Comparative Material and Mechanical Properties among Cicada Mouthparts: Cuticle Enhanced with Inorganic Elements Facilitates Piercing through Woody Stems for Feeding
Simple Summary
Abstract
1. Introduction
- The presence and accumulation of inorganic elements will vary based on the mouthpart structure, the location on the structure and by cicada species.
- Mouthpart regions with transition metals will be harder and have higher elastic modulus.
- Correlations between elements might exist, showing patterns of colocalization.
2. Materials and Methods
2.1. Species
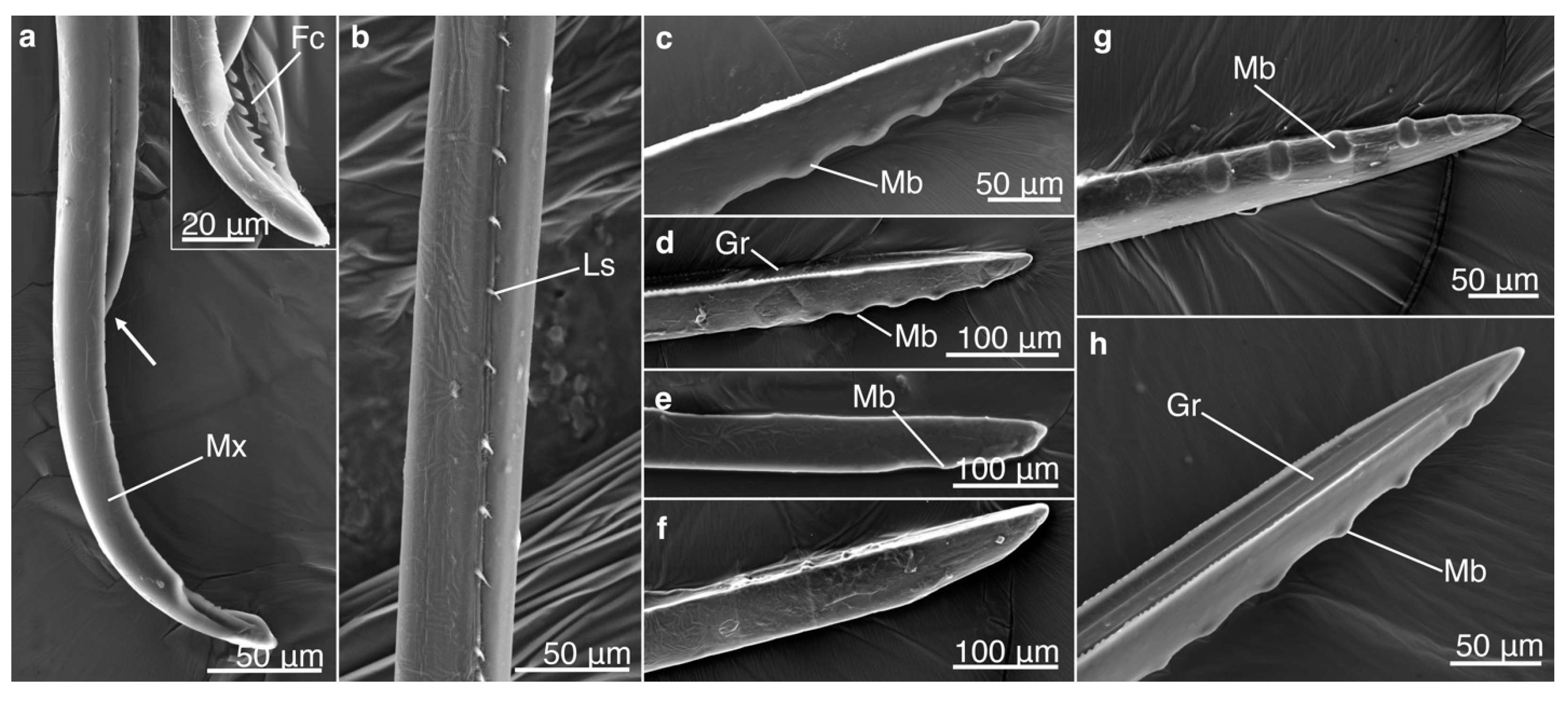
2.2. Mouthpart Morphology
2.3. Elemental Composition with Energy-Dispersive X-ray Spectroscopy
2.4. Hardness and Elastic Modulus Measurements
2.5. Statistics
3. Results
3.1. Mouthpart Morphology
3.1.1. Differences in Lengths and Widths of Mouthpart Structures among Species
3.1.2. Tip Morphology of Mandibles and Maxillae
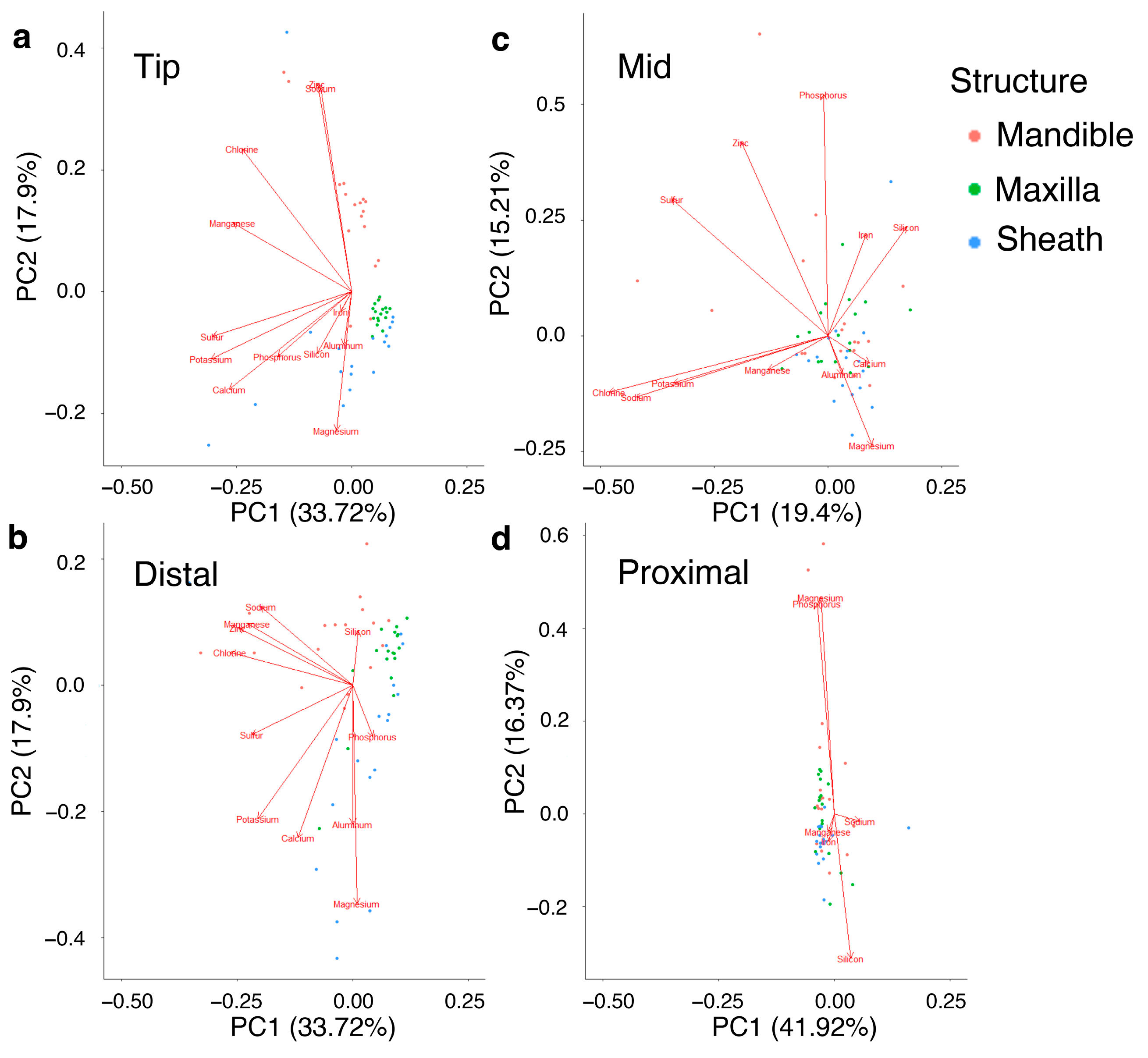
3.2. Inorganic Elements by Mouthpart Structure, Location and Cicada Species
3.2.1. Organic Nonmetals: Carbon, Oxygen
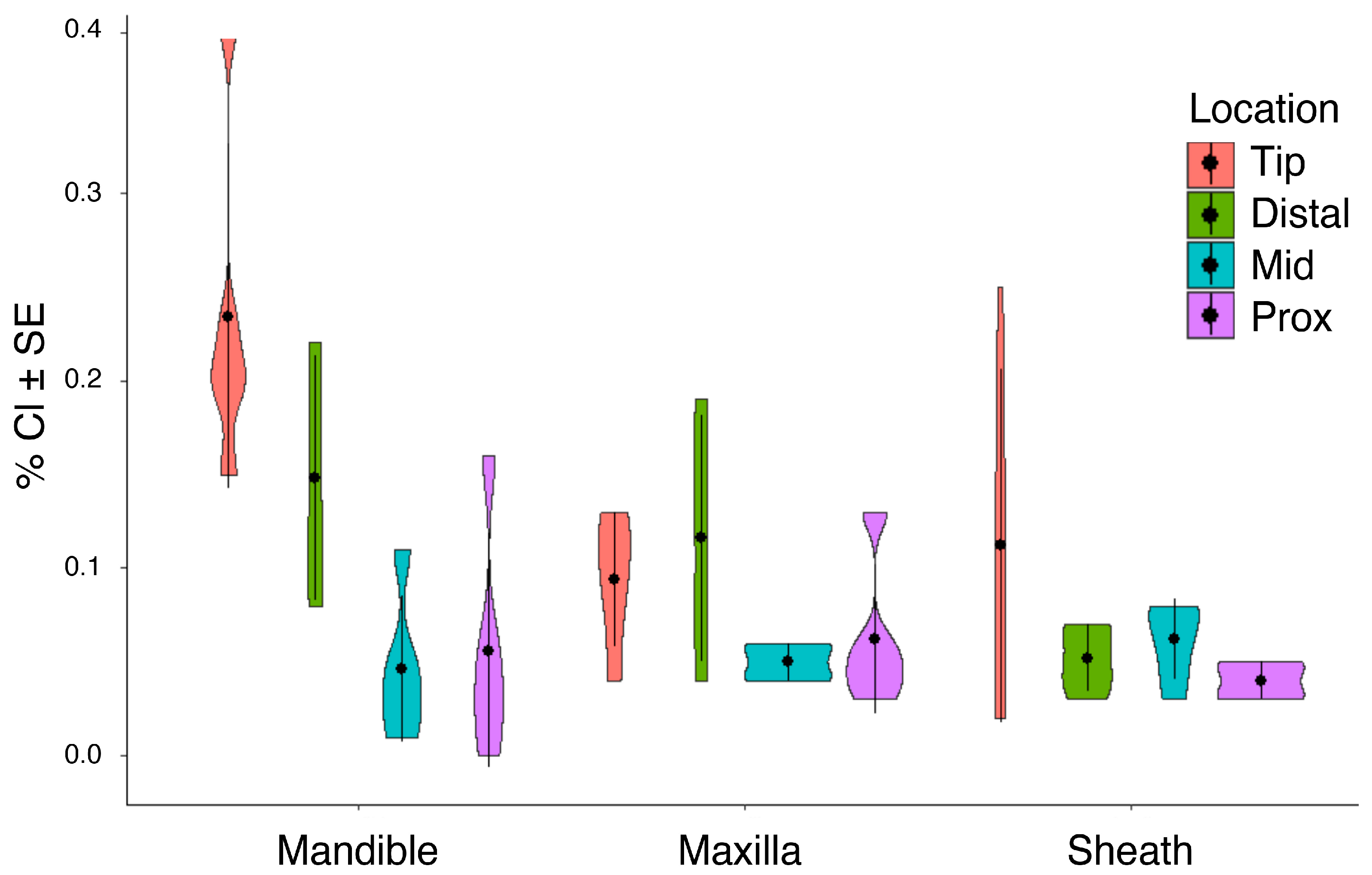
3.2.2. Alkali Metal, Halogens and Non-Metals: Chlorine, Potassium, Sodium, Sulfur, Phosphorus
3.2.3. Alkaline Earth Metals: Calcium, Magnesium
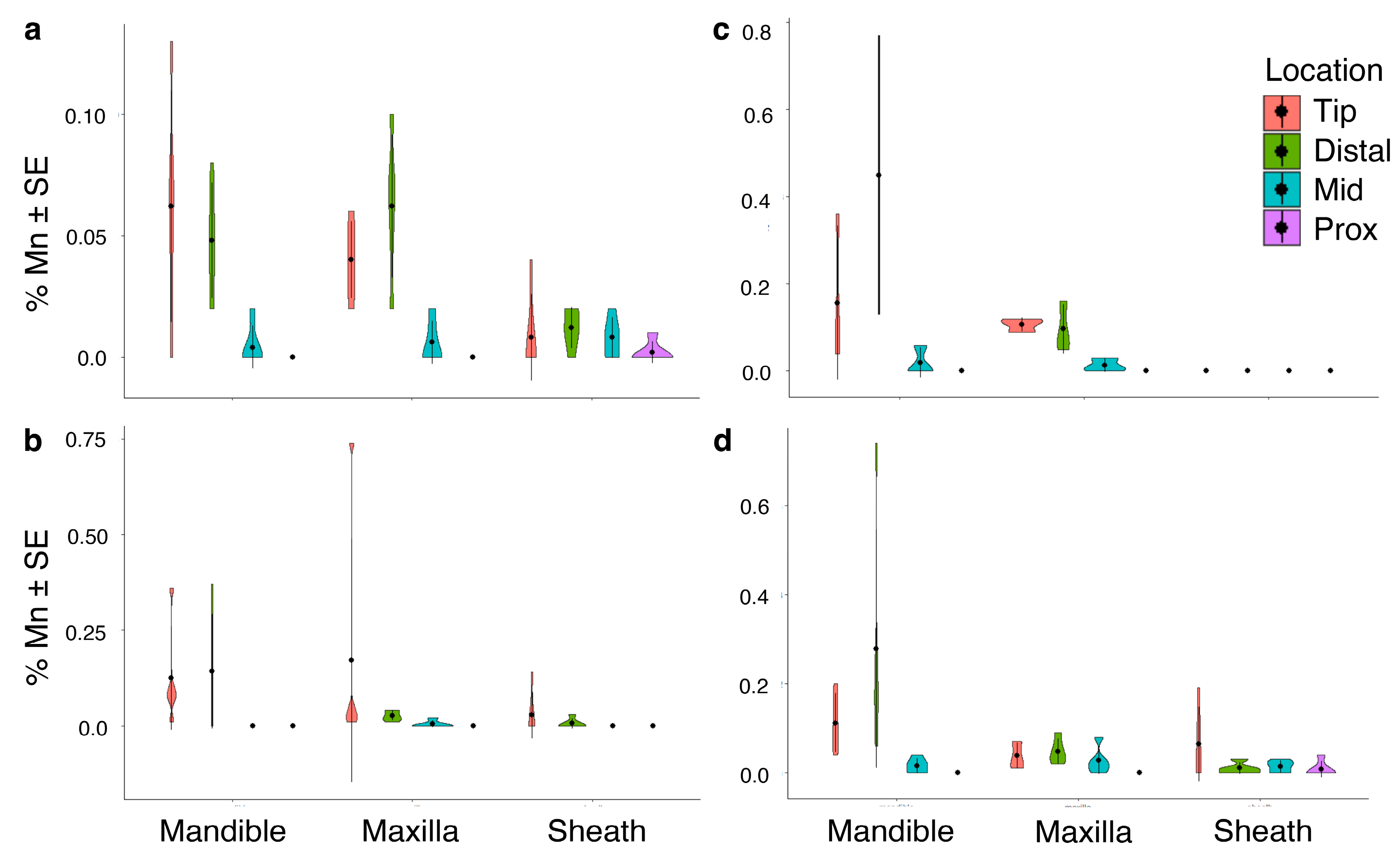
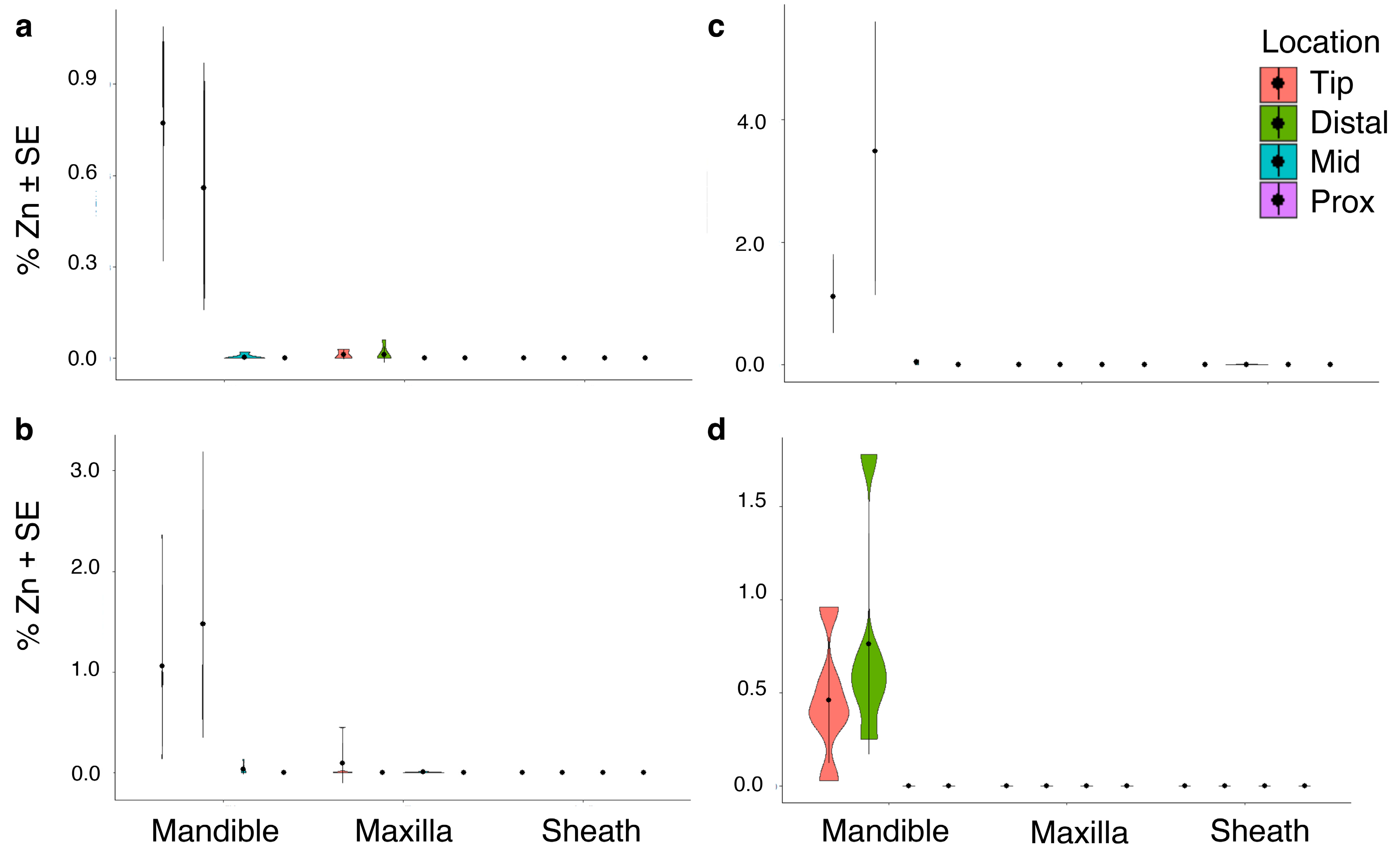
3.2.4. Transition, Post-Transition Metals and Metalloids: Aluminum, Iron, Manganese, Silicon, Zinc
3.2.5. Correlations between Organic and Inorganic Elements by Mouthpart Structure
3.2.6. Correlations between Organic and Inorganic Elements by Species
3.2.7. Generalized Patterns of Metal Bioaccumulation in Cicada Mouthparts and Locations on the Mouthparts
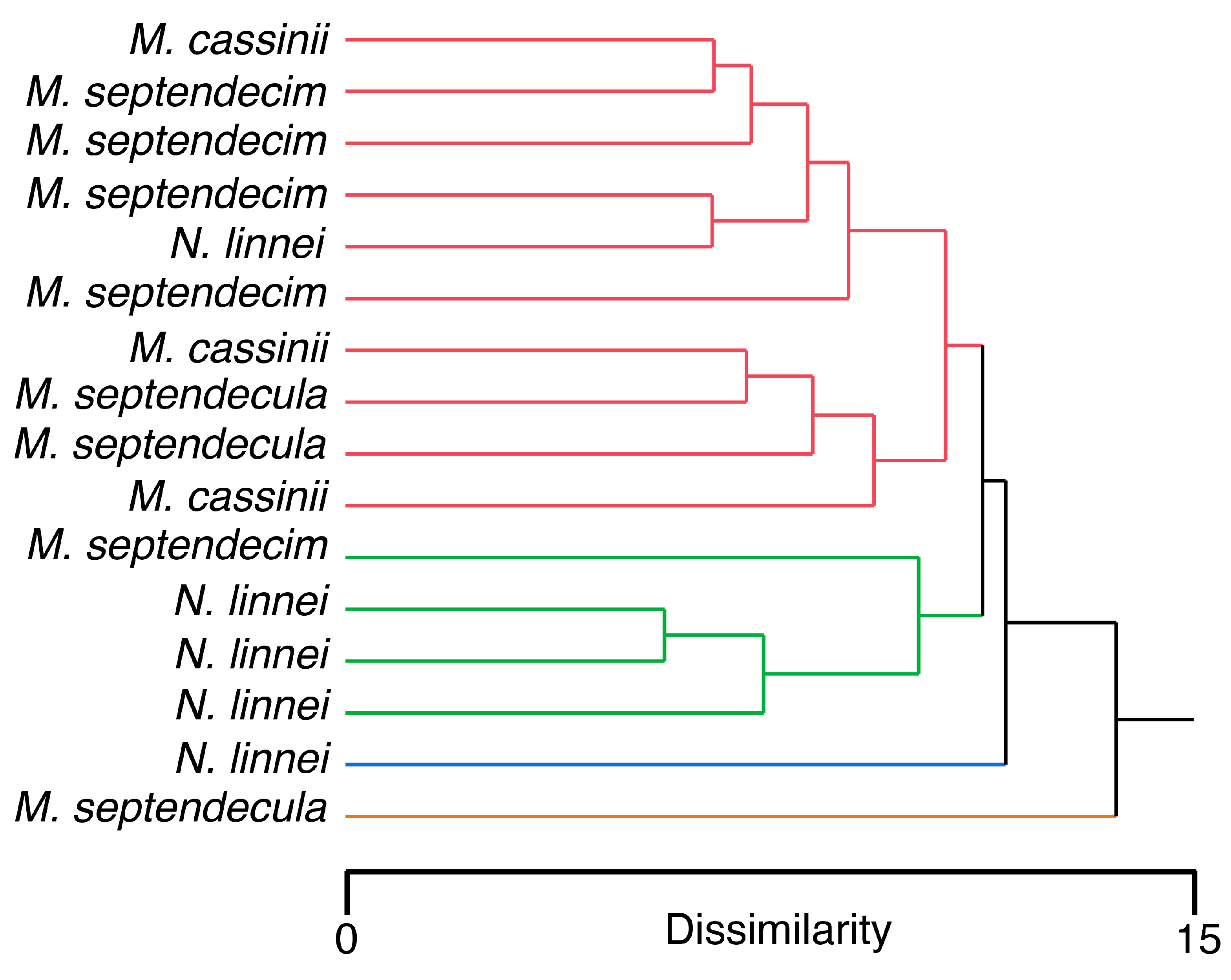
3.3. Patterns in Cicada Grouping by Morphological Measurements and EDS Results
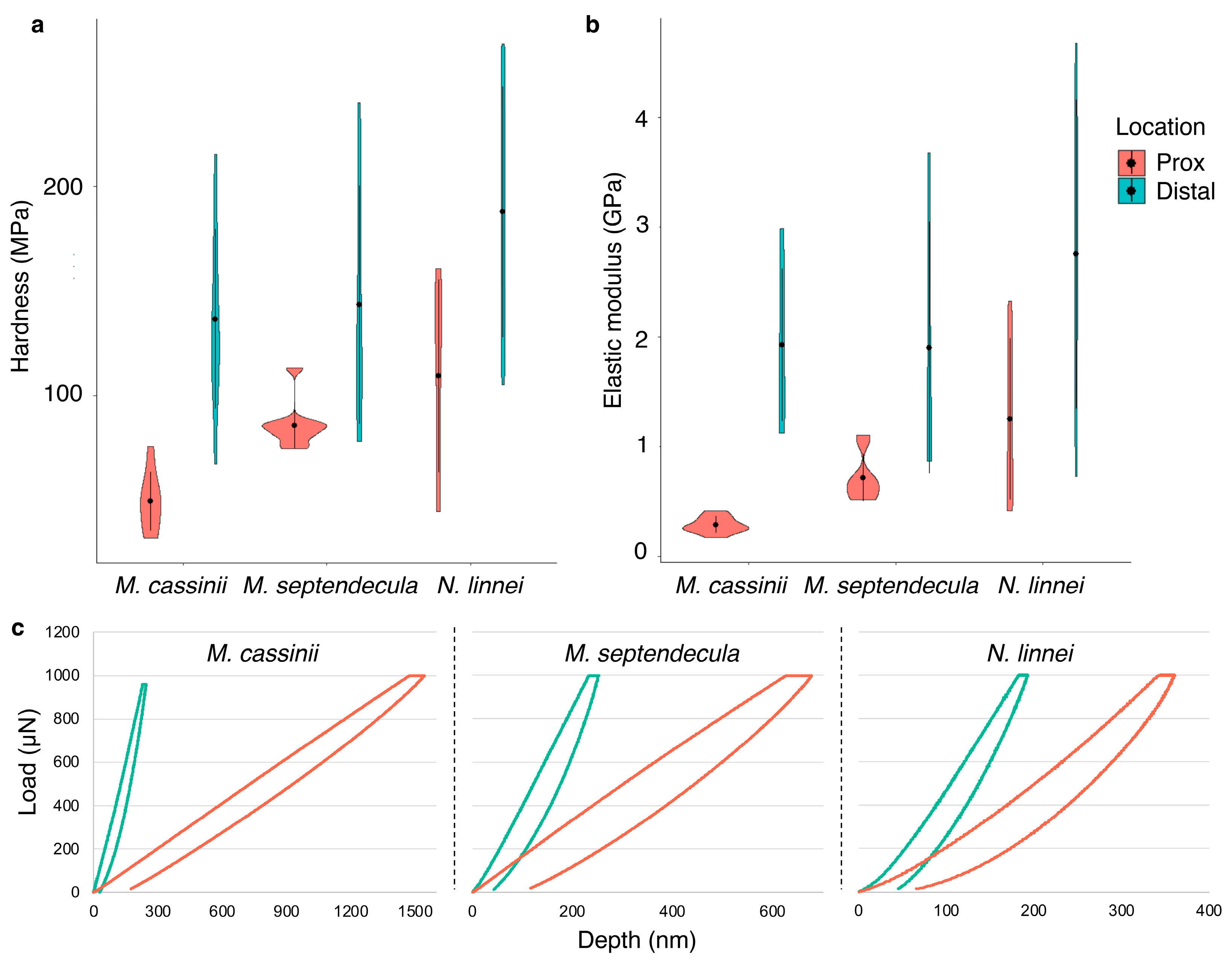
3.4. Hardness and Elastic Modulus
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: New York, NY, USA, 2005. [Google Scholar]
- Wiens, J.J.; Lapoint, R.T.; Whiteman, N.K. Herbivory increases diversification across insect clades. Nat. Commun. 2015, 6, 8370. [Google Scholar] [CrossRef] [PubMed]
- Nel, P.; Bertrand, S.; Nel, A. Diversification of insects since the Devonian: A new approach based on morphological disparity of mouthparts. Sci. Rep. 2018, 8, 3516. [Google Scholar] [CrossRef] [PubMed]
- Labandeira, C.C. Insect Mouthparts: Ascertaining the Paleobiology of Insect Feeding Strategies. Annu. Rev. Ecol. Syst. 1997, 28, 153–193. [Google Scholar] [CrossRef]
- Blanke, A.; Rühr, P.T.; Mokso, R.; Villanueva, P.; Wilde, F.; Stampanoni, M.; Uesugi, K.; Machida, R.; Misof, B. Structural mouthpart interaction evolved already in the earliest lineages of insects. Proc. R. Soc. B 2015, 282, 20151033. [Google Scholar] [CrossRef]
- Krenn, H.W. Insect Mouthparts: Form, Function, Development and Performance; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Lehnert, M.S.; Bennett, A.; Reiter, K.E.; Gerard, P.D.; Wei, Q.-H.; Byler, M.; Yan, H.; Lee, W.-K. Mouthpart conduit sizes of fluid-feeding insects determine the ability to feed from pores. Proc. R. Soc. B. 2017, 284, 20162026. [Google Scholar] [CrossRef]
