Sea Slug Mucus Production Is Supported by Photosynthesis of Stolen Chloroplasts
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sea Slug Maintenance and Experimental Setup
2.2. Sea Slug Fresh Weight
2.3. Mucus Collection and Carbohydrate Analysis
2.4. Statistical Analysis
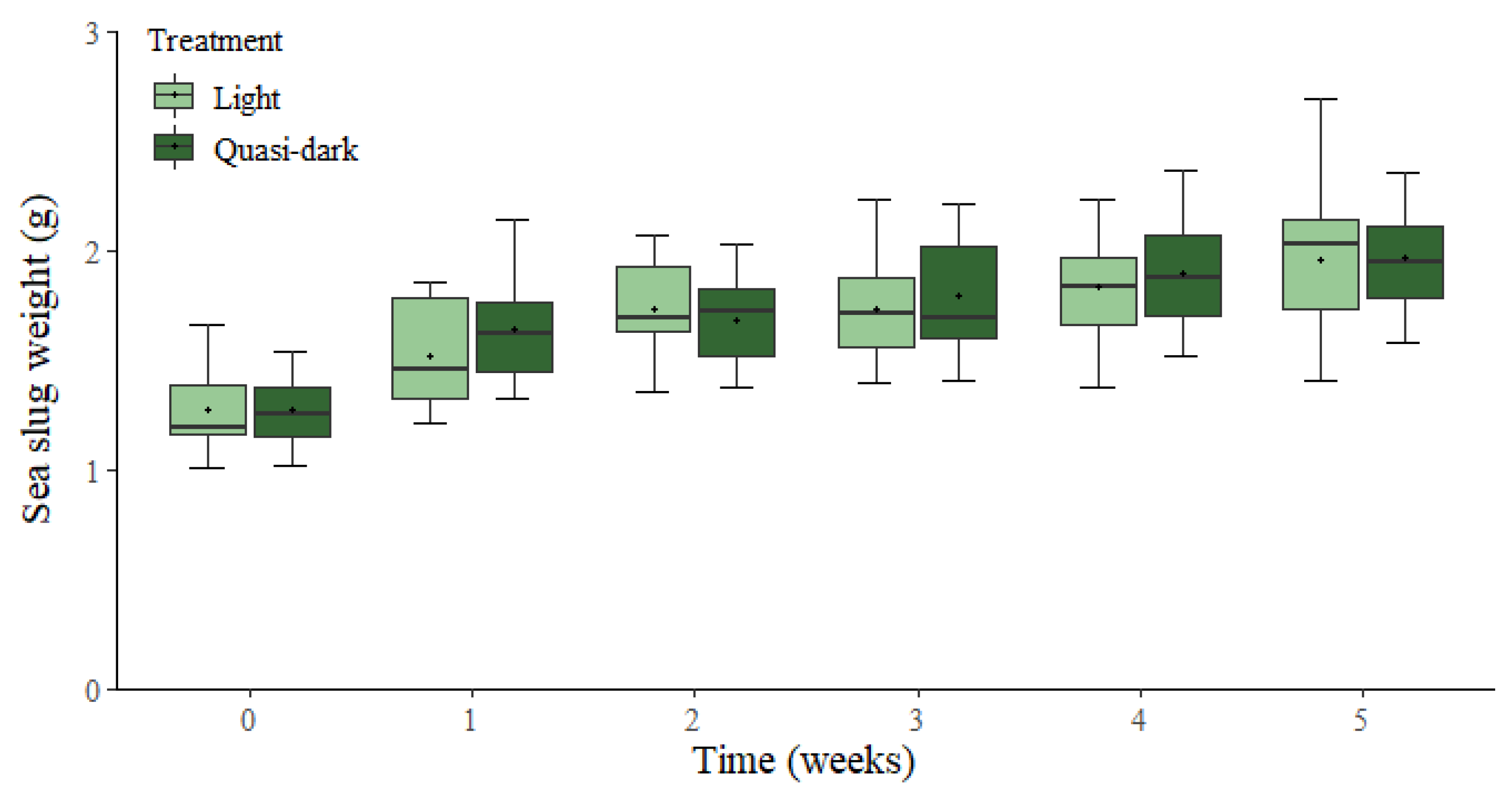
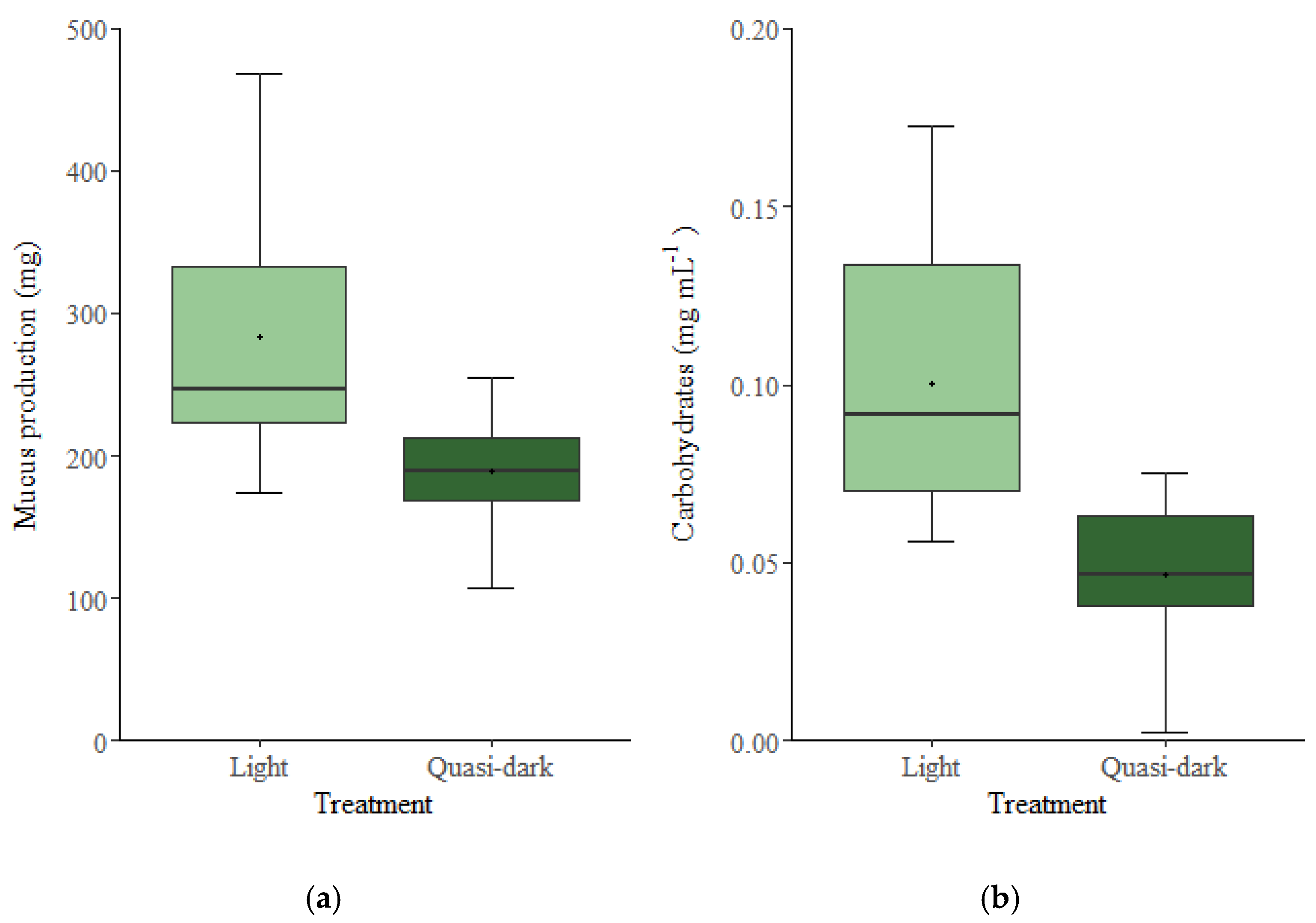
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Serôdio, J.; Cruz, S.; Cartaxana, P.; Calado, R. Photophysiology of kleptoplasts: Photosynthetic use of light by chloroplasts living in animal cells. Philos. Trans. R. Soc. B 2014, 369, 20130242. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Hirano, Y.M.; Hirano, Y.J.; Trowbridge, C.D.; Akimoto, A.; Sakai, A.; Yusa, Y. Effects of photosynthesis on the survival and weight retention of two kleptoplastic sacoglossan opisthobranchs. J. Mar. Biol. Assoc. U. K. 2013, 93, 209–215. [Google Scholar] [CrossRef]
- Cartaxana, P.; Rey, F.; LeKieffre, C.; Lopes, D.; Hubas, C.; Spangenberg, J.E.; Escrig, S.; Jesus, B.; Calado, G.; Domingues, M.R.M.; et al. Photosynthesis from stolen chloroplasts can support sea slug reproductive fitness. Proc. R. Soc. B 2021, 288, 20211779. [Google Scholar] [CrossRef]
- Händeler, K.; Grzymbowski, Y.P.; Krug, P.J.; Wägele, H. Functional chloroplasts in metazoan cells: A unique evolutionary strategy in animal life. Front. Zool. 2009, 6, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Middlebrooks, M.L.; Curtis, N.E.; Pierce, S.K. Algal sources of sequestered chloroplasts in the sacoglossan sea slug, Elysia crispata, varies by location and ecotype. Biol. Bull. 2019, 236, 88–96. [Google Scholar] [CrossRef]
