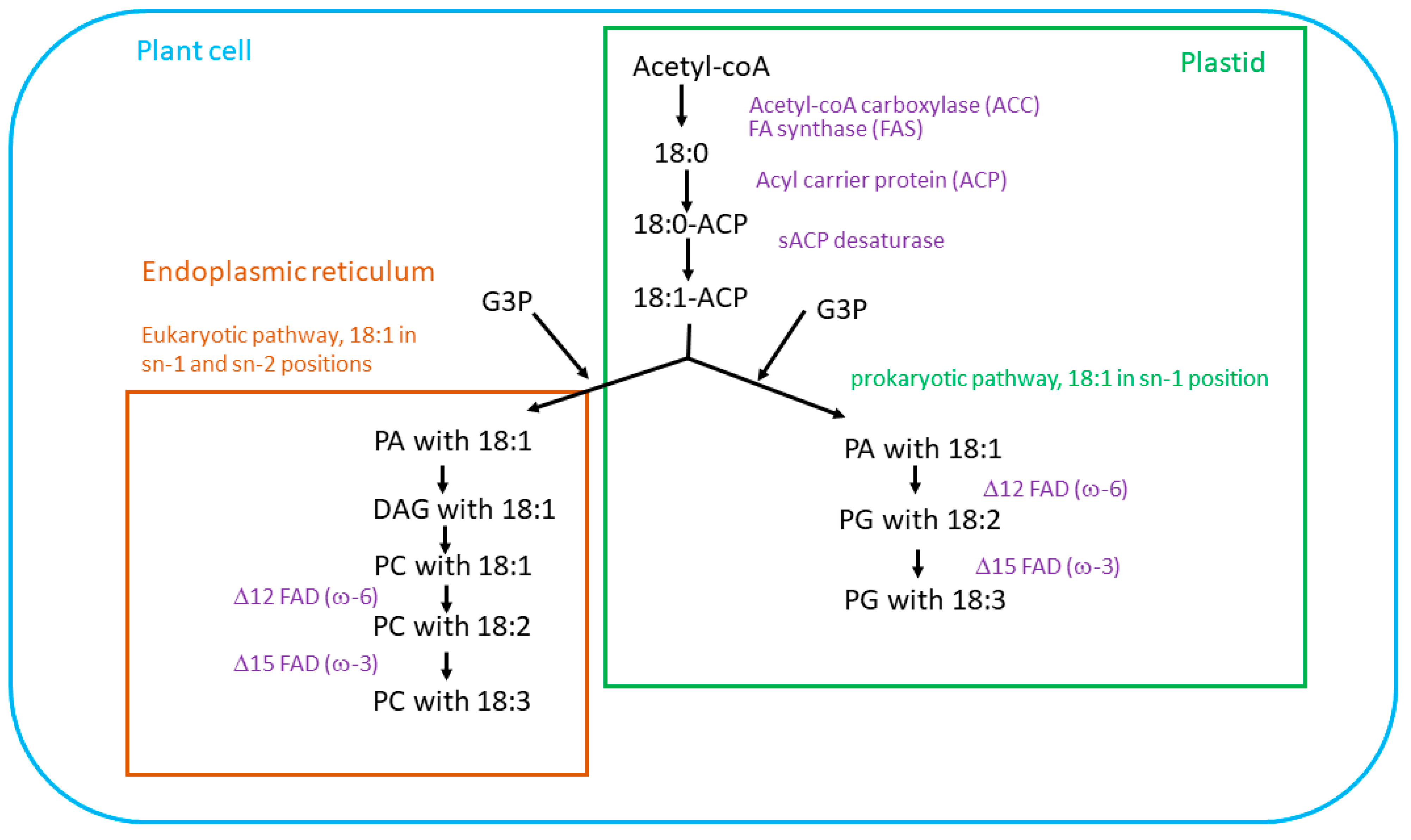
2. Biosynthesis of Essential Fatty Acids in Plants
Unsaturated Fatty Acids Synthesis
3. Dietary Plant Sources of Fatty Acids
4. Polyunsaturated Fatty Acids in Human Diet
5. Long Chain Polyunsaturated Fatty Acids (LC-PUFAs)
6. Human Clinical Trials Involving Polyunsaturated Fatty Acids
7. Anti-Inflammatory Properties of Polyunsaturated Fatty Acids
7.1. PUFAs and Neuroinflammation—Effect on Brain Microglia
7.2. PUFAs and Neuroinflammation—Effect on Brain Astrocytes
8. PUFAs and Neurological Diseases
| Rich in ω-3 and ω-6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sunflower Oil | Rapeseed Oil | Mustard Oil | Peanut Oil | Olive Oil | Avocado Oil | Grapeseed Oil | Flaxseed Oil | Walnut Oil | |
| Palmitic acid (PA) | 5.94 | 3.97 | 10.24 | 9.37 | 15.11 | 10.08 | 7.2 | 5.87 | 6.3 |
| Oleic acid (OA) | 30–80 * | 63.68 | 36.65 | 55.33 | 68.85 | 60.7 | 19.9 | 17.41 | 20.5 |
| Linoleic acid (LA, ω-6) | 21–70 * | 17.43 | 22.06 | 23.69 | 8.5 | 11.8 | 68.1 | 15.76 | 55.5 |
| α-linolenic acid (ALA, ω-3) | 0.79 | - | - | - | 0.54 | 1.2 | 0.1 | 55.40 | 14.8 |
| PUFA | Transcription Factor/Enzyme | Metabolite/ Ligand | Inflammatory Effect |
|---|---|---|---|
| Docosahexaenoic acid (DHA) | COX-2 | Resolvin D | Anti [71] |
| PLA2 | Protectin D1 | Anti [153] | |
| PPAR-α | Ligand | Anti [154] | |
| PPAR-γ | Ligand | Anti [154] | |
| Eicosapentaenoic acid (EPA) | COX-2 PPAR-α PPAR-γ | Resolvins E Ligand Ligand | Anti [72] Anti [155] Anti [156] |
| α-Linolenic acid (ALA) | PPAR-α PPAR-γ | Ligand Ligand | Anti [157] Anti [158] |
| Arachidonic acid (AA) | COX-2 COX-2 PPAR-α PPAR-δ | PGE2 PGI2 Ligand Ligand | Pro [159] Anti [160] Anti [157] Anti-Apoptotic [161] |
| Linoleic acid (LA) | PPAR-α | Ligand | Energy Control [157,162] |
| Oleic acid (OA) * | TLX-NR2E1 | Ligand | Neurogenesis, Anti [157,163] |
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry; W.H. Freeman and Company: New York, NY, USA, 2017; p. 1308. [Google Scholar]
- US Department of Health and Human Services and US Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. Available online: http://health.gov/dietaryguidelines/2015/guidelines/ (accessed on 15 January 2023).
