Macroanatomical, Histological and Microtomographic Study of the Teeth of the Komodo Dragon (Varanus komodoensis)—Adaptation to Hunting
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection for Macroscopic and Stereoscopic Examinations
2.2. Autofluorescence Analysis
2.3. Histological Analysis
2.4. Computed Microtomography
3. Results
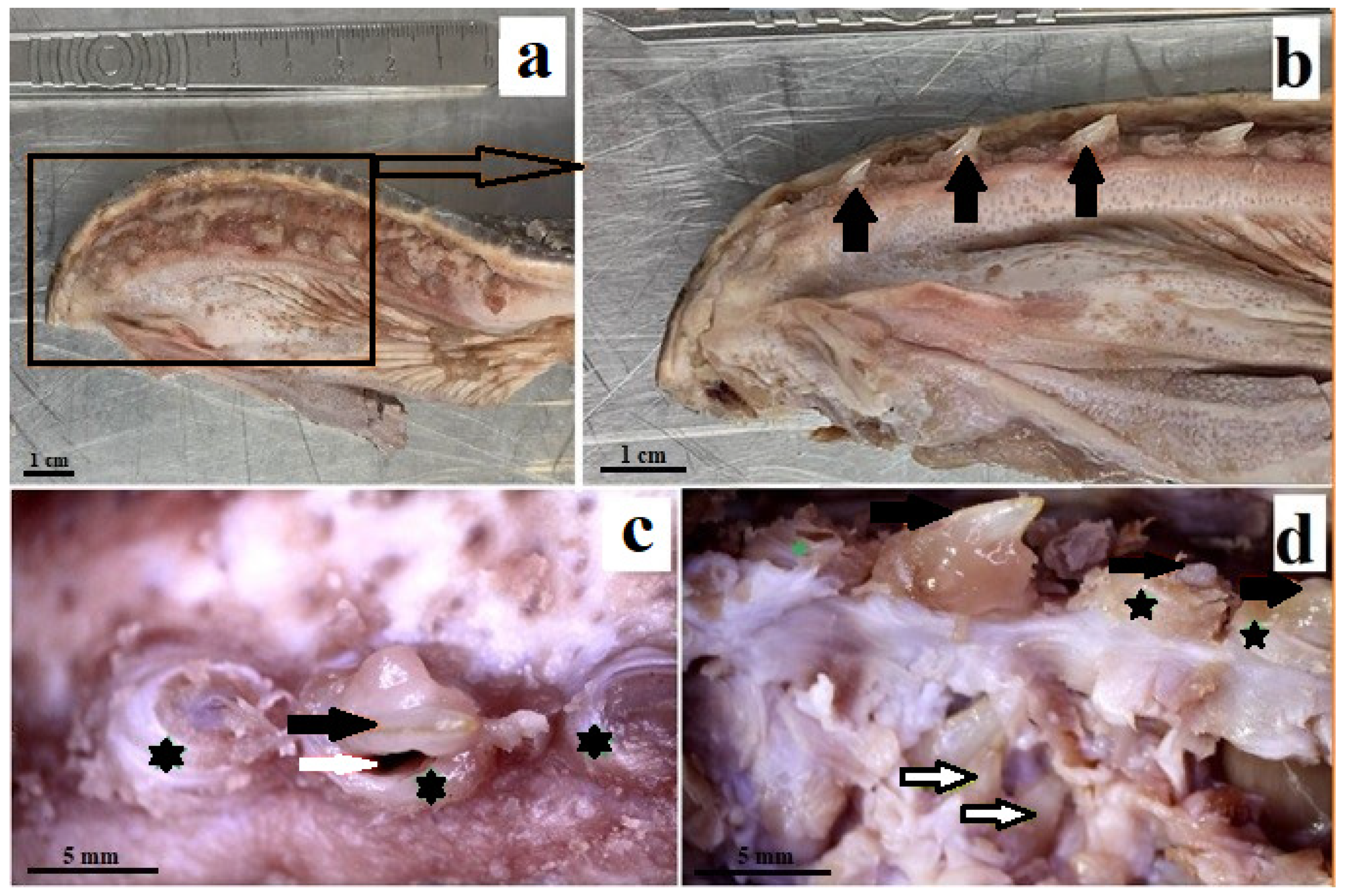
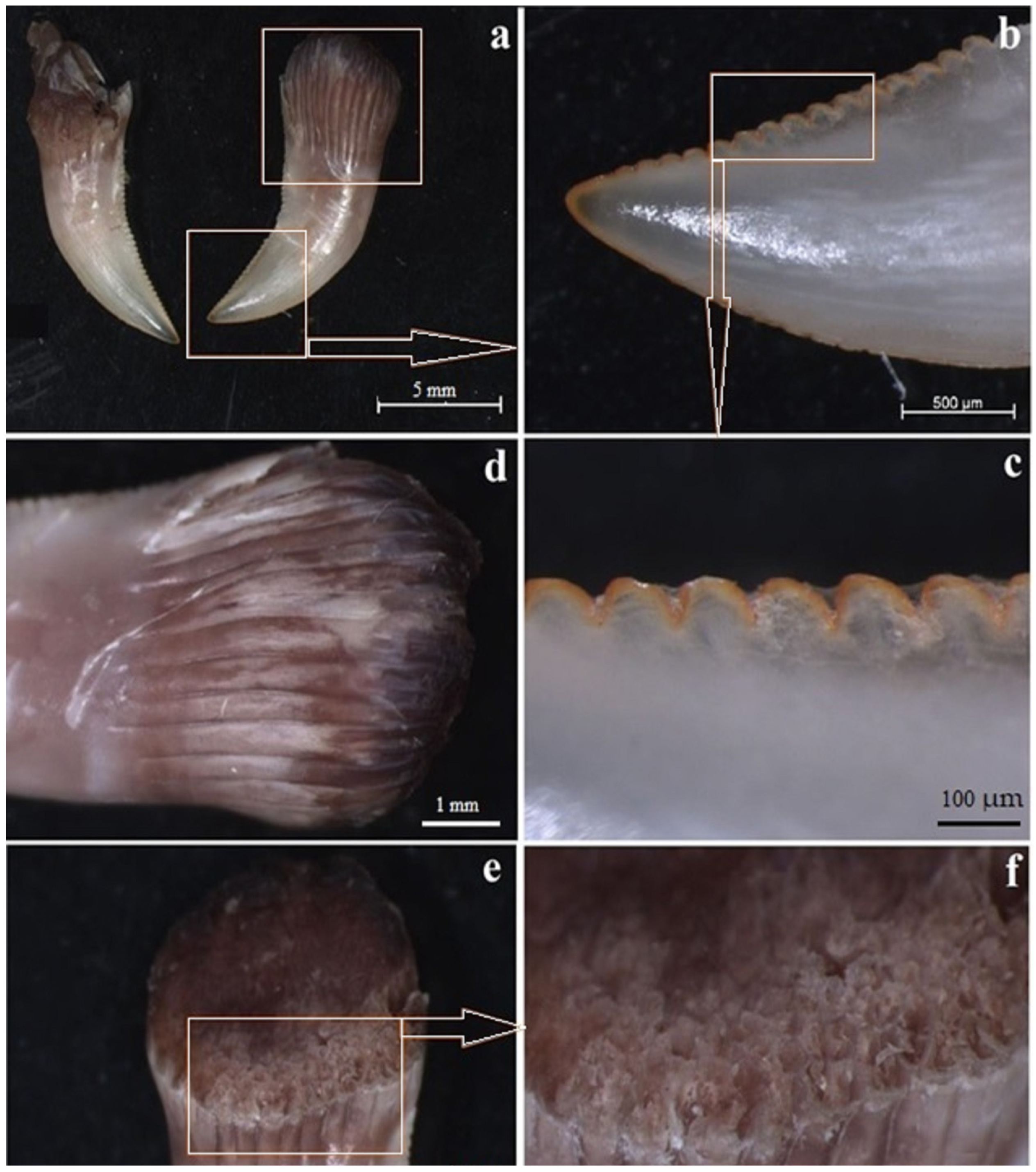
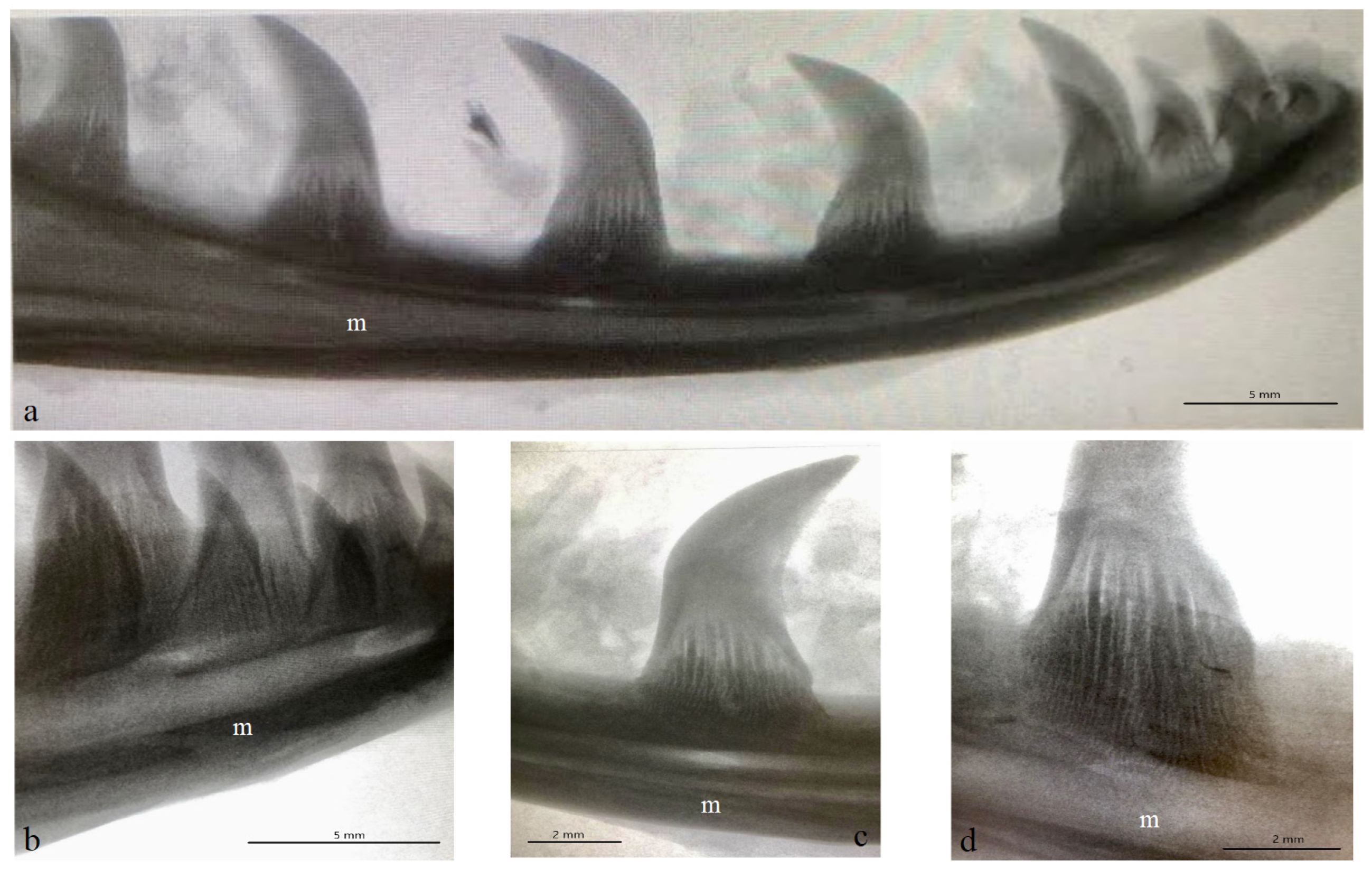
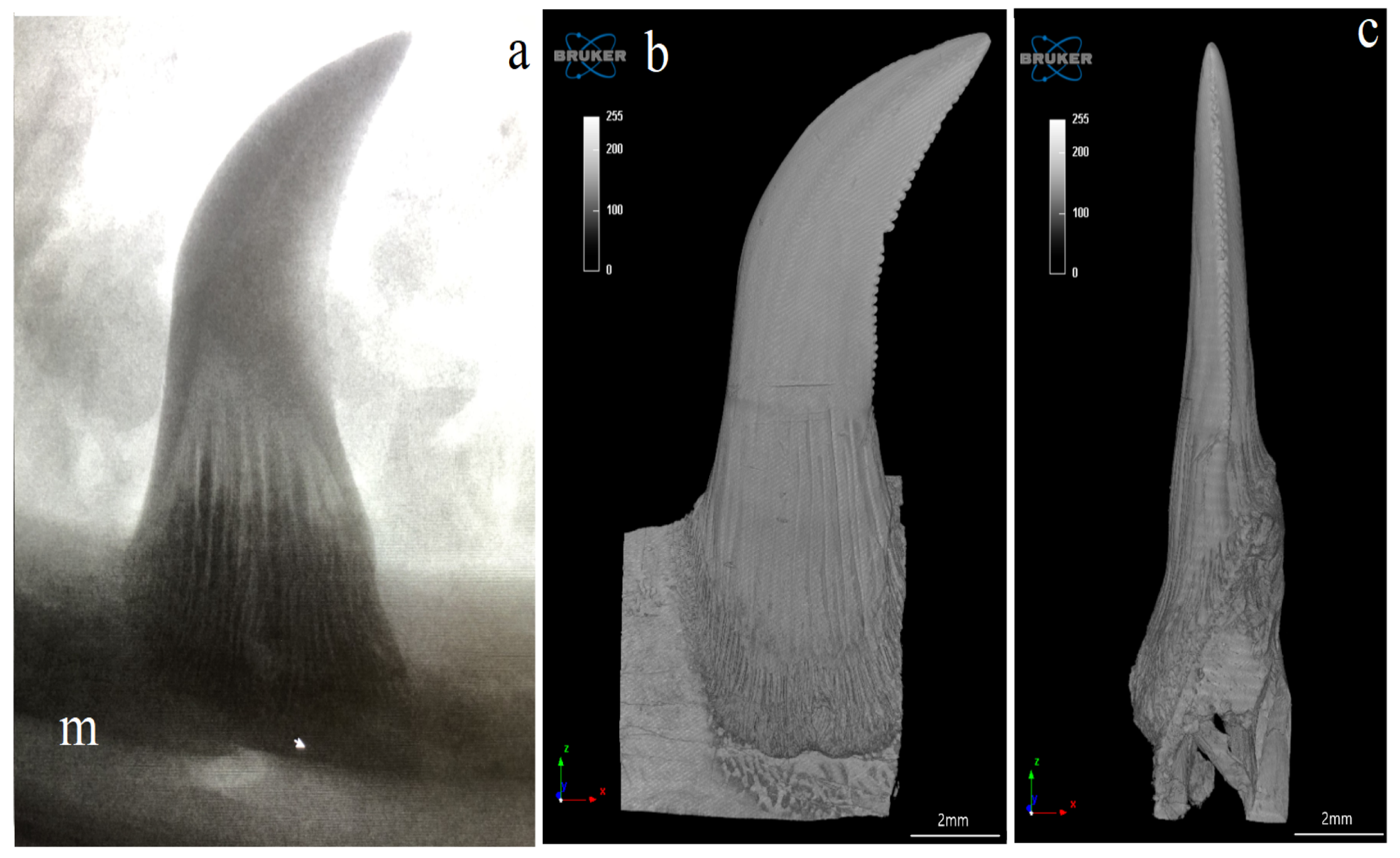
3.1. Macroscopic Analysis and X-ray Analysis
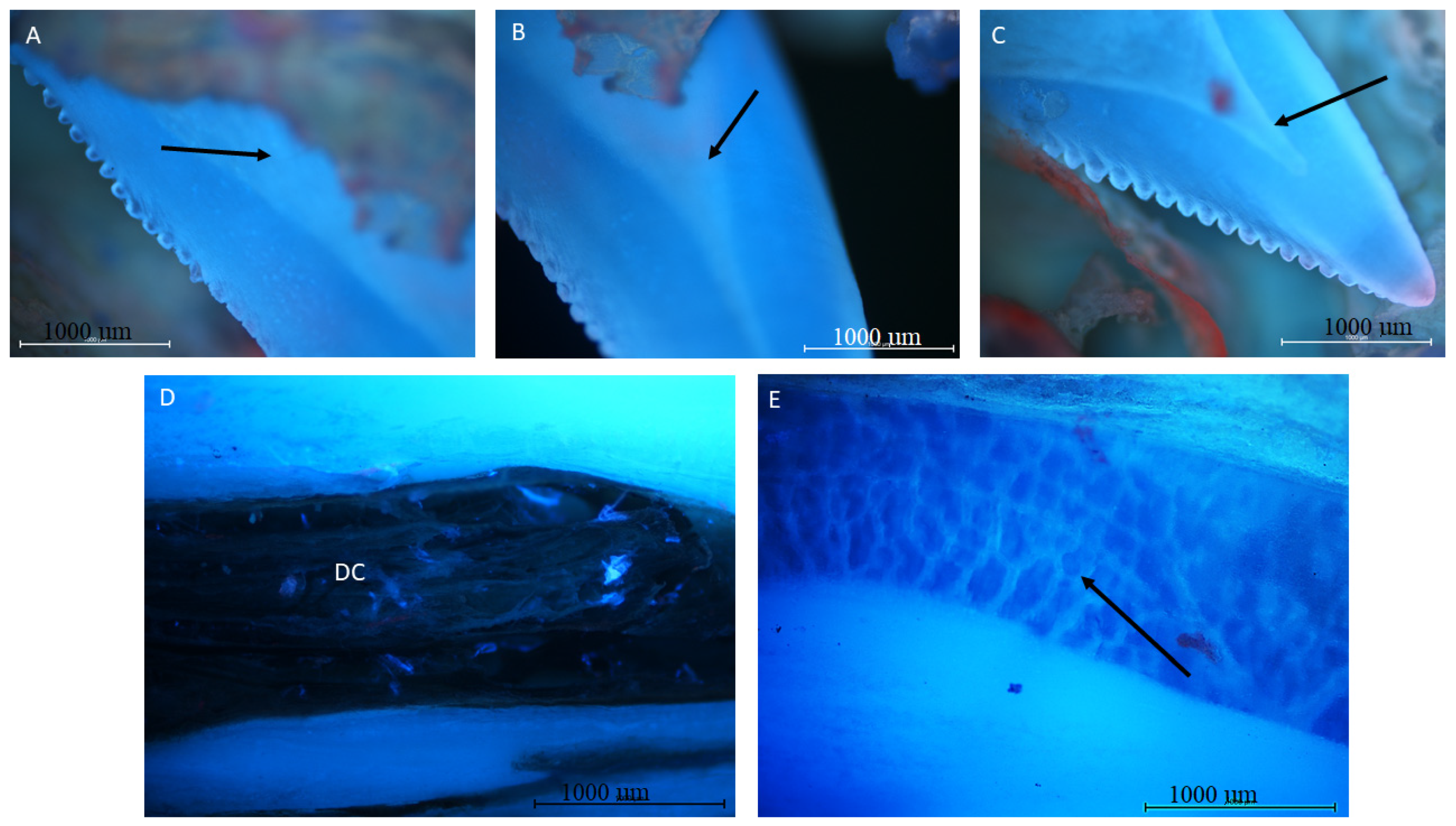
3.2. Autofluorescence
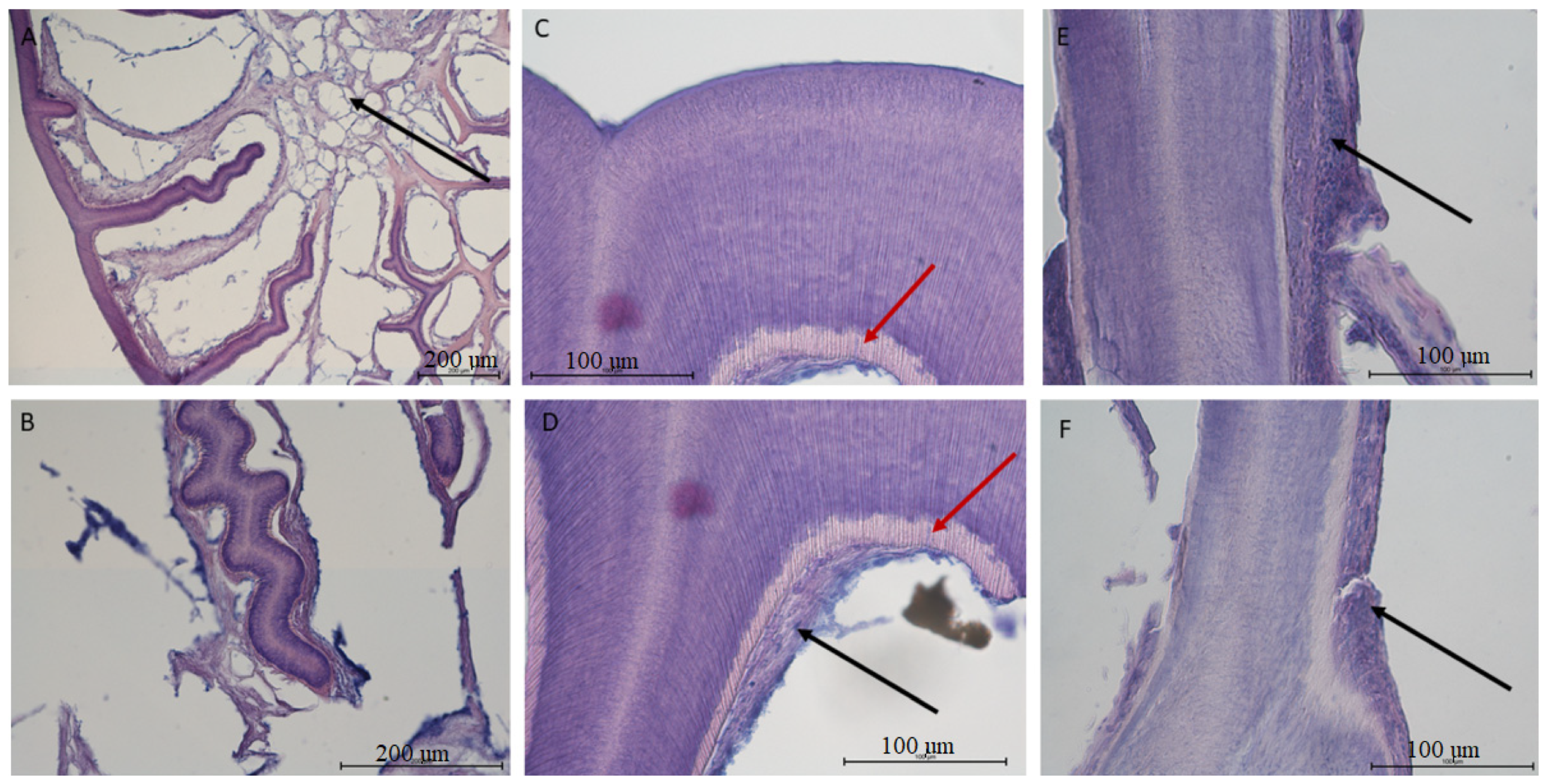
3.3. Histological Analysis
