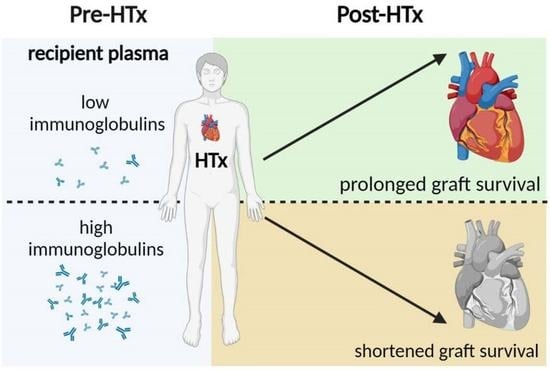
Elevated Plasma Immunoglobulin Levels Prior to Heart Transplantation Are Associated with Poor Post-Transplantation Survival
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Tissue Collection and (Immuno)histochemistry
2.3. Tissue Lysates
2.4. Multiplex Immunoassay for Immunoglobulin Detection
2.5. Statistical Analysis
3. Results
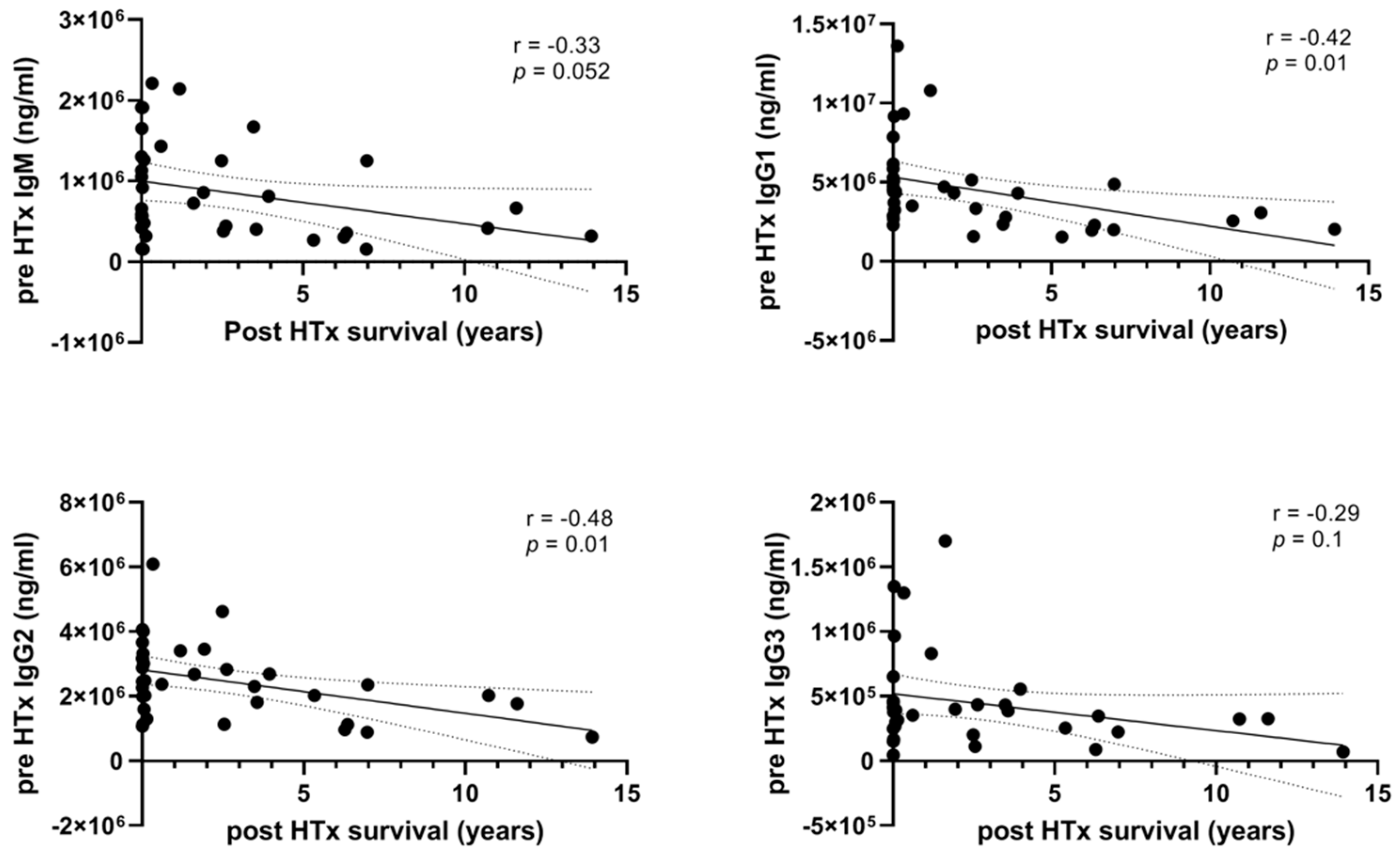
3.1. High Levels of Pre-HTx Circulating Immunoglobulins Are Associated with Shorter Survival after HTx
3.2. Patients with High Plasma Immunoglobulin Levels Pre-HTx Also Have Relatively High Plasma Immunoglobulin Levels after HTx
3.3. Immunoglobulin Tissue Levels in the Donor Heart Are Higher in Patients with Short-Term Survival Compared to Patients with Long-Term Survival
3.4. High Levels of IgG in Plasma and Epicardial Tissue Are Related to the Inflammatory CAV Phenotype
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; Lund, L.H. Global Public Health Burden of Heart Failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Theochari, C.A.; Michalopoulos, G.; Oikonomou, E.K.; Giannopoulos, S.; Doulamis, I.P.; Villela, M.A.; Kokkinidis, D.G. Heart transplantation versus left ventricular assist devices as destination therapy or bridge to transplantation for 1-year mortality: A systematic review and meta-analysis. Ann. Cardiothorac. Surg. 2018, 7, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Lund, L.H.; Khush, K.K.; Cherikh, W.S.; Goldfarb, S.; Kucheryavaya, A.Y.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Chambers, D.C.; Yusen, R.D.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Heart Transplantation Report-2017; Focus Theme: Allograft ischemic time. J. Heart Lung Transplant. 2017, 36, 1037–1046. [Google Scholar] [CrossRef]
- Chambers, D.C.; Yusen, R.D.; Cherikh, W.S.; Goldfarb, S.B.; Kucheryavaya, A.Y.; Khusch, K.; Levvey, B.J.; Lund, L.H.; Meiser, B.; Rossano, J.W.; et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung And Heart-Lung Transplantation Report-2017; Focus Theme: Allograft ischemic time. J. Heart Lung Transplant. 2017, 36, 1047–1059. [Google Scholar] [CrossRef]
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Goldfarb, S.; Hayes, D., Jr.; Kucheryavaya, A.Y.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Stehlik, J.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multiorgan Transplantation. J. Heart Lung Transplant. 2018, 37, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Huibers, M.; De Jonge, N.; Van Kuik, J.; Koning, E.S.; Van Wichen, D.; Dullens, H.; Schipper, M.; De Weger, R. Intimal fibrosis in human cardiac allograft vasculopathy. Transpl. Immunol. 2011, 25, 124–132. [Google Scholar] [CrossRef]
- Skoric, B.; Cikes, M.; Ljubas Macek, J.; Baricevic, Z.; Skorak, I.; Gasparovic, H.; Biocina, B.; Milicic, D. Cardiac allograft vasculopathy: Diagnosis, therapy, and prognosis. Croat. Med. J. 2014, 55, 562–576. [Google Scholar] [CrossRef]