- Oliver, L.; Dietrich, T.; Maranon, I.; Villaran, M.C.; Barrio, R.J. Producing Omega-3 Polyunsaturated Fatty Acids: A Review of Sustainable Sources and Future Trends for the EPA and DHA Market. Resources 2020, 9, 148. [Google Scholar] [CrossRef]
- Zhukov, A.V.; Shumskaya, M. Very-long-chain fatty acids (VLCFAs) in plant response to stress. Funct. Plant Biol. 2020, 47, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Cerone, M.; Smith, T.K. Desaturases: Structural and mechanistic insights into the biosynthesis of unsaturated fatty acids. Iubmb Life 2022, 74, 1036–1051. [Google Scholar] [CrossRef] [PubMed]
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys Acta—Biomemb 2004, 1666, 142–157. [Google Scholar] [CrossRef]
- Murata, N.; Los, D.A. Membrane fluidity and temperature perception. Plant Physiol. 1997, 115, 875–879. [Google Scholar] [CrossRef]
- Zhang, W.H.; Wang, C.X.; Qin, C.B.; Wood, T.; Olafsdottir, G.; Welti, R.; Wang, X.M. The oleate-stimulated phospholipase D, PLD delta, and phosphatidic acid decrease H2O2-induced cell death in arabidopsis. Plant Cell 2003, 15, 2285–2295. [Google Scholar] [CrossRef]
- He, M.; He, C.Q.; Ding, N.Z. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef]
- He, M.; Qin, C.-X.; Wang, X.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Biosynthesis and Regulation. Front. Plant Sci. 2020, 11, 390. [Google Scholar] [CrossRef]
- Shahid, M.; Cai, G.Q.; Zu, F.; Zhao, Q.; Qasim, M.U.; Hong, Y.Y.; Fan, C.C.; Zhou, Y.M. Comparative Transcriptome Analysis of Developing Seeds and Silique Wall Reveals Dynamic Transcription Networks for Effective Oil Production in Brassica napus L. Int. J. Mol. Sci. 2019, 20, 1982. [Google Scholar] [CrossRef]
- Ohlrogge, J.; Thrower, N.; Mhaske, V.; Stymne, S.; Baxter, M.; Yang, W.L.; Liu, J.J.; Shaw, K.; Shorrosh, B.; Zhang, M.; et al. PlantFAdb: A resource for exploring hundreds of plant fatty acid structures synthesized by thousands of plants and their phylogenetic relationships. Plant J. 2018, 96, 1299–1308. [Google Scholar] [CrossRef]
- Piccinin, E.; Cariello, M.; De Santis, S.; Ducheix, S.; Sabba, C.; Ntambi, J.M.; Moschetta, A. Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients 2019, 11, 2283. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, T.; Liu, H.; Zheng, G.C. Unsaturated fatty acid: Metabolism, synthesis and gene regulation. Afr. J. Biotechnol. 2009, 8, 1782–1785. [Google Scholar]
- Yu, C.; Wang, H.S.; Yang, S.; Tang, X.F.; Duan, M.; Meng, Q.W. Overexpression of endoplasmic reticulum omega-3 fatty acid desaturase gene improves chilling tolerance in tomato. Plant Physiol. Biochem. 2009, 47, 1102–1112. [Google Scholar] [CrossRef]
- Lee, S.H.; Ahn, S.J.; Im, Y.J.; Cho, K.; Chung, G.C.; Cho, B.H.; Han, O. Differential impact of low temperature on fatty acid unsaturation and lipoxygenase activity in figleaf gourd and cucumber roots. Biochem. Biophys. Res. Commun. 2005, 330, 1194–1198. [Google Scholar] [CrossRef]
- Roman, A.; Hernandez, M.L.; Soria-Garcia, A.; Lopez-Gomollon, S.; Lagunas, B.; Picorel, R.; Martinez-Rivas, J.M.; Alfonso, M. Non-redundant Contribution of the Plastidial FAD8 omega-3 Desaturase to Glycerolipid Unsaturation at Different Temperatures in Arabidopsis. Mol. Plant 2015, 8, 1599–1611. [Google Scholar] [CrossRef]
- Tvrzicka, E.; Kremmyda, L.S.; Stankova, B.; Zak, A. Fatty acids are biocompounds: Their role in human metabolism, health and disease—A review. Part 1: Classification, dietary sources and biological functions. Biomed. Pap. 2011, 155, 117–130. [Google Scholar] [CrossRef]
- Wu, Z.H.; Liu, H.; Zhan, W.; Yu, Z.C.; Qin, E.R.; Liu, S.; Yang, T.G.; Xiang, N.Y.; Kudrna, D.; Chen, Y.; et al. The chromosome-scale reference genome of safflower (Carthamus tinctorius) provides insights into linoleic acid and flavonoid biosynthesis. Plant Biotechnol. J. 2021, 19, 1725–1742. [Google Scholar] [CrossRef]
- Shi, Y.; Yue, X.; An, L. Integrated regulation triggered by a cryophyte omega-3 desaturase gene confers multiple-stress tolerance in tobacco. J. Exp. Bot. 2018, 69, 2131–2148. [Google Scholar] [CrossRef]
- Yin, Y.M.; Jiang, X.X.; Ren, M.Y.; Xue, M.; Nan, D.N.; Wang, Z.L.; Xing, Y.P.; Wang, M.Y. AmDREB2C, from Ammopiptanthus mongolicus, enhances abiotic stress tolerance and regulates fatty acid composition in transgenic Arabidopsis. Plant Physiol. Biochem. 2018, 130, 517–528. [Google Scholar] [CrossRef]
- Abe, K.; Araki, E.; Suzuki, Y.; Toki, S.; Saika, H. Production of high oleic/low linoleic rice by genome editing. Plant Physiol. Biochem. 2018, 131, 58–62. [Google Scholar] [CrossRef]
- Do, P.T.; Nguyen, C.X.; Bui, H.T.; Tran, L.T.N.; Stacey, G.; Gillman, J.D.; Zhang, Z.Y.J.; Stacey, M.G. Demonstration of highly efficient dual gRNA CRISPR/Cas9 editing of the homeologous GmFAD2-1A and GmFAD2-1B genes to yield a high oleic, low linoleic and alpha-linolenic acid phenotype in soybean. BMC Plant Biol. 2019, 19, 311. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Singh, S.P.; Green, A.G. High-stearic and high-oleic cottonseed oils produced by hairpin RNA-mediated post-transcriptional gene silencing. Plant Physiol. 2002, 129, 1732–1743. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic acid: Physiological role, metabolism and nutritional implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Carrero-Colon, M.; Hudson, K. Reduced palmitic acid content in soybean as a result of mutation in FATB1a. PLoS ONE 2022, 17, e0262327. [Google Scholar] [CrossRef]
- Valenzuela, B.A.; Sanhueza, C.J.; Valenzuela, B.R. Las microalgas: Una fuente renovable para la obtención de ácidos grasos omega-3 de cadena larga para la nutrición humana y animal. Rev. Chil. De Nutr. 2015, 42, 306–310. [Google Scholar] [CrossRef]
- Napier, J.A.; Usher, S.; Haslam, R.P.; Ruiz-Lopez, N.; Sayanova, O. Transgenic plants as a sustainable, terrestrial source of fish oils. Eur. J. Lipid Sci. Technol. 2015, 117, 1317–1324. [Google Scholar] [CrossRef]
- Van Name, M.A.; Savoye, M.; Chick, J.M.; Galuppo, B.T.; Feldstein, A.E.; Pierpont, B.; Johnson, C.; Shabanova, V.; Ekong, U.; Valentino, P.L.; et al. A Low ω-6 to ω-3 PUFA Ratio (n-6:n-3 PUFA) Diet to Treat Fatty Liver Disease in Obese Youth. J. Nutr. 2020, 150, 2314–2321. [Google Scholar] [CrossRef]
