Simple Summary
Soybean meal is currently one of the most widely used ingredients in aquafeed due to its consistent supply, reasonable price, and well-balanced amino acid profile. However, anti-nutritional factors such as antigen proteins and soybean agglutinin in soybean meal can also cause intestinal barrier injury and inflammatory reactions in fish, harming fish growth, immunity, and intestinal health. Intestinal health is especially important for carnivorous fish such as rainbow trout (Oncorhynchus mykiss), which have a relatively small intestinal length index. In this study, it was confirmed that dietary NaB levels of 0.20% improved the growth of juvenile O. mykiss and reduced the severity of anti-nutritional factor-induced enteropathy by enhancing digestive enzymes and improving intestinal morphology. Dietary NaB enhanced the mRNA expression of TJ proteins while decreasing the mRNA expression of pro-inflammatory cytokines, NF-κB, and MLCK-MLC, likely reinforcing the intestinal mucosal barrier and protecting against pathogenic bacterial infections. In addition, the gut microbial composition was more conducive to O. mykiss health with the inclusion of 0.20% NaB in the diet. Furthermore, when challenged with Aeromonas salmonicida, dietary 0.20% NaB increased the survival rate of O. mykiss. This study provides a theoretical basis for the development of feed additives for use in low-fish meal feeds.
Abstract
This study aimed to determine the effects of dietary sodium butyrate (NaB) on the growth and gut health of triploid Oncorhynchus mykiss juveniles (8.86 ± 0.36 g) fed a low fish meal diet for 8 weeks, including the inflammatory response, histomorphology, and the composition and functional prediction of microbiota. Five isonitrogenous and isoenergetic practical diets (15.00% fish meal and 21.60% soybean meal) were supplemented with 0.00% (G1), 0.10% (G2), 0.20% (G3), 0.30% (G4), and 0.40% NaB (G5), respectively. After the feeding trial, the mortality for G3 challenged with Aeromonas salmonicida for 7 days was lower than that for G1 and G5. The optimal NaB requirement for triploid O. mykiss based on weight gain rate (WGR) and the specific growth rate (SGR) was estimated to be 0.22% and 0.20%, respectively. The activities of intestinal digestive enzymes increased in fish fed a NaB diet compared to G1 (p < 0.05). G1 also showed obvious signs of inflammation, but this inflammation was significantly alleviated with dietary NaB supplementation. In comparison, G3 exhibited a more complete intestinal mucosal morphology. Dietary 0.20% NaB may play an anti-inflammatory role by inhibiting the NF-κB-P65 inflammatory signaling pathway. Additionally, the relative abundance of probiotics was altered by dietary NaB. In conclusion, dietary 0.20% NaB improved the intestinal health of triploid O. mykiss fed a low fish meal diet.
1. Introduction
The rapid growth of aquaculture in recent years has resulted in a global deficit of fish meal supplies, which has prompted the urgent need for alternative protein sources for fish meal []. Plant protein is considered a crucial alternate protein source to fish meal, but it contains antinutritional elements and poisonous compounds, such as cotton phenol that can cause intestinal damage [,,]. Soybean meal is currently one of the most widely used ingredients in aquafeed due to its consistent supply, fair price, and well-balanced amino acid profile []. However, anti-nutritional factors (ANF) such as antigen protein (AP) and soybean agglutinin (SBA) in soybean meal can also cause intestinal barrier injury and inflammatory reactions in fish. This can harm fish growth, immunity, and intestinal health [,,,,]. The intestinal barrier is a complex combination of interactions between the immune cells, epithelial barrier, and gut microbiota []. Intestinal health is especially important for carnivorous fish such as rainbow trout (Oncorhynchus mykiss), as they have a relatively small intestinal length index. Therefore, functional feed additives that can reduce intestinal injury caused by ANF in plant proteins have yet to be developed. Several functional ingredients, such as glutathione and arginine, have been shown to protect intestinal health in O. mykiss fed a high level of dietary soybean protein [,].
Short-chain fatty acids (SCFAs), particularly butyric acid (BA), play a crucial role in maintaining the integrity of the intestinal epithelial barrier []. BA influences the functions of various host systems, including the intestinal, hematological, neurological, and endocrine systems, and it has anti-inflammatory effects that are closely linked to intestinal inflammation []. In addition to being absorbed by transporter proteins such as MCT1 and SMCT1 to support cellular metabolism, BA can act as a signaling molecule []. Previous in vivo and in vitro studies of NaB have demonstrated its ability to suppress NF-κB, leading to a decrease in the expression and production of pro-inflammatory cytokines and the innate immune response [,,,]. Lower concentrations of BA (up to 2 mM) have been shown to reduce permeability in HT-29 and Caco-2 cell lines [,], but higher concentrations (8 mM and 10 mM) significantly increased the permeability of Caco-2 cell lines and the distal colonic mucosa of adult rats [,]. Additionally, previous research has also shown that dietary NaB may be used to protect aquatic creatures against enteropathy (enteritis) by activating antioxidant defense responses and enhancing local immunity in the intestine against invading pathogenic organisms.
The gut microbial composition is critical for growth, and a stable and rational composition of the microbial community is important for fish health. Rawls et al. found that the gut of zebrafish (Danio rerio) has 212 genes related to the microbial community in the intestine []. The gut microbial community performs various functions, such as stimulating epithelial cell renewal [], promoting nutrient metabolism [], and enhancing immune function []. By studying the intestinal microbiota of fish, researchers can use diet to manipulate the number of intestinal probiotics and improve fish productivity []. Dietary NaB has been shown to alter the composition of the gut microorganisms in turbot (Scophthalmus maximus L.), which were more similar to the fish meal (FM) group than the soybean meal (SBM) group []. However, research on common carp (Cyprinus carpio) has shown that NaB has no significant effect on the microbial populations in the intestine []. However, research on the active mechanism of BA on the gut microbiota of carnivorous fish is limited. It is unclear why NaB affects the gut microbiota of different dietary fish in different ways.
According to the Food and Agriculture Organization of the United Nations (FAO) [], farmed salmon and trout production exceeds 3 million tons annually, making it the third largest aquaculture species in the world as of 2020. In recent years, trout farming in China has grown rapidly and has become one of the main cold-water fish farming species in the country, with annual production reaching 30,000 tons []. O. mykiss has been cultivated in China since the 1950s, and its triploids can now be found in most provinces and regions of the country. Triploid O. mykiss are poorly fertile, which means that they do not use all of their energy for growth. However, they grow faster than diploid O. mykiss during the gonadal maturity stage, and they have better disease resistance and flesh quality []. This study aimed to investigate the effects of dietary NaB on growth, digestion, intestinal histology, inflammation, histomorphology, microbiota composition and function, and resistance to Aeromonas salmonicida in juvenile triploid O. mykiss (8.86 ± 0.36 g) fed a low fish meal diet. By evaluating the relative expression of genes involved in the NF-κB and MLCK-MLC signaling pathways, we examined the effects of dietary NaB on intestinal cell inflammation and mucosal permeability. This work serves as a reference for further research on the process by which BA restores intestinal mucosa. To determine whether NaB affects the gut microbiota of O. mykiss, we analyzed the hypervariable V4 region of bacteria using an Illumina MiSeq sequencer. These findings will provide a foundation for future studies on the intestinal immune system of triploid O. mykiss and the development of low-fish meal compound diets.
2. Materials and Methods
2.1. Diets
In this experiment, five groups were identified: the control group (G1) was fed a basal diet, and four additional groups (G2–G5) were each supplemented with a different amount of NaB at concentrations of 0.10%, 0.20%, 0.30%, and 0.40%, respectively. The composition of the basal diet is listed in Table 1. Wheat flour, fish meal, and soybean meal were used as carbohydrate, protein, and lipid sources, respectively. The ingredients were weighed, finely ground, and blended according to the specified formulations. Lipid was added, and the materials were mixed for 25 min. The mixture was then homogenized again by adding a sufficient amount of distilled water to reach a caking state. Using a pelletizer (HX-200G; Guanghe Instruments, Guangzhou, China), the mixture was formed into 1 mm pellets, which were air-dried at 60 °C, cooled to room temperature, and stored in sealed bags in a −20 °C refrigerator.

Table 1.
Ingredients and their contents of the test diets (air-dry basis, %).
2.2. Experimental Conditions
The O. mykiss used in this experiment were purchased from Agrimarine Holdings Inc. (Benxi, China) and acclimated by being fed a basal diet for 14 days before the start of the experiment. From a pool of 450 fish with an initial weight of 8.86 ± 0.36 g, 15 tanks (each with a volume of 500 L) containing 30 fish each were randomly chosen. The fish were fed the test diets until apparent satiation twice a day during the 8-week feeding trial, which took place at 8:00 a.m. and 4:00 p.m. The fish were weighed every 14 days to determine body weight and adjust meal amounts. The amount of feed administered per week was counted, and the total feed amount was calculated. The O. mykiss were kept in a recirculating aquaculture system with a temperature control system and water filtration system. The water was aerated tap water, which was controlled to have the following parameters: temperature 14 ± 0.5 °C, dissolved oxygen > 6.0 mg/L, pH 6.8–7.1, NO2™-N < 0.02 mg/L, and NH4+-N < 0.20 mg/L. The water quality was measured using a YSI 8500 SELECT™ Biochemistry Analyzer (YSI Inc., Yellow Springs, OH, USA), and approximately 160 L of water per tank was renewed daily to ensure suitable water quality.