- Huibers, M.M.; Vink, A.; Kaldeway, J.; Huisman, A.; Timmermans, K.; Leenders, M.; Schipper, M.E.; Lahpor, J.R.; Kirkels, H.J.; Klopping, C.; et al. Distinct phenotypes of cardiac allograft vasculopathy after heart transplantation: A histopathological study. Atherosclerosis 2014, 236, 353–359. [Google Scholar] [CrossRef]
- Huibers, M.M.; Gareau, A.J.; Vink, A.; Kruit, R.; Feringa, H.; Beerthuijzen, J.M.; Siera-de Koning, E.; Peeters, T.; de Jonge, N.; de Weger, R.A.; et al. The composition of ectopic lymphoid structures suggests involvement of a local immune response in cardiac allograft vasculopathy. J. Heart Lung Transplant. 2015, 34, 734–745. [Google Scholar] [CrossRef]
- Barten, M.J.; Schulz, U.; Beiras-Fernandez, A.; Berchtold-Herz, M.; Boeken, U.; Garbade, J.; Hirt, S.; Richter, M.; Ruhpawar, A.; Sandhaus, T.; et al. The clinical impact of donor-specific antibodies in heart transplantation. Transplant. Rev. 2018, 32, 207–217. [Google Scholar] [CrossRef]
- Barten, M.J.; Zuckermann, A. The meaning of donor-specific antibodies after heart transplant. Curr. Opin. Organ. Transplant. 2019, 24, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Jurcevic, S.; Ainsworth, M.E.; Pomerance, A.; Smith, J.D.; Robinson, D.R.; Dunn, M.J.; Yacoub, M.H.; Rose, M.L. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation 2001, 71, 886–892. [Google Scholar] [CrossRef]
- Urban, M.; Slavcev, A.; Gazdic, T.; Ivak, P.; Besik, J.; Netuka, I. The impact of angiotensin II type 1 receptor antibodies on post-heart transplantation outcome in Heart Mate II bridged recipients. Interact. Cardiovasc. Thorac. Surg. 2016, 22, 292–297. [Google Scholar] [CrossRef]
- Nath, D.S.; Ilias Basha, H.; Tiriveedhi, V.; Alur, C.; Phelan, D.; Ewald, G.A.; Moazami, N.; Mohanakumar, T. Characterization of immune responses to cardiac self-antigens myosin and vimentin in human cardiac allograft recipients with antibody-mediated rejection and cardiac allograft vasculopathy. J. Heart Lung Transplant. 2010, 29, 1277–1285. [Google Scholar] [CrossRef]
- Banasik, M.; Boratynska, M.; Koscielska-Kasprzak, K.; Krajewska, M.; Mazanowska, O.; Kaminska, D.; Bartoszek, D.; Zabinska, M.; Myszka, M.; Nowakowska, B.; et al. The impact of non-HLA antibodies directed against endothelin-1 type A receptors (ETAR) on early renal transplant outcomes. Transpl. Immunol. 2014, 30, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Habal, M.V. Current Desensitization Strategies in Heart Transplantation. Front. Immunol. 2021, 12, 702186. [Google Scholar] [CrossRef] [PubMed]
- Civieri, G.; Iop, L.; Tona, F. Antibodies against Angiotensin II Type 1 and Endothelin 1 Type A Receptors in Cardiovascular Pathologies. Int. J. Mol. Sci. 2022, 23, 927. [Google Scholar] [CrossRef]
- Villa, C.; Mesa, K.; Cristy Smith, M.; Mooney, D.M.; Coletti, A.; Klohe, E. Hyperacute graft dysfunction in an orthotopic heart transplant in the presence of non-HLA antibodies. Am. J. Transplant. 2020, 20, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Levine, R.; Patel, J.K.; Kittleson, M.; Czer, L.; Kobashigawa, J.A. Association of vimentin antibody and other non-HLA antibodies with treated antibody mediated rejection in heart transplant recipients. Hum. Immunol. 2020, 81, 671–674. [Google Scholar] [CrossRef]