- Schofield, R.M.S.; Nesson, M.H.; Richardson, K.A. Tooth hardness increases with zinc-content in mandibles of young adult leaf-cutter ants. Naturwissenschaften 2002, 89, 579–583. [Google Scholar] [CrossRef]
- Cribb, B.W.; Stewart, A.; Huang, H.; Truss, R.; Noller, B.; Rasch, R.; Zalucki, M.P. Insect mandibles—Comparative mechanical properties and links with metal incorporation. Naturwissenschaften 2008, 95, 17–23. [Google Scholar] [CrossRef]
- Vincent, J.F.V.; Wegst, U.G.K. Design and mechanical properties of insect cuticle. Arthropod Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef]
- Lehnert, M.S.; Tarver, L.A.; Feng, J. Material properties and morphology of prestomal teeth in relation to the feeding habits of Diptera (Brachycera). Insects 2022, 22, 207. [Google Scholar] [CrossRef]
- Edwards, A.J.; Fawke, J.D.; McClements, J.G.; Smith, S.A.; Wyeth, P. Correlation of zinc distribution and enhanced hardness in the mandibular cuticle of the leaf-cutting ant Atta sexdens rubropilosa. Cell Biol. Int. 1993, 17, 697–698. [Google Scholar] [CrossRef]
- Hillerton, J.E.; Robertson, B.; Vincent, J.F.V. The presence of zinc or manganese as the predominant metal in the mandibles of adult, stored-product beetles. J. Stored Prod. Res. 1984, 20, 133–137. [Google Scholar] [CrossRef]
- Cribb, B.W.; Lin, C.-L.; Rintoul, L.; Rasch, R.; Hasenpusch, J.; Huang, H. Hardness in arthropod exoskeletons in the absence of transition metals. Acta Biomater. 2010, 6, 3152–3156. [Google Scholar] [CrossRef] [PubMed]
- Fawke, J.D.; McClements, J.G.; Wyeth, P. Cuticular metals-quantification and mapping by complementary techniques. Cell Biol. Int. 1997, 21, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Kagemori, N.; Sugiyama, J.; Kawai, S.; Sera, K.; Futatsugawa, S.; Yukawa, M.; Imazeki, H. Elemental analysis of worker mandibles of Coptotermes formosanus (Isoptera: Rhinotermitidae). Sociobiology 2005, 45, 255–259. [Google Scholar]
- Hillerton, J.E.; Vincent, J.F.V. The specific location of zinc in insect mandibles. J. Exp. Biol. 1982, 101, 333–336. [Google Scholar] [CrossRef]
- Lichtenegger, H.C.; Schöberl, T.; Ruokolainen, J.T.; Cross, J.O.; Heald, S.M.; Birkedal, H.; Waite, J.H.; Stucky, G.D. Zinc and mechanical prowess in jaws of Nereis, a marine worm. Proc. Natl. Acad. Sci. USA 2003, 100, 9144–9149. [Google Scholar] [CrossRef] [PubMed]
- Tadayon, M.; Younes-Metzler, O.; Shelef, Y.; Zaslansky, P.; Rechels, A.; Berner, A.; Zolotoyabko, E.; Barth, F.G.; Fratzl, P.; Bar-On, B.; et al. Adaptations for wear resistance and damage resilience: Micromechanics of spider cuticular “tools”. Adv. Funct. Mater. 2020, 30, 2000400. [Google Scholar] [CrossRef]
- Politi, Y.; Bertinetti, L.; Fratzl, P.; Barth, F.G. The spider cuticle: A remarkable material toolbox for functional diversity. Philos Trans. R Soc. A 2021, 379, 20200332. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, R.E. Principles of Insect Morphology; McGraw-Hill Publishing, Co.: New York, NY, USA, 1935. [Google Scholar]