- Stańdo, M.; Piatek, P.; Namiecinska, M.; Lewkowicz, P.; Lewkowicz, N. Omega-3 polyunsaturated fatty acids EPA and DHA as an adjunct to non-surgical treatment of periodontitis: A randomized clinical trial. Nutrients 2020, 12, 2614. [Google Scholar] [CrossRef]
- Watson, H.; Mitra, S.; Croden, F.C.; Taylor, M.; Wood, H.M.; Perry, S.L.; Spencer, J.A.; Quirke, P.; Toogood, G.J.; Lawton, C.L.; et al. A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 2018, 67, 1974–1983. [Google Scholar] [CrossRef]
- Keim, S.A.; Gracious, B.; Boone, K.M.; Klebanoff, M.A.; Rogers, L.K.; Rausch, J.; Coury, D.L.; Sheppard, K.W.; Husk, J.; Rhoda, D.A. ω-3 and ω-6 fatty acid supplementation may reduce autism symptoms based on parent report in preterm toddlers. J. Nutr. 2018, 148, 227–235. [Google Scholar] [CrossRef]
- Davis, C.R.; Bryan, J.; Hodgson, J.M.; Woodman, R.; Murphy, K.J. A mediterranean diet reduces F(2)-isoprostanes and triglycerides among older Australian men and women after 6 months. J. Nutr. 2017, 147, 1348–1355. [Google Scholar] [CrossRef]
- Kalstad, A.A.; Myhre, P.L.; Laake, K.; Tveit, S.H.; Schmidt, E.B.; Smith, P.; Nilsen, D.W.T.; Tveit, A.; Fagerland, M.W.; Solheim, S.; et al. Effects of n-3 Fatty acid supplements in ederly patients after myocardial infarction: A randomized, controlled trial. Circulation 2021, 143, 528–539. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Giroli, M.G.; Werba, J.P.; Risé, P.; Porro, B.; Sala, A.; Amato, M.; Tremoli, E.; Bonomi, A.; Veglia, F. Effects of Mediterranean diet or low-fat diet on blood fatty acids in patients with coronary heart disease. A randomized intervention study. Nutrients 2021, 13, 2389. [Google Scholar] [CrossRef]
- Murphy, K.J.; Dyer, K.A.; Hyde, B.; Davis, C.R.; Bracci, E.L.; Woodman, R.J.; Hodgson, J.M. Long-Term Adherence to a Mediterranean Diet 1-Year after Completion of the MedLey Study. Nutrients 2022, 14, 3098. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Schneider, I.; Meyer, H.; Neubronner, J.; von Schacky, C.; Hahn, A. Incorporation of EPA and DHA into plasma phospholipids in response to different omega-3 fatty acid formulations—A comparative bioavailability study of fish oil vs. krill oil. Lipids Health Dis. 2011, 10, 145. [Google Scholar] [CrossRef]
- Ahmed Nasef, N.; Zhu, P.; Golding, M.; Dave, A.; Ali, A.; Singh, H.; Garg, M. Salmon food matrix influences digestion and bioavailability of long-chain omega-3 polyunsaturated fatty acids. Food Funct. 2021, 12, 6588–6602. [Google Scholar] [CrossRef]
- Brennan Laing, B.; Cavadino, A.; Ellett, S.; Ferguson, L.R. Effects of an Omega-3 and Vitamin D Supplement on Fatty Acids and Vitamin D Serum Levels in Double-Blinded, Randomized, Controlled Trials in Healthy and Crohn’s Disease Populations. Nutrients 2020, 12, 1139. [Google Scholar] [CrossRef]
- Telle-Hansen, V.H.; Gaundal, L.; Bastani, N.; Rud, I.; Byfuglien, M.G.; Gjøvaag, T.; Retterstøl, K.; Holven, K.B.; Ulven, S.M.; Myhrstad, M.C.W. Replacing saturated fatty acids with polyunsaturated fatty acids increases the abundance of Lachnospiraceae and is associated with reduced total cholesterol levels-a randomized controlled trial in healthy individuals. Lipids Health Dis. 2022, 21, 92. [Google Scholar] [CrossRef]
- Tindall, A.M.; McLimans, C.J.; Petersen, K.S.; Kris-Etherton, P.M.; Lamendella, R. Walnuts and Vegetable Oils Containing Oleic Acid Differentially Affect the Gut Microbiota and Associations with Cardiovascular Risk Factors: Follow-up of a Randomized, Controlled, Feeding Trial in Adults at Risk for Cardiovascular Disease. J. Nutr. 2020, 150, 806–817. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of dietary fat on gut microbiota and faecal metabolites, and their relationship with cardiometabolic risk factors: A 6-month randomised controlled-feeding trial. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Floros, S.; Toskas, A.; Pasidi, E.; Vareltzis, P. Bioaccessibility and Oxidative Stability of Omega-3 Fatty Acids in Supplements, Sardines and Enriched Eggs Studied Using a Static In Vitro Gastrointestinal Model. Molecules 2022, 2, 415. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, V.K.; Gopakumar, L.R.; Kumaran, A.K.; Chatterjee, N.S.; Soman, V.; Peeralil, S.; Mathew, S.; McClements, D.J.; Nagarajarao, R.C. Encapsulation and Protection of Omega-3-Rich Fish Oils Using Food-Grade Delivery Systems. Foods 2021, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Bower, C.K.; Hietala, K.A.; Oliveira, A.C.; Wu, T.H. Stabilizing oils from smoked pink salmon (Oncorhynchus gorbuscha). J. Food Sci. 2009, 74, C248–C257. [Google Scholar] [CrossRef] [PubMed]
- Pisaniello, A.D.; Psaltis, P.J.; King, P.M.; Liu, G.; Gibson, R.A.; Tan, J.T.; Duong, M.; Nguyen, T.; Bursill, C.A.; Worthley, M.I.; et al. Omega-3 fatty acids ameliorate vascular inflammation: A rationale for their atheroprotective effects. Atherosclerosis 2021, 324, 27–37. [Google Scholar] [CrossRef]
- Mischoulon, D.; Dunlop, B.W.; Kinkead, B.; Schettler, P.J.; Lamon-Fava, S.; Rakofsky, J.J.; Nierenberg, A.A.; Clain, A.J.; Mletzko Crowe, T.; Wong, A.; et al. Omega-3 Fatty Acids for Major Depressive Disorder With High Inflammation: A Randomized Dose-Finding Clinical Trial. J. Clin. Psychiatry 2022, 83, 42432. [Google Scholar] [CrossRef]
- Dawczynski, C.; Dittrich, M.; Neumann, T.; Goetze, K.; Welzel, A.; Oelzner, P.; Völker, S.; Schaible, A.M.; Troisi, F.; Thomas, L.; et al. Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin. Nutr. 2018, 37, 494–504. [Google Scholar] [CrossRef]
- Das, U.N. Beneficial effect of eicosapentaenoic and docosahexaenoic acids in the management of systemic lupus erythematosus and its relationship to the cytokine network. Prostaglandins Leukot. Essent. Fat. Acids 1994, 51, 207–213. [Google Scholar] [CrossRef]
- Alfaddagh, A.; Elajami, T.K.; Saleh, M.; Elajami, M.; Bistrian, B.R.; Welty, F.K. The effect of eicosapentaenoic and docosahexaenoic acids on physical function, exercise, and joint replacement in patients with coronary artery disease: A secondary analysis of a randomized clinical trial. J. Clin. Lipidol. 2018, 12, 937–947. [Google Scholar] [CrossRef]
