Chronic Corticosterone Exposure Suppresses Copper Transport through GR-Mediated Intestinal CTR1 Pathway in Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Determination of Plasma Copper Content
2.3. Determination of Liver Copper Content
2.4. Determination of Plasma and Liver Ceruloplasmin and Cu/Zn-SOD Activity
2.5. Histological Evaluation
2.6. Isolation of Primary Hepatocytes and Assessment of Reactive Oxygen Species (ROS)
2.7. Total RNA Isolation and Real-Time PCR
2.8. Protein Extraction and Western Blotting Analysis
2.9. Cell Culture and Immunofluorescence
2.10. Chromatin Immunoprecipitation (ChIP) Assay
2.11. Luciferase Reporter Assay
2.12. Statistical Analysis
3. Results
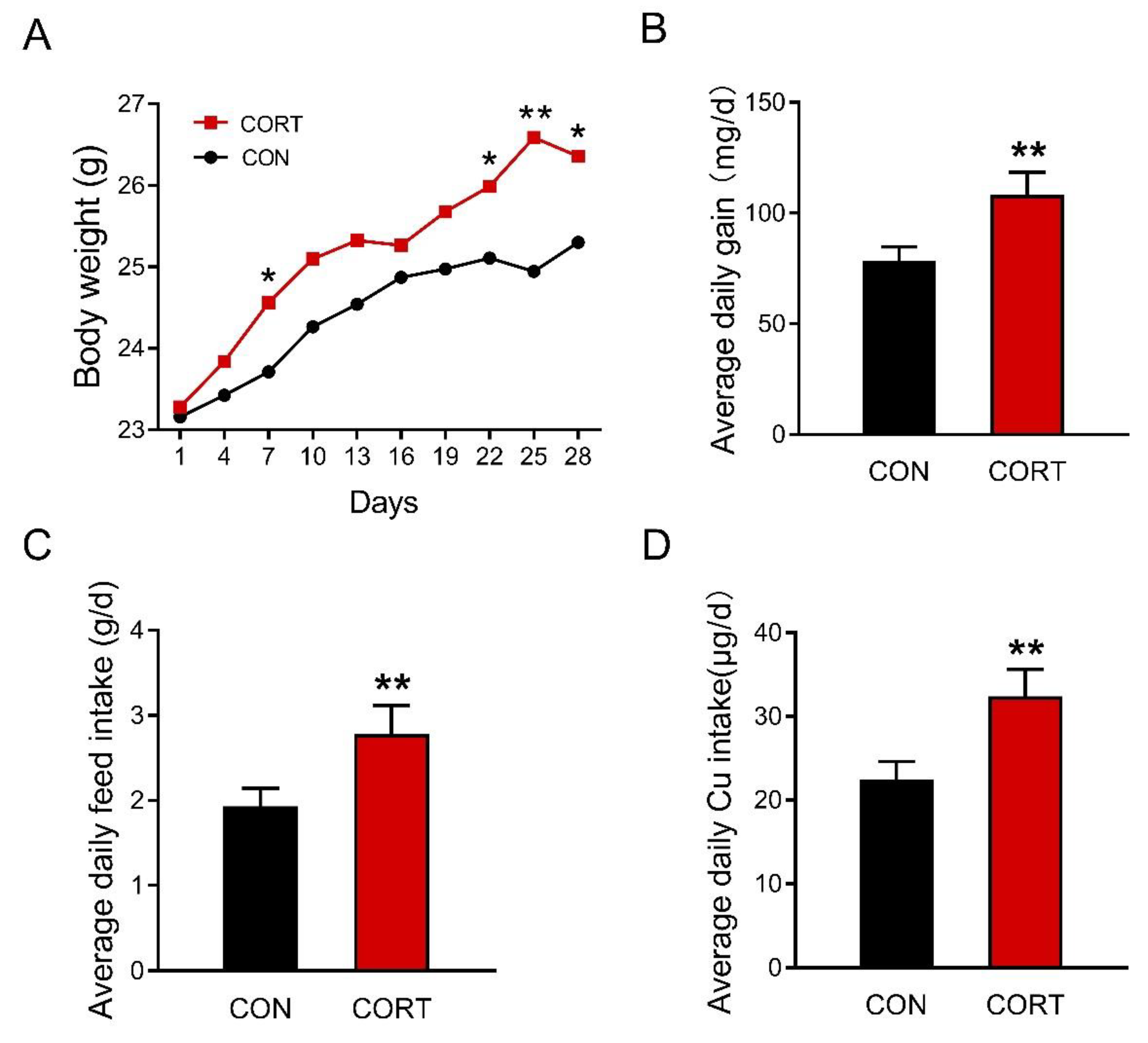
3.1. Chronic Corticosterone Exposure Markedly Increases Average Daily Food Intake and Final Body Weight in Mice
3.2. Chronic Corticosterone Exposure Significantly Reduces the Plasma and Hepatic Copper-Related Parameters in Mice
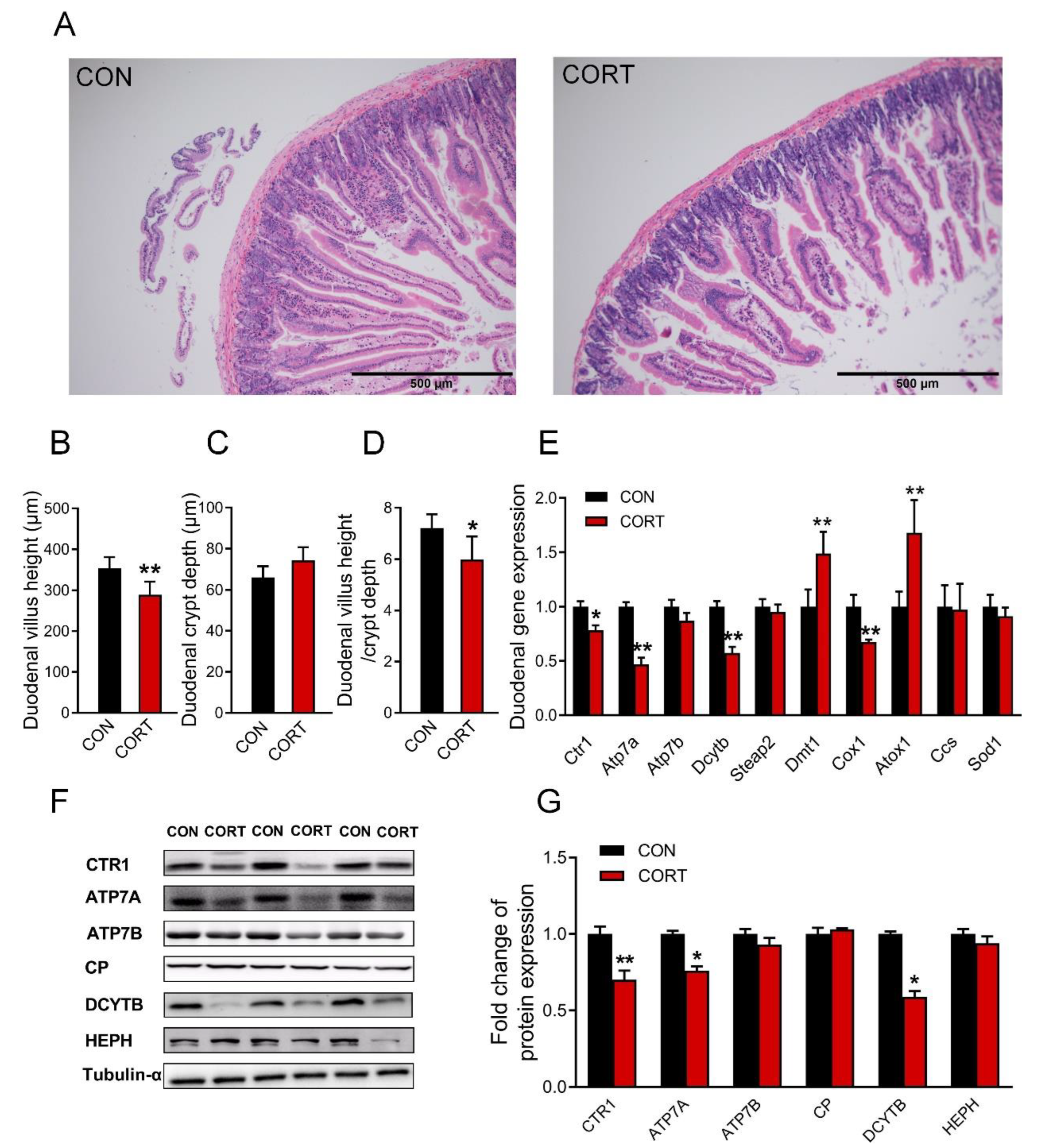
3.3. Chronic Corticosterone Exposure Damages the Duodenal Structure and Impairs the Expression of Copper Transport-Related Genes in Mice
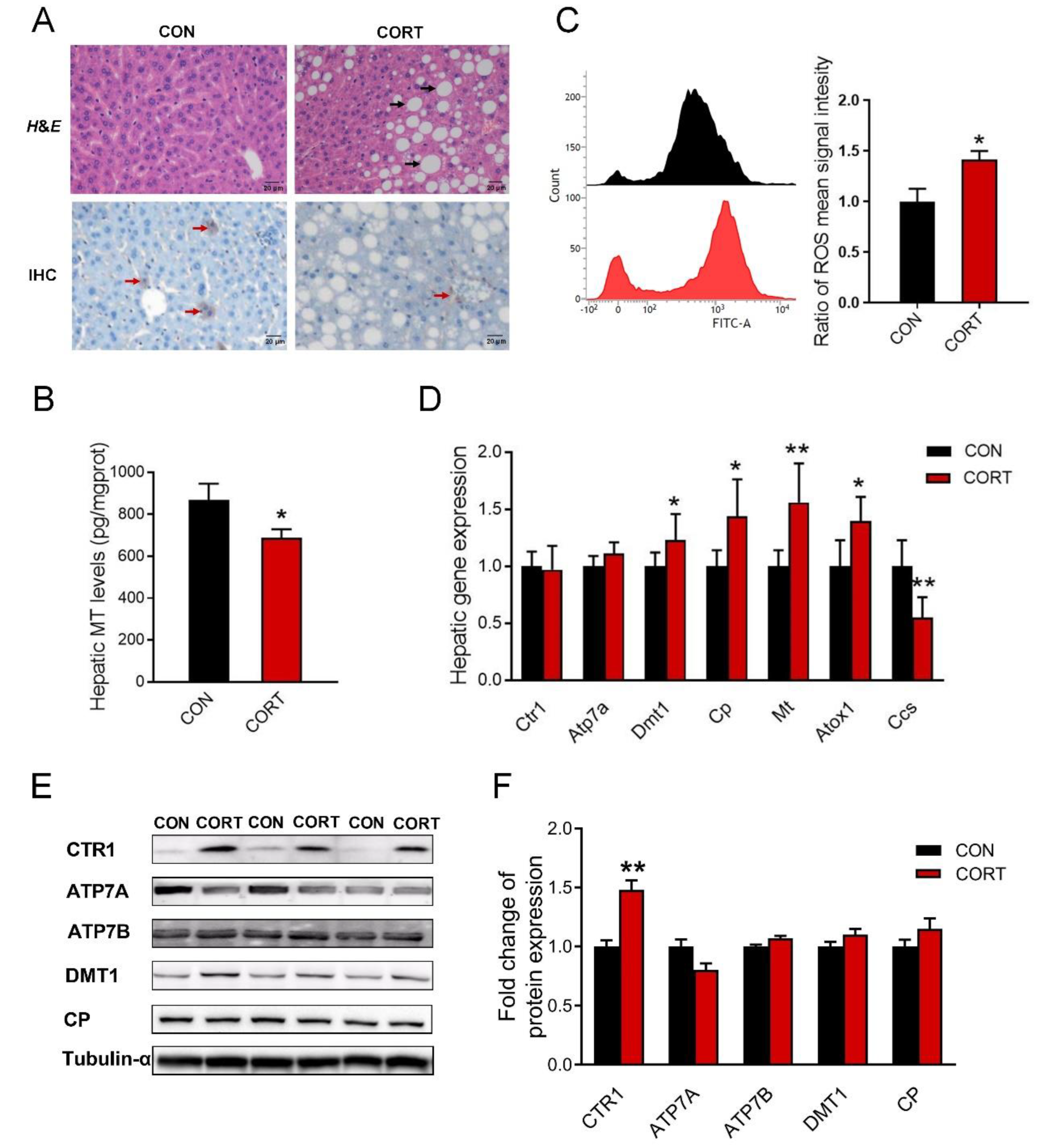
3.4. Chronic Corticosterone Exposure Induces Hepatic Damage and Disrupts Hepatic Copper Metabolism in Mice
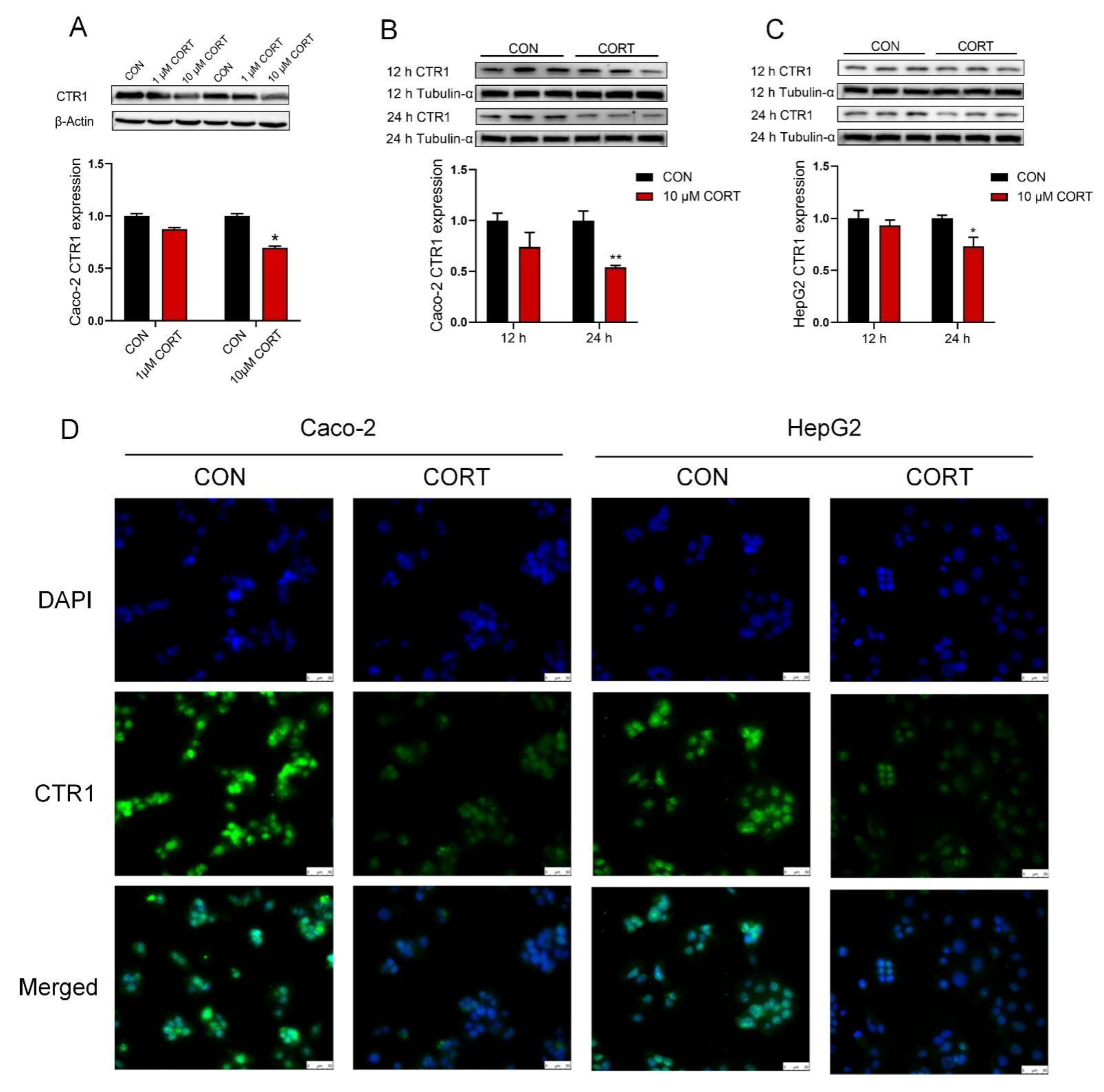
3.5. Corticosterone Down-Regulates CTR1 Protein Expression in Caco-2 Cells and HepG2 Cells
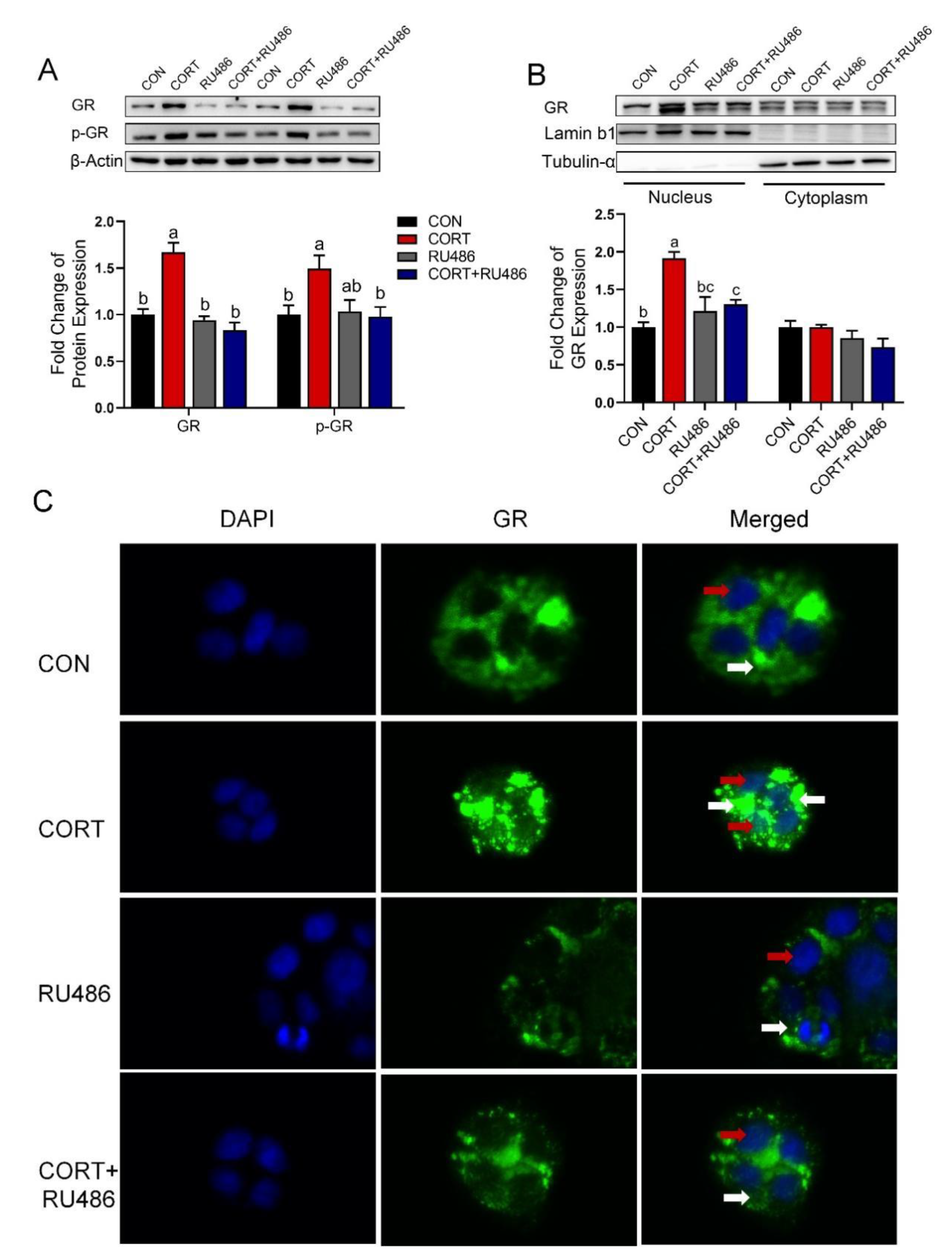
3.6. Corticosterone Inhibits CTR1 Protein Expression Accompanied by an Increase in Nuclear GR Protein in Caco-2 Cells
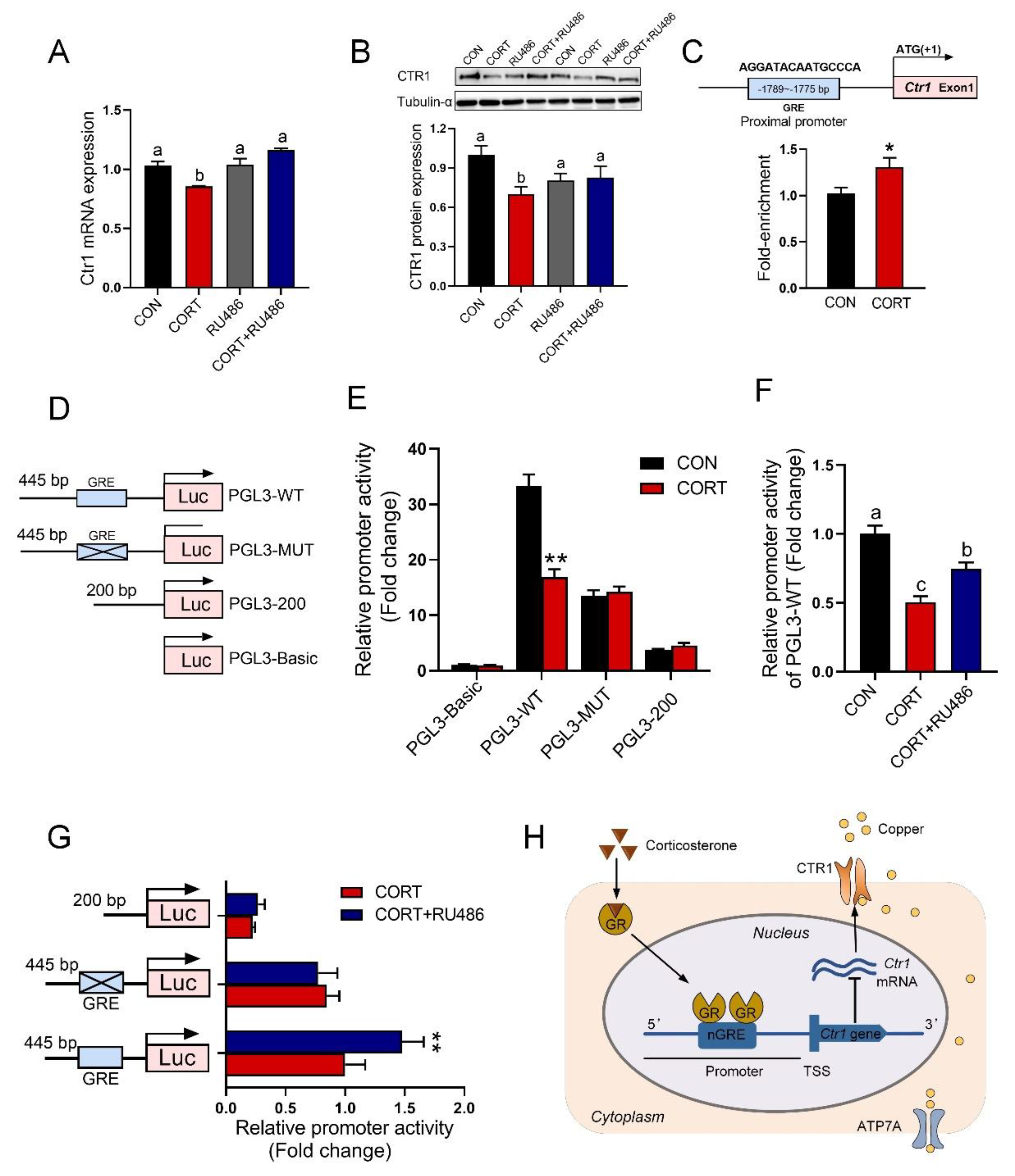
3.7. GR Binding to GRE in the Promoter of the Ctr1 Gene to Reduce Its Protein Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grubman, A.; White, A.R. Copper as a key regulator of cell signalling pathways. Expert Rev. Mol. Med. 2014, 16, e11. [Google Scholar] [CrossRef] [PubMed]