- Cerkvenik, U.; Dodou, D.; van Leeuwen, J.L.; Gussekloo, S.W.S. Functional principles of steerable multi-element probes in insects. Biol. Rev. 2019, 94, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dai, W. How does the intricate mouthpart apparatus coordinate for feeding in the hemimetabolous insect pest Erthesina fullo? Insects 2020, 11, 503. [Google Scholar] [CrossRef]
- Fontaine, A.R.; Olsen, N.; Ring, R.A.; Singla, C.L. Cuticular metal hardening of mouthparts and claws of some forest insects of British Columbia. J. Entomol. Soc. B C 1991, 88, 45–55. [Google Scholar]
- Koch, K.G.; Chapman, K.; Louis, J.; Heng-Moss, T.; Sarath, G. Plant tolerance: A unique approach to control hemipteran pests. Front. Plant. Sci. 2016, 7, 01363. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.E. Hemipteran pests of sugarcane in North America. Insects 2019, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Rijal, J.P.; Joyce, A.L.; Gyawaly, S. Biology, ecology, and management of hemipteran pests in almond orchards in the United States. J. Integr. Pest. Manag. 2021, 12, 24. [Google Scholar] [CrossRef]
- Young, D.; Bennet-Clark, H.C. The role of the tymbal in cicada sound production. J. Exp. Bio. 1995, 198, 1001–1010. [Google Scholar] [CrossRef]
- Cooley, J.R.; Kritsky, G.; Edwards, M.J.; Zyla, J.D.; Marshall, D.C.; Hill, K.B.R.; Krauss, R.; Simon, C. The distribution of periodical cicada brood X in 2004. Am. Entomol. 2009, 55, 106–112. [Google Scholar]
- Kritsky, G. Periodical Cicadas: The Brood X Edition; Ohio Biological Survey: Columbus, OH, USA, 2021. [Google Scholar]
- Karban, R. Opposite density effects of nymphal and adult mortality for periodical cicadas. Ecology 1984, 65, 1656–1661. [Google Scholar] [CrossRef]
- Cook, W.M.; Holt, R.D.; Yao, J. Spatial variability in oviposition damage by periodical cicadas in a fragmented landscape. Oecologia 2001, 127, 51–61. [Google Scholar] [CrossRef]
- Ahern, R.G.; Frank, S.D.; Raupp, M.J. Comparison of exclusion and imidacloprid for reduction of oviposition damage to young trees by periodical cicadas (Hemiptera: Cicadidae). J. Econ. Entomol. 2005, 98, 2133–2136. [Google Scholar]
- Lehnert, M.S.; Reiter, K.E.; Smith, G.A.; Kritsky, G. An augmented wood-penetrating structure: Cicada ovipositors enhanced with metals and other inorganic elements. Sci. Rep. 2019, 9, 19731. [Google Scholar] [PubMed]
- Le Lannic, J.; Nénon, J.P. Functional morphology of the ovipositor in Megarhyssa atrata (Hymenoptera, Ichneumonidae) and its penetration into wood. Zoomorphology 1999, 119, 73–79. [Google Scholar] [CrossRef]
- Polidori, C.; Garcia, A.J.; Nieves-Aldrey, J.L. Breaking up the wall: Metal-enrichment in ovipositors, but not in mandibles, co-varies with substrate hardness in gall-wasps and their associates. PLoS ONE 2013, 8, e70529. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Strehl, C.E. Xylem feeding by periodical cicada nymphs on tree roots. Ecol Entomol. 1978, 3, 323–327. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Li, N.; Zhang, Q. Effect of root moisture content and diameter on root tensile properties. PLoS ONE 2016, 11, e0151791. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Dietrich, C.; Dai, W. Development of mouthparts in the cicada Meimuna mongolica (Distant): Successive morphological patterning and sensilla differentiation from nymph to adult. Sci. Rep. 2016, 6, 38151. [Google Scholar] [CrossRef]