- Albert, C.M.; Cook, N.R.; Pester, J.; Moorthy, M.V.; Ridge, C.; Danik, J.S.; Gencer, B.; Siddiqi, H.K.; Ng, C.; Gibson, H.; et al. Effect of Marine Omega-3 Fatty Acid and Vitamin D Supplementation on Incident Atrial Fibrillation: A Randomized Clinical Trial. JAMA 2021, 325, 1061–1073. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Bratseth, V.; Laake, K.; Arnesen, H.; Solheim, S.; Schmidt, E.B.; Badimon, L.; Seljeflot, I. One year of omega 3 polyunsaturated fatty acid supplementation does not reduce circulating prothrombotic microvesicles in elderly subjects after suffering a myocardial infarction. Clin. Nutr. 2021, 40, 5674–5677. [Google Scholar] [CrossRef]
- De Borst, M.H.; Baia, L.C.; Hoogeveen, E.K.; Giltay, E.J.; Navis, G.; Bakker, S.J.L.; Geleijnse, J.M.; Kromhout, D.; Soedamah-Muthu, S.S. Effect of Omega-3 Fatty Acid Supplementation on Plasma Fibroblast Growth Factor 23 Levels in Post-Myocardial Infarction Patients with Chronic Kidney Disease: The Alpha Omega Trial. Nutrients 2017, 9, 1233. [Google Scholar] [CrossRef]
- Okereke, O.I.; Vyas, C.M.; Mischoulon, D.; Chang, G.; Cook, N.R.; Weinberg, A.; Bubes, V.; Copeland, T.; Friedenberg, G.; Lee, I.M.; et al. Effect of Long-term Supplementation With Marine Omega-3 Fatty Acids vs. Placebo on Risk of Depression or Clinically Relevant Depressive Symptoms and on Change in Mood Scores: A Randomized Clinical Trial. JAMA 2021, 326, 2385–2394. [Google Scholar] [CrossRef]
- Li, P.; Chen, Y.; Song, J.; Yan, L.; Tang, T.; Wang, R.; Fan, X.; Zhao, Y.; Qi, K. Maternal DHA-rich n-3 PUFAs supplementation interacts with FADS genotypes to influence the profiles of PUFAs in the colostrum among Chinese Han population: A birth cohort study. Nutr. Metab. 2022, 19, 48. [Google Scholar] [CrossRef]
- Harsløf, L.B.; Larsen, L.H.; Ritz, C.; Hellgren, L.I.; Michaelsen, K.F.; Vogel, U.; Lauritzen, L. FADS genotype and diet are important determinants of DHA status: A cross-sectional study in Danish infants. Am. J. Clin. Nutr. 2013, 97, 1403–1410. [Google Scholar] [CrossRef]
- Tandon, S.; Gonzalez-Casanova, I.; Barraza-Villarreal, A.; Romieu, I.; Demmelmair, H.; Jones, D.P.; Koletzko, B.; Stein, A.D.; Ramakrishnan, U. Infant Metabolome in Relation to Prenatal DHA Supplementation and Maternal Single-Nucleotide Polymorphism rs174602: Secondary Analysis of a Randomized Controlled Trial in Mexico. J. Nutr. 2021, 151, 3339–3349. [Google Scholar] [CrossRef]
- Metherel, A.H.; Irfan, M.; Klingel, S.L.; Mutch, D.M.; Bazinet, R.P. Higher Increase in Plasma DHA in Females Compared to Males Following EPA Supplementation May Be Influenced by a Polymorphism in ELOVL2: An Exploratory Study. Lipids 2021, 56, 211–228. [Google Scholar] [CrossRef]
- Burak, C.; Wolffram, S.; Zur, B.; Langguth, P.; Fimmers, R.; Alteheld, B.; Stehle, P.; Egert, S. Effect of alpha-linolenic acid in combination with the flavonol quercetin on markers of cardiovascular disease risk in healthy, non-obese adults: A randomized, double-blinded placebo-controlled crossover trial. Nutrition 2019, 58, 47–56. [Google Scholar] [CrossRef]
- Joris, P.J.; Draijer, R.; Fuchs, D.; Mensink, R.P. Effect of α-linolenic acid on vascular function and metabolic risk markers during the fasting and postprandial phase: A randomized placebo-controlled trial in untreated (pre-)hypertensive individuals. Clin. Nutr. 2020, 39, 2413–2419. [Google Scholar] [CrossRef]
- Cazzoletti, L.; Zanolin, M.E.; Spelta, F.; Bono, R.; Chamitava, L.; Cerveri, I.; Garcia-Larsen, V.; Grosso, A.; Mattioli, V.; Pirina, P.; et al. Dietary fats, olive oil and respiratory diseases in Italian adults: A population-based study. Clin. Exp. Allergy 2019, 49, 799–807. [Google Scholar] [CrossRef]
- Vassiliou, E.K.; Gonzalez, A.; Garcia, C.; Tadros, J.H.; Chakraborty, G.; Toney, J.H. Oleic acid and peanut oil high in oleic acid reverse the inhibitory effect of insulin production of the inflammatory cytokine TNF-alpha both in vitro and in vivo systems. Lipids Health Dis. 2009, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, A.R.; Sampaio, G.R.; da Silva, L.R.; Portal, V.L.; Markoski, M.M.; de Quadros, A.S.; Rogero, M.M.; da Silva Torres, E.A.F.; Marcadenti, A. Effects of extra virgin olive oil and pecans on plasma fatty acids in patients with stable coronary artery disease. Nutrition 2021, 91–92, 111411. [Google Scholar] [CrossRef] [PubMed]
- Caldas, A.P.S.; Alves, R.D.M.; Hermsdorff, H.H.M.; de Oliveira, L.L.; Bressan, J. Effects of high-oleic peanuts within a hypoenergetic diet on inflammatory and oxidative status of overweight men: A randomised controlled trial. Br. J. Nutr. 2020, 123, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Yen, J.H.; Vassiliou, E.; Adhikary, S.; Toscano, M.G.; Ganea, D. Docosahexaenoic acid prevents dendritic cell maturation and in vitro and in vivo expression of the IL-12 cytokine family. Lipids Health Dis. 2010, 9, 12. [Google Scholar] [CrossRef]
- Pettit, L.K.; Varsanyi, C.; Tadros, J.; Vassiliou, E. Modulating the inflammatory properties of activated microglia with Docosahexaenoic acid and Aspirin. Lipids Health Dis. 2013, 12, 16. [Google Scholar] [CrossRef]
- Borsini, A.; Nicolaou, A.; Camacho-Muñoz, D.; Kendall, A.C.; Di Benedetto, M.G.; Giacobbe, J.; Su, K.P.; Pariante, C.M. Omega-3 polyunsaturated fatty acids protect against inflammation through production of LOX and CYP450 lipid mediators: Relevance for major depression and for human hippocampal neurogenesis. Mol. Psychiatry 2021, 26, 6773–6788. [Google Scholar] [CrossRef]
- Naeini, Z.; Toupchian, O.; Vatannejad, A.; Sotoudeh, G.; Teimouri, M.; Ghorbani, M.; Nasli-Esfahani, E.; Koohdani, F. Effects of DHA-enriched fish oil on gene expression levels of p53 and NF-κB and PPAR-γ activity in PBMCs of patients with T2DM: A randomized, double-blind, clinical trial. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 441–447. [Google Scholar] [CrossRef]
- Dalli, J.; Winkler, J.W.; Colas, R.A.; Arnardottir, H.; Cheng, C.Y.; Chiang, N.; Petasis, N.A.; Serhan, C.N. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem. Biol. 2013, 20, 188–201. [Google Scholar] [CrossRef]
- Sun, Y.P.; Oh, S.F.; Uddin, J.; Yang, R.; Gotlinger, K.; Campbell, E.; Colgan, S.P.; Petasis, N.A.; Serhan, C.N. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J. Biol. Chem. 2007, 282, 9323–9334. [Google Scholar] [CrossRef]
- Markworth, J.F.; D’Souza, R.F.; Aasen, K.M.M.; Mitchell, S.M.; Durainayagam, B.R.; Sinclair, A.J.; Peake, J.M.; Egner, I.M.; Raastad, T.; Cameron-Smith, D.; et al. Arachidonic acid supplementation transiently augments the acute inflammatory response to resistance exercise in trained men. J. Appl. Physiol. 2018, 125, 271–286. [Google Scholar] [CrossRef]
