Long-Term Exposure to Ozone Increases Neurological Disability after Stroke: Findings from a Nationwide Longitudinal Study in China
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Exposure Assessment
2.2. Population Selection
2.3. Outcome
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Study Sample
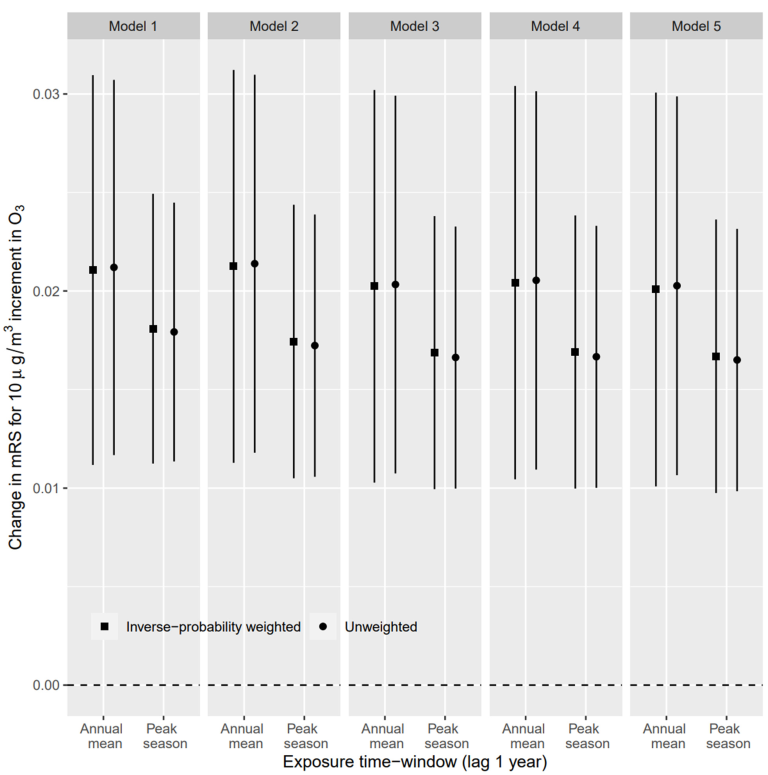
3.2. Association between O3 Exposure and mRS Score
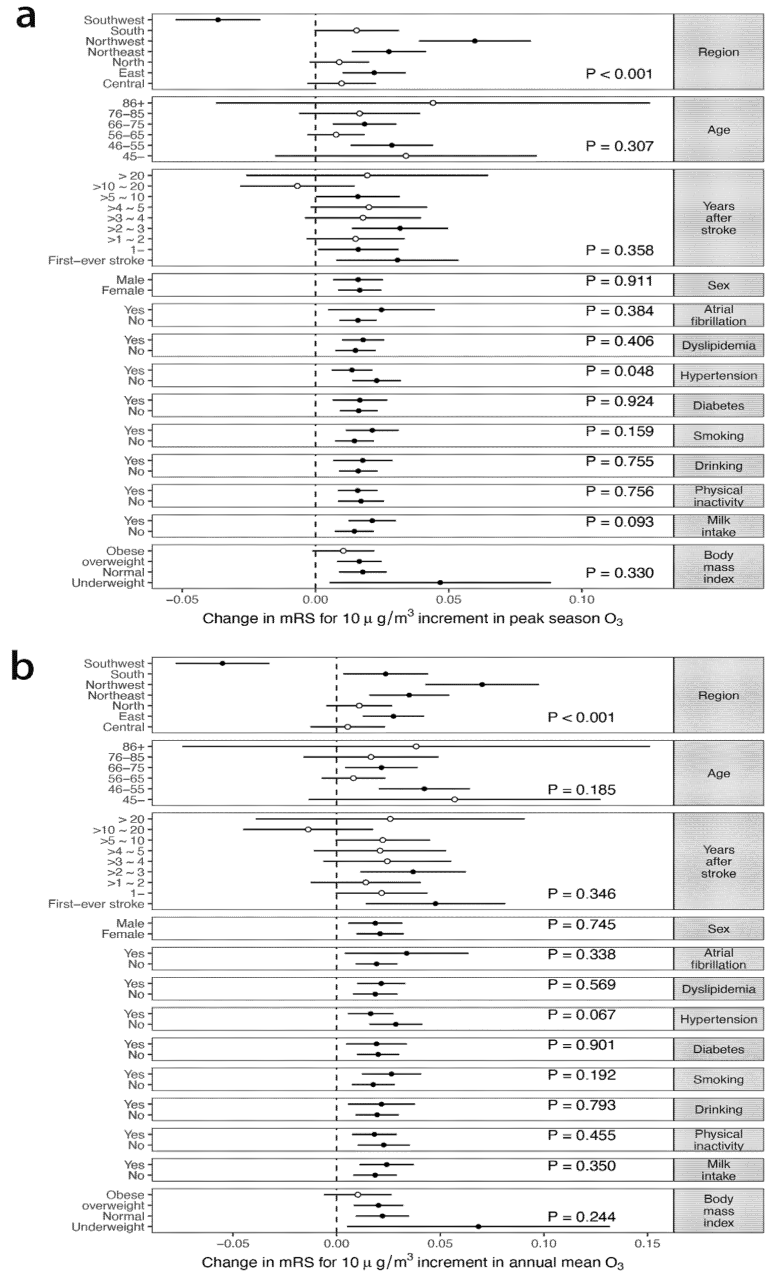
3.3. Effect Modification
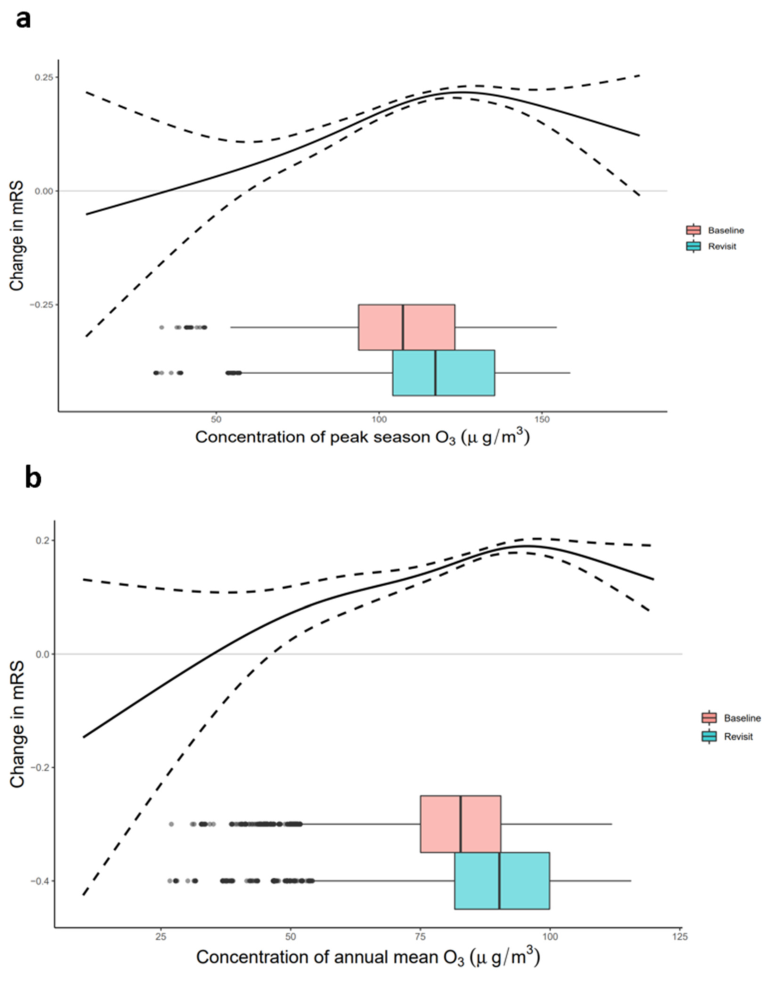
3.4. Exposure−Response Relationship
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shakir, R. The struggle for stroke reclassification. Nat. Rev. Neurol. 2018, 14, 447–448. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Bhutta, Z.A.; Carter, A.; Bell, M.L. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Feigin, V.L.; Krishnamurthi, R.V.; Parmar, P.; Norrving, B.; Mensah, G.A.; Bennett, D.A.; Barker-Collo, S.; Moran, A.E.; Sacco, R.L.; Truelsen, T. Update on the Global Burden of Ischemic and Hemorrhagic Stroke in 1990-2013: The GBD 2013 Study. Neuroepidemiology 2015, 45, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Zheng, Y.; Geng, G.; Xiao, Q.; Meng, X.; Wang, M.; Li, X.; Wu, N.; Zhang, Q.; Zhu, T. Estimating Spatiotemporal Varia-tion in Ambient Ozone Exposure during 2013-2017 Using a Data-Fusion Model. Environ. Sci. Technol. 2020, 54, 14877–14888. [Google Scholar] [CrossRef] [PubMed]
- Danesh Yazdi, M.; Wang, Y.; Di, Q.; Zanobetti, A.; Schwartz, J. Long-term exposure to PM2.5 and ozone and hospital admissions of Medicare participants in the Southeast USA. Environ. Int. 2019, 130, 104879. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, Y.; Tao, L.; Xu, Q.; Wang, C.; Wang, A.; Li, X.; Guo, J.; Yan, A. Particulate Matter and Hospital Admissions for Stroke in Beijing, China: Modification Effects by Ambient Temperature. J. Am. Heart Assoc. 2016, 5, e003437. [Google Scholar] [CrossRef]
- McClure, L.A.; Loop, M.S.; Crosson, W.; Kleindorfer, D.; Kissela, B.; Al-Hamdan, M. Fine Particulate Matter (PM(2.5)) and the Risk of Stroke in the REGARDS Cohort. J. Stroke Cerebrovasc. Dis. 2017, 26, 1739–1744. [Google Scholar] [CrossRef]
- Zhao, N.; Pinault, L.; Toyib, O.; Vanos, J.; Tjepkema, M.; Cakmak, S. Long-term ozone exposure and mortality from neurological diseases in Canada. Environ. Int. 2021, 157, 106817. [Google Scholar] [CrossRef]
