Small Intestinal Microbiota Oscillations, Host Effects and Regulation—A Zoom into Three Key Effector Molecules
Simple Summary
Abstract
1. Introduction
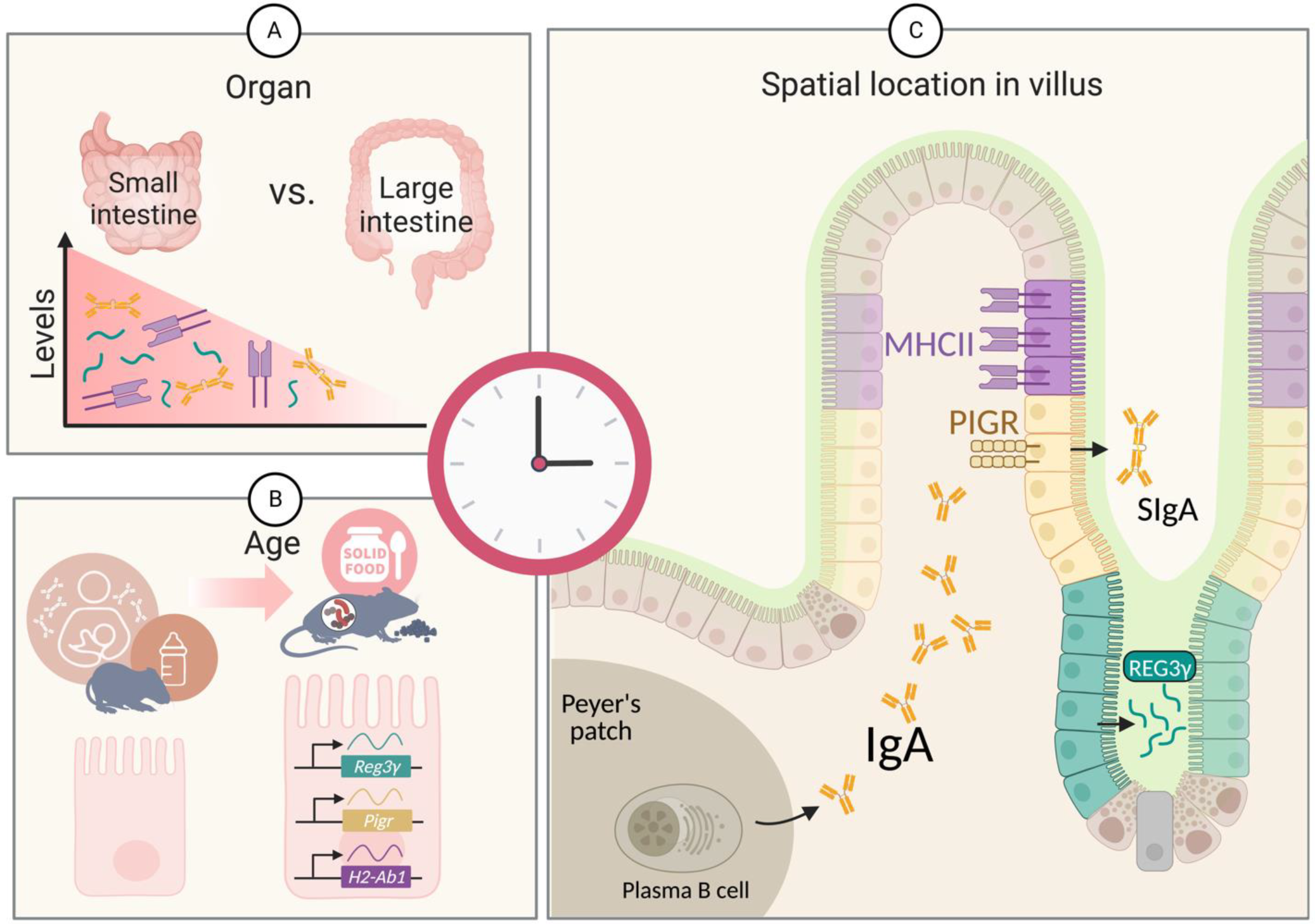
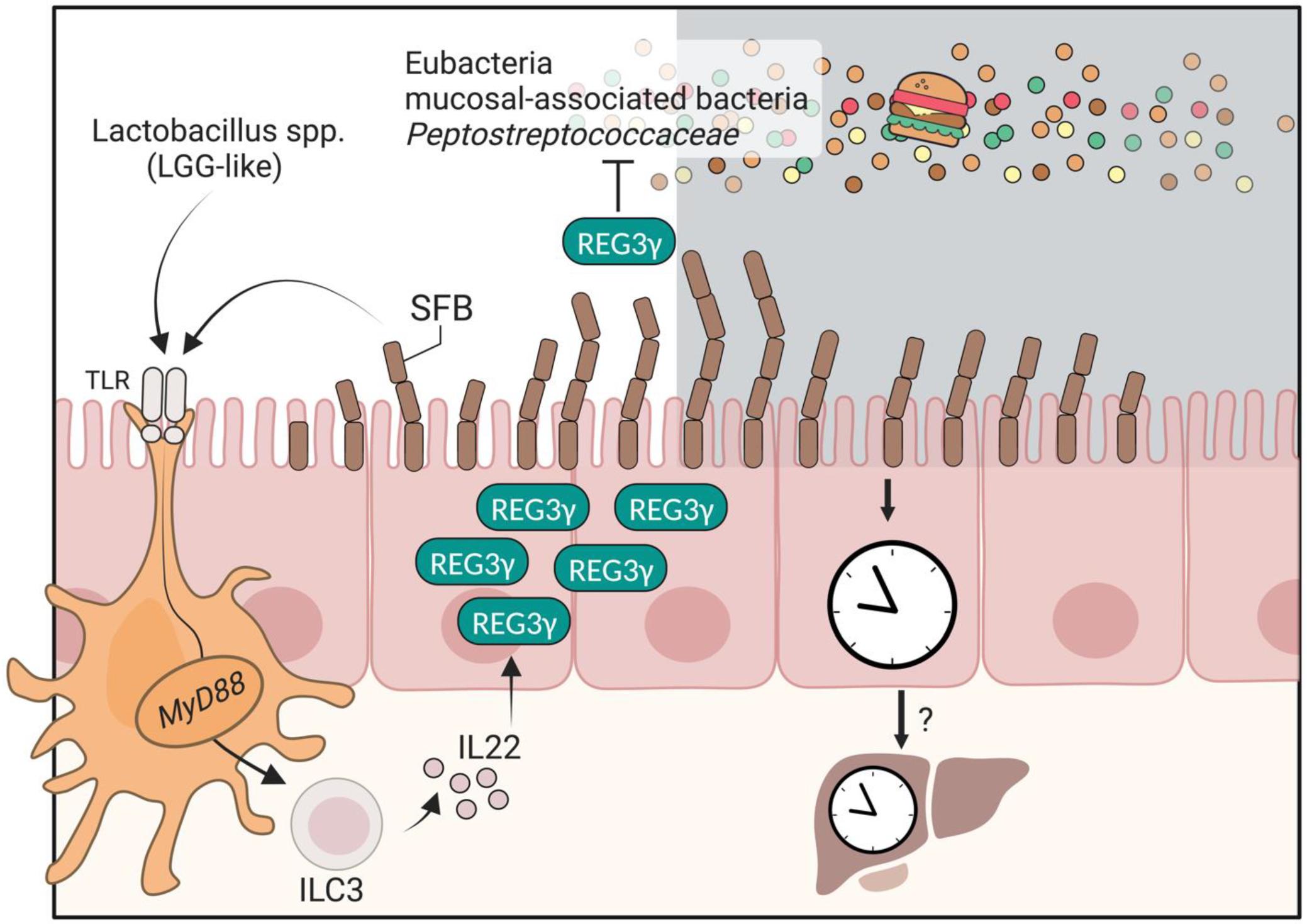
2. The Antimicrobial Peptide REG3γ
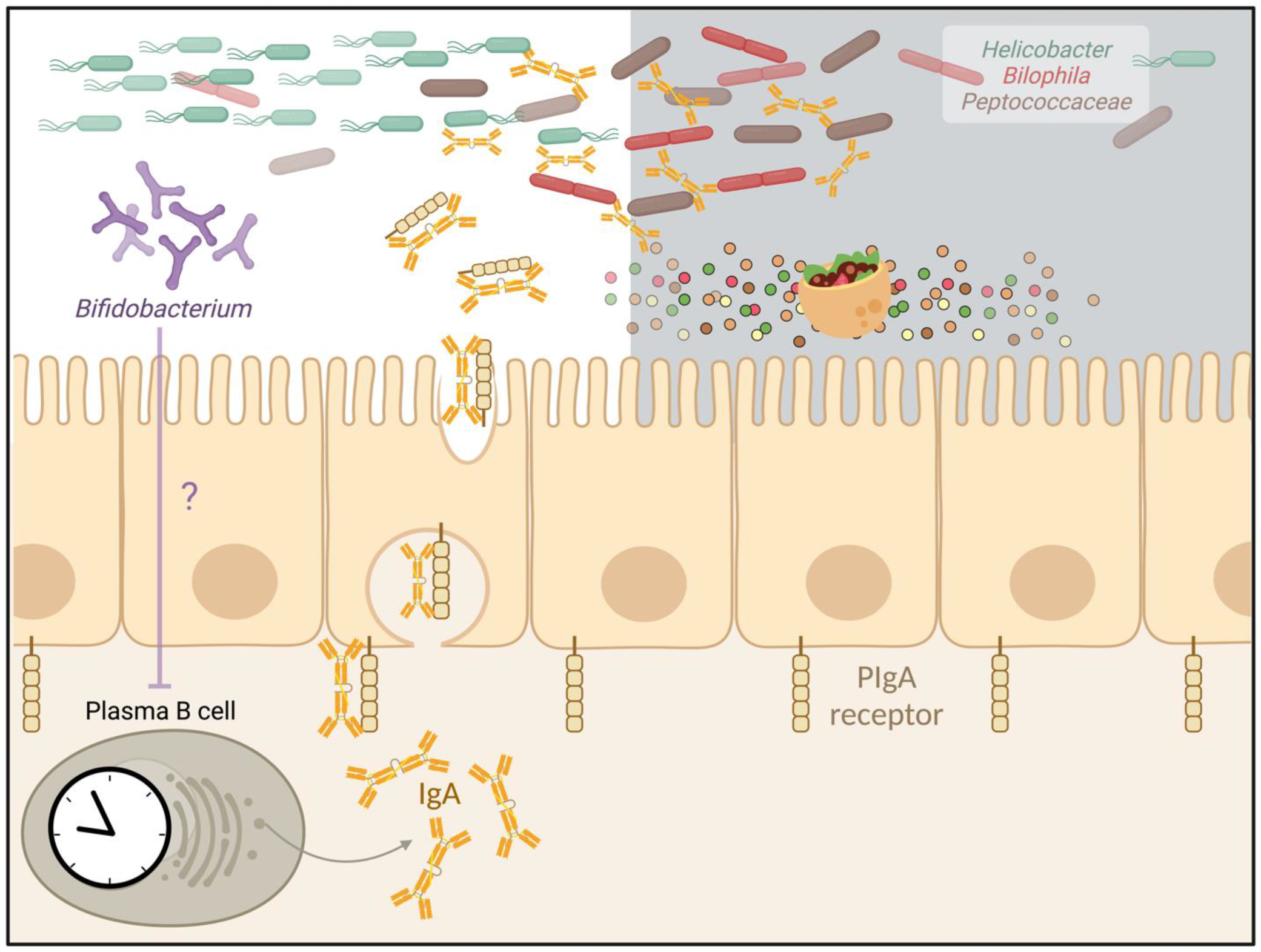
3. The Secreted Antibody IgA
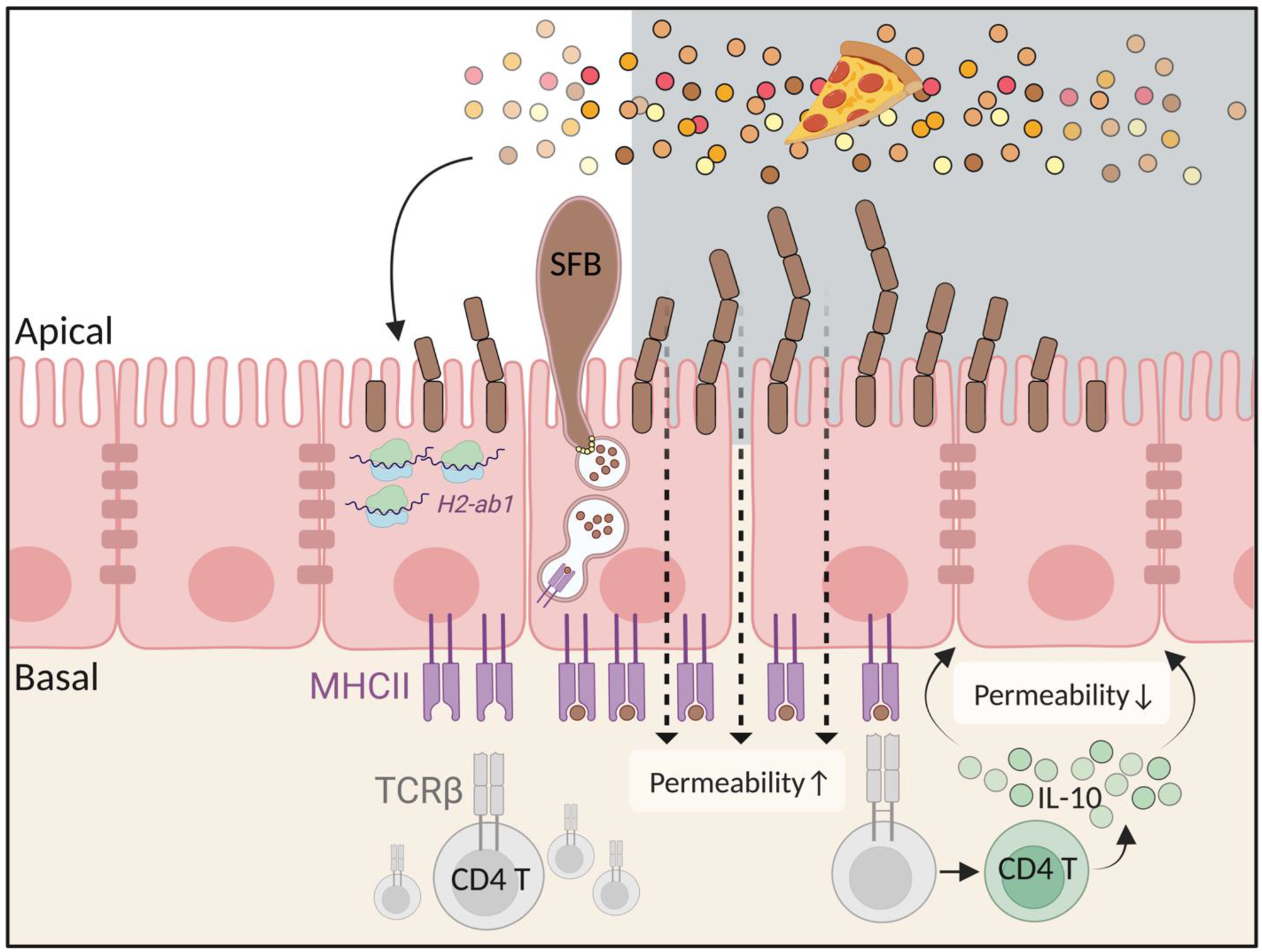
4. The Antigen Presentation Molecule MHC Class II
| REG3γ | IgA | MHCII | ||
|---|---|---|---|---|
| Oscillations (steady-state) | Mostly reported as higher at ~ZT12 [26,27] | Fecal and small intestinal IgA are higher at ZT6 [21,76] | H2-Ab1 (MHCII mRNA) peak ~ZT8Surface protein ~ZT10-ZT12 [20,54] | |
| Influencing factors | Circadian |
| ||
| Note: Rev-erbα-/- mice exhibit normal eating rhythms, ClockΔ19/19 mice are arrhythmic, whereas Per1/2 knock-out mice show perturbated rhythmic eating. Mb1-Cre for plasma and B cells and Villin-Cre for intestinal epithelial cells. | ||||
| Diet | ||||
| Microbiota |
| |||
| Function |
|
| ||
5. Challenges and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A Circadian Gene Expression Atlas in Mammals: Implications for Biology and Medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed]
- Ruben, M.D.; Wu, G.; Smith, D.F.; Schmidt, R.E.; Francey, L.J.; Lee, Y.Y.; Anafi, R.C.; Hogenesch, J.B. A Database of Tissue-Specific Rhythmically Expressed Human Genes Has Potential Applications in Circadian Medicine. Sci. Transl. Med. 2018, 10, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Mure, L.S.; Le, H.D.; Benegiamo, G.; Chang, M.W.; Rios, L.; Jillani, N.; Ngotho, M.; Kariuki, T.; Dkhissi-Benyahya, O.; Cooper, H.M.; et al. Diurnal Transcriptome Atlas of a Primate across Major Neural and Peripheral Tissues. Science 2018, 359, 1159–1160. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Hogenesch, J.B.; Kay, S.A. Circadian Rhythms from Flies to Human. Nature 2002, 417, 329–335. [Google Scholar] [CrossRef]
- Hoogerwerf, W.A.; Sinha, M.; Conesa, A.; Luxon, B.A.; Shahinian, V.B.; Cornélissen, G.; Halberg, F.; Bostwick, J.; Timm, J.; Cassone, V.M. Transcriptional Profiling of MRNA Expression in the Mouse Distal Colon. Gastroenterology 2008, 135, 2019–2029. [Google Scholar] [CrossRef]
- Tognini, P.; Murakami, M.; Liu, Y.; Eckel-Mahan, K.L.; Newman, J.C.; Verdin, E.; Baldi, P.; Sassone-Corsi, P. Distinct Circadian Signatures in Liver and Gut Clocks Revealed by Ketogenic Diet. Cell. Metab. 2017, 26, 523–538.e5. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Korem, T.; Dohnalová, L.; Shapiro, H.; Jaitin, D.A.; David, E.; Winter, D.R.; Gury-BenAri, M.; Tatirovsky, E.; et al. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 2016, 167, 1495–1510.e12. [Google Scholar] [CrossRef]
- Weger, B.D.; Gobet, C.; Yeung, J.; Martin, E.; Jimenez, S.; Betrisey, B.; Foata, F.; Berger, B.; Balvay, A.; Foussier, A.; et al. The Mouse Microbiome Is Required for Sex-Specific Diurnal Rhythms of Gene Expression and Metabolism. Cell. Metab. 2019, 29, 362–382.e8. [Google Scholar] [CrossRef]
- Mukherji, A.; Kobiita, A.; Ye, T.; Chambon, P. Homeostasis in Intestinal Epithelium Is Orchestrated by the Circadian Clock and Microbiota Cues Transduced by TLRs. Cell 2013, 153, 812–827. [Google Scholar] [CrossRef]