- Corsetto, P.A.; Cremona, A.; Montorfano, G.; Jovenitti, I.E.; Orsini, F.; Arosio, P.; Rizzo, A.M. Chemical-physical changes in cell membrane microdomains of breast cancer cells after omega-3 PUFA incorporation. Cell Biochem. Biophys. 2012, 64, 45–59. [Google Scholar] [CrossRef]
- Shaikh, S.R.; Rockett, B.D.; Salameh, M.; Carraway, K. Docosahexaenoic acid modifies the clustering and size of lipid rafts and the lateral organization and surface expression of MHC class I of EL4 cells. J. Nutr. 2009, 139, 1632–1639. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.; Li, Y.; Geng, X.; Jia, X.; Zhang, L.; Yang, H. Arachidonic Acid Metabolism Controls Macrophage Alternative Activation Through Regulating Oxidative Phosphorylation in PPARγ Dependent Manner. Front. Immunol. 2021, 12, 618501. [Google Scholar] [CrossRef]
- Zhuang, P.; Shou, Q.; Lu, Y.; Wang, G.; Qiu, J.; Wang, J.; He, L.; Chen, J.; Jiao, J.; Zhang, Y. Arachidonic acid sex-dependently affects obesity through linking gut microbiota-driven inflammation to hypothalamus-adipose-liver axis. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2715–2726. [Google Scholar] [CrossRef]
- Joffre, C.; Rey, C.; Layé, S. N-3 Polyunsaturated Fatty Acids and the Resolution of Neuroinflammation. Front. Pharmacol. 2019, 10, 1022. [Google Scholar] [CrossRef]
- Garcia Corrales, A.V.; Haidar, M.; Bogie, J.F.J.; Hendriks, J.J.A. Fatty acid synthesis in glial cells of the cns. Int. J. Mol. Sci. 2021, 22, 8159. [Google Scholar] [CrossRef]
- Pifferi, F.; Laurent, B.; Plourde, M. Lipid Transport and Metabolism at the Blood-Brain Interface: Implications in Health and Disease. Front. Physiol. 2021, 12, 645646. [Google Scholar] [CrossRef]
- Aizawa, F.; Nishinaka, T.; Yamashita, T.; Nakamoto, K.; Koyama, Y.; Kasuya, F.; Tokuyama, S. Astrocytes release polyunsaturated fatty acids by lipopolysaccharide stimuli. Biol. Pharm. Bull. 2016, 39, 1100–1106. [Google Scholar] [CrossRef]
- Ji, A.; Diao, H.; Wang, X.; Yang, R.; Zhang, J.; Luo, W.; Cao, R.; Cao, Z.; Wang, F.; Cai, T. N-3 polyunsaturated fatty acids inhibit lipopolysaccharide-induced microglial activation and dopaminergic injury in rats. NeuroToxicology 2012, 33, 780–788. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jang, J.S.; Son, D.J.; Im, H.S.; Kim, J.Y.; Park, J.E.; Choi, W.R.; Han, S.B.; Hong, J.T. Antarctic krill oil diet protects against lipopolysaccharide-induced oxidative stress, neuroinflammation and cognitive impairment. Int. J. Mol. Sci. 2017, 18, 2554. [Google Scholar] [CrossRef] [PubMed]
- Tyrtyshnaia, A.; Konovalova, S.; Bondar, A.; Ermolenko, E.; Sultanov, R.; Manzhulo, I. Anti-inflammatory activity of N-docosahexaenoylethanolamine and n-eicosapentaenoylethanolamine in a mouse model of lipopolysaccharide-induced neuroinflammation. Int. J. Mol. Sci. 2021, 22, 10728. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, D.; Wang, F.; Liu, S.; Zhao, S.; Ling, E.A.; Hao, A. Saturated fatty acids activate microglia via Toll-like receptor 4/NF-κB signalling. Br. J. Nutr. 2012, 107, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Knight, A.G.; Gupta, S.; Keller, J.N.; Bruce-Keller, A.J. Saturated long-chain fatty acids activate inflammatory signaling in astrocytes. J. Neurochem. 2012, 120, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.O.; Kim, K.C.; Jin, C.Y.; Han, M.H.; Park, C.; Lee, K.J.; Park, Y.M.; Choi, Y.H.; Kim, G.Y. Inhibitory effects of eicosapentaenoic acid on lipopolysaccharide-induced activation in BV2 microglia. Int. Immunopharmacol. 2007, 7, 222–229. [Google Scholar] [CrossRef]
- Beaulieu, J.; Costa, G.; Renaud, J.; Moitié, A.; Glémet, H.; Sergi, D.; Martinoli, M.G. The Neuroinflammatory and Neurotoxic Potential of Palmitic Acid Is Mitigated by Oleic Acid in Microglial Cells and Microglial-Neuronal Co-cultures. Mol. Neurobiol. 2021, 58, 3000–3014. [Google Scholar] [CrossRef]
- Lu, D.Y.; Tsao, Y.Y.; Leung, Y.M.; Su, K.P. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: Implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology 2010, 35, 2238–2248. [Google Scholar] [CrossRef]
- Hadad, N.; Levy, R. Combination of EPA with Carotenoids and Polyphenol Synergistically Attenuated the Transformation of Microglia to M1 Phenotype Via Inhibition of NF-κB. NeuroMolecular Med. 2017, 19, 436–451. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, Y.; Yang, Z.; Liu, M.; Zhang, C.; Zhao, Y.; Song, C. ω-3 DPA Protected Neurons from Neuroinflammation by Balancing Microglia M1/M2 Polarizations through Inhibiting NF-κB/MAPK p38 Signaling and Activating Neuron-BDNF-PI3K/AKT Pathways. Mar. Drugs 2021, 19, 587. [Google Scholar] [CrossRef]
- Antonietta Ajmone-Cat, M.; Lavinia Salvatori, M.; de Simone, R.; Mancini, M.; Biagioni, S.; Bernardo, A.; Cacci, E.; Minghetti, L. Docosahexaenoic acid modulates inflammatory and antineurogenic functions of activated microglial cells. J. Neurosci. Res. 2012, 90, 575–587. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, M.; Kalueff, A.V.; Song, C. Dietary eicosapentaenoic acid normalizes hippocampal omega-3 and 6 polyunsaturated fatty acid profile, attenuates glial activation and regulates BDNF function in a rodent model of neuroinflammation induced by central interleukin-1β administration. Eur. J. Nutr. 2018, 57, 1781–1791. [Google Scholar] [CrossRef]
- Manzhulo, O.; Tyrtyshnaia, A.; Kipryushina, Y.; Dyuizen, I.; Manzhulo, I. Docosahexaenoic acid induces changes in microglia/macrophage polarization after spinal cord injury in rats. Acta Histochem. 2018, 120, 741–747. [Google Scholar] [CrossRef]
- Chang, C.Y.; Wu, C.C.; Wang, J.D.; Li, J.R.; Wang, Y.Y.; Lin, S.Y.; Chen, W.Y.; Liao, S.L.; Chen, C.J. DHA attenuated Japanese Encephalitis virus infection-induced neuroinflammation and neuronal cell death in cultured rat Neuron/glia. Brain Behav. Immun. 2021, 93, 194–205. [Google Scholar] [CrossRef]
- Talamonti, E.; Sasso, V.; To, H.; Haslam, R.P.; Napier, J.A.; Ulfhake, B.; Pernold, K.; Asadi, A.; Hessa, T.; Jacobsson, A.; et al. Impairment of DHA synthesis alters the expression of neuronal plasticity markers and the brain inflammatory status in mice. FASEB J. 2020, 34, 2024–2040. [Google Scholar] [CrossRef]
- Willis, L.M.; Bielinski, D.F.; Fisher, D.R.; Matthan, N.R.; Joseph, J.A. Walnut extract inhibits LPS-induced activation of Bv-2 microglia via internalization of TLR4: Possible involvement of phospholipase D2. Inflammation 2010, 33, 325–333. [Google Scholar] [CrossRef]