- Vital, X.G.; Rey, F.; Cartaxana, P.; Cruz, S.; Domingues, M.R.; Calado, R.; Simões, N. Pigment and fatty acid heterogeneity in the sea slug Elysia crispata is not shaped by habitat depth. Animals 2021, 11, 3157. [Google Scholar] [CrossRef] [PubMed]
- Pierce, S.K.; Curtis, N.E.; Massey, S.E.; Bass, A.L.; Karl, S.A.; Finney, C. A morphological and molecular comparison between Elysia crispata and a new species of kleptoplastic sacoglossan sea slug (Gastropoda: Opisthobranchia) from the Florida Keys, USA. Molluscan Res. 2006, 26, 23–38. [Google Scholar]
- Krug, P.J.; Vendetti, J.E.; Valdés, Á. Molecular and morphological systematics of Elysia Risso, 1818 (Heterobranchia: Sacoglossa) from the Caribbean region. Zootaxa 2016, 4148, 1–137. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.B. Ascoglossan (=Sacoglossa) molluscs in the Florida Keys: Rare marine invertebrates at special risk. Bull. Mar. Sci. 1994, 54, 900–916. [Google Scholar]
- Davies, M.S.; Hawkins, S.J. Mucus from marine molluscs. Adv. Mar. Biol. 1998, 34, 1–71. [Google Scholar]
- Trench, M.E.; Trench, R.K.; Muscatine, L. Utilization of photosynthetic products of symbiotic chloroplasts in mucus synthesis by Placobranchus ianthobaptus (Gould), Opisthobranchia, Sacoglossa. Comp. Biochem. Physiol. 1970, 37, 113–117. [Google Scholar] [CrossRef]
- Trench, R.K.; Trench, M.E.; Muscatine, L. Symbiotic chloroplasts; their photosynthetic products and contribution to mucus synthesis in two marine slugs. Biol. Bull. 1972, 142, 335–349. [Google Scholar] [CrossRef]