2.3. Experimental Sample Collection
At the end of the 56-day feeding trial, O. mykiss were fasted for one day before sampling to empty the contents of their digestive tracts. They were then weighed after being anesthetized with MS-222 (75 mg/L), and the total weight of each tank was recorded to calculate the growth index using a balance (ME204E, METTLER TOLEDO Technology Co., Shanghai, China). Tail vein blood samples were taken, and the supernatant was collected as serum after centrifugation at 1530× g for 10 min at 4 °C. The serum was kept at −40 °C for subsequent biochemical assays. Six O. mykiss were used to obtain mid-intestine samples (between the pyloric and hindgut), which were kept at −80 °C for biochemical examination. Three different fish were dissected to obtain proximal intestine samples, which were immediately frozen in liquid nitrogen and stored at −80 °C for gene expression analysis. Two O. mykiss were sterilized with 75% alcohol, and the whole mucosa layer of the distal intestine was removed using sterile tools under an alcohol lamp for the investigation of the intestinal microbiota. Afterward, the intestines of three fish from each group were removed, the contents were cleared, and the fish were placed in Bouin’s solution for intestinal histology.
2.4. Nutrient Proximate Analysis
The nutrient levels of the whole O. mykiss and test diets were determined according to the standard methods described in AOAC (2012) []. The samples’ moisture content was determined by baking them at 105 °C for three hours. The Kjeldahl method (method 2001.11) was used to measure nitrogen in order to assess crude protein (N × 6.25; 2300, FOSS Tecator AB, Hogans, Sweden). The ash concentration was determined by burning the samples for two hours at 600 °C (method number: 942.05). Crude lipid was extracted from the O. mykiss fishmeal using the Soxhlet extraction method (method number: 920.39).
2.5. LPS, AMS, and Trypsin Activities
The activities of intestinal lipase (LPS), amylase (AMS), and trypsin were measured using commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The intestinal tissue homogenates were centrifuged at 861× g for 10 min after adding 9 volumes (w/v) of 5.3% ice-cold saline. The supernatant was then centrifuged and stored at −80 °C until needed. Lipase (LPS; A054-2-1) activity was measured at 580 nm based on the rate of production of methyl tachykinin in red. The amount of hydrolyzed starch was calculated based on the blue complex formed when the unhydrolyzed starch was mixed with iodine solution and its absorbance at 660 nm, allowing for the calculation of intestinal amylase (AMS; C016-1-1) activity. The UV colorimetric approach was used to determine the amount of trypsin (A080-2-2) present. Total protein (TP) was quantified using the Coomassie bright blue method at 595 nm. All absorbance measurements were performed using a microplate reader (Synergy 2, BoiTek Instruments, Inc., Winooski, VT, USA).
2.6. Histological Examination
Four O. mykiss intestinal segments without alimentary canal contents were randomly placed in Bouin’s solution, rinsed multiple times in water, and soaked in 70% ethanol until the ethanol did not change color. The dehydrated midguts were then embedded in normal paraffin. Using a microtome (HistoCore MULTICUT, Leica, Shanghai, China), the material was cut into 4 μm thick sections. After deparaffinization and hydration, the slides were stained with hematoxylin and eosin and mounted with neutral resin. Each group had 30 sections of the MI examined under a microscope (Echo Revolve, San Diego, CA, USA).
2.7. Gene Expression Analyses
Liquid nitrogen was used to grind O. mykiss gut samples that were stored at −80 °C. Total RNA was extracted from the resulting powder using TRIzol Reagent (Ambion, San Diego, CA, USA) and then reverse transcribed into cDNA (RR047A; TaKaRa, Beijing, China). The quality of the RNA and cDNA was assessed using agarose gel electrophoresis (visible bands) and a Thermo Scientific NanoDrop 2000 UV-Vis spectrophotometer (A260 nm/A280 nm: 1.8–2.0). cDNA was used as template DNA for RT-PCR amplification reactions. Three replicates of each amplification reaction were used for comparison (Table 2). Table 3 lists the sequences of all the primers used in this study. The internal reference gene β-actin was used to normalize cDNA loading. The 2−ΔΔCT method was used to compare the expression results of each gene mRNA [].

Table 2.
PCR amplification procedure and real-time PCR primer sequences.

Table 3.
Primer sequences.
2.8. 16S rRNA Gene Sequencing and Bioinformatic Analysis
The purity of total microbial DNA extracted from the alimentary canal contents of O. mykiss was assessed using A230 nm, A260 nm, and A280 nm. The highly variable V3-V4 region of the bacterial 16S rRNA gene was amplified according to the PCR procedure in Table 2. The PCR amplification products were purified using agarose gel electrophoresis (AxyPrep DNA Gel Extraction Kit; Axygen Biosciences, Union City, CA, USA).
Illumina adapter sequences were added to the outer end of the target region by PCR and used to construct the Miseq library (Illumina, Shanghai, China). Pair-end sequencing was performed on Miseq technology. The PE reads obtained from Miseq sequencing were first spliced according to the overlapping relationship (Fast Length Adjustment of Short Reads, FLASH 1.2.11) [], while sequence quality was quality-controlled (Uparse 11) and filtered (Usearch 11), and operational taxonomic units (OTUs) clustering analysis and species taxonomy analysis were performed after differentiating samples. Based on the results of OTU clustering analysis, various diversity indices and the detection of sequencing depth for OTUs can be analyzed, such as alpha diversity analysis (Mothur 1.30.2) and beta diversity analysis (Qiime 1.9.1) []. UCHIME was used to identify chimeric sequences []. The raw reads were submitted to the NCBI Sequence Read Archive (SRA, accession number: PRJNA874478). A statistical analysis of community structure can be performed at each taxonomic level using the taxonomic information. Based on the above analysis, a series of in-depth statistical and visualization analyses, such as multivariate analysis and significance tests, can be performed on the community composition and phylogenetic information of multiple species.
Principal component analysis (PCA) plots based on the OTU level were used to assess beta diversity. Different intestinal bacteria were found in each of the five groups, and the microbial makeup at the phylum and genus levels was examined. To identify which bacterial taxa were different between groups, a linear discriminant analysis (LDA) effect size (LEfSe) investigation was carried out using the Python LEfSe module []. Non-binary co-expression network analysis was used to measure the intra-order interaction of variables (weighted topological, wTO). The wTO technique calculates a link-to-link threshold of significance with a p-value adjusted for multiple tests of 0.05 []. Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2) was used to predict functional pathways in KEGG Orthology (KOs) [] using the 16S rRNA gene sequencing data. The phenotypic characteristics of the bacterial community were predicted using BugBase (http://bugbase.cs.umn.edu accessed on 3 November 2017).
2.9. Aeromonas Salmonicida Challenge
After sampling, the experimental fish were fed test diets for 7 days. Sixty fish from each group were randomly separated into three tanks, with 20 fish from each tank examined for A. salmonicida infection. Bacteria were incubated for 14 h at 28 °C in trypticase soy broth (TSB). A. salmonicida was diluted to 1.08 × 108 CFU/mL (LD50) in phosphate-buffered saline (PBS), and 100 μL of the bacteria suspension was injected into the base of the pectoral fin of each fish. The number of dead fish was recorded every 24 h for 7 days after A. salmonicida infection and starvation.
2.10. Calculations
The growth performance parameters were calculated as follows:
- weight gain rate (WGR; %) = 100 × (W56 − W0)/W0;
- specific growth rate (SGR; %/d) = 100 × (lnW56 − lnW0)/56 days;
- protein efficiency ratio (PER) = (W56 − W0)/ (Wf × feed protein content);
- feed conversion ratio (FCR) = Wf/ (W56 − W0);
- hepatosomatic index (HSI; %) = 100 × (liver weight (g)/body weight (g));
- condition factor (CF; %) = 100 × W56/L563;
- viscerosomatic index (VSI; %) = 100 × (viscera (g)/body weight (g));
- survival rate (SR, %) = 100 × W56/W0,
- where W0 represents the initial body weight (g), W56 represents the final body weight (g), Wf represents the feed intake (g), and L56 represents the final body length (cm).
A one-way analysis of variance, a covariance analysis, and Tukey multiple comparisons were conducted on the data, and the group and initial weight were tested for their effect on the WGR and SGR using covariance analysis (SPSS 20.0, SPSS Inc., Chicago, IL, USA). The data showed substantial variations within the group, as indicated by a p-value of less than 0.05. The mean and standard error (S.E.) were used to express the data, and a broken-line analysis was performed using Graphpad Prism 8.0 to estimate the optimum range of NaB requirements for triploid O. mykiss under low fish meal conditions based on the WGR and SGR.
3. Results
3.1. Growth and Feeding Parameters
There was no significant difference in SR and CF among the groups (p > 0.05) (Table 4). The WGR, SGR, and PER were significantly higher in the G3 group compared to the G1 group (p < 0.05). The value of FCR was significantly lower in the G3 group (p < 0.05). The WGR and SGR rose and then declined as dietary NaB levels increased, reaching a maximum in the 0.20% group and being significantly higher than those of the control group (p < 0.05) (Figure 1). Based on the broken-line analysis of SGR and WGR, the optimal NaB requirement for triploid O. mykiss was 0.20%.

Table 4.