- Eeva, T.; Sorvari, J.; Koivunen, V. Effects of heavy metal pollution on red wood ant (Formica s. str.) populations. Environ. Pollut. 2004, 132, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.G. Uptake of cadmium, copper, lead, and zinc from sediments by an aquatic macrophyte and by terrestrial arthropods in a freshwater wetland ecosystem. Arch. Environ. Contam. Toxicol. 2016, 71, 198–209. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Rajabi, H.; Jafarpour, M.; Darvizeh, A.; Dirks, J.H.; Gorb, S.N. Stiffness distribution in insect cuticle: A continuous or a discontinuous profile? J. R. Soc. Interface 2017, 14, 20170310. [Google Scholar] [CrossRef]
- Jafarpour, M.; Eshghi, S.; Darvizeh, A.; Gorb, S.N.; Rajabi, H. Functional significance of graded properties of insect cuticle supported by an evolutionary analysis. J. R. Soc. Interface 2020, 17, 20200378. [Google Scholar] [CrossRef]
- R Core Team, R. A Language and Environment for Statistical Computing; R foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Wickham, H.; Francois, R.; Muller, H.K. R Package Version 1.0.9; Dplyr: A Grammar of Data Manipulation. 2022. Available online: https://dplyr.tidyverse.org (accessed on 22 December 2022).
- Tang, Y.; Horikoshi, M.; Li, W. Ggfortify: Unified Interface to Visualize Statistical Result of Popular R Packages. R J. 2016, 8, 474–485. [Google Scholar] [CrossRef]
- Borgognone, M.G.; Bussi, J.; Hough, G. Principal component analysis in sensory analysis: Covariance or correlation matrix? Food Qual Prefer. 2001, 12, 5–7. [Google Scholar] [CrossRef]
- Quicke, D.L.J.; Wyeth, P.; Fawke, J.D.; Basibuyuk, H.H.; Vincent, J.F.V. Manganese and zinc in the ovipositors and mandibles of hymenopterous insects. Zool J. Linn. Soc. 1998, 124, 387–396. [Google Scholar] [CrossRef]
- Schofield, R.M.S.; Bailey, J.; Coon, J.J.; Devaraj, A.; Garrett, R.W.; Goggans, M.S.; Hebner, M.G.; Lee, B.S.; Lee, D.; Lovern, N.; et al. The homogenous alternative to biomineralization: Zn- and Mn-rich materials enable sharp organismal “tools” that reduce force requirements. Sci. Rep. 2021, 11, 17481. [Google Scholar] [CrossRef] [PubMed]
- Schofield, R.M.S.; Lefevre, H. High concentrations of zinc in the fangs and manganese in the teeth of spiders. J. Exp. Biol. 1998, 114, 577–581. [Google Scholar] [CrossRef]
- Broomell, C.C.; Mattoni, M.A.; Zok, F.W.; Waite, J.H. Critical role of zinc in hardening of Nereis jaws. J. Exp. Biol. 2006, 209, 3219–3225. [Google Scholar] [CrossRef]
- Broomell, M.A.; Zok, F.W.; Waite, J.H. Role of transition metals in sclerotization of biological tissue. Acta Biomater. 2008, 4, 2045–2051. [Google Scholar] [CrossRef]