- Shah, A.S.V.; Lee, K.K.; McAllister, D.; Hunter, A.; Nair, H.; Whiteley, W.; Langrish, J.P.; Newby, D.E.; Mills, N. Short term exposure to air pollution a.and stroke: Systematic review and meta-analysis. BMJ 2015, 350, h1295. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Air Quality Guidelines: Particulate Matter (PM2.5 and PM10), Ozone, Nitrogen Dioxide, Sulfur Dioxide and Carbon Monoxide. World Health Organization. 2021. Available online: https://apps.who.int/iris/handle/10665/345329 (accessed on 31 May 2022).
- Wing, J.J.; Sánchez, B.N.; Adar, S.D.; Meurer, W.J.; Morgenstern, L.B.; Smith, M.A.; Lisabeth, L.D. Synergism of Short-Term Air Pollution Exposures and Neighborhood Disadvantage on Initial Stroke Severity. Stroke 2017, 48, 3126–3129. [Google Scholar] [CrossRef]
- Gao, Q.; Zang, E.; Bi, J.; Dubrow, R.; Lowe, S.R.; Chen, H.; Zeng, Y.; Shi, L.; Chen, K. Long-term ozone exposure and cognitive impairment among Chinese older adults: A cohort study. Environ. Int. 2022, 160, 107072. [Google Scholar] [CrossRef] [PubMed]
- Ilango, S.D.; Gonzalez, K.; Gallo, L.; Allison, M.A.; Cai, J.; Isasi, C.R.; Hosgood, D.H.; Vasquez, P.M.; Zeng, D.; Mortamais, M.; et al. Long-Term Exposure to Ambient Air Pollution and Cognitive Function Among Hispanic/Latino Adults in San Diego, California. J. Alzheimer Dis. 2021, 79, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Broderick, J.P.; Adeoye, O.; Elm, J. Evolution of the Modified Rankin Scale and Its Use in Future Stroke Trials. Stroke 2017, 48, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Ma, J.; Li, M.; Xue, T.; Lan, Z.; Guo, J.; Shen, Y.; Chao, B.; Tian, G.; Zhang, Q.; et al. Rapid transitions in the epidemiology of stroke and its risk factors in China from 2002 to 2013. Neurology 2017, 89, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Wang, L.D.; Chao, B.H.; Liu, Y.L. Prevalence of stroke in China: An epidemiological study based on the National Stroke Screening Survey. Lancet 2015, 386, 49. [Google Scholar] [CrossRef]
- Qi, W.; Ma, J.; Guan, T.; Zhao, D.; Abu-Hanna, A.; Schut, M.; Chao, B.; Wang, L.; Liu, Y. Risk Factors for Incident Stroke and Its Subtypes in China: A Prospective Study. J. Am. Heart Assoc. 2020, 9, e016352. [Google Scholar] [CrossRef]