- Lutsenko, S. Human copper homeostasis: A network of interconnected pathways. Curr. Opin. Chem. Biol. 2010, 14, 211–217. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; Mangan, D.; O’Keefe, J.H. Copper deficiency may be a leading cause of ischaemic heart disease. Open Heart 2018, 5, e000784. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.F.; Prohaska, J.R.; Knutson, M.D. Metabolic crossroads of iron and copper. Nutr. Rev. 2010, 68, 133–147. [Google Scholar] [CrossRef] [PubMed]
- White, A.R.; Huang, X.D.; Jobling, M.F.; Barrow, C.J.; Beyreuther, K.; Masters, C.L.; Bush, A.I.; Cappai, R. Homocysteine potentiates copper- and amyloid beta peptide-mediated toxicity in primary neuronal cultures: Possible risk factors in the Alzheimer’s-type neurodegenerative pathways. J. Neurochem. 2001, 76, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- de Romana, D.L.; Olivares, M.; Uauy, R.; Araya, M. Risks and benefits of copper in light of new insights of copper homeostasis. J. Trace Elem. Med. Biol. 2011, 25, 3–13. [Google Scholar] [CrossRef]
- Ishida, S.; Andreux, P.; Poitry-Yamate, C.; Auwerx, J.; Hanahan, D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 19507–19512. [Google Scholar] [CrossRef] [PubMed]
- Hermann, W. Classification and differential diagnosis of Wilson’s disease. Ann. Transl. Med. 2019, 7, s63. [Google Scholar] [CrossRef]
- Medici, V.; LaSalle, J.M. Genetics and epigenetic factors of Wilson disease. Ann. Transl. Med. 2019, 7, s58. [Google Scholar] [CrossRef]
- Comstra, H.S.; McArthy, J.; Rudin-Rush, S.; Hartwig, C.; Gokhale, A.; Zlatic, S.A.; Blackburn, J.B.; Werner, E.; Petris, M.; D’Souza, P.; et al. The interactome of the copper transporter ATP7A belongs to a netowrk of neurodevelopmental and neurodegeneration factors. Elife 2017, 6, e24722. [Google Scholar] [CrossRef]
- De Feo, C.J.; Aller, S.G.; Siluvai, G.S.; Blackburn, N.J.; Unger, V.M. Three-dimensional structure of the human copper transporter hCTR1. Proc. Natl. Acad. Sci. USA 2009, 106, 4237–4242. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.W.; Prohaska, J.R.; Thiele, D.J. Essential role for mammalian copper transporter Ctr1 in copper homeostasis and embryonic development. Proc. Natl. Acad. Sci. USA 2001, 98, 6842–6847. [Google Scholar] [CrossRef] [PubMed]