- Degtyar, E.; Harrington, M.J.; Politi, Y.; Fratzl, P. The mechanical role of metal ions in biogenic protein-based materials. Angew. Chem. Int. Ed. 2014, 53, 12026–12044. [Google Scholar] [CrossRef]
- Christianson, D.W. Structural chemistry and biology of manganese metalloenzymes. Prog. Biophys. Mol. Biol. 1997, 67, 217–252. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.O. Chlorinated tyrosine derivatives in insect cuticle. Insect Biochem. Mol. Biol. 2004, 34, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Clerc, D.; Ledbetter, H. Mechanical hardness: A semiempirical theory based on screened electrostatics and elastic shear. J. Phys. Chem Solids 1998, 59, 1071–1095. [Google Scholar] [CrossRef]
- Shaw, M.C.; DeSalvo, G.J. The role of elasticity in hardness testing. Met. Microstruct. Anal. 2012, 1, 310–317. [Google Scholar] [CrossRef]
- Bustamante, J.; Panzarino, J.F.; Rupert, T.; Loudon, C. Forces to pierce cuticle of tarsi and material properties determined by nanoindentation: The Achilles’ heel of bed bugs. Biol. Open 2017, 6, 1541–1551. [Google Scholar] [CrossRef]
- Barbakadze, N.; Enders, S.; Gorb, S.N.; Arzt, E. Local mechanical properties of the head articulation cuticle in the beetle Pachnoda marginata (Coleoptera, Scarabaeidae). J. Exp. Biol. 2006, 209, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Tong, J.; Ma, Y.H. Nanomechanical behaviours of cuticle of three kinds of beetle. J. Bionic. Eng. 2008, 5, 152–157. [Google Scholar] [CrossRef]
- Wang, L.Y.; Rajabi, H.; Ghoroubi, N.; Lin, C.-P.; Gorb, S.N. Biomechanical strategies underlying the robust body armour of an aposematic weevil. Front. Physiol. 2018, 9, 1410. [Google Scholar] [CrossRef]
- Stamm, K.; Saltin, B.D.; Dirks, J.-H. Biomechanics of insect cuticle: An interdisciplinary experimental challenge. Appl. Phys. A. 2021, 127, 329. [Google Scholar] [CrossRef]
- Khun, N.W.; Liu, E. Thermal, mechanical and tribological properties of polycarbonate/acrylonitrile-butadiene-styrene blends. J. Polym. Eng. 2013, 33, 535–543. [Google Scholar] [CrossRef]
- Klocke, D.; Schmitz, H. Water as a major modulator of the mechanical properties of insect cuticle. Acta Biomater. 2011, 7, 2935–2942. [Google Scholar] [CrossRef]
- Schofield, R.M.S.; Nesson, M.H.; Richardson, K.A. Zinc is incorporated into cuticular “tools” after ecdysis: The time course of zinc accumulation in “tools” and whole bodies of an ant and a scorpion. J. Insect Physiol. 2003, 49, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Politi, Y.; Pippel, E.; Licuco-Massouh, A.C.; Bertinetti, L.; Blumtritt, H.; Barth, F.G.; Fratzl, P. Nano-channels in the spider fang for the transport of Zn ions to cross-link His-rich proteins pre-deposited in the cuticle matrix. Arthropod Struct. Dev. 2017, 46, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, C.-Y.; Fang, Y.; Carlson, C.M.; Xu, H.; Ješovnik, A.; Sosa-Calvo, J.; Zarnowski, R.; Bechtel, H.A.; Fournelle, J.H.; et al. Biomineral armor in leaf-cutter ants. Nat. Comm. 2020, 11, 5792. [Google Scholar] [CrossRef] [PubMed]