- Chao, B.H.; Tu, W.J.; Wang, L.D. Initial establishment of a stroke management model in China: 10 years (2011–2020) of Stroke Prevention Project Committee, National Health Commission. Chin. Med. J. 2021, 134, 2418–2420. [Google Scholar] [CrossRef]
- Yi, X.; Luo, H.; Zhou, J.; Yu, M.; Chen, X.; Tan, L.; Wei, W.; Li, J. Prevalence of stroke and stroke related risk factors: A population based cross sectional survey in southwestern China. BMC Neurol. 2020, 20, 5. [Google Scholar] [CrossRef]
- Longde, W.; Ling, Y.; Yang, H.; Yi, Z.; Yong, W.; Xun, J.; Xiao, N.; Qiu, Q.; Li, H.; Yu, X.; et al. Fixed-dose combination treatment after stroke for secondary prevention in China: A national community-based study. Stroke 2015, 46, 1295–1300. [Google Scholar] [CrossRef]
- Mpandzou, G.; Aït Ben Haddou, E.; Regragui, W.; Benomar, A.; Yahyaoui, M. Vitamin D deficiency and its role in neurological conditions: A review. Rev. Neurol. 2016, 172, 109–122. [Google Scholar] [CrossRef]
- Kerac, M.; Postels, D.; Mallewa, M.; Jalloh, A.A.; Voskuijl, W.; Groce, N.; Gladstone, M.; Molyneux, E. The interaction of malnutrition and neurologic disability in Africa. Semin. Pediatric Neurol. 2014, 21, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jiang, B.; Sun, H.; Ru, X.; Sun, D.; Wang, L.; Wang, L.; Jiang, Y.; Li, Y.; Wang, Y.; et al. Prevalence, Incidence, and Mortality of Stroke in China: Results from a Nationwide Population-Based Survey of 480 687 Adults. Circulation 2017, 135, 759–771. [Google Scholar] [CrossRef]
- Chen, G.; Wang, A.; Li, S.; Zhao, X.; Wang, Y.; Li, H.; Meng, X.; Knibbs, L.; Bell, M.L.; Abramson, M.J.; et al. Long-Term Exposure to Air Pollution and Survival After Ischemic Stroke. Stroke 2019, 50, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Hoffmann, B.; Andersen, Z.J.; Atkinson, R.W.; Bauwelinck, M.; Bellander, T.; Brandt, J.; Brunekreef, B.; Cesaroni, G.; Chen, J.; et al. Long-term exposure to low-level ambient air pollution and incidence of stroke and coronary heart disease: A pooled analysis of six European cohorts within the ELAPSE project. Lancet Planet. Health 2021, 5, e620–e632. [Google Scholar] [CrossRef]
- Mumby, S.; Chung, K.F.; Adcock, I.M. Transcriptional Effects of Ozone and Impact on Airway Inflammation. Front. Immunol. 2019, 10, 1610. [Google Scholar] [CrossRef] [PubMed]