- Carey, A.N.; Fisher, D.R.; Bielinski, D.F.; Cahoon, D.S.; Shukitt-Hale, B. Walnut-Associated Fatty Acids Inhibit LPS-Induced Activation of BV-2 Microglia. Inflammation 2020, 43, 241–250. [Google Scholar] [CrossRef]
- Lowry, J.R.; Marshall, N.; Wenzel, T.J.; Murray, T.E.; Klegeris, A. The dietary fatty acids α-linolenic acid (ALA) and linoleic acid (LA) selectively inhibit microglial nitric oxide production. Mol. Cell. Neurosci. 2020, 109, 103569. [Google Scholar] [CrossRef]
- Inoue, T.; Tanaka, M.; Masuda, S.; Ohue-Kitano, R.; Yamakage, H.; Muranaka, K.; Wada, H.; Kusakabe, T.; Shimatsu, A.; Hasegawa, K.; et al. Omega-3 polyunsaturated fatty acids suppress the inflammatory responses of lipopolysaccharide-stimulated mouse microglia by activating SIRT1 pathways. Biochim. Et Biophys. Acta—Mol. Cell Biol. Lipids 2017, 1862, 552–560. [Google Scholar] [CrossRef]
- Yang, Z.H.; Amar, M.; Sampson, M.; Courville, A.B.; Sorokin, A.V.; Gordon, S.M.; Aponte, A.M.; Stagliano, M.; Playford, M.P.; Fu, Y.P.; et al. Comparison of Omega-3 Eicosapentaenoic Acid Versus Docosahexaenoic Acid-Rich Fish Oil Supplementation on Plasma Lipids and Lipoproteins in Normolipidemic Adults. Nutrients 2020, 12, 749. [Google Scholar] [CrossRef]
- Yang, B.; Ren, X.L.; Li, Z.H.; Shi, M.Q.; Ding, F.; Su, K.P.; Guo, X.J.; Li, D. Lowering effects of fish oil supplementation on proinflammatory markers in hypertension: Results from a randomized controlled trial. Food Funct. 2020, 11, 1779–1789. [Google Scholar] [CrossRef]
- Li, L.; Wu, Y.; Wang, Y.; Wu, J.; Song, L.; Xian, W.; Yuan, S.; Pei, L.; Shang, Y. Resolvin D1 promotes the interleukin-4-induced alternative activation in BV-2 microglial cells. J. Neuroinflammation 2014, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Krashia, P.; Cordella, A.; Nobili, A.; La Barbera, L.; Federici, M.; Leuti, A.; Campanelli, F.; Natale, G.; Marino, G.; Calabrese, V.; et al. Blunting neuroinflammation with resolvin D1 prevents early pathology in a rat model of Parkinson’s disease. Nat. Commun. 2019, 10, 3945. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, M.; Miyamoto, Y.; Ikeshima-Kataoka, H. Astrocytic Neuroimmunological Roles Interacting with Microglial Cells in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 1599. [Google Scholar] [CrossRef]
- Zgorzynska, E.; Dziedzic, B.; Markiewicz, M.; Walczewska, A. Omega-3 pufas suppress il-1β-induced hyperactivity of immunoproteasomes in astrocytes. Int. J. Mol. Sci. 2021, 22, 5410. [Google Scholar] [CrossRef]
- Badia-Soteras, A.; de Vries, J.; Dykstra, W.; Broersen, L.M.; Verkuyl, J.M.; Smit, A.B.; Verheijen, M.H.G. High-Throughput Analysis of Astrocyte Cultures Shows Prevention of Reactive Astrogliosis by the Multi-Nutrient Combination Fortasyn Connect. Cells 2022, 11, 1428. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Rogers, J. Inflammation in Alzheimer disease-A brief review of the basic science and clinical literature. Cold Spring Harb. Perspect. Med. 2012, 2, a006346. [Google Scholar] [CrossRef]
- Villegas-Llerena, C.; Phillips, A.; Garcia-Reitboeck, P.; Hardy, J.; Pocock, J.M. Microglial genes regulating neuroinflammation in the progression of Alzheimer’s disease. Curr. Opin. Neurobiol. 2016, 36, 74–81. [Google Scholar] [CrossRef]
- Venigalla, M.; Sonego, S.; Gyengesi, E.; Sharman, M.J.; Münch, G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 2016, 95, 63–74. [Google Scholar] [CrossRef]
- Oksman, M.; Iivonen, H.; Hogyes, E.; Amtul, Z.; Penke, B.; Leenders, I.; Broersen, L.; Lütjohann, D.; Hartmann, T.; Tanila, H. Impact of different saturated fatty acid, polyunsaturated fatty acid and cholesterol containing diets on beta-amyloid accumulation in APP/PS1 transgenic mice. Neurobiol. Dis. 2006, 23, 563–572. [Google Scholar] [CrossRef]
- Van de Rest, O.; Berendsen, A.A.M.; Haveman-Nies, A.; de Groot, L.C.P.G.M. Dietary patterns, cognitive decline, and dementia: A systematic review. Adv. Nutr. 2015, 6, 154–168. [Google Scholar] [CrossRef]
- Ma, Q.L.; Zhu, C.; Morselli, M.; Su, T.; Pelligrini, M.; Lu, Z.; Jones, M.; Denver, P.; Castro, D.; Gu, X.; et al. The Novel Omega-6 Fatty Acid Docosapentaenoic Acid Positively Modulates Brain Innate Immune Response for Resolving Neuroinflammation at Early and Late Stages of Humanized APOE-Based Alzheimer’s Disease Models. Front. Immunol. 2020, 11, 558036. [Google Scholar] [CrossRef]
- Gao, J.; Wang, L.; Zhao, C.; Wu, Y.; Lu, Z.; Gu, Y.; Ba, Z.; Wang, X.; Wang, J.; Xu, Y. Peony seed oil ameliorates neuroinflammation-mediated cognitive deficits by suppressing microglial activation through inhibition of NF-kappa B pathway in presenilin 1/2 conditional double knockout mice. J. Leukoc. Biol. 2021, 110, 1005–1022. [Google Scholar] [CrossRef]
- Moura, E.L.R.; Dos Santos, H.; Celes, A.P.M.; Bassani, T.B.; Souza, L.C.; Vital, M.A.B.F. Effects of a Nutritional Formulation Containing Caprylic and Capric Acid, Phosphatidylserine, and Docosahexaenoic Acid in Streptozotocin-Lesioned Rats. J. Alzheimer’s Dis. Rep. 2020, 4, 353–363. [Google Scholar] [CrossRef]
- Fujita, Y.; Kano, K.; Kishino, S.; Nagao, T.; Shen, X.; Sato, C.; Hatakeyama, H.; Ota, Y.; Niibori, S.; Nomura, A.; et al. Dietary cis-9, trans-11-conjugated linoleic acid reduces amyloid β-protein accumulation and upregulates anti-inflammatory cytokines in an Alzheimer’s disease mouse model. Sci. Rep. 2021, 11, 9749. [Google Scholar] [CrossRef]
- Geng, X.; Yang, B.; Li, R.; Teng, T.; Ladu, M.J.; Sun, G.Y.; Greenlief, C.M.; Lee, J.C. Effects of Docosahexaenoic Acid and Its Peroxidation Product on Amyloid-β Peptide-Stimulated Microglia. Mol. Neurobiol. 2020, 57, 1085–1098. [Google Scholar] [CrossRef]
- Minogue, A.M.; Lynch, A.M.; Loane, D.J.; Herron, C.E.; Lynch, M.A. Modulation of amyloid-β-induced and age-associated changes in rat hippocampus by eicosapentaenoic acid. J. Neurochem. 2007, 103, 914–926. [Google Scholar] [CrossRef]
- Kelly, L.; Grehan, B.; Chiesa, A.D.; O’Mara, S.M.; Downer, E.; Sahyoun, G.; Massey, K.A.; Nicolaou, A.; Lynch, M.A. The polyunsaturated fatty acids, EPA and DPA exert a protective effect in the hippocampus of the aged rat. Neurobiol. Aging 2011, 32, e2311–e2318. [Google Scholar] [CrossRef]