- Nose, Y.; Kim, B.-E.; Thiele, D.J. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006, 4, 235–244. [Google Scholar] [CrossRef]
- Kuo, Y.M.; Zhou, B.; Cosco, D.; Gitschier, J. The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc. Natl. Acad. Sci. USA 2001, 98, 6836–6841. [Google Scholar] [CrossRef] [PubMed]
- Bandmann, O.; Weiss, K.H.; Kaler, S.G. Wilson’s disease and other neurological copper disorders. Lancet Neurol. 2015, 14, 103–113. [Google Scholar] [CrossRef]
- Chang, I.J.; Hahn, S.H. The genetics of Wilson disease. Handb. Clin. Neurol. 2017, 142, 19–34. [Google Scholar] [PubMed]
- Tumer, Z. An overview and update of ATP7A mutations leading to menkes disease and occipital horn syndrome. Hum. Mutat. 2013, 34, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.O.; Kamilaris, T.C.; Chrousos, G.P.; Gold, P.W. Mechanisms of stress—A dynamic overview of hormonal and behavioral homeostasis. Neurosci. Biobehav. Rev. 1992, 16, 115–130. [Google Scholar] [CrossRef]
- Chen, J.; Shen, H.; Chen, C.; Wang, W.; Yu, S.; Zhao, M.; Li, M. The effect of psychological stress on iron absorption in rats. BMC Gastroenterol. 2009, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.; Zhao, M.; Ma, L.; Li, M. Psychological stress induces dysregulation of iron metabolism in rat brain. Neuroscience 2008, 155, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Chen, J.; Wang, W.; Wang, L.; Ma, L.; Shen, H.; Li, M. Psychological stress induces hypoferremia through the IL-6-hepcidin axis in rats. Biochem. Biophys. Res. Commun. 2008, 373, 90–93. [Google Scholar] [CrossRef]
- Guo, S.; Yang, C.; Jiang, S.; Ni, Y.; Zhao, R.; Ma, W. Repeated restraint stress enhances hepatic TFR2 expression and induces hepatic iron accumulation in rats. Biol. Trace Elem. Res. 2020, 196, 590–596. [Google Scholar] [CrossRef]