- Haydon, M.J.; Cobbett, C.S. Transporters of ligands for essential metal ions in plants. New Phytol. 2007, 174, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Kandziora-Ciupa, M.; Ciepa, R.; Nadgórska-Socha, A.; Barczyk, G. A comparative study of heavy metal accumulation and antioxidant responses in Vaccinium myrtillus L. leaves in polluted and non-polluted areas. Environ. Sci. Pollut. Res. 2013, 20, 4920–4932. [Google Scholar] [CrossRef]
- Kasseney, B.D.; Deng, T.; Mo, J. Effect of wood hardness and secondary compounds on feeding preference of Odontotermes formosanus (Isoptera: Termitidae). J. Econ. Entomol. 2011, 104, 862–867. [Google Scholar] [CrossRef]
- Matushkina, N.; Gorb, S. Mechanical properties of the endophytic ovipositor in damselflies (Zygoptera, Odonata) and their oviposition substrates. Zoology 2007, 110, 167–175. [Google Scholar] [CrossRef]
- Kritsky, G. Periodical Cicadas: The Plague and the Puzzle; Indiana Academy of Science: Indianapolis, IN, USA, 2004. [Google Scholar]

| M. cassinii | M. septendecim | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mouthpart | Location | Mouthpart × Location | Mouthpart | Location | Mouthpart × Location | |||||||
| F | P | F | P | F | P | F | P | F | P | F | P | |
| Al | 2.94 | 0.062 | 0.353 | 0.787 | 0.35 | 0.905 | 2.78 | 0.072 | 2.56 | 0.066 | 2.56 | 0.031 |
| Ca | 1.47 | 0.239 | 2.37 | 0.082 | 2.17 | 0.062 | 0.43 | 0.654 | 1.22 | 0.313 | 0.79 | 0.586 |
| Cl | 5.46 | 0.007 | 10.62 | <0.001 | 2.95 | 0.016 | 3.06 | 0.056 | 3.94 | 0.014 | 1.26 | 0.292 |
| Fe | 1.68 | 0.198 | 1.51 | 0.223 | 0.59 | 0.733 | - | - | - | - | - | - |
| K | 6.97 | 0.002 | 3.18 | 0.032 | 2.29 | 0.051 | 0.31 | 0.733 | 2.67 | 0.058 | 0.72 | 0.637 |
| Mg | 0.87 | 0.426 | 0.56 | 0.646 | 2.44 | 0.038 | 4.55 | 0.015 | 2.58 | 0.065 | 5.72 | <0.001 |
| Mn | 7.16 | 0.002 | 16.58 | <0.001 | 3.73 | 0.004 | 1.43 | 0.248 | 3.33 | 0.027 | 0.96 | 0.464 |
| Na | 22.36 | <0.001 | 13.36 | <0.001 | 6.74 | <0.001 | 42.93 | <0.001 | 11.94 | <0.001 | 9.54 | <0.001 |
| P | 0.58 | 0.561 | 0.55 | 0.651 | 1.05 | 0.401 | 2.50 | 0.093 | 0.89 | 0.451 | 1.17 | 0.340 |
| S | 0.19 | 0.831 | 0.06 | 0.980 | 0.685 | 0.663 | 0.20 | 0.822 | 0.36 | 0.780 | 0.88 | 0.517 |
| Si | 0.68 | 0.514 | 0.36 | 0.853 | 0.52 | 0.792 | 0.98 | 0.382 | 0.75 | 0.529 | 0.84 | 0.543 |
| Zn | 43.21 | <0.001 | 15.75 | <0.001 | 14.97 | <0.001 | 16.10 | <0.001 | 5.97 | 0.002 | 5.40 | <0.001 |
| M. septendecula | N. linnei | |||||||||||
| Mouthpart | Location | Mouthpart ×Location | Mouthpart | Location | Mouthpart ×Location | |||||||
| F | P | F | P | F | P | F | P | F | P | F | P | |
| Al | - | - | - | - | - | - | 0.72 | 0.494 | 0.95 | 0.424 | 1.66 | 0.152 |
| Ca | 5.23 | 0.013 | 6.02 | 0.003 | 2.06 | 0.097 | 0.57 | 0.572 | 0.67 | 0.573 | 1.27 | 0.291 |
| Cl | 2.06 | 0.150 | 0.92 | 0.447 | 0.34 | 0.911 | 0.19 | 0.830 | 0.56 | 0.641 | 1.01 | 0.430 |
| Fe | - | - | - | - | - | - | 1.16 | 0.323 | 1.28 | 0.291 | 1.16 | 0.346 |
| K | 4.16 | 0.028 | 2.84 | 0.060 | 0.42 | 0.859 | 0.73 | 0.485 | 0.90 | 0.449 | 1.06 | 0.398 |
| Mg | 0.01 | 0.994 | 0.15 | 0.930 | 1.45 | 0.237 | 10.53 | <0.001 | 1.75 | 0.170 | 4.32 | 0.001 |
| Mn | 6.58 | 0.005 | 5.53 | 0.005 | 3.23 | 0.018 | 5.24 | 0.009 | 5.17 | 0.004 | 3.45 | 0.007 |
