Soft Tissue and Biomolecular Preservation in Vertebrate Fossils from Glauconitic, Shallow Marine Sediments of the Hornerstown Formation, Edelman Fossil Park, New Jersey
Abstract
:Simple Summary
Abstract
1. Introduction
| Citation | Specimen Number | Taxon | Age | Formation | Rock Type |
|---|---|---|---|---|---|
| Barker et al. [34] | PAS11-02 | Cetacea indet. | Miocene | Pungo River | Unconsolidated Silt and Clay |
| Barker et al. [34] | PAS11-03 | Cetacea indet. | Miocene | Pungo River | Unconsolidated Silt and Clay |
| Barker et al. [34] | PAS11-01 | Cetacea indet. | Miocene | Calvert | Unconsolidated Silt and Clay |
| Barker et al. [34] | PAS11-04 | Dugongidae indet. | Miocene | Torreya | Unconsolidated Silt and Clay |
| Cadena and Schweitzer [35] | CCMFC01 | Pelomedusoides indet. | Paleogene | Los Cuervos | Sandstone |
| Lindgren et al. [6] | IRSNB 1624 | Prognathodon | Cretaceous | Ciply | Phosphatic Chalk |
| Macauley et al. [36] | RU-CAL-00001 | Testudines indet. | Miocene | Calvert | Unconsolidated Silt and Clay |
| Schweitzer et al. [3] | WCBa-20 | Balaenoptera | Miocene | Pisco | Mudstone |
| Surmik et al. [16] | WNoZ/s/7/166 | Nothosaurus | Triassic | Gogolin | Limestone |
| Surmik et al. [16] | SUT-MG/F/Tvert/15 | Nothosaurus | Triassic | Gogolin | Limestone |
| Surmik et al. [16] | SUT-MG/F/Tvert/2 | Protanystropheus | Triassic | Gogolin | Limestone |
| Wiemann et al. [10] | YPM 656 | Crocodylia indet. | Cretaceous | Navesink | Unconsolidated Greensand |
| Wiemann et al. [10] | YMP VP 059362 | Ichthyosaurus | Jurassic | Posidonia Shale | Mudstone |
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Histology
2.2.2. X-ray Diffraction (XRD)
2.2.3. Demineralization
2.2.4. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDX)
2.2.5. Protein Extraction
2.2.6. Polyacrylamide Gel Electrophoresis (PAGE) with Silver-Staining
2.2.7. Enzyme-Linked Immunosorbant Assay (ELISA)
2.2.8. Immunofluorescence
3. Results
3.1. Histology
3.2. X-ray Diffraction
3.3. Demineralization
3.4. Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
3.5. Polyacrylamide Gel Electrophoresis with Silver-Staining
3.6. Enzyme-Linked Immunosorbent Assay
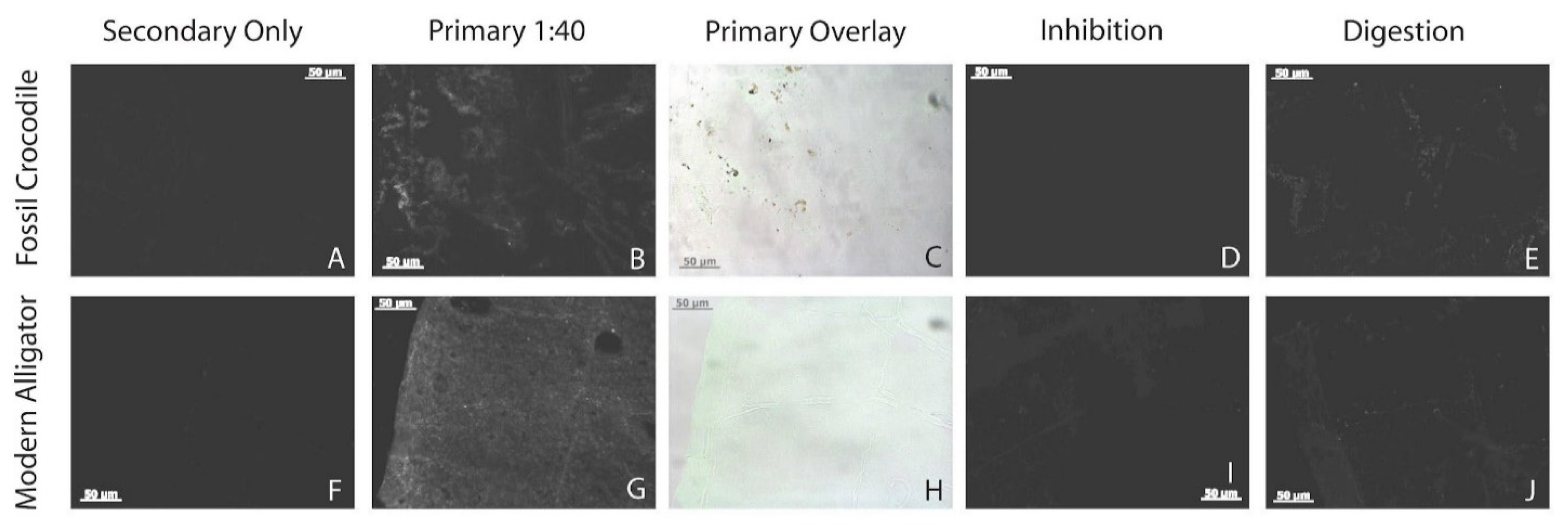
3.7. Immunofluorescence
4. Discussion
4.1. Overall Preservation
4.2. Soft Tissue Preservation
4.3. Biomolecular Preservation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schroeter, E.R.; Cleland, T.P.; Schweitzer, M.H. Deep Time Paleoproteomics: Looking Forward. J. Proteome Res. 2021, 21, 9–19. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Wittmeyer, J.L.; Horner, J.R.; Toporski, J.K. Soft-tissue vessels and cellular preservation in Tyrannosaurus rex. Science 2005, 307, 1952–1955. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.H.; Wittmeyer, J.L.; Horner, J.R. Soft tissue and cellular preservation in vertebrate skeletal elements from the Cretaceous to the present. Proc. R. Soc. B 2007, 274, 183–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweitzer, M.H.; Zheng, W.; Organ, C.L.; Avci, R.; Suo, Z.; Freimark, L.M.; Lebleu, V.S.; Duncan, M.B.; Heiden, M.G.V.; Neveu, J.M.; et al. Biomolecular characterization and protein sequences of the Campanian Hadrosaur B. canadensis. Science 2009, 324, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.H.; Zheng, W.; Cleland, T.P.; Bern, M. Molecular analyses of dinosaur osteocytes support the presence of endogenous molecules. Bone 2013, 52, 414–423. [Google Scholar] [CrossRef]