- Cox, K.H.; Takahashi, J.S. Circadian Clock Genes and the Transcriptional Architecture of the Clock Mechanism. J. Mol. Endocrinol. 2019, 63, R93–R102. [Google Scholar] [CrossRef]
- Challet, E. The Circadian Regulation of Food Intake. Nat. Rev. Endocrinol. 2019, 15, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Dibner, C.; Schibler, U.; Albrecht, U. The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks. Annu. Rev. Physiol. 2009, 72, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Sassone-Corsi, P. Time for Food: The Intimate Interplay between Nutrition, Metabolism, and the Circadian Clock. Cell 2015, 161, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Ratiner, K.; Shapiro, H.; Goldenberg, K.; Elinav, E. Time-limited Diets and the Gut Microbiota in Cardiometabolic Disease. J. Diabetes 2022, 14, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.L.; Kiersgaard, M.K.; Sørensen, D.B.; Mikkelsen, L.F. Fasting of Mice: A Review. Lab. Anim. 2013, 47, 225–240. [Google Scholar] [CrossRef]
- Andreatta, G.; Allen, C.N. How Neurons Adjust to Diurnality. eLife 2021, 10, 74704. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Chaix, A.; Yooseph, S.; Panda, S. Diet and Feeding Pattern Affect the Diurnal Dynamics of the Gut Micro-biome. Cell. Metab. 2014, 20, 1006–1017. [Google Scholar] [CrossRef]
- Leone, V.; Gibbons, S.M.; Martinez, K.; Hutchison, A.L.; Huang, E.Y.; Cham, C.M.; Pierre, J.F.; Heneghan, A.F.; Nadim-palli, A.; Hubert, N.; et al. Effects of Diurnal Variation of Gut Microbes and High-Fat Feeding on Host Circadian Clock Function and Metabolism. Cell Host Microbe 2015, 17, 681–689. [Google Scholar] [CrossRef]
- Liang, X.; Bushman, F.D.; FitzGerald, G.A. Rhythmicity of the Intestinal Microbiota Is Regulated by Gender and the Host Circadian Clock. Proc. Natl. Acad. Sci. USA 2015, 112, 10479–10484. [Google Scholar] [CrossRef]
- Dantas Machado, A.C.; Brown, S.D.; Lingaraju, A.; Sivaganesh, V.; Martino, C.; Chaix, A.; Zhao, P.; Pinto, A.F.M.; Chang, M.W.; Richter, R.A.; et al. Diet and Feeding Pattern Modulate Diurnal Dynamics of the Ileal Microbiome and Transcriptome. Cell Rep. 2022, 40, 111008. [Google Scholar] [CrossRef]
- Penny, H.A.; Domingues, R.G.; Krauss, M.Z.; Melo-Gonzalez, F.; Lawson, M.A.E.; Dickson, S.; Parkinson, J.; Hurry, M.; Purse, C.; Jegham, E.; et al. Rhythmicity of Intestinal IgA Responses Confers Oscillatory Commensal Microbiota Mutualism. Sci. Immunol. 2022, 7, 75. [Google Scholar] [CrossRef]
- Lee, C.; Liang, F.; Lee, I.; Lu, T.; Shan, Y.; Jeng, C.; Zou, Y.; Yu, H.; Chen (Alen), S. External Light-dark Cycle Shapes Gut Microbiota through Intrinsically Photosensitive Retinal Ganglion Cells. EMBO Rep. 2022, 23, 52316. [Google Scholar] [CrossRef]
- Heddes, M.; Altaha, B.; Niu, Y.; Reitmeier, S.; Kleigrewe, K.; Haller, D.; Kiessling, S. The Intestinal Clock Drives the Mi-crobiome to Maintain Gastrointestinal Homeostasis. Nat. Commun. 2022, 13, 6068. [Google Scholar] [CrossRef]
- Allaband, C.; Lingaraju, A.; Flores Ramos, S.; Kumar, T.; Javaheri, H.; Tiu, M.D.; Carolina Dantas Machado, A.; Richter, R.A.; Elijah, E.; Haddad, G.G.; et al. Time of Sample Collection Critical for Microbiome Replicability. bioRxiv 2022. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef]
