Micropropagation Protocol and Genetic Stability of the Salix myrtilloides Plants Cultivated In Vitro
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Culture Initiation and Stabilization
2.2. Multiplication
2.3. Rooting and Acclimatization
2.4. ISSR Analysis
2.5. Flow Cytometry
2.6. Statistical Analysis
3. Results and Discussion
3.1. Tissue Culture Initiation and Stabilization
3.2. Multiplication
3.3. Rooting and Acclimatization
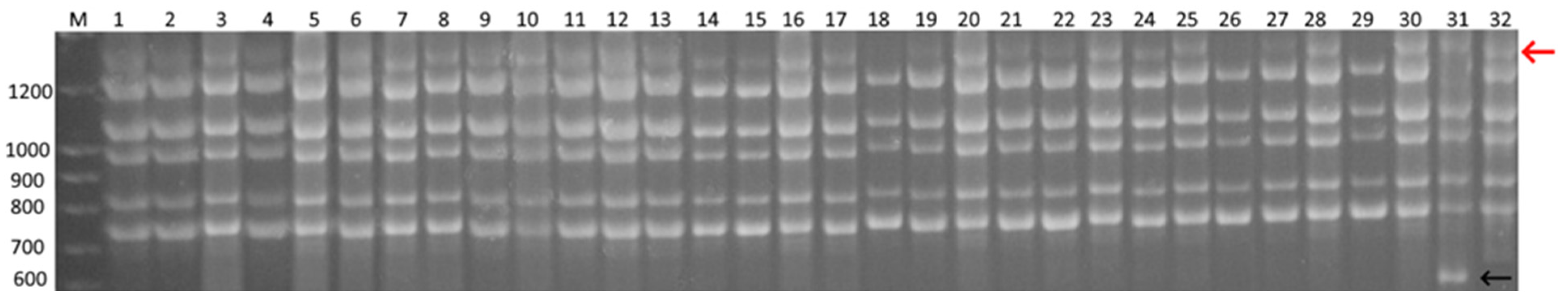
3.4. ISSR Analysis
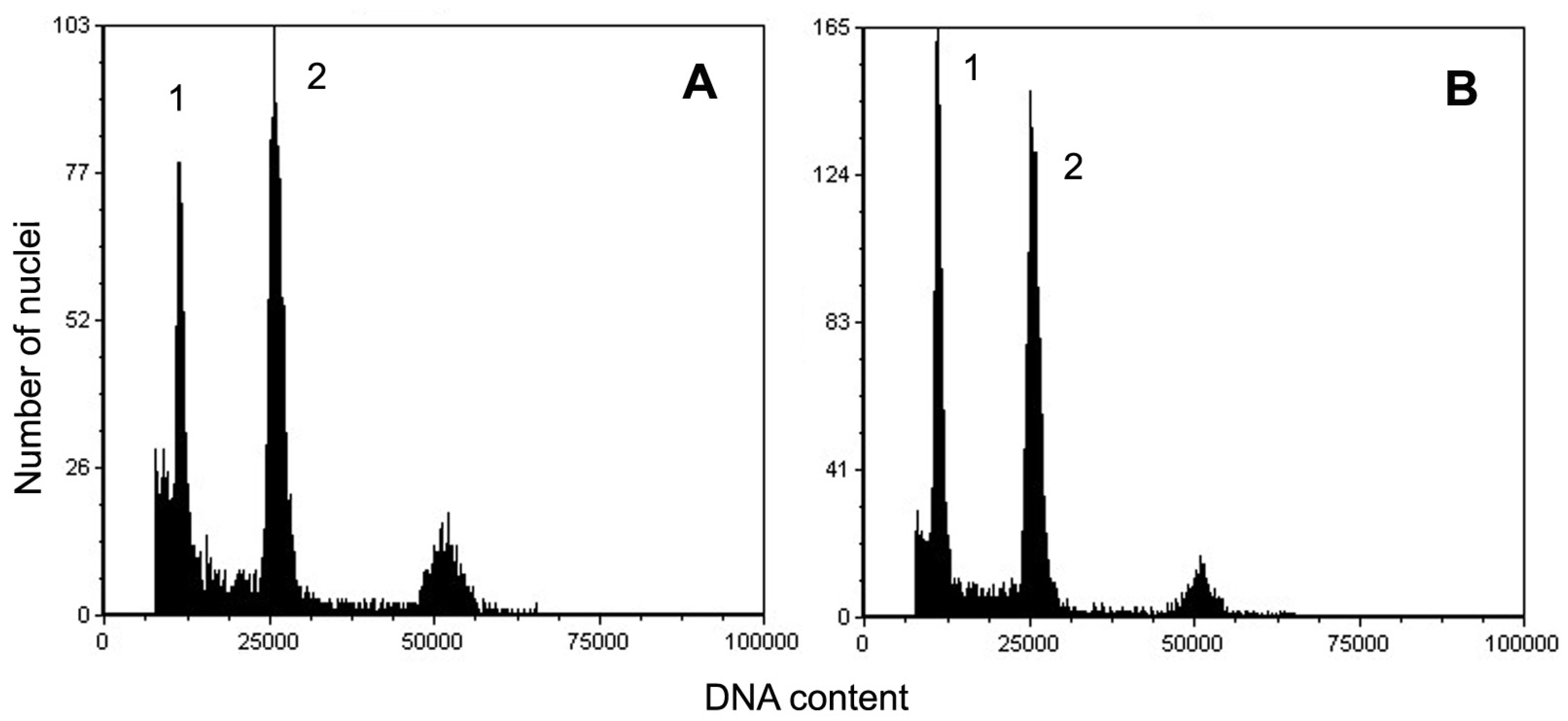
3.5. Flow Cytometry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IUCN. Guidelines for Reintroductions and Other Conservation Translocations; Version 1.0; IUCN Species Survival Commission: Gland, Switzerland, 2013; Available online: https://portals.iucn.org/library/efiles/documents/2013-009.pdf (accessed on 5 November 2022).
- IUCN. IUCN Red List of Threatened Species: Threats Classification Scheme Version 3.2. 2017. Available online: https://www.iucnredlist.org/resources/threat-classification-scheme (accessed on 3 November 2022).
- Gómez, J.M.; González-Megías, A.; Lorite, J.; Abdelaziz, M.; Perfectti, F. The silent extinction: Climate change and the potential hybridization-mediated extinction of endemic high-mountain plants. Biodivers. Conserv. 2015, 24, 1843–1857. [Google Scholar] [CrossRef]