- Lindgren, J.; Uvdal, P.; Engdahl, A.; Lee, A.H.; Alwmark, C.; Bergquist, K.E.; Nilsson, E.; Ekström, P.; Rasmussen, M.; Douglas, D.A.; et al. Microspectroscopic Evidence of Cretaceous Bone Proteins. PLoS ONE 2011, 6, e19445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armitage, M.H.; Anderson, K.L. Soft sheets of fibrillar bone from a fossil of the supraorbital horn of the dinosaur Triceratops horridus. Acta Histochem. 2013, 115, 603–608. [Google Scholar] [CrossRef]
- Cadena, E.A. Microscopical and elemental FESEM and phenom ProX-SEM-EDS analysis of osteocyte- and blood vessel-like microstructures obtained from fossil vertebrates of the eocene messel pit, germany. PeerJ 2016, 4, e1618. [Google Scholar] [CrossRef] [Green Version]
- Cadena, E.A. In situ SEM/EDS compositional characterization of osteocytes and blood vessels in fossil and extant turtles on untreated bone surfaces; different preservational pathways microns away. PeerJ 2020, 8, e9833. [Google Scholar] [CrossRef]
- Wiemann, J.; Fabbri, M.; Yang, T.R.; Stein, K.; Sander, P.M.; Norell, M.A.; Briggs, D.E.G. Fossilization transforms vertebrate hard tissue proteins into N-heterocyclic polymers. Nat. Commun. 2018, 9, 4741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullmann, P.V.; Pandya, S.H.; Nellermoe, R. Patterns of soft tissue and cellular preservation in relation to fossil bone microstructure and overburden depth at the Standing Rock Hadrosaur Site, Maastrichtian Hell Creek Formation, South Dakota, USA. Cretac. Res. 2019, 99, 1–13. [Google Scholar] [CrossRef]
- Bailleul, A.M.; Zheng, W.; Horner, J.R.; Hall, B.K.; Holliday, C.M.; Schweitzer, M.H. Evidence of proteins, chromosomes and chemical markers of DNA in exceptionally preserved dinosaur cartilage. Natl. Sci. Rev. 2020, 7, 815–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Reest, A.J.; Currie, P.J. Preservation frequency of tissue-like structures in vertebrate remains from the upper Campanian of Alberta: Dinosaur Park Formation. Cretac. Res. 2020, 109, 104370. [Google Scholar] [CrossRef]
- Wiersma, K.; Läbe, S.; Sander, P.M. Organic Phase Preservation in Fossil Dinosaur and Other Tetrapod Bone from Deep Time. In Fossilization: Understanding the Material Nature of Ancient Plants and Animals; Gee, C.T., McCoy, V.E., Sander, P.M., Eds.; Johns Hopkins University Press: Baltimore, MD, USA, 2021; pp. 16–54. [Google Scholar]