- Frazier, K.; Kambal, A.; Zale, E.A.; Pierre, J.F.; Hubert, N.; Miyoshi, S.; Miyoshi, J.; Ringus, D.L.; Harris, D.; Yang, K.; et al. High-Fat Diet Disrupts REG3γ and Gut Microbial Rhythms Promoting Metabolic Dysfunction. Cell Host Microbe 2022, 30, 809–823. [Google Scholar] [CrossRef]
- Brooks, J.F.; Behrendt, C.L.; Ruhn, K.A.; Lee, S.; Raj, P.; Takahashi, J.S.; Hooper, L.V. The Microbiota Coordinates Diur-nal Rhythms in Innate Immunity with the Circadian Clock. Cell 2021, 184, 4154–4167.e12. [Google Scholar] [CrossRef]
- Altaha, B.; Heddes, M.; Pilorz, V.; Niu, Y.; Gigl, M.; Kleigrewe, K.; Oster, H.; Haller, D. Genetic and Environmental Cir-cadian Disruption Induce Metabolic Impairment through Changes in the Gut Microbiome. bioRxiv 2022. [Google Scholar] [CrossRef]
- Munyoki, S.K.; Goff, J.P.; Mullett, S.J.; Burns, J.K.; DePoy, L.; Wendell, S.G.; McClung, C.A.; Morrison, K.E.; Jašarević, E. The Magnitude of Sex Differences in Host-Microbe Interactions Are Time-of-Day Dependent. bioRxiv 2022. [Google Scholar] [CrossRef]
- Katya Frazier, A.; Manzoor, S.; Carroll, K.; DeLeon, O.; Miyoshi, S.; Miyoshi, J.; St George, M.; Tan, A.; Izumo, M.; Takahashi, J.S.; et al. Gut Microbes and the Liver Circadian Clock Partition Glucose and Lipid Metabolism. bioRxiv 2022. [Google Scholar] [CrossRef]
- Chakraborty, S.; Mandal, J.; Cheng, X.; Galla, S.; Hindupur, A.; Saha, P.; Yeoh, B.S.; Mell, B.; Yeo, J.Y.; Vijay-Kumar, M.; et al. Diurnal Timing Dependent Alterations in Gut Microbial Composition Are Synchronously Linked to Salt-Sensitive Hyperten-sion and Renal Damage. Hypertension 2020, 76, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Risely, A.; Wilhelm, K.; Clutton-Brock, T.; Manser, M.B.; Sommer, S. Diurnal Oscillations in Gut Bacterial Load and Com-position Eclipse Seasonal and Lifetime Dynamics in Wild Meerkats. Nat. Commun. 2021, 12, 6017. [Google Scholar] [CrossRef] [PubMed]
- Reitmeier, S.; Kiessling, S.; Clavel, T.; List, M.; Almeida, E.L.; Ghosh, T.S.; Neuhaus, K.; Grallert, H.; Linseisen, J.; Skurk, T.; et al. Arrhythmic Gut Microbiome Signatures Predict Risk of Type 2 Diabetes. Cell Host Microbe 2020, 28, 258–272.e6. [Google Scholar] [CrossRef] [PubMed]
- Skarke, C.; Lahens, N.F.; Rhoades, S.D.; Campbell, A.; Bittinger, K.; Bailey, A.; Hoffmann, C.; Olson, R.S.; Chen, L.; Yang, G.; et al. A Pilot Characterization of the Human Chronobiome. Sci. Rep. 2017, 7, 17141. [Google Scholar] [CrossRef]
- Takayasu, L.; Suda, W.; Takanashi, K.; Iioka, E.; Kurokawa, R.; Shindo, C.; Hattori, Y.; Yamashita, N.; Nishijima, S.; Oshima, K.; et al. Circadian Oscillations of Microbial and Functional Composition in the Human Salivary Microbiome. DNA Res. 2017, 24, 261–270. [Google Scholar] [CrossRef]
- Sarkar, A.; Kuehl, M.N.; Alman, A.C.; Burkhardt, B.R. Linking the Oral Microbiome and Salivary Cytokine Abundance to Circadian Oscillations. Sci. Rep. 2021, 11, 2658. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Yuan, Y.; Wang, J.; Zhang, S.; Zhu, R.; Wang, Y.; Wu, Y.; Liao, X.; Mi, J. Reducing Light Exposure Enhances the Circadian Rhythm of the Biological Clock through Interactions with the Gut Microbiota. Sci. Total Environ. 2023, 858, 160041. [Google Scholar] [CrossRef]
- Ellison, A.R.; Wilcockson, D.; Cable, J. Circadian Dynamics of the Teleost Skin Immune-Microbiome Interface. Microbiome 2021, 9, 222. [Google Scholar] [CrossRef]