Growth performance, feed conversion of triploid O. mykiss in five groups during the experiment.
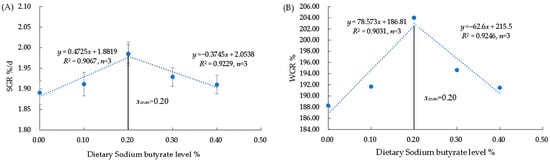
Figure 1.
Regressive analysis between dietary NaB level and SGR of O. mykiss (A). Regressive analysis between dietary NaB level and WGR of O. mykiss (B).
Table 4 demonstrates that all four treatment groups had significantly lower HSI and VSI than G1 (p < 0.05).
3.2. Body Composition
As shown in Table 5, dietary NaB did not affect whole-body moisture, crude lipid, crude protein, and ash levels (p > 0.05).

Table 5.
Body composition of triploid O. mykiss in five groups (wet weight, %).
3.3. Digestive Physiology
Table 6 shows the impact of varied dietary NaB levels on the intestinal digestive enzyme activity of triploid O. mykiss. The activity of trypsin, LPS, and AMS was significantly higher in the 0.20% group (G3) compared to the other groups (p < 0.05). These enzyme activities then decreased gradually as the NaB level increased. LPS activity was also found to be lower in the 0.40% group (G5) compared to the control group (p < 0.05).

Table 6.
The intestinal digestive enzyme and intestinal morphology in triploid O. mykiss in five groups.
3.4. Histology
According to the data in Table 6, adding NaB to the diet significantly increased the values of villi height, villi width, and muscular thickness of the O. mykiss midgut (p < 0.05). The largest values of these parameters were observed when NaB was added at 0.20% levels (G3). However, dietary 0.40% NaB resulted in significantly lower villi height and villi width compared to the control group (p < 0.05).
The intestine in the control group was structurally incomplete, with absorptive vacuoles of epithelial cells in simple mucosal foldings that were round-shaped and irregularly arranged, a loss of tissue microvilli, and blurred morphological contours of the striated border (Figure 2). The intestine also showed obvious signs of inflammation, including wizened enterocytes, enterocytes with disordered nuclei, and mixed leukocyte infiltration in the lamina propria. In contrast, the diets supplemented with 0.20% and 0.30% NaB (plates C and D) had the healthiest O. mykiss intestinal histomorphology among the five groups, with obvious goblet cells and non-vacuolated areas, well-developed microvilli arranged in an orderly fashion without fusion or exfoliation, a significant increase in the number of goblet cells, and a compactly arranged simple columnar epithelium.
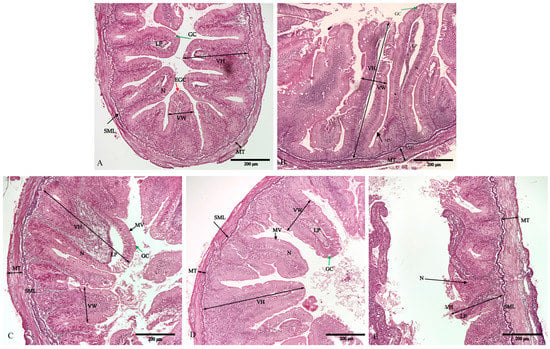
Figure 2.
Effects of dietary sodium butyrate level on intestinal morphology of triploid O. mykiss. Details of the distal intestine section from fish in the G1 (A), G2 (B), G3 (C), G4 (D), and G5 (E) groups. VH: villi height; VW: villus width; MT: muscle layer thickness; SML: submucous layer; LP: lamina propria; GC: goblet cell (green arrows); MV: microvilli (arrowheads); N: nucleus; EGC: eosinophilic granular cell (red arrow). Scale bar: 200 μm.
3.5. Intestinal Gene Expression
Gene expression analysis was performed on intestinal samples collected from O. mykiss fed low fish meal diets. It was found that the addition of NaB at a concentration of 0.20% caused a significant decrease in the expression of IL-1β, IL-8, IKK, NF-κB, IκB-α, TNF-α, MLCK, and MLC genes in the intestine of O. mykiss (p < 0.05) (Figure 3).
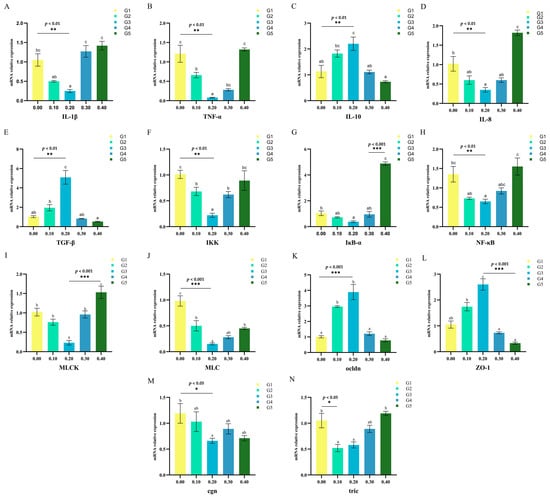
Figure 3.
Effects of NaB on the expression of IL-1β (A), TNF-α (B), IL-10 (C), IL-8 (D), TGF-β(E), IKK (F), IκB-α (G), NF-κB (H), MLCK (I), MLC (J), ocldn (K), ZO-1 (L), cgn (M) and tric (N) in intestinal mucosal barrier of juvenile triploid O. mykiss. abc different superscript letters are significantly different (p < 0.05). All error bars represent S.E.; * p < 0.05, ** p < 0.01 and *** p < 0.001.
3.6. Gut Bacterial Community Composition
The Illumina MiSeq PE300 reading system generated an average of 40,862 sequences per sample from the 30 sequenced samples, totaling 530,999,267 effective sequences after deleting low-quality reads and chimeras. The sequences ranged in length from 212 to 516 bp, with an average length of 424 bp. The percentage of good coverage for each group exceeded 99.9%, demonstrating the accuracy and reliability of the diverse results. The rarefaction curves reached a plateau, indicating that the sequencing depth was sufficient to represent the diversity of the microbial species. The similarities and overlaps of OTUs (not defined) in the various groups were evaluated using a Venn diagram. The five groups shared 612 OTUs, and each group had a unique number of OTUs: 130 (G1), 149 (G2), 90 (G3), 93 (G4), and 99 (G5) (Figure 4A).
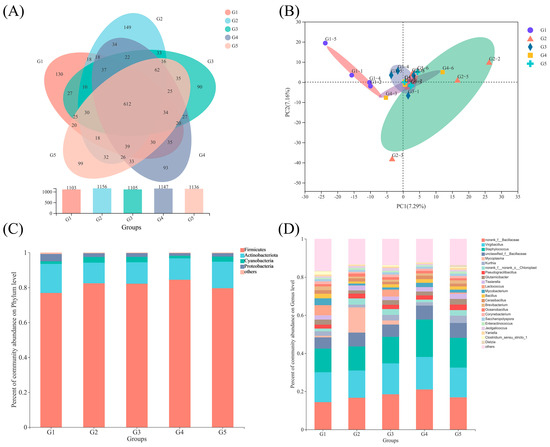
Figure 4.
Venn diagram showing the number of shared and unique OTUs in the five groups (A). The OUT level (B) is used for principal component analysis (PCA). Relative abundance of intestinal microbes in O. mykiss at the phylum (C) and genus level (D).
Furthermore, the beta diversity analysis based on weighted and unweighted UniFrac distances indicated that the five groups of gut bacteria were separated and grouped in different sections of the PCA plot (Figure 4B). According to (Figure 4B), O. mykiss in the various treatment groups had significantly different gut microbial compositions, with the PC1 and PC2 axes explaining 7.16% and 7.29%, respectively.
There were 14 distinct bacterial phyla identified. At the phylum level, the relative abundance of Firmicutes was lower in the G1 group compared to the other four groups, while Actinobacteriota and Proteobacteria had higher relative abundances (Figure 4C).
We conducted LEfSe analysis (Figure 5A) on the five groups and identified several bacterial genera as biomarkers of group difference: Bacillus, Enterobacter, Legionella, Escherichia-Shigella, Delftia, Sphingomonas, Lactobacillus, Pseudomonas, Brevundimonas, Tumebacillus, and others. Proteobacteria were the phylum-level biomarkers in G1, while Rhabdanaerobium was the genus-level biomarker in G2. Hydrogenophaga, nor-ank-f-norank-o-norank-c-KD4-96, and Erysipelothrix were the genus-level biomarkers in G3. Nocardioides were the genus-level biomarkers in G4, and Atopostipes and Bosea were the genus-level biomarkers in G5 (Figure 5B).
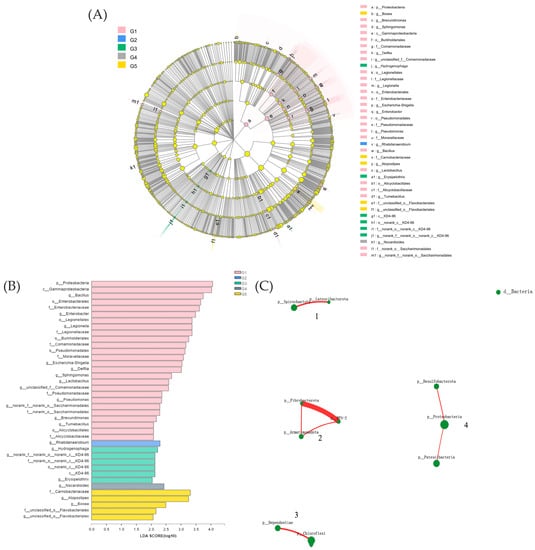
Figure 5.