- Schweitzer, M.H.; Suo, Z.; Avci, R.; Asara, J.M.; Allen, M.A.; Arce, F.T.; Horner, J.R. Analyses of soft tissue from Tyrannosaurus rex suggest the presence of protein. Science 2007, 316, 277–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surmik, D.; Boczarowski, A.; Balin, K.; Dulski, M.; Szade, J.; Kremer, B.; Pawlicki, R. Spectroscopic studies on organic matter from Triassic reptile bones, Upper Silesia, Poland. PLoS ONE 2016, 11, e0151143. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Zheng, W.; Moyer, A.E.; O’Connor, J.K.; Wang, M.; Zheng, X.; Wang, X.; Schroeter, E.R.; Zhou, Z.; Schweitzer, M.H. Molecular evidence of keratin and melanosomes in feathers of the Early Cretaceous bird Eoconfuciusornis. Proc. Natl Acad. Sci. USA 2016, 113, 7900–7907. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Zheng, W.; Sawyer, R.H.; Pennington, M.W.; Zheng, X.; Wang, X.; Wang, M.; Hu, L.; O’Connor, J.; Zhao, T.; et al. The molecular evolution of feathers with direct evidence from fossils. Proc. Natl Acad. Sci. USA 2019, 116, 3018–3023. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.C.; Chiang, C.C.; Huang, P.Y.; Chung, C.Y.; Huang, T.D.; Wang, C.C.; Chen, C.I.; Chang, R.S.; Liao, C.H.; Reisz, R.R. Evidence of preserved collagen in an Early Jurassic sauropodomorph dinosaur revealed by synchrotron FTIR microspectroscopy. Nat. Commun. 2017, 8, 14220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullmann, P.V.; Voegele, K.K.; Grandstaff, D.E.; Ash, R.D.; Zheng, W.; Schroeter, E.R.; Schweitzer, M.H.; Lacovara, K.J. Molecular tests support the viability of rare earth elements as proxies for fossil biomolecule preservation. Sci. Rep. 2020, 10, 15566. [Google Scholar] [CrossRef]
- Asara, J.M.; Schweitzer, M.H.; Freimark, L.M.; Phillips, M.; Cantley, L.C. Protein Sequences from Mastodon and Tyrannosaurus Rex Revealed by Mass Spectrometry. Science 2007, 316, 280–285. [Google Scholar] [CrossRef]
- Cappellini, E.; Jensen, L.J.; Szklarczyk, D.; Ginolhac, A.; da Fonseca, R.A.; Stafford, T.W., Jr.; Holen, S.R.; Collins, M.J.; Orlando, L.; Willerslev, E.; et al. Proteomic analysis of a pleistocene mammoth femur reveals more than one hundred ancient bone proteins. J. Proteome Res. 2012, 11, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Cleland, T.P.; Schroeter, E.R.; Zamborg, L.; Zheng, W.; Lee, J.E.; Tran, J.C.; Bern, M.; Duncan, M.B.; Lebleu, V.S.; Ahlf, D.R.; et al. Mass spectrometry and antibody-based characterization of blood vessels from Brachylophosaurus canadensis. J. Proteome Res. 2015, 14, 5252–5262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeter, E.R.; DeHart, C.J.; Cleland, T.P.; Zheng, W.; Thomas, P.M.; Kelleher, N.L.; Bern, M.; Schweitzer, M.H. Expansion for the Brachylophosaurus canadensis collagen I sequence and additional evidence of the preservation of Cretaceous protein. J. Proteome Res. 2017, 16, 920–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eglinton, G.; Logan, G. Molecular Preservation. Philos. Trans. R. Soc. B 1991, 333, 315–328. [Google Scholar]
- Collins, M.J.; Westbroek, P.; Muyzer, G.; de Leeuw, J.W. Experimental evidence for condensation reactions between sugars and proteins in carbonate skeletons. Geochim. Cosmochim. Acta 1992, 56, 1539–1544. [Google Scholar] [CrossRef]