- Guo, S.H.; Dong, Y.Y.; Cheng, X.X.; Chen, Z.J.; Ni, Y.D.; Zhao, R.Q.; Ma, W.Q. Chronic psychological stress disrupts iron metabolism and enhances hepatic mitochondrial function in mice. Biol. Trace Elem. Res. 2022. ahead of print. [Google Scholar] [CrossRef]
- Jiang, S.; Guo, T.; Guo, S.; Gao, J.; Ni, Y.; Ma, W.; Zhao, R. Chronic variable stress induces hepatic Fe(II) deposition by up-regulating ZIP14 expression via miR-181 family pathway in rats. Biology 2021, 10, 653. [Google Scholar] [CrossRef]
- Li, H.F.; Jiang, S.X.; Yang, C.; Yang, S.; He, B.; Ma, W.Q.; Zhao, R.Q. Long-term dexamethasone exposure down-regulates hepatic TFR1 and reduces liver iron concentration in rats. Nutrients 2017, 9, 617. [Google Scholar] [CrossRef]
- Gasparini, S.J.; Weber, M.-C.; Henneicke, H.; Kim, S.; Zhou, H.; Seibel, M.J. Continuous corticosterone delivery via the drinking water or pellet implantation: A comparative study in mice. Steroids 2016, 116, 76–82. [Google Scholar] [CrossRef]
- Ding, H.; Cui, X.-Y.; Cui, S.-Y.; Ye, H.; Hu, X.; Zhao, H.-L.; Liu, Y.-T.; Zhang, Y.-H. Depression-like behaviors induced by chronic corticosterone exposure via drinking water: Time-course analysis. Neurosci. Lett. 2018, 687, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Kinlein, S.A.; Shahanoor, Z.; Romeo, R.D.; Karatsoreos, I.N. Chronic corticosterone treatment during adolescence has significant effects on metabolism and skeletal development in male C57BL6/N mice. Endocrinology 2017, 158, 2239–2254. [Google Scholar] [CrossRef]
- Moda-Sava, R.N.; Murdock, M.H.; Parekh, P.K.; Fetcho, R.N.; Huang, B.S.; Huynh, T.N.; Witztum, J.; Shaver, D.C.; Rosenthal, D.L.; Alway, E.J.; et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 2019, 364, eaat8078. [Google Scholar] [CrossRef] [PubMed]
- Barbier-Torres, L.; Iruzubieta, P.; Fernandez-Ramos, D.; Delgado, T.C.; Taibo, D.; Guitierrez-de-Juan, V.; Varela-Rey, M.; Azkargorta, M.; Navasa, N.; Fernandez-Tussy, P.; et al. The mitochondrial negative regulator MCJ is a therapeutic target for acetaminophen-induced liver injury. Nat. Commun. 