- Youker, K.A.; Assad-Kottner, C.; Cordero-Reyes, A.M.; Trevino, A.R.; Flores-Arredondo, J.H.; Barrios, R.; Fernandez-Sada, E.; Estep, J.D.; Bhimaraj, A.; Torre-Amione, G. High proportion of patients with end-stage heart failure regardless of aetiology demonstrates anti-cardiac antibody deposition in failing myocardium: Humoral activation, a potential contributor of disease progression. Eur. Heart J. 2014, 35, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- van den Hoogen, P.; de Jager, S.C.A.; Huibers, M.M.H.; Schoneveld, A.H.; Puspitasari, Y.M.; Valstar, G.B.; Oerlemans, M.; de Weger, R.A.; Doevendans, P.A.; den Ruijter, H.M.; et al. Increased circulating IgG levels, myocardial immune cells and IgG deposits support a role for an immune response in pre- and end-stage heart failure. J. Cell. Mol. Med. 2019, 23, 7505–7516. [Google Scholar] [CrossRef] [PubMed]
- Colvin, M.M.; Cook, J.L.; Chang, P.P.; Hsu, D.T.; Kiernan, M.S.; Kobashigawa, J.A.; Lindenfeld, J.; Masri, S.C.; Miller, D.V.; Rodriguez, E.R.; et al. Sensitization in Heart Transplantation: Emerging Knowledge: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e553–e578. [Google Scholar] [CrossRef] [PubMed]
- Moazami, N.; Itescu, S.; Williams, M.R.; Argenziano, M.; Weinberg, A.; Oz, M.C. Platelet transfusions are associated with the development of anti-major histocompatibility complex class I antibodies in patients with left ventricular assist support. J. Heart Lung Transplant. 1998, 17, 876–880. [Google Scholar]
- Massad, M.G.; Cook, D.J.; Schmitt, S.K.; Smedira, N.G.; McCarthy, J.F.; Vargo, R.L.; McCarthy, P.M. Factors influencing HLA sensitization in implantable LVAD recipients. Ann. Thorac. Surg. 1997, 64, 1120–1125. [Google Scholar] [CrossRef] [PubMed]
- Chiu, P.; Schaffer, J.M.; Oyer, P.E.; Pham, M.; Banerjee, D.; Joseph Woo, Y.; Ha, R. Influence of durable mechanical circulatory support and allosensitization on mortality after heart transplantation. J. Heart Lung Transplant. 2016, 35, 731–742. [Google Scholar] [CrossRef]
- Drakos, S.G.; Kfoury, A.G.; Kotter, J.R.; Reid, B.B.; Clayson, S.E.; Selzman, C.H.; Stehlik, J.; Fisher, P.W.; Merida, M., 3rd; Eckels, D.D.; et al. Prior human leukocyte antigen-allosensitization and left ventricular assist device type affect degree of post-implantation human leukocyte antigen-allosensitization. J. Heart Lung Transplant. 2009, 28, 838–842. [Google Scholar] [CrossRef]
- Rickham, P.P. Human Experimentation. Code of Ethics of the World Medical Association. Declaration of Helsinki. Br. Med. J. 1964, 2, 177. [Google Scholar] [CrossRef]
- Sammani, A.; Jansen, M.; Linschoten, M.; Bagheri, A.; de Jonge, N.; Kirkels, H.; van Laake, L.W.; Vink, A.; van Tintelen, J.P.; Dooijes, D.; et al. UNRAVEL: Big data analytics research data platform to improve care of patients with cardiomyopathies using routine electronic health records and standardised biobanking. Neth. Heart J. 2019, 27, 426–434. [Google Scholar] [CrossRef]