- Parris, D.J.; Morgan, M.M.; Stewart, F.J. Feeding Rapidly Alters Microbiome Composition and Gene Transcription in the Clownfish Gut. Appl. Environ. Microbiol. 2019, 85, e02479-18. [Google Scholar] [CrossRef]
- Adamovich, Y.; Ladeuix, B.; Sobel, J.; Manella, G.; Neufeld-Cohen, A.; Assadi, M.H.; Golik, M.; Kuperman, Y.; Tarasiuk, A.; Koeners, M.P.; et al. Oxygen and Carbon Dioxide Rhythms Are Circadian Clock Controlled and Differentially Directed by Be-havioral Signals. Cell. Metab. 2019, 29, 1092–1103.e3. [Google Scholar] [CrossRef]
- Wang, Y.; Kuang, Z.; Yu, X.; Ruhn, K.A.; Kubo, M.; Hooper, L. The Intestinal Microbiota Regulates Body Composition through NFIL3 and the Circadian Clock. Science 2017, 357, 912–916. [Google Scholar] [CrossRef] [PubMed]
- Farkas, A.M.; Panea, C.; Goto, Y.; Nakato, G.; Galan-Diez, M.; Narushima, S.; Honda, K.; Ivanov, I.I. Induction of Th17 Cells by Segmented Filamentous Bacteria in the Murine Intestine. J. Immunol. Methods 2015, 421, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, H.; Hugerth, L.W.; Sundh, J.; Lundin, E.; Andersson, A.F. Genome Sequence of Segmented Filamentous Bacteria Present in the Human Intestine. Commun. Biol. 2020, 3, 485. [Google Scholar] [CrossRef] [PubMed]
- Tannock, G.W.; Miller, J.R.; Savage, D.C. Host Specificity of Filamentous, Segmented Microorganisms Adherent to the Small Bowel Epithelium in Mice and Rats. Appl. Environ. Microbiol. 1984, 47, 441–442. [Google Scholar] [CrossRef]
- Flannigan, K.L.; Denning, T.L. Segmented Filamentous Bacteria-Induced Immune Responses: A Balancing Act between Host Protection and Autoimmunity. Immunology 2018, 154, 537–546. [Google Scholar] [CrossRef]
- Prakash, T.; Oshima, K.; Morita, H.; Fukuda, S.; Imaoka, A.; Kumar, N.; Sharma, V.K.; Kim, S.-W.; Takahashi, M.; Saitou, N.; et al. Complete Genome Sequences of Rat and Mouse Segmented Filamentous Bacteria, a Potent Inducer of Th17 Cell Differenti-ation. Cell Host Microbe 2011, 10, 273–284. [Google Scholar] [CrossRef]
- Umesaki, Y.; Setoyama, H.; Matsumoto, S.; Imaoka, A.; Itoh, K. Differential Roles of Segmented Filamentous Bacteria and Clostridia in Development of the Intestinal Immune System. Infect. Immun. 1999, 67, 3504–3511. [Google Scholar] [CrossRef]
- Ivanov, I.I.; de Llanos Frutos, R.; Manel, N.; Yoshinaga, K.; Rifkin, D.B.; Sartor, R.B.; Finlay, B.B.; Littman, D.R. Specific Mi-crobiota Direct the Differentiation of IL-17-Producing T-Helper Cells in the Mucosa of the Small Intestine. Cell Host Microbe 2008, 4, 337–349. [Google Scholar] [CrossRef]
- Gaboriau-Routhiau, V.; Rakotobe, S.; Lécuyer, E.; Mulder, I.; Lan, A.; Bridonneau, C.; Rochet, V.; Pisi, A.; de Paepe, M.; Brandi, G.; et al. The Key Role of Segmented Filamentous Bacteria in the Coordinated Maturation of Gut Helper T Cell Responses. Immunity 2009, 31, 677–689. [Google Scholar] [CrossRef]
- Bhakta, N.R. Pitfalls of Probiotics. Sci. Transl. Med. 2016, 8, 368. [Google Scholar] [CrossRef]
- Chen, B.; Chen, H.; Shu, X.; Yin, Y.; Li, J.; Qin, J.; Chen, L.; Peng, K.; Xu, F.; Gu, W.; et al. Presence of Segmented Filamentous Bacteria in Human Children and Its Potential Role in the Modulation of Human Gut Immunity. Front. Microbiol. 2018, 9, 1403. [Google Scholar] [CrossRef] [PubMed]