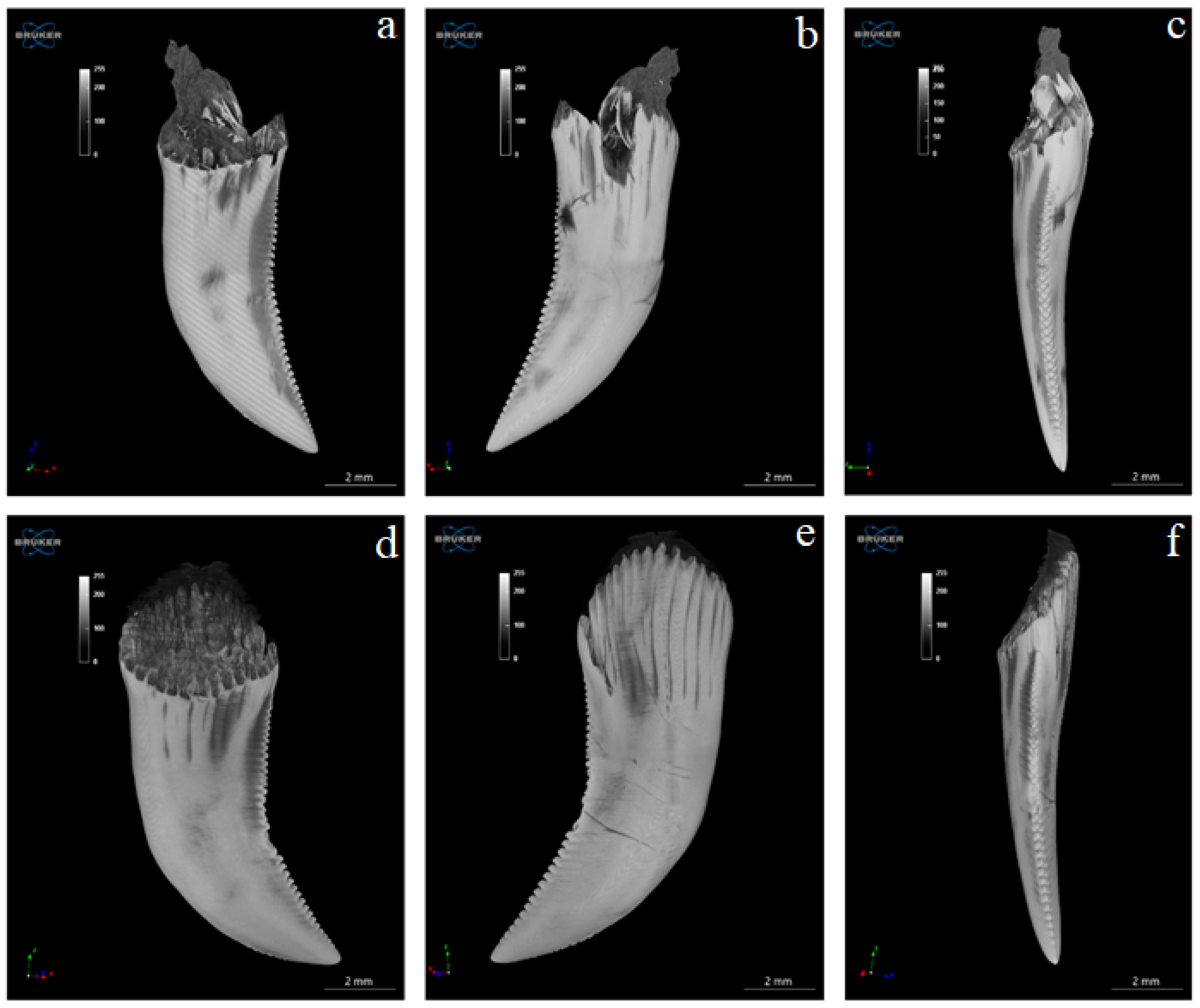
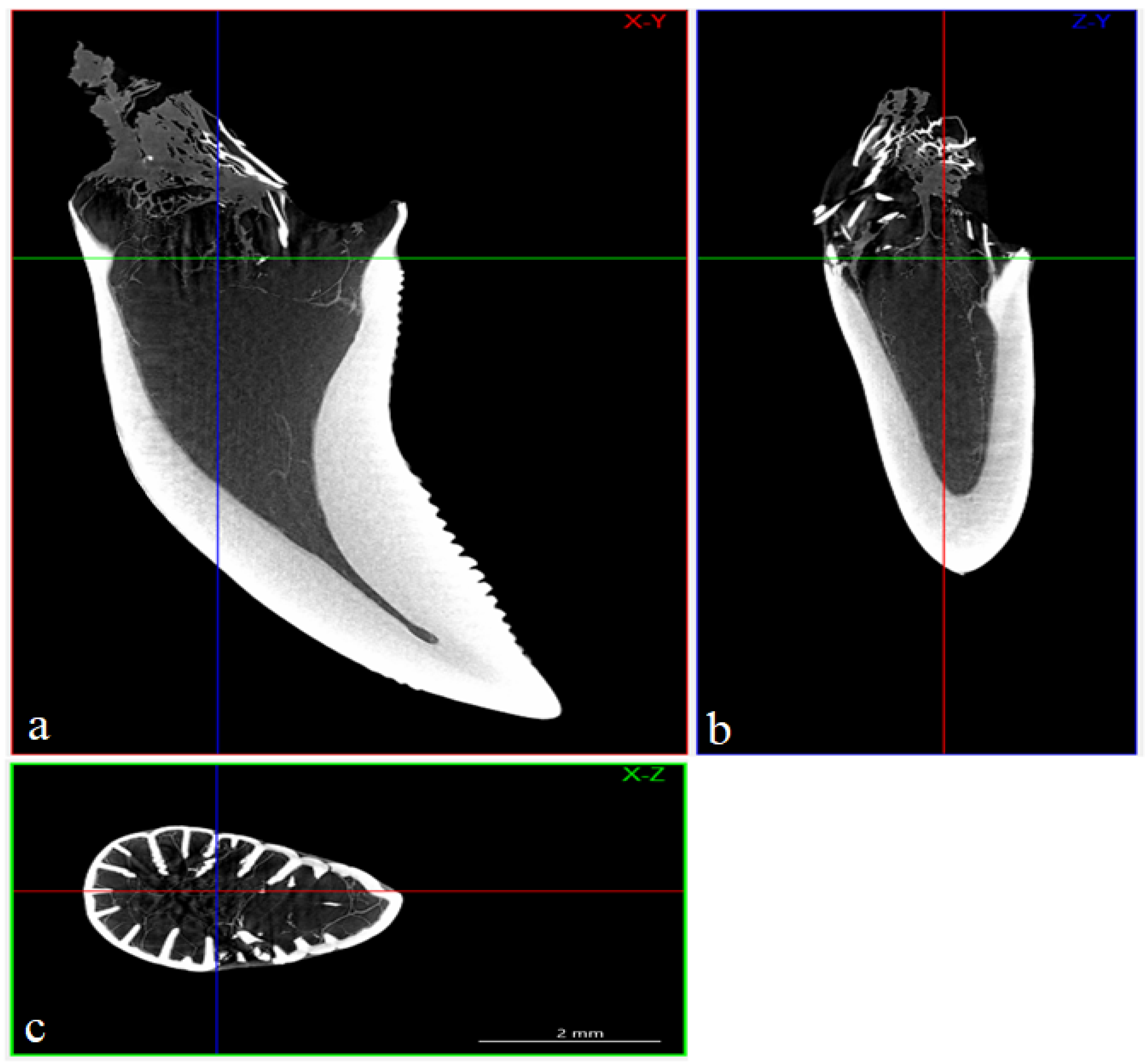
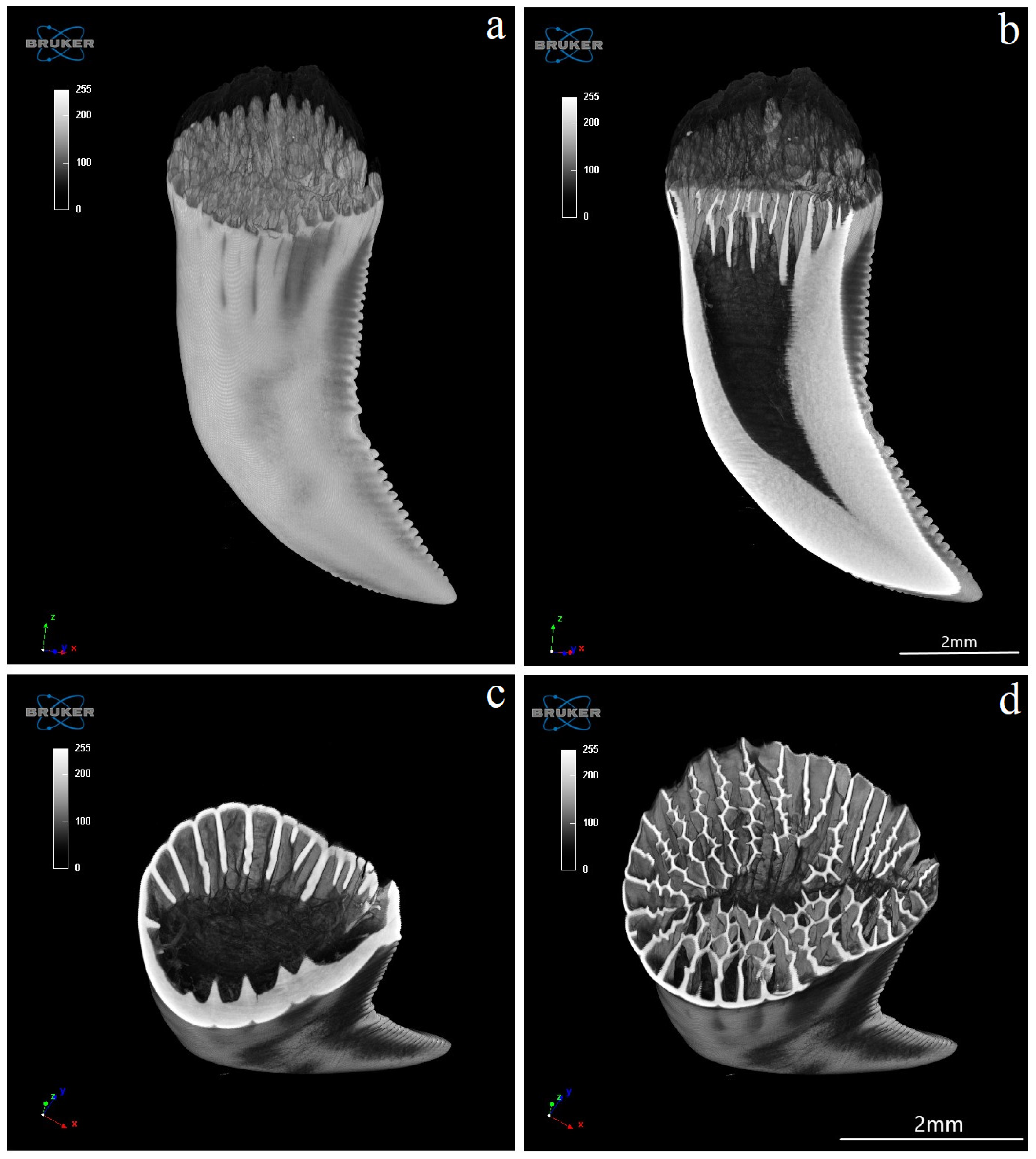
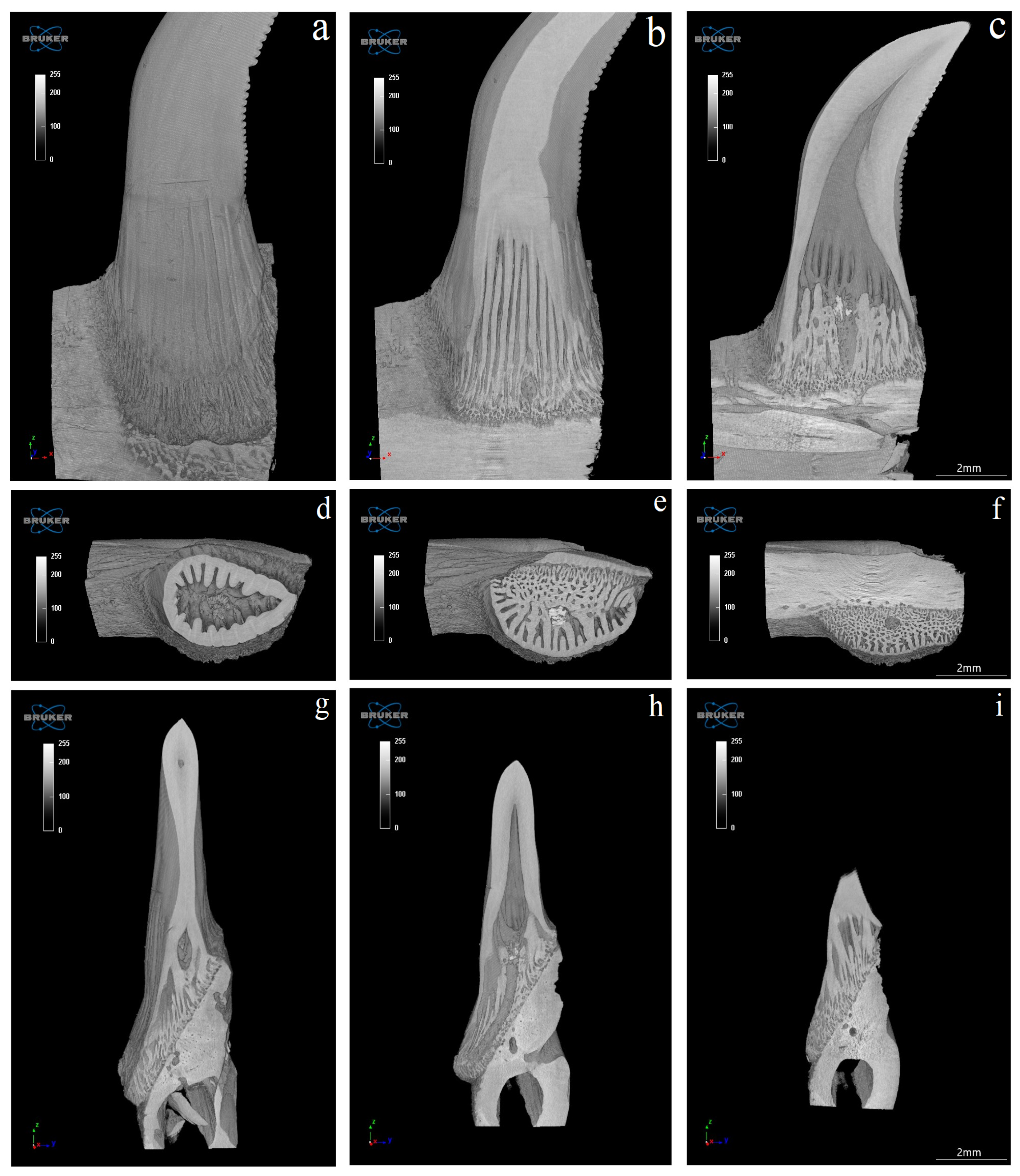
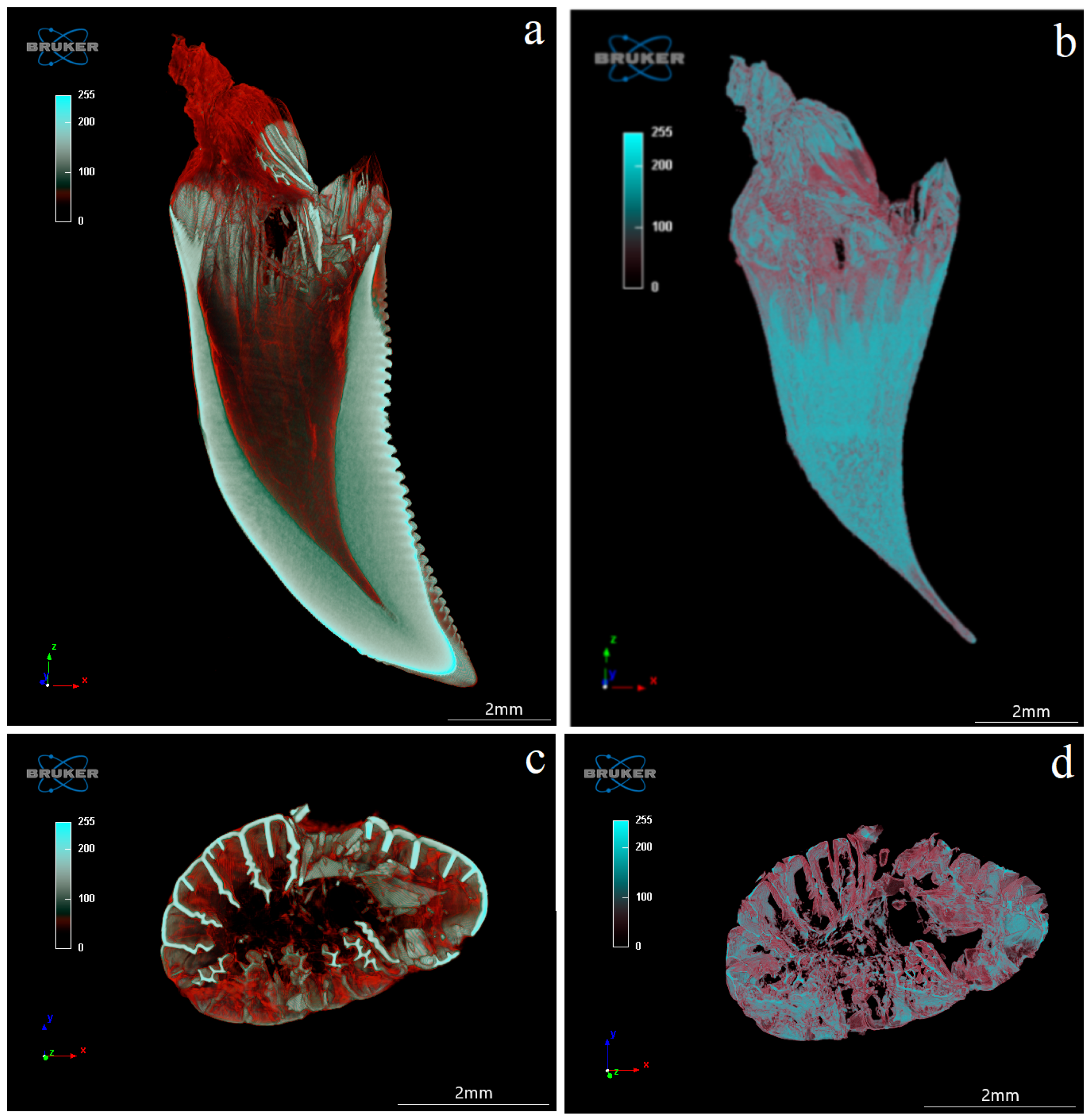
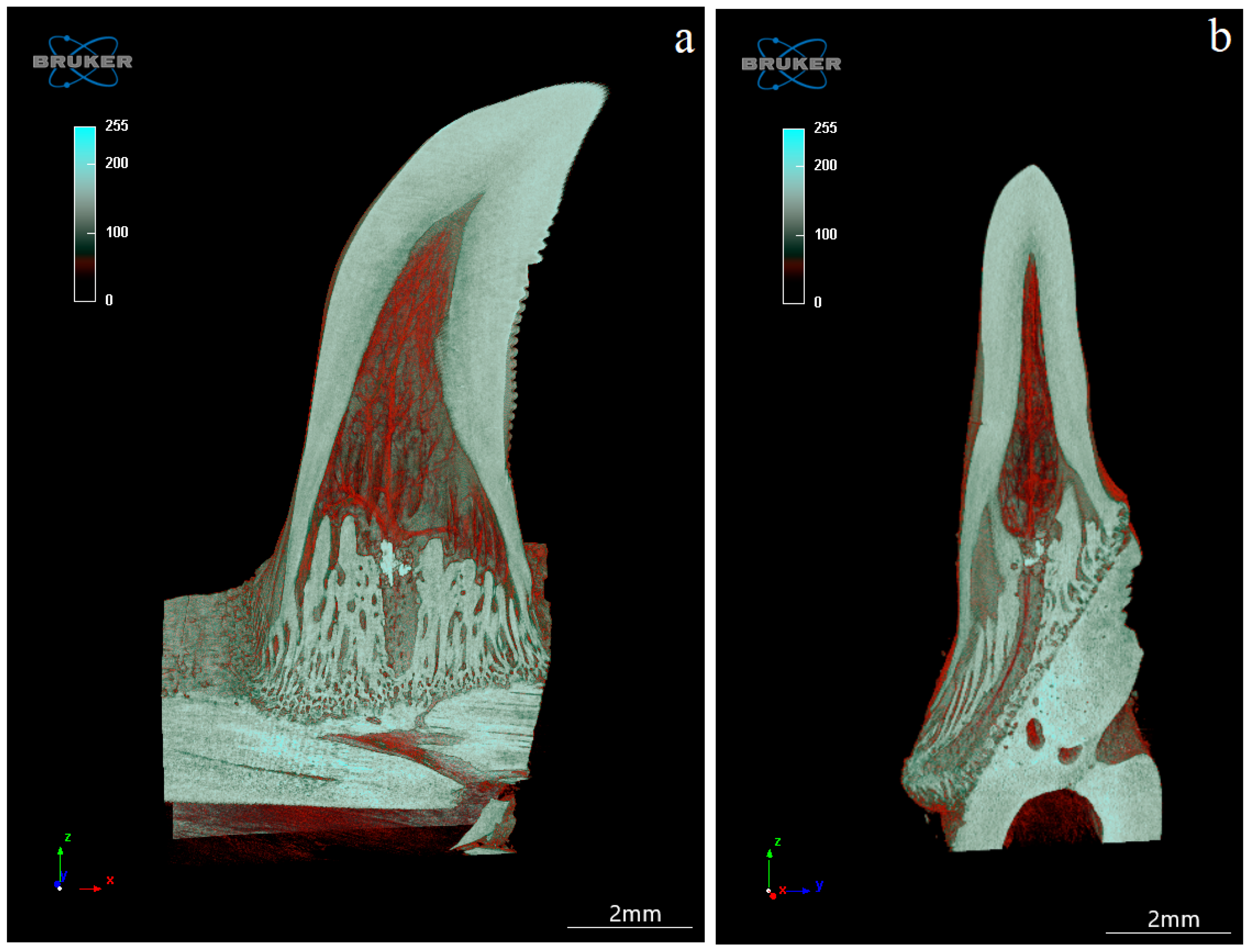
3.4. Computed Microtomography
4. Discussion
5. Conclusions
- -
- all the teeth are laterally flattened along their entire length and their crows are hooked caudally;
- -
- the distal margin of each tooth is serrated, apart from the base area;
- -
- the teeth are surrounded by cuffs with visible grooves around the teeth where the oral mucosa is, containing the opening of the venom gland;
- -