Inter-group variation in the relative abundance of intestinal microbial communities (A). LDA score from Lefse-PICRUSt (B). Network interaction graph for the hindgut microbial communities at the order level, using weighted topological (wTO) network analysis (C). Note: Red-colored links are positive correlations, while negative correlations are in green-colored links. The thickness of the line indicates the magnitude of the correlation coefficient.
The gut microbiota was divided into four clusters, all of which had primarily positive interactions. The majority of the microbes in Cluster 4 were from the Proteobacteria phylum, while those in Cluster 1 came from the Spirochaetota phylum. The Fibrobacteria phylum and the WPS-2 phylum had the highest positive correlation and a symbiotic relationship (Figure 5C). The lowest relative abundance values for potential pathogenicity were predicted in G3, according to BugBase results for triploid O. mykiss gut microbiota, but these values were not significantly different from those of the other four groups (Figure 6D). Using the PICRUSt2 algorithm and deductions from the KEGG databases, the 16S rRNA gene-based microbial compositions were used to estimate bacterial gene functions. The abundance of the G3 immune system was significantly greater than that of the other four groups (p < 0.05) (Figure 6B). There were no significant differences in the abundance values for the digestive system and disease resistance among the five groups, but G3 had the highest values (Figure 6A,C).
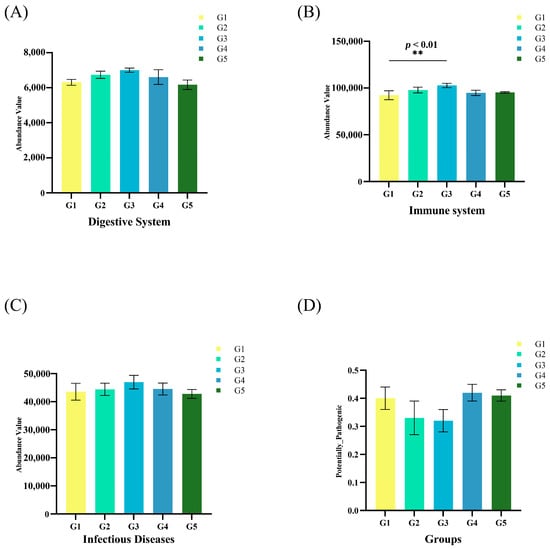
Figure 6.
Predictive metagenomic analysis of O. mykiss fecal microbiota functional profiling. Bacterial gene functions were predicted from the 16S rRNA gene-based microbial compositions using the PICRUSt2 algorithm and inferences from KEGG databases. Abundance values of the digestive system (A), immune system (B), and infectious diseases (C) in the gut microbiota of O. mykiss. Relative abundance values of potential pathogenicity in the gut microbiota of O. mykiss (D). All error bars represent S.E.; ** p < 0.01).
3.7. Survival against the A. salmonicida Challenge
After being challenged with A. salmonicida, fish that were given a diet supplemented with 0.20% NaB (G3) had a lower average mortality rate of 33.33% compared to the 66.67% rate for fish fed a non-supplemented diet (G1) and the 78.33% rate for fish fed a diet supplemented with 0.40% NaB (G5). The death occurred from the first to the fifth day following A. salmonicida inoculation (Figure 7).
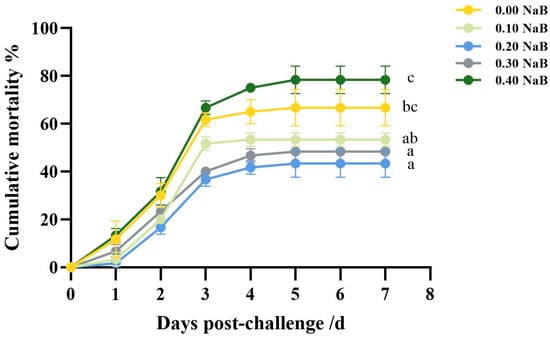
Figure 7.
Effect of NaB supplementation on survival against bacterial infection. Different superscript letters indicate significant difference (p < 0.05).
4. Discussion
4.1. Growth Parameters
The optimal amount of NaB to add varies by species and has different effects on its growth performance. Elmazni et al. found that 0.05% NaB significantly improved the body weight of male New Zealand rabbits []. Diets with 1.00% and 2.00% NaB significantly improved the survival and weight gain of juvenile Chinese mitten crabs (Eriocheir sinensis) compared to those in the 0% NaB group []. The inclusion of 0.117% NaB in diets for Arapaima gigas juveniles improves growth parameters []. The equation for the relationship between the concentration of NaB added (x) and WGR (y) was obtained by fitting a linear, quadratic, and cubic model: y = 78.573 x + 186.81 (R2 = 0.9031), y = −248.56 x2 + 108.77 x + 187.18 (R2 = 0.6578), y = −227.07 x3 − 112.32 x2 + 89.245 x + 187.46 (R2 = 0.6629). The equation for the relationship between the concentration of NaB added (x) and SGR (y) was obtained by fitting a linear, quadratic, and cubic model: y = 0.4725 x + 1.8819 (R2 = 0.9067), y = −1.93 x2 + 0.7674 x + 1.8815 (R2 = 0.6629), y = −1.25 x3 − 0.7436 x2 + 0.5464 x + 1.8856 (R2 = 0.6652). Among them, the R2 value of the linear equation was the highest. However, this study used the linear regression method and the SGR and WGR as evaluation indices to calculate that the NaB requirements for juvenile triploid O. mykiss were 0.20% (Figure 1).
Liu et al. found that adding either 0.10% or 0.20% NaB to the diet significantly increased the SGR of grass carp (Ctenopharyngodon idella) []. It was also discovered that adding 0.20% NaB to the diet significantly altered the immune-related gene expression and improved the immunity of European seabass (Dicentrarchus labrax) without significantly affecting the WGR and SGR of the fish []. This study found that adding 0.20% NaB to the diet increased the weight gain, feed efficiency, and SGR of juvenile O. mykiss. This may be because NaB is a direct energy source for intestinal cells. When it enters the intestine, it can stimulate the rapid repair of damaged villi by renewing intermediate cells, making the villi longer, and improving the ability to digest and absorb nutrients. The addition of NaB also increases the ability of juvenile O. mykiss to absorb nutrients and strengthens its antioxidant defenses. Additionally, NaB can promote the proliferation and maturation of intestinal cells, improve the morphology and barrier function of the intestine, and have bactericidal, antibacterial, and anti-inflammatory effects on the intestine, helping to maintain intestinal health and improve growth performance.
The addition of excess NaB in this study suppressed the growth of O. mykiss, which may be due to the low pH in the intestine caused by the excess NaB, which inhibits the functioning of neutral and alkaline digestive enzymes. Additionally, excessive amounts of BA prevented the normal development of the digestive tract in O. mykiss and made nutrients in the intestine less digestible. Zhang et al. found that NaB did not affect feed palatability and that its growth-promoting effect on fish was achieved not by increasing feeding amounts, but by improving feed utilization [].
4.2. Body Composition
A previous study found that the whole-body composition of grass carp was not affected by dietary NaB []. Similarly, this experiment found that the crude protein, crude lipid, and ash levels of juvenile triploid O. mykiss were not affected by dietary NaB supplementation. However, it was discovered that dietary NaB significantly enhanced the crude protein content of whole tilapia (Oreochromis mossambicus). This is likely due to NaB promoting a more efficient conversion of ingested food into structural proteins, which increases fish muscle production, although the exact mechanism is not known [].
4.3. Digestive Physiology and Histological Analyses
Poor-quality protein sources can significantly reduce the height of the intestinal mucosa, blur epithelial cell borders, or even cause separation, which limits the utilization of protein sources and hinders the ability of fish to grow healthily [,]. Therefore, it is important to protect the intestinal health of the fish. Zhou et al. found that Trachinotus ovatus had significantly higher intestinal protease, amylase, and alkaline protease activities when 2.0 and 4.0 g/kg of NaB were added to its diet []. Aalamifar et al. found that adding 5.0 or 10.0 g/kg of NaB to the feed of Latescalcarifer significantly increased the overall intestine alkaline protease and lipase activities compared to the control group []. Similar results were found with triploid O. mykiss, where the addition of more NaB initially caused an increase and then a decrease in the activity of intestinal digestive enzymes. O. mykiss has a shorter digestive system, fewer active digestive enzymes, and a shorter feed residence time in the colon than omnivorous fish. The main purpose of adding NaB to low fish meal feeds is to improve fish digestion by promoting the production of digestive enzymes and improving gut absorption, which will improve fish growth and development [].