- Gho, J.M.; van Es, R.; Stathonikos, N.; Harakalova, M.; te Rijdt, W.P.; Suurmeijer, A.J.; van der Heijden, J.F.; de Jonge, N.; Chamuleau, S.A.; de Weger, R.A.; et al. High resolution systematic digital histological quantification of cardiac fibrosis and adipose tissue in phospholamban p.Arg14del mutation associated cardiomyopathy. PLoS ONE 2014, 9, e94820. [Google Scholar] [CrossRef]
- Billingham, M.E. Histopathology of graft coronary disease. J. Heart Lung Transplant. 1992, 11, S38–S44. [Google Scholar] [PubMed]
- Huibers, M.M.; Gareau, A.J.; Beerthuijzen, J.M.; Siera-de Koning, E.; van Kuik, J.; Kamburova, E.G.; Vink, A.; de Jonge, N.; Lee, T.D.; Otten, H.G.; et al. Donor-Specific Antibodies Are Produced Locally in Ectopic Lymphoid Structures in Cardiac Allografts. Am. J. Transplant. 2017, 17, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L., Jr. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Deswal, A.; Petersen, N.J.; Feldman, A.M.; Young, J.B.; White, B.G.; Mann, D.L. Cytokines and cytokine receptors in advanced heart failure: An analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 2001, 103, 2055–2059. [Google Scholar] [CrossRef] [PubMed]
- Cordero-Reyes, A.M.; Youker, K.A.; Torre-Amione, G. The role of B-cells in heart failure. Methodist Debakey Cardiovasc. J. 2013, 9, 15–19. [Google Scholar] [CrossRef]
- Smolgovsky, S.; Ibeh, U.; Tamayo, T.P.; Alcaide, P. Adding insult to injury—Inflammation at the heart of cardiac fibrosis. Cell. Signal. 2021, 77, 109828. [Google Scholar] [CrossRef]
- Van Linthout, S.; Tschope, C. Inflammation—Cause or Consequence of Heart Failure or Both? Curr. Heart Fail. Rep. 2017, 14, 251–265. [Google Scholar] [CrossRef]
- Labarrere, C.A.; Woods, J.R.; Hardin, J.W.; Jaeger, B.R.; Zembala, M.; Deng, M.C.; Kassab, G.S. Early inflammatory markers are independent predictors of cardiac allograft vasculopathy in heart-transplant recipients. PLoS ONE 2014, 9, e113260. [Google Scholar] [CrossRef]
- Briasoulis, A.; Inampudi, C.; Pala, M.; Asleh, R.; Alvarez, P.; Bhama, J. Induction immunosuppressive therapy in cardiac transplantation: A systematic review and meta-analysis. Heart Fail. Rev. 2018, 23, 641–649. [Google Scholar] [CrossRef]
- Sarmiento, E.; Jaramillo, M.; Calahorra, L.; Fernandez-Yanez, J.; Gomez-Sanchez, M.; Crespo-Leiro, M.G.; Paniagua, M.; Almenar, L.; Cebrian, M.; Rabago, G.; et al. Evaluation of humoral immunity profiles to identify heart recipients at risk for development of severe infections: A multicenter prospective study. J. Heart Lung Transplant. 2017, 36, 529–539. [Google Scholar] [CrossRef]