- McLaughlin, B.C.; Ackerly, D.D.; Klos, P.Z.; Natali, J.; Dawson, T.E.; Thompson, S.E. Hydrologic refugia, plants, and climate change. Glob. Chang. Biol. 2017, 23, 2941–2961. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.P.; Seddon, P.J. Directions in reintroduction biology. Trends Ecol. Evol. 2008, 23, 20–25. [Google Scholar] [CrossRef]
- Zimmer, H.C.; Auld, T.D.; Cuneo, P.; Offord, C.A.; Commander, L.E. Conservation translocation—An increasingly viable option for managing threatened plant species. Aust. J. Bot. 2019, 67, 501–509. [Google Scholar] [CrossRef]
- Seddon, P.J.; Armstrong, D.P. Reintroduction and other conservation translocations: History and future developments. In Reintroduction of Fish and Wildlife Populations; Jackowski, D.S., Millspaugh, J.J., Angermeier, P.L., Stotow, R., Eds.; University of California Press: Oakland, CA, USA, 2016; pp. 7–27. [Google Scholar]
- Convention on the Conservation of European Wildlife and Natural Habitats (Convention Relative à la Conservation de la vie Sauvage et du Milieu Naturel de l’Europe) Bern/Berne, 19.IX.1979 Appendix I—Strictly Protected Flora Species. Available online: http://www.bgci.org.uk/files/7/0/global_strategy.pdf (accessed on 3 November 2022).
- IUCN. The IUCN Red List of Threatened Species. Version 2022-1. 2022. Available online: https://www.iucnredlist.org (accessed on 5 November 2022).