- Day, D.B.; Xiang, J.; Mo, J.; Li, F.; Chung, M.K.; Gong, J.; Weschler, C.J.; Ohman-Strickland, P.A.; Sundell, J.; Weng, W.; et al. Association of Ozone Exposure With Cardiorespiratory Pathophysiologic Mechanisms in Healthy Adults. JAMA Intern. Med. 2017, 177, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Niu, Y.; Cai, J.; Lin, Z.; Liu, C.; Li, H.; Chen, C.; Song, W.; Zhao, Z.; Chen, R.; et al. Effects of Personal Short-Term Exposure to Ambient Ozone on Blood Pressure and Vascular Endothelial Function: A Mechanistic Study Based on DNA Methylation and Metabolomics. Environ. Sci. Technol. 2018, 52, 12774–12782. [Google Scholar] [CrossRef]
- Rich, D.Q.; Kipen, H.M.; Huang, W.; Wang, G.; Wang, Y.; Zhu, P.; Ohman-Strickland, P.; Hu, M.; Philipp, C.; Diehl, S.R.; et al. Association Between Changes in Air Pollution Levels During the Beijing Olympics and Biomarkers of Inflammation and Thrombosis in Healthy Young Adults. JAMA 2012, 307, 2068–2078. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Xu, H.; Brook, R.D.; Liu, S.; Yi, T.; Wang, Y.; Feng, B.; Zhao, M.; Wang, X.; et al. Ambient Air Pollution Is Associated With HDL (High-Density Lipoprotein) Dysfunction in Healthy Adults. Arter. Thromb. Vasc. Biol. 2019, 39, 513–522. [Google Scholar] [CrossRef]
- Martinez-Lazcano, J.C.; González-Guevara, E.; del Carmen Rubioubio, M.; Franco-Pérez, J.; Custodio, V.; Hernández-Cerón, M.; Livera, C.; Paz, C. The effects of ozone exposure and associated injury mechanisms on the central nervous system. Rev. Neurosci. 2013, 24, 337–352. [Google Scholar] [CrossRef]
- Croze, M.L.; Zimmer, L. Ozone Atmospheric Pollution and Alzheimer’s Disease: From Epidemiological Facts to Molecular Mechanisms. J. Alzheimer Dis. 2018, 62, 503–522. [Google Scholar] [CrossRef] [PubMed]

| Overall | O3 First Quartile | O3 Second Quartile | O3 Third Quartile | O3 Fourth Quartile | p-Value | |
|---|---|---|---|---|---|---|
| (≤93.70 μg/m3) | (93.70–107.50 μg/m3) | (107.50–123.27 μg/m3) | (>123.27 μg/m3) | |||
| Age Group | <0.01 | |||||
| ≤45 | 585 (2.09) | 197 (2.49) | 161 (2.29) | 125 (1.78) | 102 (1.46) | —— |
| 45–55 | 4288 (15.28) | 1176 (14.84) | 1016 (14.48) | 1066 (15.15) | 1030 (14.76) | —— |
| 55–65 | 9857 (35.13) | 2438 (30.77) | 2405 (34.27) | 2486 (35.33) | 2528 (36.22) | —— |