- Brabec, T.; Schwarzer, M.; Kováčová, K.; Dobešová, M.; Schierová, D.; Březina, J.; Pacáková, I.; Šrůtková, D.; Ben-Nun, O.; Goldfarb, Y.; et al. Epithelial Antigen Presentation Controls Commensal-Specific Intraepithelial T-Cells in the Gut. bioRxiv 2022. [Google Scholar] [CrossRef]
- Moor, A.E.; Harnik, Y.; Ben-Moshe, S.; Massasa, E.E.; Rozenberg, M.; Eilam, R.; Bahar Halpern, K.; Itzkovitz, S. Spatial Reconstruction of Single Enterocytes Uncovers Broad Zonation along the Intestinal Villus Axis. Cell 2018, 175, 1156–1167.e15. [Google Scholar] [CrossRef]
- Tuganbaev, T.; Mor, U.; Bashiardes, S.; Liwinski, T.; Nobs, S.P.; Leshem, A.; Dori-Bachash, M.; Thaiss, C.A.; Pinker, E.Y.; Ratiner, K.; et al. Diet Diurnally Regulates Small Intestinal Microbiome-Epithelial-Immune Homeostasis and Enteritis. Cell 2020, 182, 1441–1459.e21. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.T. Toll-like Receptor Signalling in the Intestinal Epithelium: How Bacterial Recognition Shapes Intestinal Func-tion. Nat. Rev. Immunol. 2010, 10, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.L.; Hooper, L.V. Epithelial Antimicrobial Defence of the Skin and Intestine. Nat. Rev. Immunol. 2012, 12, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Zheng, H.; Derebe, M.G.; Callenberg, K.M.; Partch, C.L.; Rollins, D.; Propheter, D.C.; Rizo, J.; Grabe, M.; Jiang, Q.-X.; et al. Antibacterial Membrane Attack by a Pore-Forming Intestinal C-Type Lectin. Nature 2014, 505, 103–107. [Google Scholar] [CrossRef]
- Loonen, L.M.; Stolte, E.H.; Jaklofsky, M.T.; Meijerink, M.; Dekker, J.; van Baarlen, P.; Wells, J.M. REG3γ-Deficient Mice Have Altered Mucus Distribution and Increased Mucosal Inflammatory Responses to the Microbiota and Enteric Pathogens in the Ileum. Mucosal Immunol. 2014, 7, 939–947. [Google Scholar] [CrossRef]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The Antibacterial Lectin RegIIIγ Promotes the Spatial Segregation of Microbiota and Host in the Intestine. Science 2011, 334, 255–258. [Google Scholar] [CrossRef]
- El Aidy, S.; van Baarlen, P.; Derrien, M.; Lindenbergh-Kortleve, D.J.; Hooiveld, G.; Levenez, F.; Doré, J.; Dekker, J.; Sam-som, J.N.; Nieuwenhuis, E.E.S.; et al. Temporal and Spatial Interplay of Microbiota and Intestinal Mucosa Drive Establishment of Immune Homeostasis in Conventionalized Mice. Mucosal Immunol. 2012, 5, 567–579. [Google Scholar] [CrossRef]
- Vaishnava, S.; Behrendt, C.L.; Ismail, A.S.; Eckmann, L.; Hooper, L.V. Paneth Cells Directly Sense Gut Commensals and Maintain Homeostasis at the Intestinal Host-Microbial Interface. Proc. Natl. Acad. Sci. USA 2008, 105, 20858–20863. [Google Scholar] [CrossRef] [PubMed]
- Talbot, J.; Hahn, P.; Kroehling, L.; Nguyen, H.; Li, D.; Littman, D.R. Feeding-Dependent VIP Neuron–ILC3 Circuit Regu-lates the Intestinal Barrier. Nature 2020, 579, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Woof, J.M.; Kerr, M.A. The Function of Immunoglobulin A in Immunity. J. Pathol. 2006, 208, 270–282. [Google Scholar] [CrossRef]
- Owen, J.A.; Punt, J.; Stranford, A.S. Kuby Immunology, 7th ed.; Macmillan Education: New York, NY, USA, 2013. [Google Scholar]
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Mackiewicz, A.; Schmidt, M. Secretory IgA in Intestinal Mucosal Secretions as an Adaptive Barrier against Microbial Cells. Int. J. Mol. Sci. 2020, 21, 9254. [Google Scholar] [CrossRef]