- Salama, A.; Shukla, M.R.; Popova, E.; Fisk, N.S.; Jones, M.P.; Saxena, P.K. In vitro propagation and reintroduction of golden paintbrush (Castilleja levisecta), a critically imperilled plant species. Can. J. Plant Sci. 2018, 98, 762–770. [Google Scholar] [CrossRef]
- Schäfer, D.; Vincent, H.; Fischer, M.; Kempel, A. The importance of genetic diversity for the translocation of eight threatened plant species into the wild. Glob. Ecol. Conserv. 2020, 24, e01240. [Google Scholar] [CrossRef]
- Sheikholeslami, B.; Shukla, M.; Turi, C.; Harpur, C.; Saxena, P.K. Saving threatened plant species: Reintroduction of Hill’s thistle (Cirsium hillii (Canby) Fernald) to its natural habitat. PLoS ONE 2020, 15, e0231741. [Google Scholar] [CrossRef]
- Kułak, V.; Longboat, S.; Brunet, N.D.; Shukla, M.; Saxena, P. In vitro technology in plant conservation: Relevance to biocultural diversity. Plants 2022, 11, 503. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic plant species conservation: Biotechnological approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef]
- Sarasan, V.A.; Cripps, R.; Ramsay, M.M.; Atherton, C.; McMichen, M.; Prendercast, G.; Rowntree, J. Conservation in vitro of threatened plants—Progress in the past decade. In Vitro Cell. Dev. Biol.-Plant 2006, 42, 206–214. [Google Scholar] [CrossRef]
- Phillips, R.L.; Kaeppler, S.M.; Olhoft, P. Genetic instability of plant tissue cultures: Breakdown of normal controls. Proc. Natl. Acad. Sci. USA 1994, 91, 5222–5226. [Google Scholar] [CrossRef] [PubMed]
- Endemann, M.; Hristoforoglu, K.; Stauber, T.; Wilhelm, E. Assessment of age-related polyploidy in Quercus robur L. somatic embryos and regenerated plants using DNA flow cytometry. Biol. Plant 2001, 44, 339–345. [Google Scholar] [CrossRef]
- Rodrigues, P.H.V. Somaclonal variation in micropropagated Heliconia bihai cv. Lobster Claw I plantlets (Heliconiaceae). Sci. Agric. 2008, 65, 681–684. [Google Scholar] [CrossRef]
- Hossain, A.; Konisho, K.; Minami, M.; Nemoto, K. Somaclonal variation of regenerated plants in chili pepper (Capsicum annuum L.). Euphytica 2003, 130, 233–239. [Google Scholar] [CrossRef]
- Kawiak, A.; Łojkowska, E. Application of rapd in the determination of genetic fidelity in micropropagated Drosera plantlets. In Vitro Cell. Dev. Biol. Anim. 2004, 40, 592–595. [Google Scholar] [CrossRef]
- Modgil, M.; Mahajan, K.; Chakrabarti, S.; Sharma, D.; Sobti, R. Molecular analysis of genetic stability in micropropagated apple rootstock MM106. Sci. Hortic. 2005, 104, 151–160. [Google Scholar] [CrossRef]
- Thiem, B.; Śliwińska, E. Flow cytometric analysis of nuclear DNA content in cloudberry (Rubus chamaemorus L.) in vitro cultures. Plant Sci. 2003, 164, 129–134. [Google Scholar] [CrossRef]
- Ochatt, S.J.; Patat-Ochatt, E.M.; Moessner, A. Ploidy level determination within the context of in vitro breeding. Plant Cell Tiss. Organ Cult. (PCTOC) 2011, 104, 329–341. [Google Scholar] [CrossRef]
- Singh, S.R.; Dalal, S.; Singh, R.; Dhawan, A.K.; Kalia, R.K. Evaluation of genetic fidelity of in vitro raised plants of Dendrocalamus asper (Schult. & Schult. F.) Backer ex K. Heyne using DNA-based markers. Acta Physiol. Plant. 2012, 35, 419–430. [Google Scholar] [CrossRef]