- Cadena, E.A.; Schweitzer, M.H. Variation in osteocytes morphology vs. bone type in turtle shell and their exceptional preservation from the Jurassic to the present. Bone 2012, 51, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Voegele, K.K.; Ullmann, P.V.; Lonsdorf, T.; Christman, Z.; Heierbacher, M.; Kibelstis, B.J.; Putnam, I.; Boles, Z.; Walsh, S.; Lacovara, K.J. Microstratigraphic analysis of fossil distribution in the lower Hornerstown and upper Navesink formations at the Edelman Fossil Park, NJ. Front. Earth Sci. 2021, 9, 756655. [Google Scholar] [CrossRef]
- Gallagher, W.B. The Cretaceous/Tertiary mass extinction event in the northern Atlantic Coastal Plain. Mosasaur 1993, 5, 75–154. [Google Scholar]
- Obasi, C.C.; Terry, D.O., Jr.; Myer, G.H.; Grandstaff, D.E. Glauconite composition and morphology, shocked quartz, and the origin of the Cretaceous(?) Main Fossiliferous Layer (MFL) in southern New Jersey, U.S.A. J. Sediment. Res. 2011, 81, 479–494. [Google Scholar] [CrossRef]
- Gallagher, W.B. Oligotrophic oceans and minimalist organisms: Collapse of the Maastrichtian marine ecosystem and Paleocene recovery in the Cretaceous-Tertiary sequence of New Jersey. Neth. J. Geosci. 2003, 82, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Schweitzer, M.H.; Zheng, W.; Cleland, T.P.; Goodwin, M.B.; Boatman, E.; Theil, E.; Marcus, M.A.; Fakra, S.C. A role for iron and oxygen chemistry in preserving soft tissues, cells and molecules from deep time. Proc. R. Soc. B 2014, 281, 20132741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boatman, E.M.; Goodwin, M.B.; Holman, H.-Y.N.; Fakra, S.; Zheng, W.; Gronsky, R.; Schweitzer, M.H. Mechanisms of soft tissue and protein preservation in Tyrannosaurus rex. Sci. Rep. 2019, 9, 15678. [Google Scholar] [CrossRef] [PubMed]
- Barker, K.; Terry, D.O.; Ullmann, P.V. Recovering endogenous cells and soft tissues from fossil bones: Does the depositional environment matter? In Proceedings of the GSA Connect, Portland, Oregon, 10–13 October 2021; 53, p. 225-10. [Google Scholar]
- Cadena, E.A.; Schweitzer, M.H. A Pelomedusoid turtle from the Paleocene–Eocene of Colombia exhibiting preservation of blood vessels and osteocytes. J. Herpetol. 2014, 48, 461–465. [Google Scholar] [CrossRef]
- Macauley, K.W.; Ash, R.D.; Ullmann, P.V. Relating rare earth element uptake to soft tissue preservation in Cretaceous and Miocene fossil bones. In Proceedings of the Joint 69th Annual Southeastern/55th Annual Northeastern GSA Section Meeting—2020, Reston, Virginia, 20–22 March 2020; The Geological Society of America: Boulder, CO, USA; 52, p. 59-5. [Google Scholar]
- Boles, Z.M. Taphonomy and Paleoecology of a Cretaceous-Paleogene Marine Bone Bed. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, 2016. [Google Scholar]
- Hedges, R.E.M. Bone diagenesis: An overview of processes. Archaeometry 2002, 44, 319–328. [Google Scholar] [CrossRef]
- Schweitzer, M.H. Molecular paleontology: Some current advances and problems. Ann. Paléontologie 2004, 90, 81–102. [Google Scholar] [CrossRef]
- Amber, R.P.; Daniel, M. Proteins and molecular paleontology. Philos. Trans. R. Soc. B 1991, 333, 381–389. [Google Scholar]
- Child, A.M. Microbial taphonomy of archaeological bone. Stud. Conserv. 1995, 40, 19–30. [Google Scholar]