- Cartaxana, P.; Rey, F.; Ribeiro, M.; Moreira, A.S.P.; Domingues, M.R.M.; Calado, R.; Cruz, S. Nutritional state determines reproductive investment in the mixotrophic sea slug Elysia viridis. Mar. Ecol. Prog. Ser. 2019, 611, 167–177. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, A.K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Maeda, T.; Hirose, E.; Chikaraishi, Y.; Kawato, M.; Takishita, K.; Yoshida, T.; Verbruggen, H.; Tanaka, J.; Shimamura, S.; Takaki, Y.; et al. Algivore or phototroph? Plakobranchus ocellatus (Gastropoda) continuously acquires kleptoplasts and nutrition from multiple algal species in nature. PLoS ONE 2012, 7, e42024. [Google Scholar] [CrossRef]
- Cruz, S.; LeKieffre, C.; Cartaxana, P.; Hubas, C.; Thiney, N.; Jakobsen, S.; Escrig, S.; Jesus, B.; Kühl, M.; Calado, R.; et al. Functional kleptoplasts intermediate incorporation of carbon and nitrogen in cells of the Sacoglossa sea slug Elysia viridis. Sci. Rep. 2020, 10, 10548. [Google Scholar] [CrossRef]
- Trench, R.K.; Greene, R.W.; Bystrom, B.G. Chloroplasts as functional organelles in animal tissues. J. Cell Biol. 1969, 42, 404–417. [Google Scholar] [CrossRef]
- Dubinsky, Z.; Jokiel, P.L. Ratio of energy and nutrient fluxes regulates symbiosis between zooxanthellae and corals. Pac. Sci. 1994, 48, 313–324. [Google Scholar]
- Davies, P.S. Effect of daylight variations on the energy budgets of shallow-water corals. Mar. Biol. 1991, 108, 137–144. [Google Scholar]
- Davies, P.S. The role of zooxanthellae in the nutritional requirements of Pocillopora eydouxi. Coral Reefs 1984, 2, 181–186. [Google Scholar]