- Sarmiento, E.; Rodriguez-Molina, J.; Munoz, P.; Fernandez-Yanez, J.; Palomo, J.; Fogueda, M.; Fernandez-Cruz, E.; Bouza, E.; Carbone, J. Decreased levels of serum immunoglobulins as a risk factor for infection after heart transplantation. Transplant. Proc. 2005, 37, 4046–4049. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.C.S.; Huibers, M.M.H.; Gaykema, L.H.; Siera-de Koning, E.; Ramjankhan, F.Z.; Maisel, A.S.; de Jonge, N. Soluble ST2 in end-stage heart failure, before and after support with a left ventricular assist device. Eur. J. Clin. Investig. 2018, 48, e12886. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.H.; Brann, C.N.; Becker, K.; Thohan, V.; Koerner, M.M.; Loebe, M.; Noon, G.P.; Torre-Amione, G. Dynamic expression of the membrane attack complex (MAC) of the complement system in failing human myocardium. Am. J. Cardiol. 2006, 97, 1626–1629. [Google Scholar] [CrossRef]
- Prescimone, T.; D’Amico, A.; Caselli, C.; Cabiati, M.; Viglione, F.; Caruso, R.; Verde, A.; Del Ry, S.; Trivella, M.G.; Giannessi, D. Caspase-1 transcripts in failing human heart after mechanical unloading. Cardiovasc. Pathol. 2015, 24, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Lok, S.I.; Winkens, B.; Goldschmeding, R.; van Geffen, A.J.; Nous, F.M.; van Kuik, J.; van der Weide, P.; Klopping, C.; Kirkels, J.H.; Lahpor, J.R.; et al. Circulating growth differentiation factor-15 correlates with myocardial fibrosis in patients with non-ischaemic dilated cardiomyopathy and decreases rapidly after left ventricular assist device support. Eur. J. Heart Fail. 2012, 14, 1249–1256. [Google Scholar] [CrossRef]
- Yip, N.H.; Lederer, D.J.; Kawut, S.M.; Wilt, J.S.; D’Ovidio, F.; Wang, Y.; Dwyer, E.; Sonett, J.R.; Arcasoy, S.M. Immunoglobulin G levels before and after lung transplantation. Am. J. Respir. Crit. Care Med. 2006, 173, 917–921. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Hoogen, P.; Huibers, M.M.H.; van den Dolder, F.W.; de Weger, R.; Siera-de Koning, E.; Oerlemans, M.I.F.; de Jonge, N.; van Laake, L.W.; Doevendans, P.A.; Sluijter, J.P.G.; et al. Elevated Plasma Immunoglobulin Levels Prior to Heart Transplantation Are Associated with Poor Post-Transplantation Survival. Biology 2023, 12, 61. https://doi.org/10.3390/biology12010061
van den Hoogen P, Huibers MMH, van den Dolder FW, de Weger R, Siera-de Koning E, Oerlemans MIF, de Jonge N, van Laake LW, Doevendans PA, Sluijter JPG, et al. Elevated Plasma Immunoglobulin Levels Prior to Heart Transplantation Are Associated with Poor Post-Transplantation Survival. Biology. 2023; 12(1):61. https://doi.org/10.3390/biology12010061
Chicago/Turabian Stylevan den Hoogen, Patricia, Manon M. H. Huibers, Floor W. van den Dolder, Roel de Weger, Erica Siera-de Koning, Marish I. F. Oerlemans, Nicolaas de Jonge, Linda W. van Laake, Pieter A. Doevendans, Joost. P. G. Sluijter, and et al. 2023. "Elevated Plasma Immunoglobulin Levels Prior to Heart Transplantation Are Associated with Poor Post-Transplantation Survival" Biology 12, no. 1: 61. https://doi.org/10.3390/biology12010061
APA Stylevan den Hoogen, P., Huibers, M. M. H., van den Dolder, F. W., de Weger, R., Siera-de Koning, E., Oerlemans, M. I. F., de Jonge, N., van Laake, L. W., Doevendans, P. A., Sluijter, J. P. G., Vink, A., & de Jager, S. C. A. (2023). Elevated Plasma Immunoglobulin Levels Prior to Heart Transplantation Are Associated with Poor Post-Transplantation Survival. Biology, 12(1), 61. https://doi.org/10.3390/biology12010061