- Hedges, R.E.M.; Millard, A.R. Measurements and relationships of diagenetic alteration of bone from three archaeological sites. J. Archaeol. Sci. 1995, 22, 201–209. [Google Scholar] [CrossRef]
- Sweeney, S.M.; Orgel, J.P.; Fertala, A.; McAuliffe, J.D.; Turner, K.R.; Di Lullo, G.A.; Chen, S.; Antipova, O.; Perumal, S.; Ala-Kokko, L.; et al. Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates. J. Biol. Chem. 2008, 283, 21187–21197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- San Antonio, J.D.; Schweitzer, M.H.; Jensen, S.T.; Kalluri, R.; Buckley, M.; Orgel, J.P.R.O. Dinosaur peptides suggest mechanisms of protein survival. PLoS ONE 2011, 6, e20381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.Y.; Cappellini, E.; Zhang, H.Y. Why collagens best survived in fossils? Clues from amino acid thermal stability. Biochem. Biophys. Res. Commun. 2012, 422, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Perumal, S.; Antipova, O.; Orgel, J.P.R.O. Collagen fibril architecture, domain organization, and triple-helical conformation govern its proteolysis. Proc. Natl. Acad. Sci. USA 2008, 105, 2824–2829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, M.J.; Nielsen-Marsh, C.M.; Hiller, J.; Smith, C.I.; Roberts, J.P. The survival of organic matter in bone: A review. Archaeometry 2002, 44, 383–394. [Google Scholar] [CrossRef] [Green Version]
- Trueman, C.N.; Martill, D.M. Long Term Survival of Bone: The Role of Bioerosion. Archaeometry 2002, 44, 371–382. [Google Scholar] [CrossRef]
- Chinsamy, A.; Raath, M.A. Preparation of fossil bone for histological examination. Palaeontol. Afr. 1992, 29, 39–44. [Google Scholar]
- Cleland, T.P.; Voegele, K.K.; Schweitzer, M.H. Empirical evaluation of methodology related to the extraction of bone proteins. PLoS ONE 2012, 7, e31443. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Schweitzer, M.H. Chemical Analyses of Fossil Bone. In Forensic Microscopy for Skeletal Tissues; Bell, L.S., Ed.; Humana Press: Burnaby, BC, Canada, 2012; pp. 153–172. [Google Scholar]
- Pellegrini, R.A.; Callahan, W.R.; Hastings, A.K.; Parris, D.C.; McCauley, J.D. Skeletochronology and Paleohistology of Hyposaurus rogersii (Crocodyliformes, Dyrosauridae) from the Early Paleogene of New Jersey, USA. Animals 2021, 11, 3067. [Google Scholar] [CrossRef] [PubMed]
- Jans, M.M.E. Microbial bioerosion of bone—A review. In Current Developments in Bioerosion; Wisshak, M., Tapanila, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 397–413. [Google Scholar]
- Pawlicki, R. Histochemical demonstration of DNA in osteocytes from dinosaur bones. Folia Histochem. Chytobiologica 1995, 33, 183–186. [Google Scholar]
- Surmik, D.; Rothschild, B.M.; Pawlicki, R. Unusual intraosseous fossilized soft tissues from the Middle Triassic Nothosaurus bone. Sci. Nat. 2017, 104, 25. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, M.H. Soft tissue preservation in terrestrial Mesozoic vertebrates. Annu. Rev. Earth Planet. Sci. 2011, 39, 187–216. [Google Scholar] [CrossRef]