- Wooldridge, S.A. A new conceptual model for the enhanced release of mucus in symbiotic reef corals during ‘bleaching’ conditions. Mar. Ecol. Prog. Ser. 2009, 396, 145–152. [Google Scholar] [CrossRef][Green Version]
- Ireland, C.; Scheuer, P.J. Photosynthetic marine mollusks: In vivo 14C incorporation into metabolites of the sacoglossan Placobranchus ocellatus. Science 1979, 205, 922–923. [Google Scholar] [CrossRef]
- Torres, J.P.; Lin, Z.; Winter, J.M.; Krug, P.J.; Schmidt, E.W. Animal biosynthesis of complex polyketides in a photosynthetic partnership. Nat. Commun. 2020, 11, 2882. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, D.; Cruz, S.; Martins, P.; Ferreira, S.; Nunes, C.; Domingues, P.; Cartaxana, P. Sea Slug Mucus Production Is Supported by Photosynthesis of Stolen Chloroplasts. Biology 2022, 11, 1207. https://doi.org/10.3390/biology11081207
Lopes D, Cruz S, Martins P, Ferreira S, Nunes C, Domingues P, Cartaxana P. Sea Slug Mucus Production Is Supported by Photosynthesis of Stolen Chloroplasts. Biology. 2022; 11(8):1207. https://doi.org/10.3390/biology11081207
Chicago/Turabian StyleLopes, Diana, Sónia Cruz, Patrícia Martins, Sónia Ferreira, Cláudia Nunes, Pedro Domingues, and Paulo Cartaxana. 2022. "Sea Slug Mucus Production Is Supported by Photosynthesis of Stolen Chloroplasts" Biology 11, no. 8: 1207. https://doi.org/10.3390/biology11081207
APA StyleLopes, D., Cruz, S., Martins, P., Ferreira, S., Nunes, C., Domingues, P., & Cartaxana, P. (2022). Sea Slug Mucus Production Is Supported by Photosynthesis of Stolen Chloroplasts. Biology, 11(8), 1207. https://doi.org/10.3390/biology11081207