2017, 8, 2068. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Hu, Y.; Hou, Z.; Sun, Q.; Jia, Y.; Zhao, R. Chronic corticosterone exposure induces liver inflammation and fibrosis in association with m(6)A-linked post-transcriptional suppression of heat shock proteins in chicken. Cell Stress Chaperones 2020, 25, 47–56. [Google Scholar] [CrossRef]
- Feng, Y.; Dong, H.; Sun, B.; Hu, Y.; Yang, Y.; Jia, Y.; Jia, L.; Zhong, X.; Zhao, R. METTL3/METTL14 transactivation and m(6)A-dependent TGF-beta 1 translation in activated kupffer cells. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 839–856. [Google Scholar] [CrossRef]
- Fardet, L.; Nazareth, I.; Petersen, I. Long-term systemic glucocorticoid therapy and weight gain: A population-based cohort study. Rheumatology 2021, 60, 1502–1511. [Google Scholar] [CrossRef]
- Karatsoreos, I.N.; Bhagat, S.M.; Bowles, N.P.; Weil, Z.M.; Pfaff, D.W.; McEwen, B.S. Endocrine and physiological changes in response to chronic corticosterone: A potential model of the metabolic syndrome in mouse. Endocrinology 2010, 151, 2117–2127. [Google Scholar] [CrossRef]
- Luijten, I.H.N.; Brooks, K.; Boulet, N.; Shabalina, I.G.; Jaiprakash, A.; Carlsson, B.; Fischer, A.W.; Cannon, B.; Nedergaard, J. Glucocorticoid-induced obesity develops independently of UCP1. Cell Rep. 2019, 27, 1686–1698.e5. [Google Scholar] [CrossRef]
- Tataranni, P.A.; Larson, D.E.; Snitker, S.; Young, J.B.; Flatt, J.P.; Ravussin, E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am. J. Physiol. -Endocrinol. Metab. 1996, 271, E317–E325. [Google Scholar] [CrossRef]
- Stopa, L.R.S.; de Souza, C.F.; Santos, G.F.; Martins, A.B.; Ferreira, R.N.; de Andrade, F.G.; Leite, C.M.; Zaja, D.A.M.; Zaia, C.; Uchoa, E.T. Sex differences in glucocorticoids-induced anabolic effects in rats. Physiol. Behav. 2019, 209, 112587. [Google Scholar] [CrossRef]
- Harno, E.; Sefton, C.; Wray, J.R.; Allen, T.-J.; Davies, A.; Coll, A.P.; White, A. Chronic glucocorticoid treatment induces hepatic lipid accumulation and hyperinsulinaemia in part through actions on AgRP neurons. Sci. Rep. 2021, 11, 13776. [Google Scholar] [CrossRef]