- Rossen, R.D.; Schade, A.L.; Butler, W.T.; Kasel, J.A. The Proteins in Nasal Secretion: A Longitudinal Study of the Gam-maA-Globulin, GammaG-Globulin, Albumin, Siderophilin, and Total Protein Concentrations in Nasal Washings from Adult Male Volunteers. J. Clin. Investig. 1966, 45, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.C.; Johnson, R.L. Circadian and Interpersonal Variability of Iga in Nasal Secretions. Ann. Otol. Rhinol. Laryngol. 1973, 82, 216–222. [Google Scholar] [CrossRef]
- Menzio, P.; Morra, B.; Sartoris, A.; Molino, R.; Bussi, M.; Cortesina, G. Nasal Secretory IgA Circadian Rhythm: A Single-Dose Suppression Test. Ann. Otol. Rhinol. Laryngol. 1980, 89, 173–175. [Google Scholar] [CrossRef]
- Harada, T.; Hamaguchi, Y.; And, Y.S.; Miyoshi, Y. Circadian Variation of Secretory IgA in Nasal Secretions from Normal Subjects. Acta Otolaryngol. 1984, 97, 359–362. [Google Scholar] [CrossRef]
- Massmann, P.F.; França, E.L.; de Souza, E.G.; Souza, M.S.; Brune, M.F.S.S.; Honorio-França, A.C. Maternal Hypertension Induces Alterations in Immunological Factors of Colostrum and Human Milk. Front. Life Sci. 2013, 7, 155–163. [Google Scholar] [CrossRef][Green Version]
- França, E.L.; dos Reis Nicomedes, T.; de Mattos Paranhos Calderon, I.; França, A.C.H. Time-Dependent Alterations of Soluble and Cellular Components in Human Milk. Biol. Rhythm Res. 2010, 41, 333–347. [Google Scholar] [CrossRef]
- Wada, M.; Orihara, K.; Kamagata, M.; Hama, K.; Sasaki, H.; Haraguchi, A.; Miyakawa, H.; Nakao, A.; Shibata, S. Cir-cadian Clock-Dependent Increase in Salivary IgA Secretion Modulated by Sympathetic Receptor Activation in Mice. Sci. Rep. 2017, 7, 8802. [Google Scholar] [CrossRef]
- Dimitriou, L.; Sharp, N.C.C.; Doherty, M. Circadian Effects on the Acute Responses of Salivary Cortisol and IgA in Well Trained Swimmers. Br. J. Sports Med. 2002, 36, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Tokura, H. Bright Light Exposure during the Daytime Affects Circadian Rhythms of Urinary Melatonin and Salivary Immunoglobulin A. Chronobiol. Int. 1999, 16, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, T.; Sakaguchi, H.; Hatayama, T.; Takata, A.; Hyodoh, F.; Tsujita, S.; Ueki, A.; Morimoto, K. Secretory IgA in Saliva and Academic Stress. Int. J. Immunopathol. Pharmacol. 2004, 17, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.; Oddi, S.; Forzani, L.; Tabacman, E.; Reinheimer, J.; Vinderola, G. Variability in Gut Mucosal Secretory IgA in Mice along a Working Day. BMC Res. Notes 2018, 11, 98. [Google Scholar] [CrossRef]
- Eriksson, E.; Royo, F.; Lyberg, K.; Carlsson, H.E.; Hau, J. Effect of Metabolic Cage Housing on Immunoglobulin A and Corticosterone Excretion in Faeces and Urine of Young Male Rats. Exp. Physiol. 2004, 89, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Fagarasan, S.; Kawamoto, S.; Kanagawa, O.; Suzuki, K. Adaptive Immune Regulation in the Gut: T Cell–Dependent and T Cell–Independent IgA Synthesis. Annu. Rev. Immunol. 2010, 28, 243–273. [Google Scholar] [CrossRef]
- Cerutti, A. The Regulation of IgA Class Switching. Nat. Rev. Immunol. 2008, 8, 421–434. [Google Scholar] [CrossRef]