The surface area of intestinal absorption for carnivorous fish such as O. mykiss, which have relatively short intestinal length indices, is closely related to their ability to digest and absorb nutrients. Therefore, the expansion of the microvilli and the increased height and width of the intestinal villi can enhance the absorption area of the intestine, improving the efficiency of nutrient absorption []. The addition of 0.20% NaB to the diet of low fish meal D. labrax for 60 days reduced the inflammatory response in the posterior segment intestine and restored normal morphology []. When 0.20% microencapsulated NaB was added to the diet, it accelerated the proliferation of intestinal epithelial cells, increased the length and density of the villi, and encouraged goblet cell mucus production, all of which helped to maintain the development and integrity of the gut morphological structure []. The addition of 0.05% NaB to the feed of C. idella significantly increased the height of the intestinal villi []. Low fish meal A. gigas diets supplemented with 0.20% NaB for 12 weeks improved intestinal pathology, including an increase in the intestinal villi absorption surface area, a reduction in the inflammatory infiltration of intestinal leukocytes, and an increase in enzyme activity in the brush border of the intestine []. This study obtained similar results, and the potential mechanism by which dietary NaB improves intestinal morphology, digestion, and absorption is by providing energy to the intestinal epithelial cells. This can stimulate normal intestinal epithelial cell development and enzyme production that aids in mucosal repair and intestinal mucosa repair. NaB also stimulates intracellular mRNA and protein synthesis, increases villi height, promotes digestion and absorption, and improves growth performance.
4.4. Intestinal Microbial Diversity Analyses and Functional Prediction
The regular intestinal flora of fish is essential for their growth and development because it not only helps with nutrient digestion and absorption from the intestines but also helps the fish maintain its overall health by activating its immune system []. The operation of the intestinal barrier is directly linked to the gut bacteria and their metabolites. The findings of this study, which are in line with earlier research on fish or terrestrial animals, showed that the gut bacterial compositions of fish are highly responsive to diets [,,]. In this study, α-diversity, Sobs, Ace, and Chao significantly increased with increasing dietary NaB concentrations. NaB has also been shown to alter the composition of the intestinal microbiota and increase the diversity of the intestinal microbiota in tests with carp (Cyprinus carpio) and gilthead sea bream (Sparus aurata). However, adding 0.40% NaB was able to prevent developmental delays in parasitized fish and increase the diversity of the gut microbiota by increasing the number of bacteria that produce butyrate []. In comparison to humans and other mammals, the potential mechanisms of NaB on fish gut microbiota are largely unknown. This may be because NaB enters bacterial cells as non-dissociated butyric acid, which then dissociates into CH3-CH2-CH2-COO™ and H+. Harmful bacteria such as Escherichia coli and Salmonella, which are poorly tolerant of H+, die in large numbers, while helpful bacteria such as Lactobacillus and Bifidobacterium, which are highly tolerant of H+, proliferate []. One type of organic acid, BA, decreases the pH in the fish digestive tract and prevents the overgrowth of harmful bacteria that are sensitive to pH []. Furthermore, based on the Venn diagram results and the α-diversity of the gut microbiota, it is possible to conclude that the addition of 0.2% NaB (G3) resulted in a healthier microbial composition and structure compared to G1, indicating that NaB positively contributes to the maintenance of the balance between the various microbial populations in the intestine. Previous studies have shown that probiotics in the gut produce enzymes that help with digestion and nutrient absorption []. This may be an important mode of interaction between the host and the indigenous microbiota []. Proteobacteria, for example, can utilize external environmental reservoirs to proliferate and reach a relatively high prevalence in fish guts [,]. Bacteria from the Actinobacteria genus produce secondary metabolites and extracellular enzymes []. The relative abundance of Proteobacteria and Actinobacteria was significantly reduced (G1) when 0.20% NaB was added to the diet. In the phylum Bacteroidetes, Bacteroides can secrete enzymes for breaking down and metabolizing sugars and polysaccharides [,]. The increased population of helpful bacteria in the gut helps O. mykiss digest plant proteins.
In the long term, there were differences in the predicted functional profiles between gut tract fractions. The nutrition and immunity of the host may be affected by the gut microbiota. According to the microbial makeup and functional capabilities, the five groups in this investigation varied in their predictions of functional pathways. Our findings showed that the diet containing 0.20% NaB had higher levels of genes related to the immune system and the digestive system (G3). Furthermore, O. mykiss used protein as a source of energy for growth. The gut microbiota, on the other hand, have a digestive system that may boost metabolic power and increase the abundance of the Firmicutesto phylum to compensate for the host deficiencies. Because dietary NaB significantly reduced the abundance of the Proteobacteria phylum in O. mykiss gut microorganisms, the gut microbiota exhibited immune responses. The lowest relative abundance values for potential pathogenicity were predicted in the 0.20% NaB-supplemented diet (G3) based on BugBase results for triploid O. mykiss gut microbiota. This was also an important factor that adding NaB can improve the immunity and disease resistance of O. mykiss. There are still few reports on the prediction of KEGG function in the gut microbiota of fish, and its effects and specific mechanisms require further study.
4.5. Gut immunity Gene Expression
Dietary NaB supplements may regulate the function of immune cells, including mucosal immune cells and epithelial cells, which may help the body fight off the invasion of pathogens. Interleukins, growth factors, cell-stimulating factors, and tumor necrosis factors are examples of peptide-based cellular regulatory molecules collectively referred to as cytokines []. Normally, immunological and inflammatory responses involve cytokines. Tumor necrosis factor (TNF), which has important biological functions, increases IL-1 and IL-8 release and promotes neutrophil adherence to endothelial cells, resulting in an inflammatory response in the body []. NaB can improve the defense and regulation of animals against pathogens through the control of innate and adaptive immunity and the maintenance of immunological homeostasis [,]. Xiao et al. found that NaB was able to reduce IL-8 expression while increasing IL-10 expression in acute pancreatitis of Pelteobagrus fulvidraco []. In this study, gene expression analysis of intestinal samples taken after O. mykiss was fed low fish meal diets revealed that dietary 0.20% NaB significantly affected gene expression of IL-1β, IL-8, TNF-α, nuclear factor κB (NF-κB), IKK, and IκB in the intestine of O. mykiss. The expression of TNF-α and IL-8 genes decreased as NaB concentration increased.
Unfavorable stimuli in animals first trigger the immune system, which then mildly expresses a variety of cytokines to defend the organism. However, an overactive immune response might result in inflammation. In this experiment, dietary NaB enhanced the expression of both pro-inflammatory and anti-inflammatory cytokines and regulated the expression of pathway genes associated with cytokine expression, therefore influencing the immune response in O. mykiss in both positive and negative ways. TNF-α and NF-κB expression were found to be reduced in turbot (Scophthalmus maximus L.) after 0.2% dietary NaB supplementation []. Infections caused by bacterial enteritis can compromise the integrity of the intestinal barrier in fish []. Aeromonas hydrophila can induce intestinal inflammation in grass carp, and it can also be disrupted by inflammatory processes caused by the excessive substitution of fish meal and fish oil in the feed []. Enteritis can also be caused by damage to the intestinal mucosa due to frequent consumption of a diet high in soybean meal []. Therefore, dietary NaB may play an anti-inflammatory role in reducing intestinal inflammation by inhibiting the NF-κB-P65 inflammatory signaling pathway, thus protecting the intestinal mucosa (Figure 8) [,].
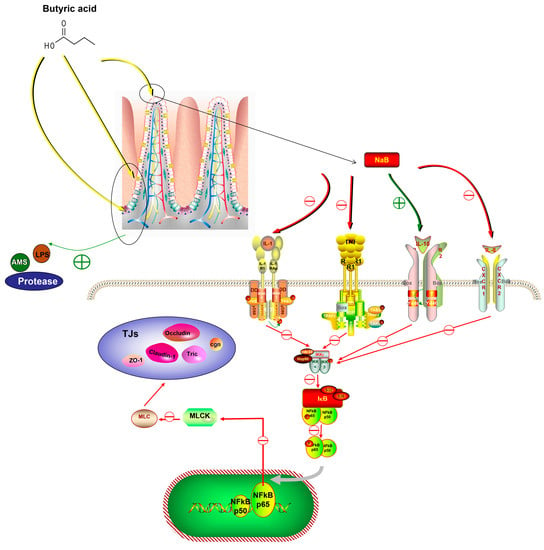
Figure 8.
A general summary of the effect of NaB on immune function and its potential signalling pathways in the intestine of O. mykiss.
Animals with healthy intestines have a variety of bacteria, some of which produce endotoxins that cause harmful reactions and trigger the production of intestinal inflammatory mediators and cytokines, leading to a disruption of the intestinal barrier [,]. An increase in intestinal mucosal and vascular permeability, which is an indication of intestinal barrier damage, increases the risk of pathogens and endotoxins moving from the intestine to local lymph nodes, portal veins, and the peripheral blood system. This can cause pancreatic necrosis and organ failure in O. mykiss. Tight junctions (TJ), which prevent bacteria, lipopolysaccharides, and other potentially dangerous substances from entering the bloodstream from the intestinal lumen, make up a significant part of the intestinal barrier in the fish gut []. Cldn-1, Ocln, and ZO-1 are the three primary TJ proteins, and they improve the intestinal barrier by reducing paracellular permeability []. TNF-α, which increases TJ permeability by controlling the expression of the MLCK gene in Caco-2 cells through the NF-κB signaling pathway, has also been linked to intestinal barrier failure []. In this study, dietary NaB may protect intestinal barrier function by increasing the expression of TJ-related proteins, Ocln, and ZO-1 genes. Some scientists propose that intestinal epithelial NF-κB regulates MLCK activity and directly targets MLCK. Specifically, activated NF-κB releases p65, which translocates to the cytosol of the nucleus and binds to the MLCK promoter to increase MLCK. MLCK-mediated MLC phosphorylation subsequently causes myosin contraction and TJ dysregulation. NaB prevents the master inflammatory regulator NF-κB and also prevents MLCK-mediated barrier erosion (Figure 8). Therefore, this study showed that dietary NaB may protect the integrity of the intestinal epithelium in O. mykiss by preventing TJ dysfunction and barrier degradation caused by MLCK.