- Ostlund, E.N.; Crom, R.L.; Pedersen, D.D.; Johnson, D.J.; Williams, W.O.; Schmitt, B.J. Equine west nile encephalitis, United States. Emerg. Infect. Dis. 2001, 7, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Appiah, A.S.; Amoatey, H.M.; Klu, G.Y.P.; Afful, N.T.; Azu, E.; Owusu, G.K. Spread of African cassava mosaic virus from cassava (Manihot esculenta Crantz) to physic nut (Jatropha curcas L.) in Ghana. J. Phytol. 2012, 4, 31–37. [Google Scholar]
- Schroeter, E.A.R. The Morphology, Histology, and Molecular Preservation of an Exceptionally Complete Titanosaur from Southernmost Patagonia. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, 2013. [Google Scholar]
- Cleland, T.P.; Schroeter, E.R.; Feranec, R.S.; Vashishth, D. Peptide sequences from the first Castoroides ohioensis skull and the utility of old museum collections for palaeoproteomics. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsson, R.K.; Miller, K.G.; Browning, J.V.; Wright, J.D.; Cramer, B.S. Sequence stratigraphy and sea-level change across the Cretaceous-Tertiary boundary. In Catastrophic Events and Mass Extinctions: Impacts and Beyond; Koeberl, C., MacLeod, K.G., Eds.; Geological Society of America: Boulder, CO, USA, 2002; Volume 356, pp. 97–108. [Google Scholar]
- Zheng, X.; Bailleul, A.M.; Li, Z.; Wang, X.; Zhou, Z. Nuclear preservation in the cartilage of the Jehol dinosaur Caudipteryx. Commun. Biol. 2021, 4, 1125. [Google Scholar] [CrossRef] [PubMed]
- Hagelberg, E.; Bell, L.S.; Allen, T.; Boyde, A.; Jones, S.J.; Clegg, J.B. Analysis of ancient bone DNA: Techniques and applications. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1991, 333, 399–407. [Google Scholar]
- Turner-Walker, G.; Jans, M. Reconstructing taphonomic histories using histological analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 266, 227–235. [Google Scholar] [CrossRef]
- Fernandez-Jalvo, Y.; Andrews, P.; Pesquero, D.; Smith, C.; Marin-Monfort, D.; Sanchez, B.; Geigl, E.M.; Alonso, A. Early bone diagenesis in temperate environments Part I: Surface features and histology. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 288, 62–81. [Google Scholar] [CrossRef]
- Danise, S.; Cavalazzi, B.; Dominici, S.; Westall, F.; Monechi, S.; Guioli, S. Evidence of microbial activity from a shallow water whale fall (Voghera, northern Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 317, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, A.M.H.; Nielsen, T.K.; Matthiesen, H.; Carøe, C.; Hansen, L.H.; Gregory, D.J.; Turner-Walker, G.; Collins, M.J.; Gilbert, M.T.P. Bio biodeterioration—The effect of marine and terrestrial depositional environments on early diagenesis and bone bacterial community. PLoS ONE 2020, 15, e0240512. [Google Scholar] [CrossRef] [PubMed]
- Norris, S.J.; Paredes, A.M.; Immega, N.; Temple, D.; Bakker, R.T. Release of osteocytes and lamellae through chemical dissolution reveals the intricate, anastomosing circumferential pattern of blood vessels in Dimetrodon neural spines. Soc. Vertebr. Paleontol. Programs Abstr. 2017, 169. [Google Scholar]