- Thiem, B.; Kikowska, M.; Krawczyk, A.; Więckowska, B.; Śliwinska, E. Phenolic acid and DNA contents of micropropagated Eryngium planum L. Plant Cell Tiss. Organ Cult. (PCTOC) 2013, 114, 197–206. [Google Scholar] [CrossRef]
- Nybom, H.; Weising, K.; Rotter, B. DNA fingerprinting in botany: Past, present, future. Investig. Genet. 2014, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.G.; Kubelik, A.R.; Livak, K.J.; Rafalski, J.; Tingey, S.V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990, 18, 6531–6535. [Google Scholar] [CrossRef] [PubMed]
- Ziętkiewicz, E.; Rafalski, A.; Labuda, D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 1994, 20, 176–183. [Google Scholar] [CrossRef]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; Van De Lee, T.; Hornes, M.; Friters, A.; Pot, J.; Paleman, J.; Kuiper, M. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995, 23, 4407–4414. [Google Scholar] [CrossRef] [PubMed]
- Rewers, M.; Kisiala, A.B.; Drouin, J.; Śliwinska, E.; Cholewa, E. In vitro-regenerated wetland sedge Eriophorum vaginatum L. is genetically stable. Acta Physiol. Plant. 2012, 34, 2197–2206. [Google Scholar] [CrossRef]
- Picó, F.X.; Abdelaziz, M.; Castilla, A.R. Introduction to the special issue: The ecology and genetics of population differentiation in plants. AoB Plants 2021, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Pogorzelec, M.; Głębocka, K.; Hawrylak-Nowak, B.; Bronowicka-Mielniczuk, U. Assessment of chosen reproductive cycle processes and genetic diversity of Salix myrtilloides L. in wetlands of Polesie Lubelskie: The prospects of its survival in the region. Pol. J. Ecol. 2015, 63, 291–303. [Google Scholar]
- Pogorzelec, M.; Parzymies, M.; Bronowicka-Mielniczuk, U.; Banach-Albińska, B.; Serafin, A. Pollen viability and tissue culture initiation of Salix lapponum, an endangered species in Poland. Acta Sci. Pol. Hortorum Cultus 2015, 14, 151–161. [Google Scholar]
- Pogorzelec, M.; Serafin, A.; Banach-Albińska, B.; Szczurowska, A.; Parzymies, M.; Bronowicka-Mielniczuk, U. Pollen viability of an endangered species in Poland—Salix myrtilloides L. Acta Agrobot. 2016, 69, 1679. [Google Scholar] [CrossRef][Green Version]
- Churski, M.; Danielewicz, W. Salix myrtilloides in north central Poland. Distribution, threats and conservation. Dendrobiology 2008, 60, 3–9. [Google Scholar]
- Serafin, A.; Pogorzelec, M.; Banach-Albińska, B.; Mielniczuk, J. Habitat conditions of the endangered species Salix myrtilloides in Eastern Poland. Dendrobiology 2015, 73, 55–64. [Google Scholar] [CrossRef]
- Kozub, Ł.; Pawlikowski, P. New locality of Salix myrtilloides (Salicaceae) in the Pojezierze Mazurskie lake district. Fragm. Flor. Geobot. Pol. 2016, 23, 349–352. [Google Scholar]
- Turis, P.; Kliment, J.; Feráková, V.; Dítě, D.; Eliáš, P.; Hrivnák, R.; Bernátová, D. Red list of vascular plants of the Carpathian part of Slovakia. Thaiszia J. Bot. 2014, 24, 35–87. [Google Scholar]
- Kaźmierczakowa, R.; Zarzycki, K.; Mirek, Z. Polska Czerwona Księga Roślin. Paprotniki i Rośliny Kwiatowe (Polish Red Data Book of Plants. Pteridophytes and Flowering Plants), 3rd ed.; Instytut Ochrony Przyrody PAN: Kraków, Poland, 2014; p. 895. [Google Scholar]