| 65–75 | 9681 (34.51) | 2308 (29.13) | 2447 (34.87) | 2437 (34.64) | 2489 (35.66) | —— |
| 75–85 | 3388 (12.08) | 829 (10.46) | 912 (13.00) | 865 (12.29) | 782 (11.21) | —— |
| >85 | 257 (0.92) | 76 (0.96) | 76 (1.08) | 57 (0.81) | 48 (0.69) | —— |
| Sex | <0.01 | |||||
| Female | 13,094 (46.67) | 3398 (48.38) | 3353 (47.78) | 3267 (46.43) | 3076 (44.08) | —— |
| Male | 14,842 (52.90) | 3610 (51.40) | 3659 (52.14) | 3713 (52.77) | 3860 (55.31) | —— |
| Missing | 120 (0.43) | 16 (0.23) | 5 (0.07) | 56 (0.80) | 43 (0.62) | —— |
| Atrial Fibrillation | <0.01 | |||||
| No | 26,649 (94.99) | 6661 (94.83) | 6537 (93.16) | 6736 (95.74) | 6715 (96.22) | —— |
| Yes | 1401 (4.99) | 363 (5.17) | 480 (6.84) | 294 (4.18) | 264 (3.78) | —— |
| Missing | 6 (0.02) | 0 (0.00) | 0 (0.00) | 6 (0.09) | 0 (0.00) | —— |
| Dyslipidemia | <0.01 | |||||
| No | 15,179 (54.1) | 3484 (49.6) | 3959 (56.42) | 4068 (57.82) | 3668 (52.56) | —— |
| Yes | 9715 (34.63) | 1902 (27.08) | 2242 (31.95) | 2609 (37.08) | 2962 (42.44) | —— |
| Missing | 3162 (11.27) | 1638 (23.32) | 816 (11.63) | 359 (5.10) | 349 (5.00) | —— |
| Hypertension | <0.01 | |||||
| No | 9021 (32.15) | 2493 (35.49) | 2174 (30.98) | 2249 (31.96) | 2105 (30.16) | —— |
| Yes | 19,029 (67.83) | 4531 (64.51) | 4843 (69.02) | 4781 (67.95) | 4874 (69.84) | —— |
| Missing | 6 (0.02) | 0 (0.00) | 0 (0.00) | 6 (0.09) | 0 (0.00) | —— |
| Diabetes Mellitus | <0.01 | |||||
| No | 20,847 (74.30) | 4908 (69.87) | 5289 (75.37) | 5383 (76.51) | 5267 (75.47) | —— |
| Yes | 5272 (18.79) | 1027 (14.62) | 1278 (18.21) | 1484 (21.09) | 1483 (21.25) | —— |
| Missing | 1937 (6.90) | 1089 (15.50) | 450 (6.41) | 169 (2.40) | 229 (3.28) | —— |
| Smoke | <0.01 | |||||
| No | 18,217 (64.93) | 4154 (59.14) | 4626 (65.93) | 4796 (68.16) | 4641 (66.50) | —— |
| Yes | 7133 (25.42) | 1779 (25.33) | 1824 (25.99) | 1793 (25.48) | 1737 (24.89) | —— |
| Missing | 2706 (9.64) | 1091 (15.53) | 567 (8.08) | 447 (6.35) | 601 (8.61) | —— |
| Drink | <0.01 | |||||
| No | 23,133 (82.45) | 5812 (82.74) | 5862 (83.54) | 5845 (83.07) | 5614 (80.44) | —— |
| Yes | 4910 (17.50) | 1210 (17.23) | 1152 (16.42) | 1184 (16.83) | 1364 (19.54) | —— |
| Missing | 13 (0.05) | 2 (0.03) | 3 (0.04) | 7 (0.10) | 1 (0.01) | —— |
| Sport | <0.01 | |||||