- Lynch, A.M.; Loane, D.J.; Minogue, A.M.; Clarke, R.M.; Kilroy, D.; Nally, R.E.; Roche, Ó.J.; O’Connell, F.; Lynch, M.A. Eicosapentaenoic acid confers neuroprotection in the amyloid-β challenged aged hippocampus. Neurobiol. Aging 2007, 28, 845–855. [Google Scholar] [CrossRef]
- Ivkovic, S.; Major, T.; Mitic, M.; Loncarevic-Vasiljkovic, N.; Jovic, M.; Adzic, M. Fatty acids as biomodulators of Piezo1 mediated glial mechanosensitivity in Alzheimer’s disease. Life Sci. 2022, 297, 120470. [Google Scholar] [CrossRef]
- Hjorth, E.; Zhu, M.; Toro, V.C.; Vedin, I.; Palmblad, J.; Cederholm, T.; Freund-Levi, Y.; Faxen-Irving, G.; Wahlund, L.O.; Basun, H.; et al. Omega-3 fatty acids enhance phagocytosis of alzheimer’s disease-related amyloid-β42 by human microglia and decrease inflammatory markers. J. Alzheimer’s Dis. 2013, 35, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Desale, S.E.; Chinnathambi, S. α– Linolenic acid modulates phagocytosis and endosomal pathways of extracellular Tau in microglia. Cell Adhes. Migr. 2021, 15, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Litwiniuk, A.; Domańska, A.; Chmielowska, M.; Martyńska, L.; Bik, W.; Kalisz, M.; Mendonça Junior, F.J.B. The Effects of Alpha-Linolenic Acid on the Secretory Activity of Astrocytes and β Amyloid-Associated Neurodegeneration in Differentiated SH-SY5Y Cells: Alpha-Linolenic Acid Protects the SH-SY5Y cells against β Amyloid Toxicity. Oxidative Med. Cell. Longev. 2020, 2020, 8908901. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.X.; Luo, C.M.; Feng, Y.Q.; Yao, X.L.; Shi, Z.; Liang, F.Y.; Kang, J.X.; Wan, J.B.; Pei, Z.; Su, H.X. Omega-3 polyunsaturated fatty acids promote amyloid-beta clearance from the brain through mediating the function of the glymphatic system. Faseb J. 2017, 31, 282–293. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, X.; Hjorth, E.; Colas, R.A.; Schroeder, L.; Granholm, A.C.; Serhan, C.N.; Schultzberg, M. Pro-Resolving Lipid Mediators Improve Neuronal Survival and Increase Aβ42 Phagocytosis. Mol. Neurobiol. 2016, 53, 2733–2749. [Google Scholar] [CrossRef]
- Wang, Y.; Leppert, A.; Tan, S.; van der Gaag, B.; Li, N.; Schultzberg, M.; Hjorth, E. Maresin 1 attenuates pro-inflammatory activation induced by β-amyloid and stimulates its uptake. J. Cell. Mol. Med. 2021, 25, 434–447. [Google Scholar] [CrossRef]
- Yin, P.; Wang, X.; Wang, S.; Wei, Y.; Feng, J.; Zhu, M. Maresin 1 Improves Cognitive Decline and Ameliorates Inflammation in a Mouse Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2019, 13, 466. [Google Scholar] [CrossRef]
- Kantarci, A.; Aytan, N.; Palaska, I.; Stephens, D.; Crabtree, L.; Benincasa, C.; Jenkins, B.G.; Carreras, I.; Dedeoglu, A. Combined administration of resolvin E1 and lipoxin A4 resolves inflammation in a murine model of Alzheimer’s disease. Exp. Neurol. 2018, 300, 111–120. [Google Scholar] [CrossRef]
- Emre, C.; Arroyo-García, L.E.; Do, K.V.; Jun, B.; Ohshima, M.; Alcalde, S.G.; Cothern, M.L.; Maioli, S.; Nilsson, P.; Hjorth, E.; et al. Intranasal delivery of pro-resolving lipid mediators rescues memory and gamma oscillation impairment in App NL-G-F/NL-G-F mice. Commun. Biol. 2022, 5, 245. [Google Scholar] [CrossRef]
- Little, J.P.; Madeira, J.M.; Klegeris, A. The saturated fatty acid palmitate induces human monocytic cell toxicity toward neuronal cells: Exploring a possible link between obesity-related metabolic impairments and neuroinflammation. J. Alzheimer’s Dis. 2012, 30, 179–183. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, H.; Pu, H.; Wang, G.; Li, W.; Leak, R.K.; Chen, J.; Liou, A.K.; Hu, X. N-3 PUFA supplementation benefits microglial responses to myelin pathology. Sci. Rep. 2014, 4, 7458. [Google Scholar] [CrossRef]
- Poisson, L.M.; Suhail, H.; Singh, J.; Datta, I.; Deni, A.; Labuzek, K.; Hoda, N.; Shankar, A.; Kumar, A.; Cerghet, M.; et al. Untargeted plasma metabolomics identifies endogenous metabolite with drug-like properties in chronic animal model of multiple sclerosis. J. Biol. Chem. 2015, 290, 30697–30712. [Google Scholar] [CrossRef]
- Mancera, P.; Wappenhans, B.; Cordobilla, B.; Virgili, N.; Pugliese, M.; Rueda, F.; Espinosa-Parrilla, J.F.; Domingo, J.C. Natural docosahexaenoic acid in the triglyceride form attenuates in vitro microglial activation and ameliorates autoimmune encephalomyelitis in mice. Nutrients 2017, 9, 681. [Google Scholar] [CrossRef]
- Cai, W.; Liu, S.; Hu, M.; Sun, X.; Qiu, W.; Zheng, S.; Hu, X.; Lu, Z. Post-stroke DHA Treatment Protects Against Acute Ischemic Brain Injury by Skewing Macrophage Polarity Toward the M2 Phenotype. Transl. Stroke Res. 2018, 9, 669–680. [Google Scholar] [CrossRef]
- Jiang, W.; Whellan, D.J.; Adams, K.F.; Babyak, M.A.; Boyle, S.H.; Wilson, J.L.; Patel, C.B.; Rogers, J.G.; Harris, W.S.; O’Connor, C.M. Long-Chain Omega-3 Fatty Acid Supplements in Depressed Heart Failure Patients: Results of the OCEAN Trial. JACC Heart Fail. 2018, 6, 833–843. [Google Scholar] [CrossRef]
- Okabe, N.; Nakamura, T.; Toyoshima, T.; Miyamoto, O.; Lu, F.; Itano, T. Eicosapentaenoic acid prevents memory impairment after ischemia by inhibiting inflammatory response and oxidative damage. J. Stroke Cerebrovasc. Dis. 2011, 20, 188–195. [Google Scholar] [CrossRef]
- Lalancette-Hébert, M.; Julien, C.; Cordeau, P.; Bohacek, I.; Weng, Y.C.; Calon, F.; Kriz, J. Accumulation of dietary docosahexaenoic acid in the brain attenuates acute immune response and development of postischemic neuronal damage. Stroke 2011, 42, 2903–2909. [Google Scholar] [CrossRef]
- Eady, T.N.; Belayev, L.; Khoutorova, L.; Atkins, K.D.; Zhang, C.; Bazan, N.G. Docosahexaenoic Acid Signaling Modulates Cell Survival in Experimental Ischemic Stroke Penumbra and Initiates Long-Term Repair in Young and Aged Rats. PLoS ONE 2012, 7, e46151. [Google Scholar] [CrossRef]
- Eady, T.N.; Khoutorova, L.; Obenaus, A.; Mohd-Yusof, A.; Bazan, N.G.; Belayev, L. Docosahexaenoic acid complexed to albumin provides neuroprotection after experimental stroke in aged rats. Neurobiol. Dis. 2014, 62, 1–7. [Google Scholar] [CrossRef]
- Belayev, L.; Hong, S.H.; Freitas, R.S.; Menghani, H.; Marcell, S.J.; Khoutorova, L.; Mukherjee, P.K.; Reid, M.M.; Oria, R.B.; Bazan, N.G. DHA modulates MANF and TREM2 abundance, enhances neurogenesis, reduces infarct size, and improves neurological function after experimental ischemic stroke. CNS Neurosci. Ther. 2020, 26, 1155–1167. [Google Scholar] [CrossRef]