4.6. Survival upon the A. salmonicida Challenge of Triploid O. mykiss
A. salmonicida is a short, Gram-negative bacterium of the genus Aeromonas that causes boils or ulcers in salmon and trout. Typical symptoms of the disease include the formation of characteristic abscesses on the side or tail of the fish; in severe cases, the abscesses ulcerate, ulcers form, and the liver and adipose tissue bleed on autopsy []. It has been found that the virulence of A. salmonicida is associated with various virulence factors, including the A-layer protein, glycerophospholipid cholesterol acyltransferase (GCAT), proteases, and siderophores. GCAT, in particular, has been found to significantly influence the pathogenicity of A. salmonicida, exhibiting cytotoxicity, hemolytic activity, and thermal stability []. According to Scott et al., salmon with scabies exhibit an enlarged spleen, subhepatic perithelial hemorrhage, pitting hemorrhage in parenchymal tissues, anorexia or oligophagia, and a stomach and intestine filled with blood and mucus, all of which are connected to the hemolytic and cytotoxic properties of A. salmonicida []. In this study, the group fed the 0.20% NaB diet and challenged with A. salmonicida had a significantly lower cumulative mortality compared to group G1 (p < 0.05). Piazzon et al. [] obtained similar results in the teleostean gilthead sea bream (Sparus aurata). Butyric acid, a direct source of energy for intestinal cells, promotes the proliferation of intestinal epithelial cells, restores the ANF-damaged intestinal barrier, reduces colitis, and may be responsible for the decrease in cumulative mortality. NaB also significantly increased the mRNA expression of TJ proteins and protected the intestinal barrier in O. mykiss []. The increased colonization of probiotic bacteria in the intestine following NaB administration may also contribute to the resistance of O. mykiss to A. salmonicida infection. Furthermore, more research has to be done to determine the precise mechanism by which NaB protects against bacterial infection.
5. Conclusions
Dietary 0.20%–0.22% NaB levels improved the WGR and SGR of juvenile O. mykiss fed a 15% fish meal diet and reduced the severity of ANF-induced enteropathy by enhancing digestion enzymes and improving intestinal morphology. Dietary NaB increased mRNA expression of TJ proteins (Ocln, Cldn-3, and ZO-1) while decreasing mRNA expression of pro-inflammatory cytokines (IL-1β, IL-8, and TNF-α), NF-κB, and MLCK-MLC, suggesting that it protects against pathogenic bacterial infections by strengthening the intestinal mucosal barrier. In addition, the gut microbial composition was more favorable for O. mykiss health with the inclusion of 0.20% NaB in the diet. Furthermore, when challenged with A. salmonicida, dietary 0.20% NaB increased the survival rate of O. mykiss.
Author Contributions
Conceptualization, S.L. (Siyuan Liu) and C.W.; methodology, S.L. (Siyuan Liu); resources, S.Z. and S.H.; data curation, S.L. (Siyuan Liu); writing—original draft preparation, S.L. (Siyuan Liu); writing—review and editing, S.L. (Siyuan Liu) and C.W.; visualization, Y.W.; supervision, C.W., Y.L., H.J. and S.L. (Shaoxia Lu); project administration, C.W. and H.L.; funding acquisition, C.W. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Central Public-interest Scientific Institution Basal Research Fund, grant number HRFRI (HSY202202M), the China Agriculture Research System of MOF and MARA, grant number CARS-46, the Key Scientific Research Project of Heilongjiang Province, grant number JD22A017, the National Postdoctoral Fund, grant number 2022MD713817, and the China Scholarship Council, grant number 202003260012.
Institutional Review Board Statement
The animal study protocol was approved by the Committee for the Welfare and Ethics of the Laboratory Animals of the Heilongjiang River Fisheries Research Institute, CAFS.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors thank the participants who gave their time to the trial.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nasopoulou, C.; Zabetakis, I. Benefits of fish oil replacement by plant originated oils in compounded fish feeds. A Review. LWT 2012, 47, 217–224. [Google Scholar] [CrossRef]
- Opstvedt, J.; Aksnes, A.; Hope, B.; Pike, I.H. Efficiency of feed utilization in Atlantic Salmon (Salmo salar L.) fed diets with increasing substitution of fish meal with vegetable proteins. Aquaculture 2003, 221, 365–379. [Google Scholar] [CrossRef]
- Torstensen, B.E.; Espe, M.; Sanden, M.; Stubhaug, I.; Waagbø, R.; Hemre, G.-I.; Fontanillas, R.; Nordgarden, U.; Hevrøy, E.M.; Olsvik, P.; et al. Novel production of Atlantic Salmon (Salmo salar) protein based on combined replacement of fish meal and fish oil with plant meal and vegetable oil blends. Aquaculture 2008, 285, 193–200. [Google Scholar] [CrossRef]
- Moreno-Arias, A.; López-Elías, J.A.; Miranda-Baeza, A.; Rivas-Vega, M.E.; Martínez-Córdova, L.R.; Ramírez-Suárez, J.C. Replacement of fish meal by vegetable meal mix in the diets of Litopenaeus vannamei reared in low-salinity biofloc system: Effect on digestive enzymatic activity. Aquac. Nutr. 2017, 23, 236–245. [Google Scholar] [CrossRef]
- Refstie, S.; Korsøen, Ø.J.; Storebakken, T.; Baeverfjord, G.; Lein, I.; Roem, A.J. Differing nutritional responses to dietary soybean meal in rainbow trout (Oncorhynchus mykiss) and Atlantic salmon (Salmo salar). Aquaculture 2000, 190, 49–63. [Google Scholar] [CrossRef]
- Buttle, L.G.; Burrells, A.C.; Good, J.E.; Williams, P.D.; Southgate, P.J.; Burrells, C. The binding of soybean agglutinin (SBA) to the intestinal epithelium of Atlantic salmon, Salmo salar and rainbow trout, Oncorhynchus mykiss, fed high levels of soybean meal. Vet. Immunol. Immunopathol. 2001, 80, 237–244. [Google Scholar] [CrossRef]
- Heikkinen, J.; Vielma, J.; Kemiläinen, O.; Tiirola, M.; Eskelinen, P.; Kiuru, T.; Navia-Paldanius, D.; von Wright, A. Effects of soybean meal based diet on growth performance, gut histopathology and intestinal microbiota of juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 2006, 261, 259–268. [Google Scholar] [CrossRef]
- Lin, S.; Luo, L. Effects of different levels of soybean meal inclusion in replacement for fish meal on growth, digestive enzymes and transaminase activities in practical diets for juvenile tilapia, Oreochromis niloticus × O. aureus. Anim. Feed Sci. Technol. 2011, 168, 80–87. [Google Scholar] [CrossRef]
- Röhe, I.; Göbel, T.W.; Goodarzi Boroojeni, F.; Zentek, J. Effect of feeding soybean meal and differently processed peas on the gut mucosal immune system of broilers. Poult. Sci. 2017, 96, 2064–2073. [Google Scholar] [CrossRef]
- Storebakken, T.; Kvien, I.S.; Shearer, K.D.; Grisdale-Helland, B.; Helland, S.J.; Berge, G.M. The apparent digestibility of diets containing fish meal, soybean meal or bacterial meal fed to Atlantic salmon (Salmo salar): Evaluation of different faecal collection methods. Aquaculture 1998, 169, 195–210. [Google Scholar] [CrossRef]
- McCracken, V.J.; Lorenz, R.G. The gastrointestinal ecosystem: A precarious alliance among epithelium, immunity and microbiota. Cell Microbiol. 2001, 3, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Liu, S.; Zhang, S.; Lu, S.; Liu, H.; Han, S.; Jiang, H.; Zhang, Y. Effects of dietary arginine on growth performance, digestion, absorption ability, antioxidant capability, gene expression of intestinal protein synthesis, and inflammation-related genes of triploid juvenile Oncorhynchus Mykiss fed a low-fish meal diet. Aquac. Nutr. 2022, 2022, 3793727. [Google Scholar] [CrossRef]
- Wang, C.; Su, B.; Lu, S.; Han, S.; Jiang, H.; Li, Z.; Liu, Y.; Liu, H.; Yang, Y. Effects of glutathione on growth, intestinal antioxidant capacity, histology, gene expression, and microbiota of juvenile triploid Oncorhynchus mykiss. Front. Physiol. 2021, 12, 784852. [Google Scholar] [CrossRef] [PubMed]
- Van der Beek, C.M.; Dejong, C.H.C.; Troost, F.J.; Masclee, A.A.M.; Lenaerts, K. Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr. Rev. 2017, 75, 286–305. [Google Scholar] [CrossRef] [PubMed]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef]