- Grulich, V.; Chobot, K.; Plesník, J. Červený seznam ohrožených druhů České republiky: CÉVNATÉ ROSTLINY: Red List of threatened species of Czech Republic: VASCULAR PLANTS. Příroda 2017, 35, 178. [Google Scholar]
- Elven, R.; Karlsson, T.; Salix, L. Flora Nordica; Swedish Royal Academy of Sciences: Stockholm, Sweden, 2000; Volume 1, pp. 117–188. [Google Scholar]
- Urban, D.; Wawer, M. Salix lapponum L. and S. myrtilloides L. in the area of Sobibor in the Leczynsko-Wlodawskie Lake District (Poland). Ann. UMCS Sect. E Agric. 2001, 56, 83–93. [Google Scholar]
- Skálová, D.; Navrátilová, B.; Richterová, L.; Knit, M.; Sochor, M.; Vasut, R.J. Biotechnological methods of in vitro propagation in willows (Salix spp.). Open Life Sci. 2012, 7, 931–940. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Galbraith, D.W.; Harkins, K.R.; Maddox, J.M.; Ayres, N.M.; Sharma, D.P.; Firoozabady, E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 1983, 220, 1049–1051. [Google Scholar] [CrossRef]
- Doležel, J.; Sgorbati, S.; Lucretti, S. Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiol. Plant. 1992, 85, 625–631. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GenAlex 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Mashkina, O.; Tabatskaya, T.M.; Gorobets, A.I.; Shestibratov, K.A. Method of clonal micropropagation of different willow species and hybrids. Appl. Biochem. Microbiol. 2010, 46, 769–775. [Google Scholar] [CrossRef]
- Parzymies, M.; Pogorzelec, M.; Głębocka, K.; Śliwińska, E. Genetic stability of the endangered species Salix lapponum L. regenerated in vitro during the reintroduction process. Biology 2020, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Chalupa, V. In vitro propagation of willows (Salix spp.), European mountain-ash (Sorbus aucuparia L.) and black locust (Robinia pseudoacacia L.). Biol. Plant. 1983, 25, 305–307. [Google Scholar] [CrossRef]
- Grendysz, J.; Wróbel, J.; Kulpa, D. Influence of micropropagation with addition of kinetin on development of a willow (Salix viminalis L.). World Sci. News 2017, 70, 201–215. [Google Scholar]
- Brandova, B.; Hrones, M.; Knitl, M.; Richterova, L.; Skálová, D.; Navratilowa, B.; Vasut, R.J. Biotechnologicke in vitro metody u ohrożenych druhu vrb. Opera Corcon. 2011, 48, 79–88. [Google Scholar]
- Shenoy, V.B.; Vasil, I.K. Biochemical and molecular analysis of plants derived from embryogenic cultures of Napier grass (Pennisetum purpureum K. Schum.). Theor. Appl. Genet. 1992, 83, 947–955. [Google Scholar] [CrossRef]
- Godwin, I.D.; Sangduen, N.; Kunanuvatchaidach, R.; Piperidis, G.; Adkins, S.W. RAPD polymorphisms among variant and phenotypically normal rice (Oryza sativa var. indica) somaclonal progenies. Plant Cell Rep. 1997, 16, 320–324. [Google Scholar] [CrossRef]