- histologically, the teeth of V. komodoensis show great heterogeneity in their structure, and plicidentine produces numerous secondary lamellae.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zahradnicek, O.; Buchtova, M.; Dosedelova, H.; Tucker, A.S. The development of complex tooth shape in reptiles. Front. Physiol. 2014, 5, 74. [Google Scholar] [CrossRef] [PubMed]
- Ungar, P.S. Mammal Teeth: Origin, Evolution, and Diversity; Johns Hopkins University Press: Baltimore, MD, USA, 2010. [Google Scholar]
- Benton, M.J. Vertebrate Palaeontology, 3rd ed.; Blackwell: Oxford, UK, 2005; p. 455. [Google Scholar]
- Prasad, G.V.R.; De Broin, F.L. Late Cretaceous crocodile remains from Naskal (India): Comparisons and biogeographic affinities. Ann. Paleontol. 2002, 88, 19–71. [Google Scholar] [CrossRef]
- D’Amore, D.C. Illustrating ontogenetic change in the dentition of the Nile monitor lizard, Varanus niloticus: A case study in the application of geometric morphometric methods for the quantification of shape–size heterodonty. J. Anat. 2015, 226, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K. Dental homologies in lamniform sharks (Chondrichthyes: Elasmobranchii). J. Morphol. 2002, 251, 38–72. [Google Scholar] [CrossRef] [PubMed]
- King, D.; Green, B. Goannas: The Biology of Varanid Lizards, 2nd ed.; NSW University Press: Sydney, NSW, USA, 1999. [Google Scholar]