- Sina, C.; Gavrilova, O.; Förster, M.; Till, A.; Derer, S.; Hildebrand, F.; Raabe, B.; Chalaris, A.; Scheller, J.; Rehmann, A.; et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. 2009, 183, 7514–7522. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-S.; Lu, J.-J.; Zou, X.-T. Effects of sodium butyrate on the intestinal morphology and DNA-binding activity of intestinal nuclear factor-κB in weanling pigs. J. Anim. Vet. Adv. 2012, 11, 814–821. [Google Scholar]
- Weber, T.E.; Kerr, B.J. Effect of sodium butyrate on growth performance and response to lipopolysaccharide in weanling pigs1. J. Anim. Sci. 2008, 86, 442–450. [Google Scholar] [CrossRef]
- Song, M.; Xia, B.; Li, J. Effects of topical treatment of sodium butyrate and 5-aminosalicylic acid on expression of trefoil factor 3, interleukin 1β, and nuclear factor κB in trinitrobenzene sulphonic acid induced colitis in rats. Postgrad. Med. J. 2006, 82, 130–135. [Google Scholar] [CrossRef]
- Rodríguez-Cabezas, M.E.; Gálvez, J.; Camuesco, D.; Lorente, M.D.; Concha, A.; Martinez-Augustin, O.; Redondo, L.; Zarzuelo, A. Intestinal anti-inflammatory activity of dietary fiber (Plantago ovata) seeds in HLA-B27 transgenic rats. Clin. Nutr. 2003, 22, 463–471. [Google Scholar] [CrossRef]
- Peng, L.; He, Z.; Chen, W.; Holzman, I.R.; Lin, J. Effects of butyrate on intestinal barrier function in a Caco-2 cell monolayer model of intestinal barrier. Pediatr. Res. 2007, 61, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Mariadason, J.M.; Barkla, D.H.; Gibson, P.R. Effect of short-chain fatty acids on paracellular permeability in Caco-2 intestinal epithelium model. Am. J. Physiol.-Gastrointest. Liver Physiol. 1997, 272, G705–G712. [Google Scholar] [CrossRef] [PubMed]
- Mariadason, J.M.; Kilias, D.; Catto-Smith, A.; Gibson, P.R. Effect of butyrate on paracellular permeability in rat distal colonic mucosa ex vivo. J. Gastroenterol. Hepatol. 1999, 14, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Rawls, J.F.; Samuel, B.S.; Gordon, J.I. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc. Natl. Acad. Sci. USA 2004, 101, 4596–4601. [Google Scholar] [CrossRef]
- Rawls, J.F.; Mahowald, M.A.; Ley, R.E.; Gordon, J.I. Reciprocal gut microbiota transplants from zebrafish and mice to germ-free recipients reveal host habitat selection. Cell 2006, 127, 423–433. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef]
- Lazado, C.C.; Caipang, C.M.A.; Gallage, S.; Brinchmann, M.F.; Kiron, V. Expression profiles of genes associated with immune response and oxidative stress in Atlantic cod, Gadus morhua head kidney leukocytes modulated by live and heat-inactivated intestinal bacteria. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 155, 249–255. [Google Scholar] [CrossRef]
- Rimoldi, S.; Gliozheni, E.; Ascione, C.; Gini, E.; Terova, G. Effect of a specific composition of short- and medium-chain fatty acid 1-Monoglycerides on growth performances and gut microbiota of gilthead sea bream (Sparus aurata). Peer J. 2018, 6, e5355. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Dai, J.; Yang, P.; Xu, W.; Ai, Q.; Zhang, W.; Zhang, Y.; Zhang, Y.; Mai, K. Sodium butyrate supplementation in high-soybean meal diets for turbot (Scophthalmus maximus L.): Effects on inflammatory status, mucosal barriers and microbiota in the intestine. Fish Shellfish Immunol. 2019, 88, 65–75. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.; Zhang, J.; Gatlin, D.M.; Ringø, E.; Zhou, Z. Effects of dietary microencapsulated sodium butyrate on growth, intestinal mucosal morphology, immune response and adhesive bacteria in juvenile common carp (Cyprinus carpio) pre-fed with or without oxidised oil. Br. J. Nutr. 2014, 112, 15–29. [Google Scholar] [CrossRef]
- Pauly, D.; Zeller, D. Comments on FAOs State of World Fisheries and Aquaculture (SOFIA 2016). Mar. Policy 2017, 77, 176–181. [Google Scholar] [CrossRef]
- Ma, R.; Liu, X.; Meng, Y.; Wu, J.; Zhang, L.; Han, B.; Qian, K.; Luo, Z.; Wei, Y.; Li, C. Protein nutrition on sub-adult triploid rainbow trout (1): Dietary requirement and effect on anti-oxidative capacity, protein digestion and absorption. Aquaculture 2019, 507, 428–434. [Google Scholar] [CrossRef]
- Meiler, K.A.; Kumar, V. Organic and inorganic zinc in the diet of a commercial strain of diploid and triploid rainbow trout (Oncorhynchus mykiss): Effects on performance and mineral retention. Aquaculture 2021, 545, 737126. [Google Scholar] [CrossRef]
- George, W. Latimer. In Official methods of analysis of AOAC international, 20th ed.; AOAC international: Rockville, ML, USA, 2016; pp. 30–31. [Google Scholar]
- Guo, Y.; Huang, D.; Chen, F.; Ma, S.; Zhou, W.; Zhang, W.; Mai, K. Lipid deposition in abalone Haliotis discus hannai affected by dietary lipid levels through AMPKα2/PPARα and JNK/mTOR/SREBP-1c pathway. Aquaculture 2021, 532, 736040. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Gysi, D.M.; Voigt, A.; de Fragoso, T.M.; Almaas, E.; Nowick, K. WTO: An R package for computing weighted topological overlap and a consensus network with integrated visualization tool. BMC Bioinform. 2018, 19, 392. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Elmazni, A.H.; Tony, M.A.; Sawiress, F.A.R.; Abdl-Rahman, M.A.; Saleh, S.Y. Influence of dietary supplementation of coated sodium butyrate and/or synbiotic on growth performances, caecal fermentation, intestinal morphometry and metabolic profile of growing rabbits. Res. Dev. Agric. Sci. 2020, 2, 94–105. [Google Scholar]
- Han, F.; Xu, C.; Qi, C.; Lin, Z.; Li, E.; Wang, C.; Wang, X.; Qin, J.G.; Chen, L. Sodium butyrate can improve intestinal integrity and immunity in juvenile Chinese mitten crab (Eriocheir sinensis) fed glycinin. Fish Shellfish Immunol. 2020, 102, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Luz, J.R.; Ramos, A.P.S.; Melo, J.F.B.; Braga, L.G.T. Use of sodium butyrate in the feeding of Arapaima gigas (Schinz, 1822) juvenile. Aquaculture 2019, 510, 248–255. [Google Scholar] [CrossRef]
- Liu, M.; Guo, W.; Wu, F.; Qu, Q.; Tan, Q.; Gong, W. Dietary supplementation of sodium butyrate may benefit growth performance and intestinal function in juvenile grass carp (Ctenopharyngodon idellus). Aquac. Res. 2017, 48, 4102–4111. [Google Scholar] [CrossRef]
- Terova, G.; Díaz, N.; Rimoldi, S.; Ceccotti, C.; Gliozheni, E.; Piferrer, F. Effects of sodium butyrate treatment on histone modifications and the expression of genes related to epigenetic regulatory mechanisms and immune response in European sea bass (Dicentrarchus labrax) fed a plant-based diet. PLoS ONE 2016, 11, e0160332. [Google Scholar] [CrossRef] [PubMed]
- Robles, R.; Lozano, A.B.; Sevilla, A.; Márquez, L.; Nuez-Ortín, W.; Moyano, F.J. Effect of partially protected butyrate used as feed additive on growth and intestinal metabolism in sea bream (Sparus aurata). Fish Physiol. Biochem. 2013, 39, 1567–1580. [Google Scholar] [CrossRef]
- Ahmed, H.; Sadek, K. Impact of dietary supplementation of sodium butyrate and/or protein on the growth performance, some blood parameters, and immune response of Oreochromis niloticus. Int. J. Agric. Innov. Res. 2015, 22, 579–584. [Google Scholar]
- Maruyama, N.; Katsube, T.; Wada, Y.; Oh, M.H.; Barba De La Rosa, A.P.; Okuda, E.; Nakagawa, S.; Utsumi, S. The roles of the N-linked glycans and extension regions of soybean β-conglycinin in folding, assembly and structural features. Eur. J. Biochem. 1998, 258, 854–862. [Google Scholar] [CrossRef]
- Rimoldi, S.; Finzi, G.; Ceccotti, C.; Girardello, R.; Grimaldi, A.; Ascione, C.; Terova, G. Butyrate and taurine exert a mitigating effect on the inflamed distal intestine of European sea bass fed with a high percentage of soybean meal. Fish. Aquat. Sci. 2016, 19, 40. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Huang, Z.; Wang, J.; Wang, Y.; Yu, W. Effect of dietary sodium butyrate on growth performance, enzyme activities and intestinal proliferation-related gene expression of juvenile golden pompano Trachinotus ovatus. Aquac. Nutr. 2019, 25, 1261–1271. [Google Scholar] [CrossRef]