- Black, E.K.; Phillips, J.K.; Seminetta, J.; Bailes, J.; Lee, J.M.; Finan, J.D. The effect of dietary supplementation with high- or low-dose omega-3 fatty acid on inflammatory pathology after traumatic brain injury in rats. Transl. Neurosci. 2021, 12, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Harvey, L.D.; Yin, Y.; Attarwala, I.Y.; Begum, G.; Deng, J.; Yan, H.Q.; Dixon, C.E.; Sun, D. Administration of DHA reduces endoplasmic reticulum stress-associated inflammation and alters microglial or macrophage activation in traumatic brain injury. ASN Neuro 2015, 7, 1759091415618969. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, E.; Sun, G.; Yan, H.Q.; Foley, L.M.; Andrzejczuk, L.A.; Attarwala, I.Y.; Hitchens, T.K.; Kiselyov, K.; Dixon, C.E.; et al. Effects of DHA on Hippocampal Autophagy and Lysosome Function After Traumatic Brain Injury. Mol. Neurobiol. 2018, 55, 2454–2470. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Chen, L.; Wang, Y.; Wei, X.; Zeng, S.; Zheng, Y.; Gao, C.; Liu, H. Activation of the Omega-3 Fatty Acid Receptor GPR120 Protects against Focal Cerebral Ischemic Injury by Preventing Inflammation and Apoptosis in Mice. J. Immunol. 2019, 202, 747–759. [Google Scholar] [CrossRef]
- Liu, G.J.; Tao, T.; Wang, H.; Zhou, Y.; Gao, X.; Gao, Y.Y.; Hang, C.H.; Li, W. Functions of resolvin D1-ALX/FPR2 receptor interaction in the hemoglobin-induced microglial inflammatory response and neuronal injury. J. Neuroinflammation 2020, 17, 239. [Google Scholar] [CrossRef]
- Mastromarino, M.; Favia, M.; Schepetkin, I.A.; Kirpotina, L.N.; Trojan, E.; Niso, M.; Carrieri, A.; Leśkiewicz, M.; Regulska, M.; Darida, M.; et al. Design, Synthesis, Biological Evaluation, and Computational Studies of Novel Ureidopropanamides as Formyl Peptide Receptor 2 (FPR2) Agonists to Target the Resolution of Inflammation in Central Nervous System Disorders. J. Med. Chem. 2022, 65, 5004–5028. [Google Scholar] [CrossRef]
- Konuskan, D.B.; Arslan, M.; Oksuz, A. Physicochemical properties of cold pressed sunflower, peanut, rapeseed, mustard and olive oils grown in the Eastern Mediterranean region. Saudi J. Biol. Sci. 2019, 26, 340–344. [Google Scholar] [CrossRef]
- Agaev, S.G.; Baida, A.A.; Georgiev, O.V.; Maiorova, O.O.; Mozyrev, A.G. Dielectric Spectroscopy of Vegetable Oils. Russ. J. Appl. Chem. 2020, 93, 748–756. [Google Scholar] [CrossRef]
- Alves, A.Q.; da Silva, V.A.; Goes, A.J.S.; Silva, M.S.; de Oliveira, G.G.; Bastos, I.; Neto, A.G.D.; Alves, A.J. The Fatty Acid Composition of Vegetable Oils and Their Potential Use in Wound Care. Adv. Ski. Wound Care 2019, 32, 1–8. [Google Scholar] [CrossRef]
- Muradoglu, F.; Oguz, H.I.; Yildiz, K.; Yilmaz, H. Some chemical composition of walnut (Juglans regia L.) selections from Eastern Turkey. Afr. J. Agric. Res. 2010, 5, 2379–2385. [Google Scholar]
- Ariel, A.; Li, P.L.; Wang, W.; Tang, W.X.; Fredman, G.; Hong, S.; Gotlinger, K.H.; Serhan, C.N. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J. Biol. Chem. 2005, 280, 43079–43086. [Google Scholar] [CrossRef]
- Li, H.; Ruan, X.Z.; Powis, S.H.; Fernando, R.; Mon, W.Y.; Wheeler, D.C.; Moorhead, J.F.; Varghese, Z. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: Evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005, 67, 867–874. [Google Scholar] [CrossRef]
- Zúñiga, J.; Cancino, M.; Medina, F.; Varela, P.; Vargas, R.; Tapia, G.; Videla, L.A.; Fernández, V. N-3 PUFA supplementation triggers PPAR-α activation and PPAR-α/NF-κB interaction: Anti-inflammatory implications in liver ischemia-reperfusion injury. PLoS ONE 2011, 6, e28502. [Google Scholar] [CrossRef]
- Magee, P.; Pearson, S.; Whittingham-Dowd, J.; Allen, J. PPARγ as a molecular target of EPA anti-inflammatory activity during TNF-α-impaired skeletal muscle cell differentiation. J. Nutr. Biochem. 2012, 23, 1440–1448. [Google Scholar] [CrossRef]
- Kliewer, S.A.; Sundseth, S.S.; Jones, S.A.; Brown, P.J.; Wisely, G.B.; Koble, C.S.; Devchand, P.; Wahli, W.; Willson, T.M.; Lenhard, J.M.; et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc. Natl. Acad. Sci. USA 1997, 94, 4318–4323. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, J.; Liu, L.; Shi, C.; Wang, L.; Tian, F.; Liu, F.; Wang, H.; Shao, C.; Zhang, Q.; et al. α-linolenic acid inhibits human renal cell carcinoma cell proliferation through PPAR-γ activation and COX-2 inhibition. Oncol. Lett. 2013, 6, 197–202. [Google Scholar] [CrossRef]
- Smith, W.L.; Urade, Y.; Jakobsson, P.J. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 2011, 111, 5821–5865. [Google Scholar] [CrossRef]
- Moncada, S.; Higgs, E.A.; Vane, J.R. Human arterial and venous tissues generate prostacyclin (prostaglandin x), a potent inhibitor of platelet aggregation. Lancet 1977, 1, 18–20. [Google Scholar] [CrossRef]
- Bell, E.; Ponthan, F.; Whitworth, C.; Westermann, F.; Thomas, H.; Redfern, C.P. Cell survival signalling through PPARδ and arachidonic acid metabolites in neuroblastoma. PLoS ONE 2013, 8, e68859. [Google Scholar] [CrossRef]
- Moya-Camarena, S.Y.; Vanden Heuvel, J.P.; Blanchard, S.G.; Leesnitzer, L.A.; Belury, M.A. Conjugated linoleic acid is a potent naturally occurring ligand and activator of PPARalpha. J. Lipid Res. 1999, 40, 1426–1433. [Google Scholar] [CrossRef]
- Kandel, P.; Semerci, F.; Mishra, R.; Choi, W.; Bajic, A.; Baluya, D.; Ma, L.; Chen, K.; Cao, A.C.; Phongmekhin, T.; et al. Oleic acid is an endogenous ligand of TLX/NR2E1 that triggers hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA 2022, 119, e2023784119. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coniglio, S.; Shumskaya, M.; Vassiliou, E. Unsaturated Fatty Acids and Their Immunomodulatory Properties. Biology 2023, 12, 279. https://doi.org/10.3390/biology12020279
Coniglio S, Shumskaya M, Vassiliou E. Unsaturated Fatty Acids and Their Immunomodulatory Properties. Biology. 2023; 12(2):279. https://doi.org/10.3390/biology12020279
Chicago/Turabian StyleConiglio, Salvatore, Maria Shumskaya, and Evros Vassiliou. 2023. "Unsaturated Fatty Acids and Their Immunomodulatory Properties" Biology 12, no. 2: 279. https://doi.org/10.3390/biology12020279
APA StyleConiglio, S., Shumskaya, M., & Vassiliou, E. (2023). Unsaturated Fatty Acids and Their Immunomodulatory Properties. Biology, 12(2), 279. https://doi.org/10.3390/biology12020279