- Caba-Flores, M.D.; Ramos-Ligonio, A.; Camacho-Morales, A.; Martínez-Valenzuela, C.; Viveros-Contreras, R.; Caba, M. Breast Milk and the Importance of Chrononutrition. Front. Nutr. 2022, 9, 867507. [Google Scholar] [CrossRef]
- Gopalakrishna, K.P.; Macadangdang, B.R.; Rogers, M.B.; Tometich, J.T.; Firek, B.A.; Baker, R.; Ji, J.; Burr, A.H.P.; Ma, C.; Good, M.; et al. Maternal IgA Protects against the Development of Necrotizing Enterocolitis in Preterm Infants. Nat. Med. 2019, 25, 1110–1115. [Google Scholar] [CrossRef]
- Xu, Q.; Jian, H.; Zhao, W.; Li, J.; Zou, X.; Dong, X. Early Weaning Stress Induces Intestinal Microbiota Disturbance, Mu-cosal Barrier Dysfunction and Inflammation Response Activation in Pigeon Squabs. Front. Microbiol. 2022, 13, 877866. [Google Scholar] [CrossRef] [PubMed]
- Bowman, E.P.; Kuklin, N.A.; Youngman, K.R.; Lazarus, N.H.; Kunkel, E.J.; Pan, J.; Greenberg, H.B.; Butcher, E.C. The Intestinal Chemokine Thymus-Expressed Chemokine (CCL25) Attracts IgA Antibody-Secreting Cells. J. Exp. Med. 2002, 195, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Rollenske, T.; Burkhalter, S.; Muerner, L.; von Gunten, S.; Lukasiewicz, J.; Wardemann, H.; Macpherson, A.J. Parallelism of Intestinal Secretory IgA Shapes Functional Microbial Fitness. Nature 2021, 598, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Klaasen, H.L.; van der Heijden, P.J.; Stok, W.; Poelma, F.G.; Koopman, J.P.; van den Brink, M.E.; Bakker, M.H.; Eling, W.M.; Beynen, A.C. Apathogenic, Intestinal, Segmented, Filamentous Bacteria Stimulate the Mucosal Immune System of Mice. Infect. Immun. 1993, 61, 303–306. [Google Scholar] [CrossRef]
- Sanchez-Russo, L.; Rajasekaran, A.; Bin, S.; Faith, J.; Cravedi, P. The Gut and Kidney Crosstalk in Immunoglobulin A Nephropathy. Kidney360 2022, 3, 1630–1639. [Google Scholar] [CrossRef]
- Heuberger, C.; Pott, J.; Maloy, K.J. Why Do Intestinal Epithelial Cells Express MHC Class II? Immunology 2021, 162, 357–367. [Google Scholar] [CrossRef]
- Wosen, J.E.; Mukhopadhyay, D.; Macaubas, C.; Mellins, E.D. Epithelial MHC Class II Expression and Its Role in Antigen Presentation in the Gastrointestinal and Respiratory Tracts. Front. Immunol. 2018, 9, 2144. [Google Scholar] [CrossRef]
- Koyama, M.; Mukhopadhyay, P.; Schuster, I.S.; Henden, A.S.; Hülsdünker, J.; Varelias, A.; Vetizou, M.; Kuns, R.D.; Robb, R.J.; Zhang, P.; et al. MHC Class II Antigen Presentation by the Intestinal Epithelium Initiates Graft-versus-Host Disease and Is Influenced by the Microbiota. Immunity 2019, 51, 885–898.e7. [Google Scholar] [CrossRef]
- Ladinsky, M.S.; Araujo, L.P.; Zhang, X.; Veltri, J.; Galan-Diez, M.; Soualhi, S.; Lee, C.; Irie, K.; Pinker, E.Y.; Narushima, S.; et al. Endocytosis of Commensal Antigens by Intestinal Epithelial Cells Regulates Mucosal T Cell Homeostasis. Science 2019, 363, 6431. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Ando, M.; Kamada, N.; Nagano, Y.; Narushima, S.; Suda, W.; Imaoka, A.; Setoyama, H.; Nagamori, T.; et al. Th17 Cell Induction by Adhesion of Microbes to Intestinal Epithelial Cells. Cell 2015, 163, 367–380. [Google Scholar] [CrossRef]
- Biton, M.; Haber, A.L.; Rogel, N.; Burgin, G.; Beyaz, S.; Schnell, A.; Ashenberg, O.; Su, C.-W.; Smillie, C.; Shekhar, K.; et al. T Helper Cell Cytokines Modulate Intestinal Stem Cell Renewal and Differentiation. Cell 2018, 175, 1307–1320.e22. [Google Scholar] [CrossRef] [PubMed]