- Aalamifar, H.; Soltanian, S.; Vazirzadeh, A.; Akhlaghi, M.; Morshedi, V.; Gholamhosseini, A.; Torfi Mozanzadeh, M. Dietary butyric acid improved growth, digestive enzyme activities and humoral immune parameters in Barramundi (Lates calcarifer). Aquac. Nutr. 2020, 26, 156–164. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Dadar, M.; Ringø, E. Modulation of nutrient digestibility and digestive enzyme activities in aquatic animals: The functional feed additives scenario. Aquac. Res. 2017, 48, 3987–4000. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; Elbialy, Z.I.; Abdelhamid, A.I. Dietary sodium butyrate ameliorated the blood stress biomarkers, heat shock proteins, and immune response of Nile tilapia (Oreochromis niloticus) exposed to heat stress. J. Therm. Biol. 2020, 88, 102500. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Mohsen, H.H.; Wassef, E.A.; El-Bermawy, N.M.; Abdel-Meguid, N.E.; Saleh, N.E.; Barakat, K.M.; Shaltout, O.E. Advantageous effects of dietary butyrate on growth, immunity response, intestinal microbiota and histomorphology of European Seabass (Dicentrarchus labrax) fry. Egypt. J. Aquat. Biol. Fish. 2018, 22, 93–110. [Google Scholar] [CrossRef]
- Zhou, J.S.; Guo, P.; Yu, H.B.; Ji, H.; Lai, Z.W.; Chen, Y.A. Growth performance, lipid metabolism, and health status of grass carp (Ctenopharyngodon idella) fed three different forms of sodium butyrate. Fish Physiol. Biochem. 2019, 45, 287–298. [Google Scholar] [CrossRef]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.H.; Lin, G.; Fu, G.H.; Wan, Z.Y.; Lee, M.; Wang, L.; Liu, X.J.; Yue, G.H. The intestinal microbiome of fish under starvation. BMC Genomics 2014, 15, 266. [Google Scholar] [CrossRef]
- Pop, M. We Are What We Eat: We are what we eat: How the diet of infants affects their gut microbiome. Genome Biol. 2012, 13, 152. [Google Scholar] [CrossRef]
- Schwartz, S.; Friedberg, I.; Ivanov, I.V.; Davidson, L.A.; Goldsby, J.S.; Dahl, D.B.; Herman, D.; Wang, M.; Donovan, S.M.; Chapkin, R.S. A metagenomic study of diet-dependent interaction between gut microbiota and host in infants reveals differences in immune response. Genome Biol. 2012, 13, r32. [Google Scholar] [CrossRef]
- Piazzon de Haro, M.C.; Calduch-Giner, J.A.; Fouz, B.; Estensoro, I.; Simó Mirabet, P.; Puyalto, M.; Karalazos, V.; Palenzuela, O.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J. Under control: How a dietary additive can restore the gut microbiome and proteomic profile, and improve disease resilience in a marine teleostean fish fed vegetable diets. Microbiome 2017, 5, 164. [Google Scholar] [CrossRef]
- Lückstädt, C. The use of acidifiers in fish nutrition. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2008, 3, 044. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Sun, Y.-Z.; Caipang, C.M. Short-chain fatty acids as feed supplements for sustainable aquaculture: An updated view. Aquac. Res. 2017, 48, 1380–1391. [Google Scholar] [CrossRef]
- Ray, A.K.; Ghosh, K.; Ringø, E. Enzyme-producing bacteria isolated from fish gut: A review. Aquac. Nutr. 2012, 18, 465–492. [Google Scholar] [CrossRef]
- Silva, F.C.P.; Brito, M.F.G.; Farias, L.M.; Nicoli, J.R. Composition and antagonistic activity of the indigenous intestinal microbiota of Prochilodus argenteus Agassiz. J. Fish Biol. 2005, 67, 1686–1698. [Google Scholar] [CrossRef]
- Rawls, J.F.; Mahowald, M.A.; Goodman, A.L.; Trent, C.M.; Gordon, J.I. In vivo imaging and genetic analysis link bacterial motility and symbiosis in the zebrafish gut. Proc. Natl. Acad. Sci. USA 2007, 104, 7622–7627. [Google Scholar] [CrossRef]
- Ventura, M.; Canchaya, C.; Tauch, A.; Chandra, G.; Fitzgerald, G.F.; Chater, K.F.; van Sinderen, D. Genomics of actinobacteria: Tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 2007, 71, 495–548. [Google Scholar] [CrossRef]
- Xu, J.; Bjursell, M.K.; Himrod, J.; Deng, S.; Carmichael, L.K.; Chiang, H.C.; Hooper, L.V.; Gordon, J.I. A genomic view of the human-bacteroides thetaiotaomicron symbiosis. Science 2003, 299, 2074–2076. [Google Scholar] [CrossRef] [PubMed]
- Fedorak, R.N.; Madsen, K.L. Probiotics and prebiotics in gastrointestinal disorders. Curr. Opin. Gastroenterol. 2004, 20, 146–155. [Google Scholar] [CrossRef]
- Jia, W.; Li, H.; Zhao, L.; Nicholson, J.K. Gut microbiota: A potential new territory for drug targeting. Nat. Rev. Drug Discov. 2008, 7, 123–129. [Google Scholar] [CrossRef]
- Haller, D.; Bode, C.; Hammes, W.P.; Pfeifer, A.M.; Schiffrin, E.J.; Blum, S. Non-pathogenic bacteria elicit a differential cytokine response by intestinal epithelial cell/leucocyte co-cultures. Gut 2000, 47, 79–87. [Google Scholar] [CrossRef]
- McCracken, V.J.; Chun, T.; Baldeón, M.E.; Ahrné, S.; Molin, G.; Mackie, R.I.; Gaskins, H.R. TNF-alpha sensitizes HT-29 colonic epithelial cells to intestinal lactobacilli. Exp. Biol. Med. Maywood NJ 2002, 227, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yu, L.; Gui, G.; Gong, Y.; Wen, X.; Xia, W.; Yang, H.; Zhang, L. Molecular cloning and expression analysis of interleukin-8 and -10 in yellow catfish and in response to bacterial pathogen infection. BioMed Res. Int. 2019, 2019, e9617659. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, J.; Bo, Y.; Liu, Z.; Wu, K.; Gong, C. Aeromonas hydrophila induces intestinal inflammation in grass carp (Ctenopharyngodon idella): An experimental model. Aquaculture 2014, 434, 171–178. [Google Scholar] [CrossRef]
- Estensoro, I.; Ballester-Lozano, G.; Benedito-Palos, L.; Grammes, F.; Martos-Sitcha, J.A.; Mydland, L.-T.; Calduch-Giner, J.A.; Fuentes, J.; Karalazos, V.; Ortiz, Á.; et al. Dietary butyrate helps to restore the intestinal status of a marine teleost (Sparus aurata) fed extreme diets low in fish meal and fish oil. PLoS ONE 2016, 11, e0166564. [Google Scholar] [CrossRef]
- Baeverfjord, G.; Krogdahl, A. Development and regression of soybean meal induced enteritis in Atlantic salmon, Salmo salar L., distal intestine: A comparison with the intestines of fasted fish. J. Fish Dis. 1996, 19, 375–387. [Google Scholar] [CrossRef]
- Meijer, K.; de Vos, P.; Priebe, M.G. Butyrate and other short-chain fatty acids as modulators of immunity: What relevance for health. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 715–721. [Google Scholar] [CrossRef]
- Segain, J.-P.; de la Blétière, D.R.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottière, H.M.; Galmiche, J.-P. Butyrate inhibits inflammatory responses through NF-κB inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.E. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp. Biol. Med. 2003, 228, 882–890. [Google Scholar] [CrossRef]
- Rao, R. Oxidative stress-induced disruption of epithelial and endothelial tight junctions. Front. Biosci. J. Virtual Libr. 2008, 13, 7210–7226. [Google Scholar] [CrossRef]
- Niklasson, L.; Sundh, H.; Fridell, F.; Taranger, G.L.; Sundell, K. Disturbance of the intestinal mucosal immune system of farmed Atlantic salmon (Salmo salar), in response to long-term hypoxic conditions. Fish Shellfish Immunol. 2011, 31, 1072–1080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Jia, L.; Yan, Q.; Deng, Q.; Wei, B. Effect of Clostridium butyricum and butyrate on intestinal barrier functions: Study of a rat model of severe acute pancreatitis with intra-abdominal hypertension. Front. Physiol. 2020, 11, 561061. [Google Scholar] [CrossRef]
- Ye, D.; Ma, T.Y. Cellular and molecular mechanisms that mediate basal and tumour necrosis factor-α-induced regulation of myosin light chain kinase gene activity. J. Cell Mol. Med. 2008, 12, 1331–1346. [Google Scholar] [CrossRef]
- Cipriano, R.; Bullock, G. Furunculosis and other diseases caused by Aeromonas salmonicida. Fish Dis. 2001, 66, 3–6. [Google Scholar]
- Lee, K.K.; Ellis, A.E. Glycerophospholipid: Cholesterol acyltransferase complexed with lipopolysaccharide (LPS) is a major lethal exotoxin and cytolysin of Aeromonas salmonicida: LPS stabilizes and enhances toxicity of the enzyme. J. Bacteriol. 1990, 172, 5382–5393. [Google Scholar] [CrossRef] [PubMed]
- SCOTT, M. The Pathogenicity of Aeromonas salmonicida (Griffin) in sea and brackish waters. Microbiology. 1968, 50, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Fan, P.X.; Li, L.S.; Qiao, S.Y.; Zhang, G.L.; Li, D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions1. J. Anim. Sci. 2012, 90, 266–268. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).