- Chinsamy, A. Dinosaur bone histology: Implications and inferences. Paleontol. Soc. Spec. Publ. 1997, 7, 213–227. [Google Scholar] [CrossRef]
- Padian, K.; Horner, J.R. Dinosaur Physiology. In The Dinosauria, 2nd ed.; Weishampel, D.B., Dodson, P., Osmólska, H., Eds.; University of California Press: Berkeley, CA, USA, 2004; pp. 660–672. [Google Scholar]
- Woodward, H.N.; Horner, J.R.; Farlow, J.O. Quantification of intraskeletal histovariability in Alligator mississippiensis and implications for vertebrate osteohistology. PeerJ 2014, 2, e422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweitzer, M.H.; Moyer, A.E.; Zheng, W. Testing the hypothesis of biofilm as a source for soft tissue and cell-like structures preserved in dinosaur bone. PLoS ONE 2016, 11, e0150238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweitzer, M.H.; Avci, R.; Collier, T.; Goodwin, M.B. Microscopic, chemical and molecular methods for examining fossil preservation. Comptes Rendus Palevol 2008, 7, 159–184. [Google Scholar] [CrossRef]
- Schroeter, E.R.; Blackbum, K.; Goshe, M.B.; Schweitzer, M.H. Proteomic method to extract, concentrate, digest and enrich peptides from fossils with coloured (humic) substances for mass spectrometry analyses. R. Soc. Open Sci. 2019, 6, 181433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunkelog, R.; Rüttinger, H.-H.; Peisker, K. Comparative study for the separation of aquatic humic substances by electrophoresis. J. Chromatogr. A. 1997, 777, 355–362. [Google Scholar] [CrossRef]
- Evans, R.D.; Villeneuve, J.Y. A method for characterization of humic and fulvic acids by gel electrophoresis laser ablation inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 1999, 15, 157–161. [Google Scholar] [CrossRef]
- Avci, R.; Schweitzer, M.H.; Boyd, R.D.; Wittmeyer, J.L.; Arce, F.T.; Calvo, J.O. Preservation of bone collagen from the Late Cretaceous period studied by immunological techniques and atomic force microscopy. Langmuir 2005, 21, 3584–3590. [Google Scholar] [CrossRef] [PubMed]
- Nielsen-Marsh, C.M.; Hedges, R.E.M. Patterns of diagenesis in bone I: The effects of site environments. J. Archaeol. Sci. 2000, 27, 1139–1150. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Hill, C.L.; Asara, J.M.; Lane, W.S.; Pincus, S.H. Identification of immunoreactive material in mammoth fossils. J. Mol. Evol. 2002, 55, 696–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuross, N. Alteration in fossil collagen. Archaeometry. 2002, 44, 427–434. [Google Scholar] [CrossRef]
- Cleland, T.P.; Schroeter, E.R.; Colleary, C. Diagenetiforms: A new term to explain protein changes as a result of diagenesis in paleoproteomics. Journal of Proteomics. 2021, 230, 103992. [Google Scholar] [CrossRef]
- Knauber, W.R.; Krotzky, A.J.; Schink, B. Gradient gel electrophoretic characterization of humic substances and of bound residues of the herbicide bentazon. Soil Biol. and Biochem. 1998, 30, 969–973. [Google Scholar] [CrossRef]
- van Klinken, G.J.; Hedges, R.E.M. Experiements on Collagen-Humic Interactions: Speed of humic uptake, and effects of diverse chemical treatments. J. Archaeol. Sci. 1995, 22, 263–270. [Google Scholar] [CrossRef]