| Na | 2.79 | 0.081 | 0.21 | 0.888 | 0.17 | 0.983 | 7.23 | 0.002 | 8.88 | <0.001 | 4.02 | 0.002 |
| P | 0.76 | 0.477 | 3.23 | 0.040 | 1.43 | 0.245 | 0.87 | 0.427 | 1.21 | 0.318 | 0.62 | 0.717 |
| S | 5.24 | 0.013 | 2.40 | 0.093 | 0.67 | 0.674 | 1.03 | 0.364 | 0.77 | 0.516 | 1.06 | 0.399 |
| Si | 1.99 | 0.159 | 0.16 | 0.923 | 0.58 | 0.941 | 0.27 | 0.767 | 0.154 | 0.215 | 0.27 | 0.948 |
| Zn | 13.28 | 0.001 | 6.60 | 0.002 | 6.59 | 0.003 | 16.06 | <0.001 | 6.01 | 0.001 | 6.01 | <0.001 |
| Mandible | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | C | Ca | Cl | Fe | K | Mg | Mn | Na | O | P | S | Si | Zn | |
| Al * | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| C | - | - | −0.20 | −0.51 | 0.03 | −0.27 | 0.26 | −0.52 | −0.31 | 0.85 | 0.08 | −0.18 | −0.40 | −0.52 |
| Ca | - | - | - | 0.30 | −0.12 | 0.20 | −0.17 | 0.38 | 0.14 | −0.34 | 0.18 | 0.22 | −0.06 | 0.39 |
| Cl | - | - | - | - | 0.07 | 0.65 | −0.17 | 0.79 | 0.73 | −0.85 | −0.15 | 0.21 | −0.04 | 0.87 |
| Fe | - | - | - | - | - | −0.01 | −0.06 | 0.03 | 0.14 | −0.02 | −0.06 | −0.05 | −0.04 | 0.05 |
| K | - | - | - | - | - | - | −0.07 | 0.46 | 0.32 | −0.51 | −0.19 | 0.46 | −0.11 | 0.14 |
| Mg | - | - | - | - | - | - | - | −0.21 | −0.26 | −0.63 | 0.51 | 0.03 | −0.09 | −0.21 |
| Mn | - | - | - | - | - | - | - | - | 0.60 | −0.81 | −0.19 | 0.07 | 0.07 | 0.83 |
| Na | - | - | - | - | - | - | - | - | - | −0.63 | 0.17 | −0.07 | −0.03 | 0.72 |
| O | - | - | - | - | - | - | - | - | - | - | 0.17 | −0.16 | −0.17 | −0.88 |
| P | - | - | - | - | - | - | - | - | - | - | - | 0.15 | −0.23 | −0.18 |
| S | - | - | - | - | - | - | - | - | - | - | - | - | −0.07 | 0.09 |
| Si | - | - | - | - | - | - | - | - | - | - | - | - | - | −0.06 |
| Zn | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Maxillae | ||||||||||||||
| Al | C | Ca | Cl | Fe | K | Mg | Mn | Na | O | P | S | Si | Zn | |
| Al * | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| C | - | - | −0.13 | −0.13 | −0.01 | −0.13 | −0.05 | −0.08 | 0.05 | 0.15 | −0.09 | −0.15 | −0.15 | −0.08 |
| Ca | - | - | - | 0.87 | −0.01 | 1.00 | −0.06 | 0.16 | −0.07 | −0.18 | 0.16 | 0.89 | 0.04 | 0.23 |
| Cl | - | - | - | - | −0.03 | 0.85 | −0.02 | 0.59 | −0.03 | −0.90 | 0.48 | 0.96 | 0.07 | 0.62 |
| Fe | - | - | - | - | - | −0.01 | 0.21 | −0.05 | 0.17 | 0.03 | 0.11 | 0.01 | −0.06 | −0.02 |
| K | - | - | - | - | - | - | −0.06 | 0.16 | −0.08 | −0.92 | 0.12 | 0.87 | 0.04 | 0.91 |
| Mg | - | - | - | - | - | - | - | 0.04 | 0.17 | 0.06 | −0.13 | −0.02 | −0.07 | −0.06 |
| Mn | - | - | - | - | - | - | - | - | −0.06 | −0.34 | 0.71 | 0.49 | 0.01 | 0.91 |
| Na | - | - | - | - | - | - | - | - | - | 0.07 | −0.08 | −0.01 | −0.06 | −0.04 |
| O | - | - | - | - | - | - | - | - | - | - | −0.27 | −0.91 | −0.36 | −0.39 |
| P | - | - | - | - | - | - | - | - | - | - | - | 0.15 | −0.23 | −0.18 |
| S | - | - | - | - | - | - | - | - | - | - | - | - | −0.07 | 0.09 |
| Si | - | - | - | - | - | - | - | - | - | - | - | - | - | −0.06 |
| Zn | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Sheath | ||||||||||||||