- Cioffi, C. Conservation genetics of the Komodo dragon. In Komodo Dragons: Biology and Conservation; Zoo and Aquarium Biology and Conservation Series; Murphy, J., Ciofi, C., de La Panouse, C., Walsh, T., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2002; pp. 129–164. [Google Scholar]
- Purwandana, D.; Ariefiandy, A.; Imansyah, M.J.; Rudiharto, H.; Seno, A.; Ciofi, C.; Fordham, D.A.; Tim, S.; Jessop, T.S. Demographic status of Komodo dragons populations in Komodo National Park. Biol. Conserv. 2014, 171, 29–35. [Google Scholar] [CrossRef]
- Cioffi, C. Komodo Dragon. Sci. Am. 1999, 9, 84–91. [Google Scholar] [CrossRef]
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, S.F.; Kuruppu, S.; Fung, K.; Hedges, S.B.; Richardson, M.K.; et al. Early evolution of the venom system in lizards and snakes. Nature 2006, 439, 584–588. [Google Scholar] [CrossRef] [PubMed]
- D’Amore, D.C.; Blumenschine, R.J. Komodo monitor (Varanus komodoensis) feeding behavior and dental function reflected through tooth marks on bone surfaces, and the application to ziphodont paleobiology. Paleobiology 2009, 35, 525–552. [Google Scholar] [CrossRef]
- Auffenberg, W. Behavioral Ecology of the Komodo Monitor; University Press Florida: Gainesville, FL, USA, 1981. [Google Scholar]
- Montgomery, J.M.; Gillespie, D.; Sastrawan, P.; Fredeking, T.M.; Stewart, G.L. Aerobic salivary bacteria in wild and captive Komodo dragons. J. Wildl. Dis. 2002, 38, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G.; Wroe, S.; Teeuwisse, W.; van Osch, M.J.; Moreno, K.; Ingle, J.; McHenry, C.; Ferrara, T.; Clausen, P.; Scheib, H.; et al. A central role for venom in predation by Varanus komodoensis (Komodo Dragon) and the extinct giant Varanus (Megalania) priscus. Proc. Natl. Acad. Sci. USA 2009, 106, 8969–8974. [Google Scholar] [CrossRef] [PubMed]
- Uetz, P.; Freed, P.; Hošek, J. (Eds.) The Reptile Database. 2020. Available online: http://www.reptile-database.org (accessed on 27 February 2020).