- Johnson, S.A.; Fournier, N.M.; Kalynchuk, L.E. Effect of different doses of corticosterone on depression-like behavior and HPA axis responses to a novel stressor. Behav. Brain Res. 2006, 168, 280–288. [Google Scholar] [CrossRef]
- Steiner, J.L.; Bardgett, M.E.; Wolfgang, L.; Lang, C.H.; Stocker, S.D. Glucocorticoids attenuate the central sympathoexcitatory actions of insulin. J. Neurophysiol. 2014, 112, 2597–2604. [Google Scholar] [CrossRef]
- Broderius, M.; Mostad, E.; Wendroth, K.; Prohaska, J.R. Levels of plasma ceruloplasmin protein are markedly lower following dietary copper deficiency in rodents. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2010, 151, 473–479. [Google Scholar] [CrossRef]
- Johnson, W.T.; Anderson, C.M. Cardiac cytochrome c oxidase activity and contents of subunits 1 and 4 are altered in offspring by low prenatal copper intake by rat dams. J. Nutr. 2008, 138, 1269–1273. [Google Scholar] [CrossRef]
- Chung, R.S.; Howells, C.; Eaton, E.D.; Shabala, L.; Zovo, K.; Palumaa, P.; Sillard, R.; Woodhouse, A.; Bennett, W.R.; Ray, S.; et al. The native copper- and zinc-binding protein metallothionein blocks copper-mediated a beta aggregation and toxicity in rat cortical neurons. PLoS ONE 2010, 5, e12030. [Google Scholar] [CrossRef]
- Calvo, J.; Jung, H.M.; Meloni, G. Copper metallothioneins. IUBMB Life 2017, 69, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Chen, J.K.; Zhang, Y.; Wang, X.; Qu, S.; Jiang, C.L. Chronic stress induces steatohepatitis while decreases visceral fat mass in mice. BMC Gastroenterol. 2014, 14, 106. [Google Scholar] [CrossRef]
- Lin, R.; Wang, Z.; Cao, J.; Gao, T.; Dong, Y.; Chen, Y. Role of melatonin in intestinal mucosal injury induced by restraint stress in mice. Pharm. Biol. 2020, 58, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Nose, Y.; Wood, L.K.; Kim, B.E.; Prohaska, J.R.; Fry, R.S.; Spears, J.W.; Thiele, D.J. Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J. Biol. Chem. 2010, 285, 32385–32392. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.I.; Jang, S.J.; Park, J.H.; Lee, Y.J.; Lee, T.S.; Woo, K.S.; Park, H.; Choe, J.G.; An, G.I.; Kang, J.H. Detection of increased 64Cu uptake by human copper transporter 1 gene overexpression using PET with 64CuCl2 in human breast cancer xenograft model. J. Nucl. Med. 2014, 55, 1692–1698. [Google Scholar] [CrossRef]
- Surjit, M.; Ganti, K.P.; Mukherji, A.; Ye, T.; Hua, G.; Metzger, D.; Li, M.; Chambon, P. Widespread negative response elements mediate direct repression by agonist-liganded glucocorticoid receptor. Cell 2011, 145, 224–241. [Google Scholar] [CrossRef]
- He, B.; Zhang, N.; Zhao, R. Dexamethasone downregulates SLC7A5 expression and promotes cell cycle arrest, autophagy and apoptosis in BeWo cells. J. Cell. Physiol. 2016, 231, 233–242. [Google Scholar] [CrossRef]
- Lim, H.W.; Uhlenhaut, N.H.; Rauch, A.; Weiner, J.; Hubner, S.; Hubner, N.; Won, K.J.; Lazar, M.A.; Tuckermann, J.; Steger, D.J. Genomic redistribution of GR monomers and dimers mediates transcriptional response to exogenous glucocorticoid in vivo. Genome Res. 2015, 25, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef]
- Zhang, B.; Bai, M.; Xu, X.J.; Yang, M.S.; Niu, F.; Gao, F.; Liu, B.Y. Corticosteroid receptor rebalancing alleviates critical illness-related corticosteroid insufficiency after traumatic brain injury by promoting paraventricular nuclear cell survival via Akt/CREB/BDNF signaling. J. Neuroinflammation 2020, 17, 318. [Google Scholar] [CrossRef] [PubMed]
- Datson, N.A.; Polman, J.A.E.; de Jonge, R.T.; van Boheemen, P.T.M.; van Maanen, E.M.T.; Welten, J.; McEwen, B.S.; Meiland, H.C.; Meijer, O.C. Specific regulatory motifs predict glucocorticoid responsiveness of hippocampal gene expression. Endocrinology 2011, 152, 3749–3757. [Google Scholar] [CrossRef]