- Beyaz, S.; Chung, C.; Mou, H.; Bauer-Rowe, K.E.; Xifaras, M.E.; Ergin, I.; Dohnalova, L.; Biton, M.; Shekhar, K.; Eskiocak, O.; et al. Dietary Suppression of MHC Class II Expression in Intestinal Epithelial Cells Enhances Intestinal Tumorigenesis. Cell Stem Cell 2021, 28, 1922–1935. [Google Scholar] [CrossRef] [PubMed]
- Matsu-ura, T.; Dovzhenok, A.; Aihara, E.; Rood, J.; Le, H.; Ren, Y.; Rosselot, A.E.; Zhang, T.; Lee, C.; Obrietan, K.; et al. Intercellular Coupling of the Cell Cycle and Circadian Clock in Adult Stem Cell Culture. Mol. Cell 2016, 64, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Stephens, W.Z.; Kubinak, J.L.; Ghazaryan, A.; Bauer, K.M.; Bell, R.; Buhrke, K.; Chiaro, T.R.; Weis, A.M.; Tang, W.W.; Monts, J.K.; et al. Epithelial-Myeloid Exchange of MHC Class II Constrains Immunity and Microbiota Composition. Cell Rep. 2021, 37, 109916. [Google Scholar] [CrossRef] [PubMed]
- Jašarević, E.; Morrison, K.E.; Bale, T.L. Sex Differences in the Gut Microbiome-Brain Axis across the Lifespan. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150122. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, F.; Nguyen, T.L.A.; Brinkman, B.; Yunta, R.G.; Cauwe, B.; Vandenabeele, P.; Liston, A.; Raes, J. Inflamma-tion-Associated Enterotypes, Host Genotype, Cage and Inter-Individual Effects Drive Gut Microbiota Variation in Common Labora-tory Mice. Genome Biol. 2013, 14, R4. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, M.; Raes, J.; Pelletier, E.; le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the Human Gut Microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.H.; Foster, C.M.; Vishnivetskaya, T.; Campbell, A.G.; Yang, Z.K.; Wymore, A.; Palumbo, A.V.; Chesler, E.J.; Podar, M. Host Genetic and Environmental Effects on Mouse Intestinal Microbiota. ISME J. 2012, 6, 2033–2044. [Google Scholar] [CrossRef]
- le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Ken-nedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Dollive, S.; Chen, Y.-Y.; Grunberg, S.; Bittinger, K.; Hoffmann, C.; Vandivier, L.; Cuff, C.; Lewis, J.D.; Wu, G.D.; Bushman, F.D. Fungi of the Murine Gut: Episodic Variation and Proliferation during Antibiotic Treatment. PLoS ONE 2013, 8, e71806. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratiner, K.; Fachler-Sharp, T.; Elinav, E. Small Intestinal Microbiota Oscillations, Host Effects and Regulation—A Zoom into Three Key Effector Molecules. Biology 2023, 12, 142. https://doi.org/10.3390/biology12010142
Ratiner K, Fachler-Sharp T, Elinav E. Small Intestinal Microbiota Oscillations, Host Effects and Regulation—A Zoom into Three Key Effector Molecules. Biology. 2023; 12(1):142. https://doi.org/10.3390/biology12010142
Chicago/Turabian StyleRatiner, Karina, Tahel Fachler-Sharp, and Eran Elinav. 2023. "Small Intestinal Microbiota Oscillations, Host Effects and Regulation—A Zoom into Three Key Effector Molecules" Biology 12, no. 1: 142. https://doi.org/10.3390/biology12010142
APA StyleRatiner, K., Fachler-Sharp, T., & Elinav, E. (2023). Small Intestinal Microbiota Oscillations, Host Effects and Regulation—A Zoom into Three Key Effector Molecules. Biology, 12(1), 142. https://doi.org/10.3390/biology12010142