- Abdelsalam, A.; Mahran, E.; Chowdhury, K.; Boroujerdi, A. Metabolic profiling, in vitro propagation, and genetic assessment of the endangered rare plant Anarrhinum pubescens. J. Genet. Eng. Biotechnol. 2021, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Rani, V.; Parida, A.; Raina, S.N. Random amplified polymorphic DNA (RAPD) markers for genetic analysis in micropropagated plants of Populus deltoides Marsh. Plant Cell Rep. 1995, 14, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Devarumath, R.M.; Nandy, S.; Rani, V.; Marimuthu, S.; Muraleedharan, N.; Raina, S.N. RAPD, ISSR and RFLP fingerprints as useful markers to evaluate genetic integrity of micropropagated plants of three diploid and triploid elite tea clones representing Camellia sinensis (China type) and C. assamica ssp. assamica (Assam-India type). Plant Cell Rep. 2002, 21, 166–173. [Google Scholar] [CrossRef]
- Pinto, G.; Loureiro, J.; Lopes, T.; Santos, C. Analysis of the genetic stability of Eucalyptus globulus Labill. somatic embryos by flow cytometry. Theor. Appl. Genet. 2004, 109, 580–587. [Google Scholar] [CrossRef]
- Loureiro, J.; Pinto, G.; Lopes, T.; Doležel, J.; Santos, C. Assessment of ploidy stability of the somatic embryogenesis process in Quercus ruber L. using flow cytometry. Planta 2005, 221, 815–822. [Google Scholar] [CrossRef]
- Brito, G.; Lopes, T.; Loureiro, J.; Rodriguez, E.; Santos, C. Assessment of genetic stability of two micropropagated wild olive species using flow cytometry and microsatellite markers. Trees 2010, 24, 723–732. [Google Scholar] [CrossRef]
- Escobedo-GraciaMedrano, R.M.; Maldonado-Borges, J.I.; Burgos-Tan, M.J.; Valadez-Gonzalez, N.; Ku-Cauich, J.R. Using flow cytometry and cytological analyses to assess the genetic stability of somatic embryo-derived plantlets from embryogenic Musa acuminata Colla (AA) ssp. malaccensis cell suspension cultures. Plant Cell Tiss. Organ Cult. (PCTOC) 2013, 116, 175–185. [Google Scholar] [CrossRef]
- Kikowska, M.; Włodarczyk, A.; Rewers, M.; Sliwinska, E.; Studzińska-Sroka, E.; Witkowska-Banaszczak, E.; Stochmal, A.; Żuchowski, J.; Dlugaszewska, J.; Thiem, B. Micropropagation of Chaenomeles japonica: A step towards production of polyphenol-rich extracts showing antioxidant and antimicrobial activities. Molecules 2019, 24, 1314. [Google Scholar] [CrossRef]
- Leitch, I.J.; Johnston, E.; Pellicer, J.; Hidalgo, O.; Bennett, M.D. Plant DNA C-Values Database (Release 7.1, Apr 2019). 2019. Available online: https://cvalues.science.kew.org (accessed on 5 November 2022).



| Reagent | Concentration |
|---|---|
| PCR buffer (750 mM Tris pH 8.8; 200 mM (NH4)2SO4; 0.1% Tween 20) | 1× |
| MgCl2 | 3 mM |
| dNTP | 200 μM (each) |
| Primer | 670 nM |
| Taq DNA Polymerase (Thermo Fisher Scientific Waltham, MA, USA) | 0.5 U |
| Initial Denaturation | Touchdown Cycles | Enrichment Cycles | Final Extension | |||||
|---|---|---|---|---|---|---|---|---|
| Denaturation | Annealing | Elongation | Denaturation | Annealing | Elongation | |||
| Temperature (°C) | 94 | 94 | 54→52 (−1 °C each 3 cycles) | 72 | 94 | 52 | 72 | 72 |
| Time (s) | 120 | 30 | 45 | 120 | 30 | 45 | 120 | 7 |
| Number of cycles | - | 6 | 32 | - | ||||
| Disinfection Method | Number of Regenerating Explants without Contamination n/N (%) | Number of Necrotic Explants nec/N (%) |
|---|---|---|
| NaOCl 1.5% | 66/170 (38.8%) | 44/170 (25.9%) |
| AgNO3 0.5% | 23/164 (14.0%) | 118/164 (72.0%) |
| Two-step disinfection | 24/140 (17.1%) | 53/140 (37.9%) |