- De Bosscher, K.; Vanden Berghe, W.; Vermeulen, L.; Plaisance, S.; Boone, E.; Haegeman, G. Glucocorticoids repress NF-kappa B-driven genes by disturbing the interaction of p65 with the basal transcription machinery, irrespective of coactivator levels in the cell. Proc. Natl. Acad. Sci. USA 2000, 97, 3919–3924. [Google Scholar] [CrossRef]
- Alheim, K.; Corness, J.; Samuelsson, M.K.R.; Bladh, L.G.; Murata, T.; Nilsson, T.; Okret, S. Identification of a functional glucocorticoid response element in the promoter of the cylcin-dependant kinase inhibitor p57(Kip2). J. Mol. Endocrinol. 2003, 30, 359–368. [Google Scholar] [CrossRef]
| Parameters | Treatments | p-Value | |
|---|---|---|---|
| CON | CORT | ||
| Plasma | |||
| Cu level (μmol/L) | 28.92 ± 1.23 | 25.34 ± 2.14 | 0.03 |
| Ceruloplasmin activity (U/L) | 19.44 ± 1.80 | 12.11 ± 1.41 | 0.00 |
| Cu/Zn-SOD (U/mL) | 104.13 ± 3.70 | 87.61 ± 5.97 | 0.03 |
| Liver | |||
| Cu content (μg/g) | 6.88 ± 0.21 | 4.51 ± 0.26 | 0.00 |
| Ceruloplasmin activity (U/mg protein) | 8.40 ± 0.92 | 9.50 ± 1.00 | 0.43 |
| Cu/Zn-SOD (U/mg protein) | 277.47 ± 4.24 | 251.99 ± 6.55 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Chen, Z.; Dong, Y.; Ni, Y.; Zhao, R.; Ma, W. Chronic Corticosterone Exposure Suppresses Copper Transport through GR-Mediated Intestinal CTR1 Pathway in Mice. Biology 2023, 12, 197. https://doi.org/10.3390/biology12020197
Guo S, Chen Z, Dong Y, Ni Y, Zhao R, Ma W. Chronic Corticosterone Exposure Suppresses Copper Transport through GR-Mediated Intestinal CTR1 Pathway in Mice. Biology. 2023; 12(2):197. https://doi.org/10.3390/biology12020197
Chicago/Turabian StyleGuo, Shihui, Zijin Chen, Yingying Dong, Yingdong Ni, Ruqian Zhao, and Wenqiang Ma. 2023. "Chronic Corticosterone Exposure Suppresses Copper Transport through GR-Mediated Intestinal CTR1 Pathway in Mice" Biology 12, no. 2: 197. https://doi.org/10.3390/biology12020197
APA StyleGuo, S., Chen, Z., Dong, Y., Ni, Y., Zhao, R., & Ma, W. (2023). Chronic Corticosterone Exposure Suppresses Copper Transport through GR-Mediated Intestinal CTR1 Pathway in Mice. Biology, 12(2), 197. https://doi.org/10.3390/biology12020197