- Bada, J.L.; Xueyun, S.W.; Hamilton, H. Preservation of key biomolecules in the fossil record: Current knowledge and future challenges. Phil. Trans. R. Soc. Lond. B. 1999, 354, 77–87. [Google Scholar] [CrossRef] [Green Version]

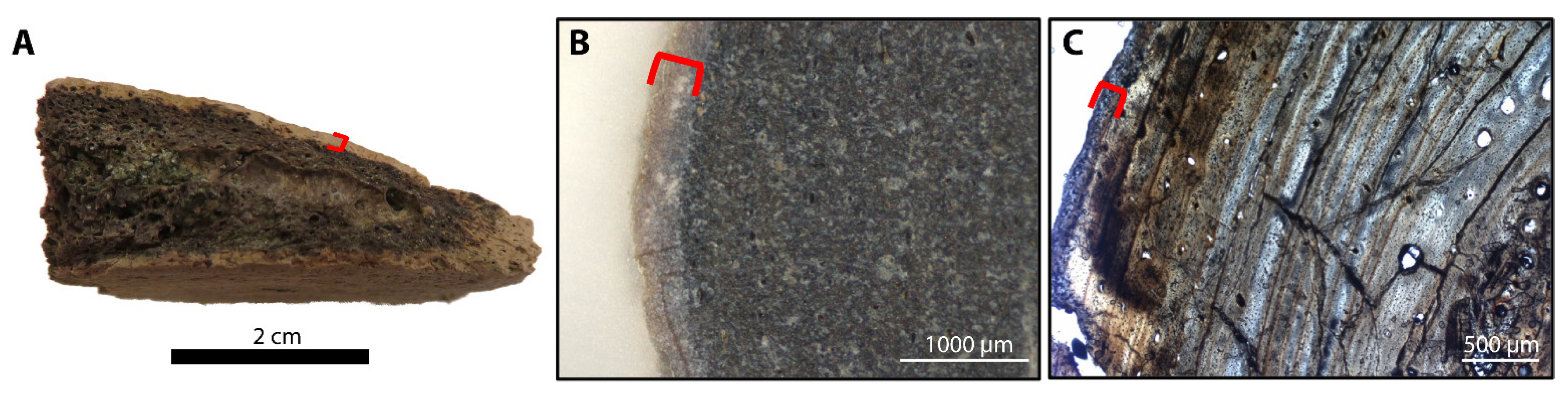
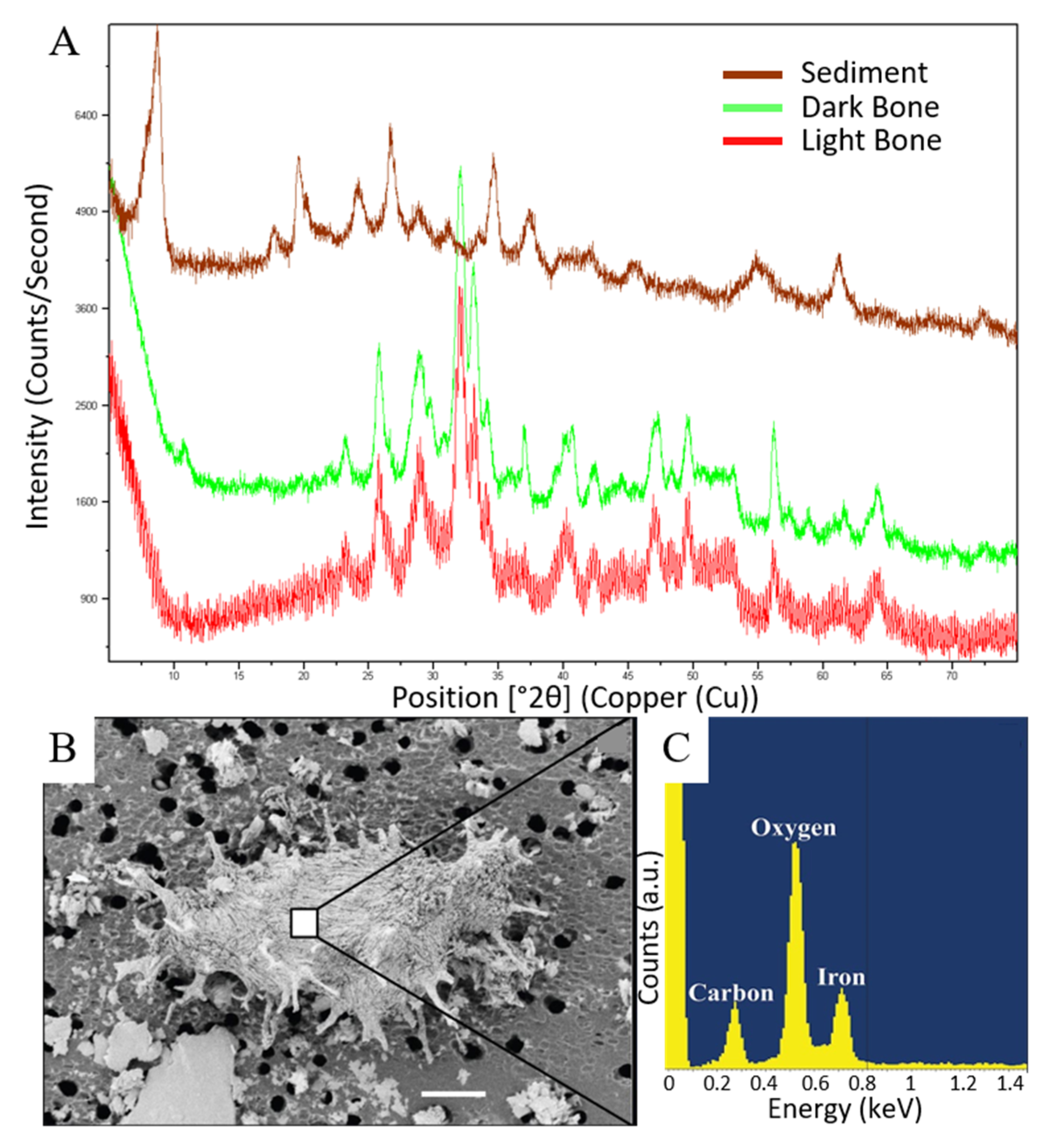

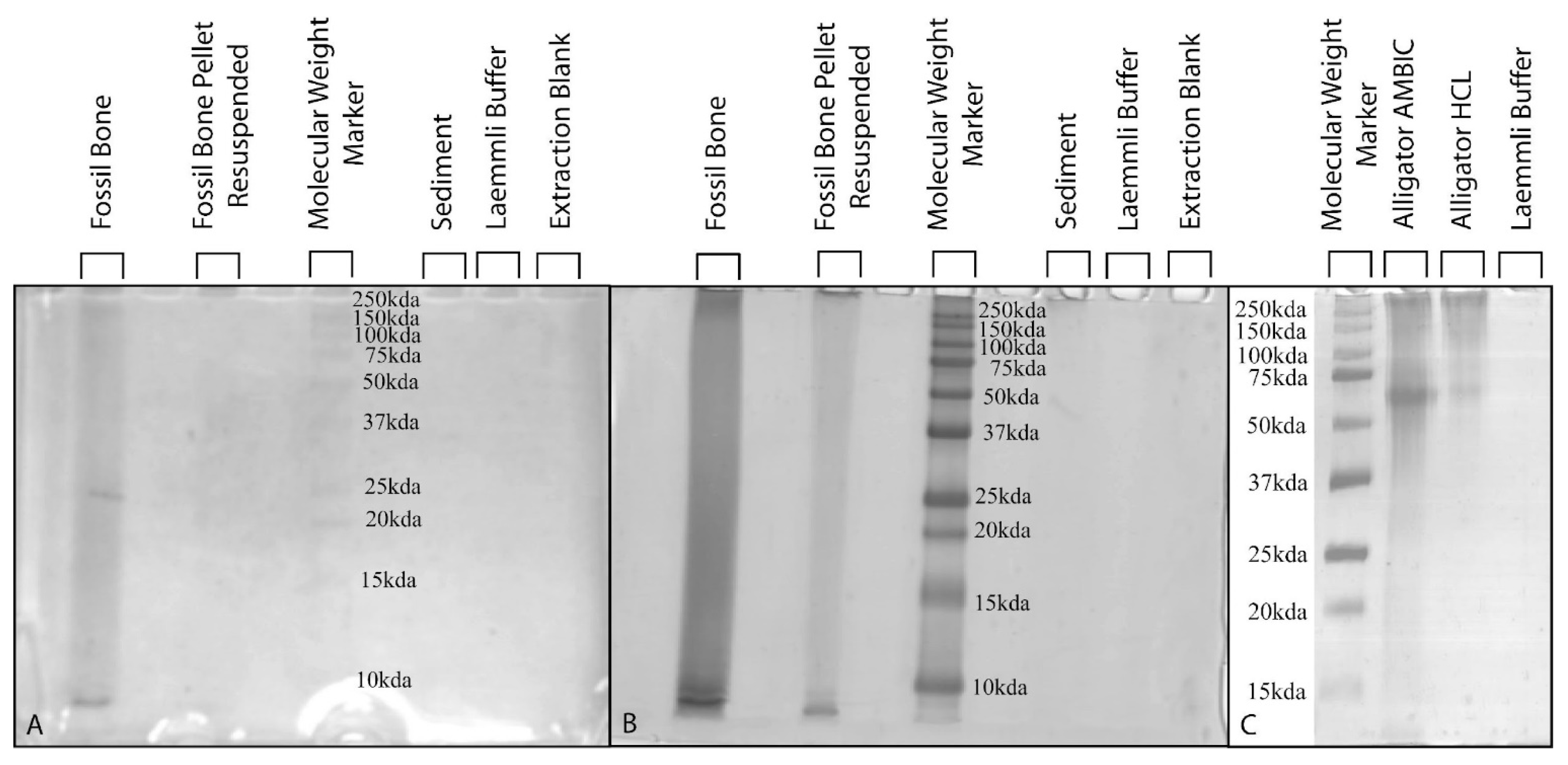
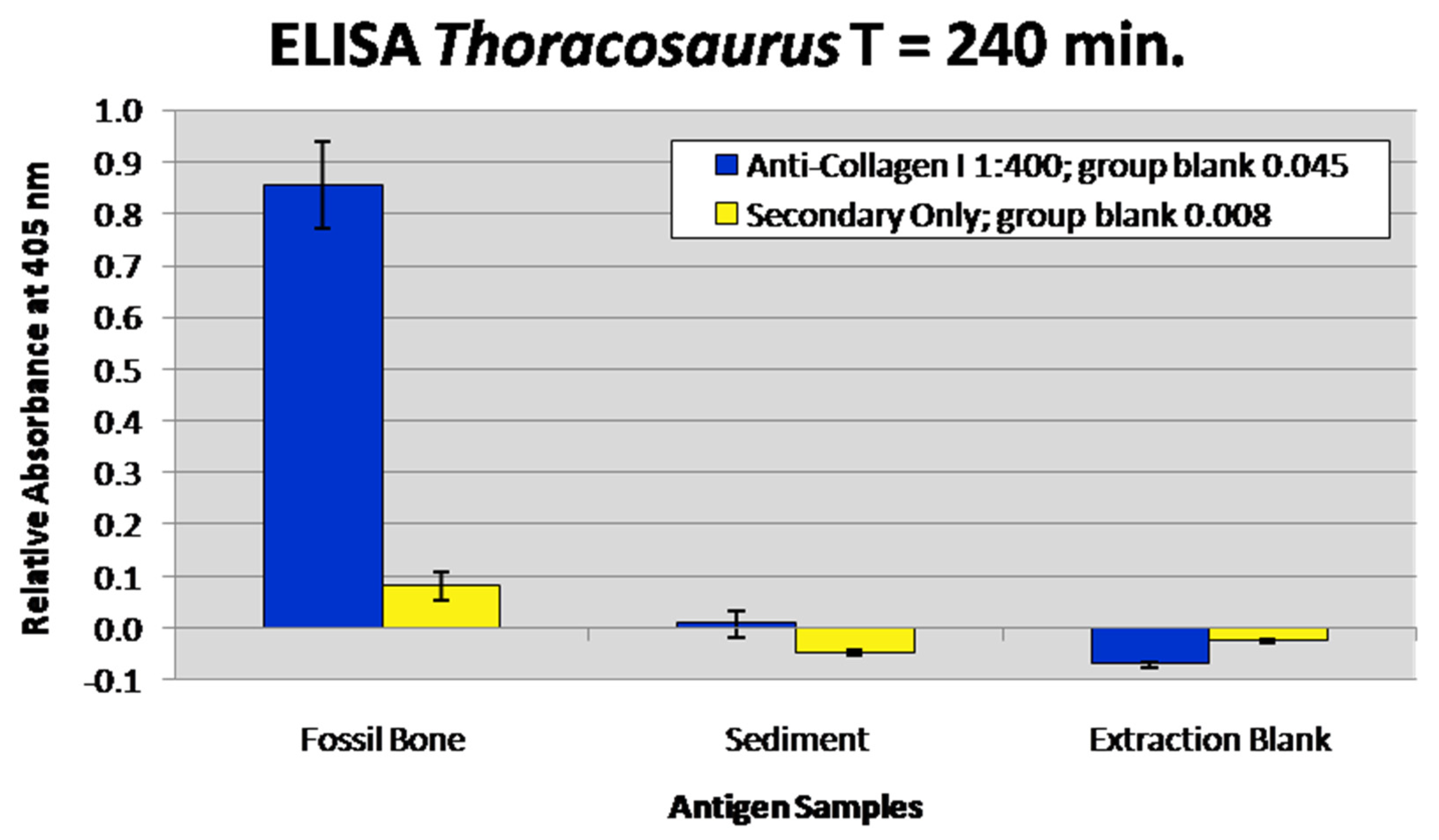
| Specimen RU-EFP-# | Taxon | Element | Source Horizon/Fm | Osteocyte Recovery | Vessel Recovery | Fibrous Matrix Recovery | Color | Histology Assessed |
|---|---|---|---|---|---|---|---|---|
| 00002-2 | Taphrosphys | Peripheral | MFL | Abundant | Frequent | Frequent | Thin Light Rim | Yes |
| 00006-8 | Thoracosaurus | Scute | MFL | Frequent | Absent | Rare | Thin Light Rim | Yes |
| 00006-11 | Thoracosaurus | Femur | MFL | Abundant | Rare | Uncommon | Thin Light Rim | Yes |
| 00018 | Euclastes | Costal | MFL | Abundant | Absent | Absent | Dark | Yes |
| 00030-1 | Crocodylia Indet. | Tibia | MFL | Absent | Absent | Absent | Light | Yes |
| 00030-2 | Crocodylia Indet. | Humerus | MFL | Rare | Absent | Absent | Medium Color | No |
| 02175 | Taphrosphys | Peripheral | MFL | Rare | Absent | Absent | Light | Yes |
| 02222 | Taphrosphys | Costal | MFL | Abundant | Absent | Absent | Dark | Yes |
| 02245 | Pan- Cheloniidae Indet. | Peripheral | MFL | Rare | Absent | Absent | Thin Light Rim | Yes |
| 02295 | Testudines Indet. | Femur | MFL | Abundant | Absent | Absent | Dark | No |
| 04161-8 | Testudines Indet. | Zeugopodial | Hornerstown | Abundant | Rare | Uncommon | Dark | No |
| 04162 | Taphrosphys | Costal | Hornerstown | Abundant | Absent | Absent | Dark | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voegele, K.K.; Boles, Z.M.; Ullmann, P.V.; Schroeter, E.R.; Zheng, W.; Lacovara, K.J. Soft Tissue and Biomolecular Preservation in Vertebrate Fossils from Glauconitic, Shallow Marine Sediments of the Hornerstown Formation, Edelman Fossil Park, New Jersey. Biology 2022, 11, 1161. https://doi.org/10.3390/biology11081161
Voegele KK, Boles ZM, Ullmann PV, Schroeter ER, Zheng W, Lacovara KJ. Soft Tissue and Biomolecular Preservation in Vertebrate Fossils from Glauconitic, Shallow Marine Sediments of the Hornerstown Formation, Edelman Fossil Park, New Jersey. Biology. 2022; 11(8):1161. https://doi.org/10.3390/biology11081161
Chicago/Turabian StyleVoegele, Kristyn K., Zachary M. Boles, Paul V. Ullmann, Elena R. Schroeter, Wenxia Zheng, and Kenneth J. Lacovara. 2022. "Soft Tissue and Biomolecular Preservation in Vertebrate Fossils from Glauconitic, Shallow Marine Sediments of the Hornerstown Formation, Edelman Fossil Park, New Jersey" Biology 11, no. 8: 1161. https://doi.org/10.3390/biology11081161
APA StyleVoegele, K. K., Boles, Z. M., Ullmann, P. V., Schroeter, E. R., Zheng, W., & Lacovara, K. J. (2022). Soft Tissue and Biomolecular Preservation in Vertebrate Fossils from Glauconitic, Shallow Marine Sediments of the Hornerstown Formation, Edelman Fossil Park, New Jersey. Biology, 11(8), 1161. https://doi.org/10.3390/biology11081161