- Langston, W., Jr. Ziphodont crocodiles: Pristichampsus vorax (Troxell), a new combination, from Eocene of North America. Fieldiana 1975, 33, 291–314. [Google Scholar]
- McCurry, M.R.; Mahony, M.; Clausen, P.D.; Quayle, M.R.; Walmsley, C.W.; Jessop, T.S.; Wroe, S.; Richards, H.; McHenry, C.R. The relationship between cranial structure, biomechanical performance and ecological diversity in varanoid lizards. PLoS ONE 2015, 10, e0130625. [Google Scholar] [CrossRef] [PubMed]
- McHenry, C.R.; Wroe, S.; Clausen, P.D.; Moreno, K.; Cunningham, E. Supermodeled sabercat, predatory behavior in Smilodon fatalis revealed by high-resolution 3D computer simulation. Proc. Natl. Acad. Sci. USA 2007, 104, 16010–16015. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, C.; De Boer, M. Distribution and conservation of the Komodo monitor (Varanus komodoensis). Herpetol. J. 2004, 14, 99–107. [Google Scholar]
- Jessop, T.S.; Madsen, T.; Ciofi, C.; Imansyah, M.J.; Purwandana, D.; Rudiharto, H.; Arifiandy, A.; Philips, J.A. Island differences in population size structure and catch per unit effort and their conservation implications for Komodo dragons. Biol. Conserv. 2007, 135, 247–255. [Google Scholar] [CrossRef]
- Imron, M.A.; Satria, R.A.; Ramlan, M.F.P. Komodo dragon predation on crab-eating macaques at the Rinca Island's Visitor Centre, Indonesia. Folia Primatol. 2018, 89, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Ariefiandy, A.; Purwandana, D.; Coulson, G.; Forsyth, D.M.; Jessop, T.S. Monitoring the ungulate prey of the Komodo dragon Varanus komodoensis: Distance sampling or faecal counts? Wildl. Biol. 2013, 19, 126–137. [Google Scholar] [CrossRef]
- Moreno, K.; Wroe, S.; Clausen, P.; McHenry, C.; D’Amore, D.C.; Rayfield, E.J.; Cunningham, E. Cranial performance in the Komodo dragon (Varanus komodoensis) as revealed by high-resolution 3-D finite element analysis. J. Anat. 2008, 212, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.; Rieppel, O. An investigation into the occurrence of plicidentine in the teeth of squamate reptiles. Copeia 2006, 3, 337–350. [Google Scholar] [CrossRef]
- Estes, R.D.; De Queiroz, K.; Gauthier, J. Phylogenetic relationships within Squamata. In Phylogenetic Relationships in Lizard Families; Estes, R., Pregill, G., Eds.; Stanford University Press: Stanford, CA, USA, 1988; pp. 119–281. [Google Scholar]
- Enax, J.; Fabritius, H.O.; Rack, A.; Prymak, O.; Raabe, D.; Epple, M. Characterization of crocodile teeth: Correlation of composition, microstructure, and hardness. J. Struct. Biol. 2013, 184, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Sander, P.M. Prismless enamel in amniotes: Terminology, function, and evolution. In Development, Function and Evolution of Teeth; Teaford, M.F., Smith, M.M., Ferguson, M.W.J., Eds.; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janeczek, M.; Goździewska-Harłajczuk, K.; Hrabska, L.; Klećkowska-Nawrot, J.; Kuropka, P.; Dobrzyński, M.; Melnyk, O.; Nikodem, A. Macroanatomical, Histological and Microtomographic Study of the Teeth of the Komodo Dragon (Varanus komodoensis)—Adaptation to Hunting. Biology 2023, 12, 247. https://doi.org/10.3390/biology12020247
Janeczek M, Goździewska-Harłajczuk K, Hrabska L, Klećkowska-Nawrot J, Kuropka P, Dobrzyński M, Melnyk O, Nikodem A. Macroanatomical, Histological and Microtomographic Study of the Teeth of the Komodo Dragon (Varanus komodoensis)—Adaptation to Hunting. Biology. 2023; 12(2):247. https://doi.org/10.3390/biology12020247
Chicago/Turabian StyleJaneczek, Maciej, Karolina Goździewska-Harłajczuk, Ludwika Hrabska, Joanna Klećkowska-Nawrot, Piotr Kuropka, Maciej Dobrzyński, Oleksii Melnyk, and Anna Nikodem. 2023. "Macroanatomical, Histological and Microtomographic Study of the Teeth of the Komodo Dragon (Varanus komodoensis)—Adaptation to Hunting" Biology 12, no. 2: 247. https://doi.org/10.3390/biology12020247
APA StyleJaneczek, M., Goździewska-Harłajczuk, K., Hrabska, L., Klećkowska-Nawrot, J., Kuropka, P., Dobrzyński, M., Melnyk, O., & Nikodem, A. (2023). Macroanatomical, Histological and Microtomographic Study of the Teeth of the Komodo Dragon (Varanus komodoensis)—Adaptation to Hunting. Biology, 12(2), 247. https://doi.org/10.3390/biology12020247