| Al | C | Ca | Cl | Fe | K | Mg | Mn | Na | O | P | S | Si | Zn | |
| Al | - | −0.30 | 0.03 | 0.01 | 0.12 | 0.30 | −0.10 | 0.30 | −0.01 | −0.04 | −0.03 | 0.20 | 0.11 | −0.02 |
| C | - | - | −0.40 | −0.36 | −0.11 | −0.59 | −0.06 | −0.21 | −0.28 | 0.82 | −0.57 | −0.53 | −0.73 | 0.11 |
| Ca | - | - | - | 0.15 | 0.20 | 0.64 | 0.04 | 0.57 | −0.01 | −0.47 | 0.08 | 0.35 | 0.11 | −0.01 |
| Cl | - | - | - | - | 0.01 | 0.27 | 0.04 | 0.06 | 0.95 | −0.76 | 0.02 | 0.51 | −0.16 | −0.03 |
| Fe | - | - | - | - | - | 0.09 | −0.09 | 0.67 | 0.01 | −0.15 | −0.02 | 0.04 | 0.04 | −0.02 |
| K | - | - | - | - | - | - | 0.51 | 0.34 | 0.14 | −0.59 | 0.39 | 0.60 | 0.12 | −0.14 |
| Mg | - | - | - | - | - | - | - | 0.13 | −0.12 | −0.29 | −0.08 | 0.03 | −0.03 | −0.08 |
| Mn | - | - | - | - | - | - | - | - | −0.03 | −0.29 | −0.38 | 0.22 | 0.02 | −0.04 |
| Na | - | - | - | - | - | - | - | - | - | −0.69 | −0.01 | 0.50 | −0.22 | −0.01 |
| O | - | - | - | - | - | - | - | - | - | - | −0.38 | −0.65 | −0.34 | 0.10 |
| P | - | - | - | - | - | - | - | - | - | - | - | 0.31 | 0.30 | −0.01 |
| S | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | −0.10 |
| Si | - | - | - | - | - | - | - | - | - | - | - | - | - | −0.10 |
| Zn | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Morphology | ||||
|---|---|---|---|---|
| Species | M. cassinii | M. septendecim | M. septendecula | N. linnei |
| M. cassinii | 3 | 0 | 0 | 0 |
| M. septendecim | 0 | 5 | 0 | 0 |
| M. septendecula | 0 | 0 | 3 | 0 |
| N. linnei | 0 | 0 | 0 | 5 |
| Inorganic elements | ||||
| species | M. cassinii | M. septendecim | M. septendecula | N. linnei |
| M. cassinii | 3 | 1 | 0 | 1 |
| M. septendecim | 2 | 3 | 0 | 0 |
| M. septendecula | 0 | 0 | 3 | 0 |
| N. linnei | 1 | 0 | 1 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reiter, K.E.; Perkovich, C.; Smith, K.N.; Feng, J.; Kritsky, G.; Lehnert, M.S. Comparative Material and Mechanical Properties among Cicada Mouthparts: Cuticle Enhanced with Inorganic Elements Facilitates Piercing through Woody Stems for Feeding. Biology 2023, 12, 207. https://doi.org/10.3390/biology12020207
Reiter KE, Perkovich C, Smith KN, Feng J, Kritsky G, Lehnert MS. Comparative Material and Mechanical Properties among Cicada Mouthparts: Cuticle Enhanced with Inorganic Elements Facilitates Piercing through Woody Stems for Feeding. Biology. 2023; 12(2):207. https://doi.org/10.3390/biology12020207
Chicago/Turabian StyleReiter, Kristen E., Cynthia Perkovich, Katelynne N. Smith, Jiansheng Feng, Gene Kritsky, and Matthew S. Lehnert. 2023. "Comparative Material and Mechanical Properties among Cicada Mouthparts: Cuticle Enhanced with Inorganic Elements Facilitates Piercing through Woody Stems for Feeding" Biology 12, no. 2: 207. https://doi.org/10.3390/biology12020207
APA StyleReiter, K. E., Perkovich, C., Smith, K. N., Feng, J., Kritsky, G., & Lehnert, M. S. (2023). Comparative Material and Mechanical Properties among Cicada Mouthparts: Cuticle Enhanced with Inorganic Elements Facilitates Piercing through Woody Stems for Feeding. Biology, 12(2), 207. https://doi.org/10.3390/biology12020207