| Cytokinin | Concentration (mg·dm−3) | Main Shoot Length (mm) | Number of Nodes | Main Shoot Weight (mg) | Plants with Axillary Shoots (%) | Number of Axillary Shoots/Plant | Mn Rate ** |
|---|---|---|---|---|---|---|---|
| K | 0 | 40.24 bd * | 11.26 a | 68.87 ac | 5 | 3.0 ab | 5.78 |
| BA | 0.1 | 17.71 df | 6.61 bc | 68.48 ac | 67 | 1.67 b | 4.43 |
| 0.5 | 61.00 ab | 10.90 a | 108.81 a | 65 | 2.08 b | 6.80 | |
| 1 | 38.00 ce | 8.50 ab | 69.53 ac | 90 | 1.94 b | 6.00 | |
| 2.5 | 23.65 df | 6.35 ad | 45.73 ac | 41 | 2.57 b | 4.23 | |
| KIN | 0.1 | 47.90 ac | 7.60 b | 94.86 ab | 25 | 1.20 b | 4.10 |
| 0.5 | 67.26 a | 9.21 ab | 124.00 a | 16 | 1.00 b | 4.77 | |
| 1 | 68.05 a | 8.42 ab | 138.66 a | 5 | 1.00 b | 4.26 | |
| 2.5 | 58.80 ab | 7.70 b | 100.47 ab | 25 | 1.40 b | 4.20 | |
| 2iP | 0.1 | 24.78 cf | 7.11 b | 62.27 ac | 79 | 4.87 a | 7.41 |
| 0.5 | 12.59 ef | 7.75 b | 56.02 ac | 100 | 2.89 b | 6.77 | |
| 1 | 38.38 ce | 3.53 d | 30.42 bc | 69 | 2.44 b | 3.56 | |
| 2.5 | 11.00 f | 3.75 cd | 23.26 c | 55 | 1.18 b | 2.42 |
| Cytokinin | Concentration (mg·dm−3) | Number of Rooted Shoots (%) | Number of Roots | Length of Roots (mm) | Weight of Roots (mg) | Number of Shoots with Callus (%) | Weight of Callus (mg) |
|---|---|---|---|---|---|---|---|
| K | 0 | 100 | 4.11 ab * | 22.75 b | 2.01 d | 0 | - |
| BA | 0.1 | 5 | 2.00 b | 23.00 ab | 1.85 d | 33 | 20.37 ab |
| 0.5 | 100 | 4.65 ab | 42.03 a | 13.23 bc | 50 | 24.43 ab | |
| 1 | 100 | 5.45 a | 52.00 a | 11.97 bc | 80 | 23.85 ab | |
| 2.5 | 95 | 5.13 a | 42.66 a | 11.24 bc | 76 | 41.06 a | |
| KIN | 0.1 | 100 | 3.30 ab | 41.15 a | 10.30 bc | 15 | 1.70 b |
| 0.5 | 100 | 3.21 ab | 41.08 a | 13.74 bc | 0 | - | |
| 1 | 95 | 3.00 ab | 47.56 a | 21.72 ab | 0 | - | |
| 2.5 | 95 | 2.94 ab | 46.71 a | 28.97 a | 0 | - | |
| 2iP | 0.1 | 68 | 5.31 a | 42.18 a | 6.71 c | 83 | 35.33 ab |
| 0.5 | 0 | - | - | - | 100 | 33.22 ab | |
| 1 | 0 | - | - | - | 72 | 38.54 ab | |
| 2.5 | 0 | - | - | - | 0 | - |
| Primer | Sequence | Products Size Range (bp) |
|---|---|---|
| SR1 | (AG)8G | 350–1150 |
| SR14 | (GA)8YG | 300–1500 |
| SR16 | (GA)8C | 320–950 |
| SR32 | (AG)8YT | 200–620 |
| SR75 | (AT)8C | 640–1600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parzymies, M.; Pogorzelec, M.; Głębocka, K.; Sliwinska, E. Micropropagation Protocol and Genetic Stability of the Salix myrtilloides Plants Cultivated In Vitro. Biology 2023, 12, 168. https://doi.org/10.3390/biology12020168
Parzymies M, Pogorzelec M, Głębocka K, Sliwinska E. Micropropagation Protocol and Genetic Stability of the Salix myrtilloides Plants Cultivated In Vitro. Biology. 2023; 12(2):168. https://doi.org/10.3390/biology12020168
Chicago/Turabian StyleParzymies, Marzena, Magdalena Pogorzelec, Katarzyna Głębocka, and Elwira Sliwinska. 2023. "Micropropagation Protocol and Genetic Stability of the Salix myrtilloides Plants Cultivated In Vitro" Biology 12, no. 2: 168. https://doi.org/10.3390/biology12020168
APA StyleParzymies, M., Pogorzelec, M., Głębocka, K., & Sliwinska, E. (2023). Micropropagation Protocol and Genetic Stability of the Salix myrtilloides Plants Cultivated In Vitro. Biology, 12(2), 168. https://doi.org/10.3390/biology12020168