| No | 11,147 (39.73) | 2824 (40.21) | 2673 (38.09) | 2660 (37.81) | 2990 (42.84) | —— |
| Yes | 16,901 (60.24) | 4198 (59.77) | 4344 (61.91) | 4370 (62.11) | 3989 (57.16) | —— |
| Missing | 8 (0.03) | 2 (0.03) | 0 (0.00) | 6 (0.09) | 0 (0.00) | —— |
| Milk | <0.01 | |||||
| No | 17,073 (60.85) | 3837 (54.63) | 4264 (60.77) | 4304 (61.17) | 4668 (66.89) | —— |
| Yes | 4449 (15.86) | 973 (13.85) | 1130 (16.10) | 1090 (15.49) | 1256 (18.00) | —— |
| Missing | 6534 (23.29) | 2214 (31.52) | 1623 (23.13) | 1642 (23.34) | 1055 (15.12) | —— |
| BMI | <0.01 | |||||
| (−Inf,18.5] | 545 (1.94) | 163 (2.32) | 175 (2.49) | 124 (1.76) | 83 (1.19) | —— |
| (18.5,24] | 10,580 (37.71) | 3092 (44.02) | 2850 (40.62) | 2435 (34.61) | 2203 (31.57) | —— |
| (24,28] | 11,849 (42.23) | 2786 (39.66) | 2906 (41.41) | 3105 (44.13) | 3052 (43.73) | —— |
| (28, Inf] | 5055 (18.02) | 967 (13.77) | 1082 (15.42) | 1370 (19.47) | 1636 (23.44) | —— |
| Missing | 27 (0.10) | 16 (0.23) | 4 (0.06) | 2 (0.03) | 5 (0.07) | —— |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Lu, H.; Cao, M.; Tong, M.; Wang, R.; Yang, X.; Liu, H.; Xiao, Q.; Chao, B.; Liu, Y.; et al. Long-Term Exposure to Ozone Increases Neurological Disability after Stroke: Findings from a Nationwide Longitudinal Study in China. Biology 2022, 11, 1216. https://doi.org/10.3390/biology11081216
Li J, Lu H, Cao M, Tong M, Wang R, Yang X, Liu H, Xiao Q, Chao B, Liu Y, et al. Long-Term Exposure to Ozone Increases Neurological Disability after Stroke: Findings from a Nationwide Longitudinal Study in China. Biology. 2022; 11(8):1216. https://doi.org/10.3390/biology11081216
Chicago/Turabian StyleLi, Jiajianghui, Hong Lu, Man Cao, Mingkun Tong, Ruohan Wang, Xinyue Yang, Hengyi Liu, Qingyang Xiao, Baohua Chao, Yuanli Liu, and et al. 2022. "Long-Term Exposure to Ozone Increases Neurological Disability after Stroke: Findings from a Nationwide Longitudinal Study in China" Biology 11, no. 8: 1216. https://doi.org/10.3390/biology11081216
APA StyleLi, J., Lu, H., Cao, M., Tong, M., Wang, R., Yang, X., Liu, H., Xiao, Q., Chao, B., Liu, Y., Xue, T., & Guan, T. (2022). Long-Term Exposure to Ozone Increases Neurological Disability after Stroke: Findings from a Nationwide Longitudinal Study in China. Biology, 11(8), 1216. https://doi.org/10.3390/biology11081216






