Changes in Liver Stiffness and Markers of Liver Synthesis and Portal Hypertension following Hepatitis C Virus Eradication in Cirrhotic Individuals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
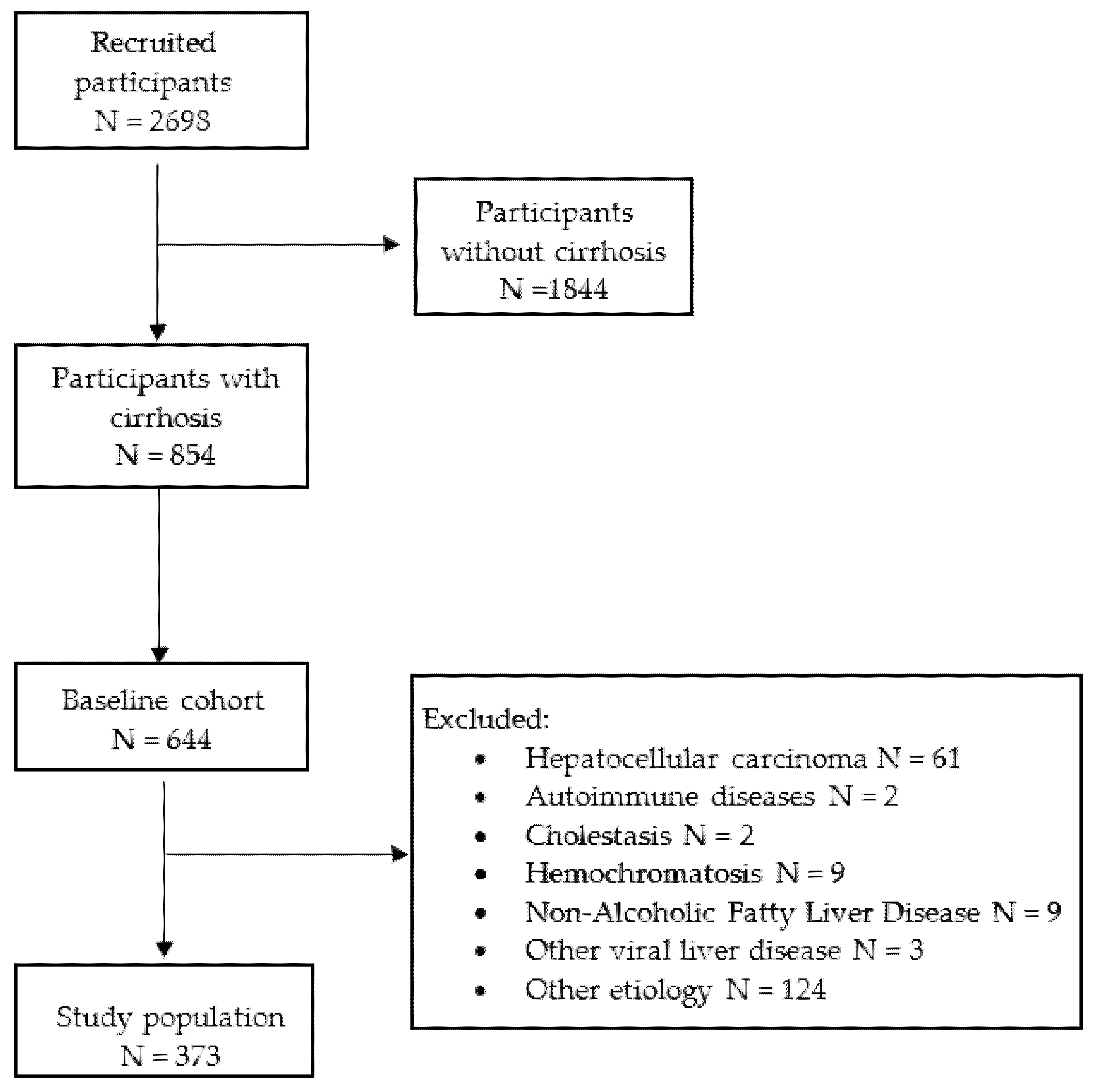
2.1. Patients
2.2. Evaluation of LS and Indirect Signs of Liver Function and Portal Hypertension
2.3. Statistical Analysis
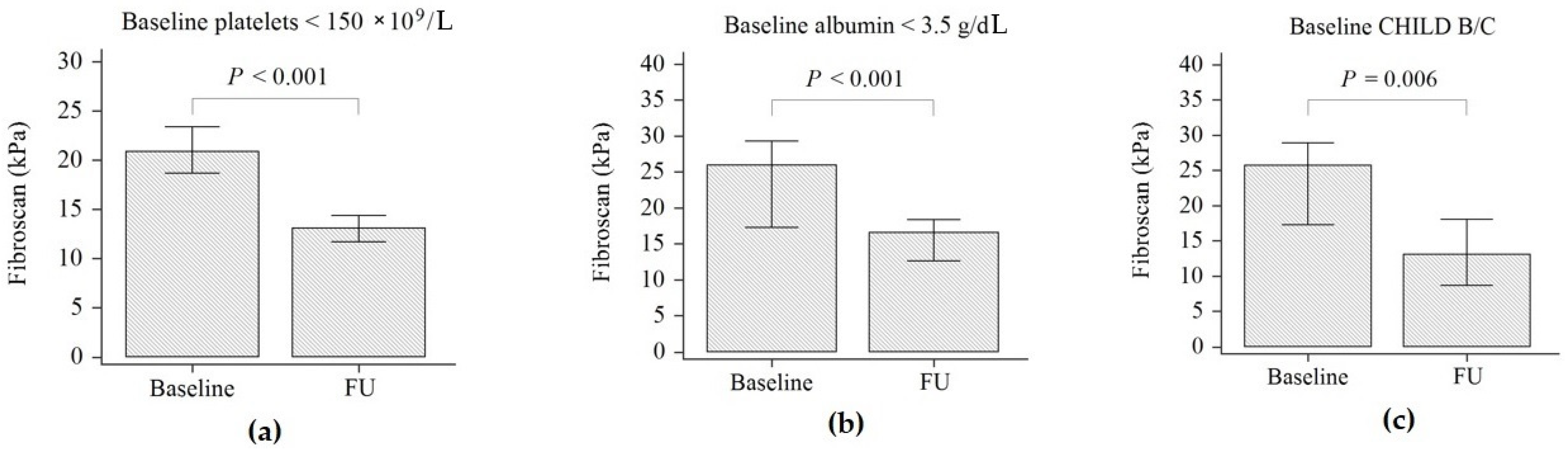
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [Google Scholar] [CrossRef] [PubMed]
- European Union HCV Collaborators. Hepatitis C virus prevalence and level of intervention required to achieve the WHO targets for elimination in the European Union by 2030: A modelling study. Lancet Gastroenterol. Hepatol. 2017, 2, 325–336. [Google Scholar] [CrossRef]
- Piedade, J.; Pereira, G.; Guimarães, L.; Duarte, J.; Victor, L.; Baldin, C.; Inacio, C.; Santos, R.; Chaves, U.; Nunes, E.P.; et al. Liver stiffness regression after sustained virological response by direct-acting antivirals reduces the risk of outcomes. Sci. Rep. 2021, 11, 11681. [Google Scholar] [CrossRef] [PubMed]
- Bachofner, J.A.; Valli, P.V.; Kröger, A.; Bergamin, I.; Künzler, P.; Baserga, A.; Braun, D.; Seifert, B.; Moncsek, A.; Fehr, J.; et al. Direct antiviral agent treatment of chronic hepatitis C results in rapid regression of transient elastography and fibrosis markers fibrosis-4 score and aspartate aminotransferase-platelet ratio index. Liver Int. 2017, 37, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Piecha, F.; Gänßler, M.; Ozga, A.; Wehmeyer, M.H.; Kluwe, J.; Lampalzer, S.; Creutzfeldt, A.M.; Buescher, G.; Horvatits, T.; Sterneck, M.; et al. Evolution of liver stiffness and post-treatment surveillance by liver elastography for HCV patients in the DAA era. Scand. J. Gastroenterol. 2021, 56, 840–848. [Google Scholar] [CrossRef] [PubMed]
- McPhail, J.; Sims, O.Y.; Guo, Y.; Wooten, D.; Herndon, J.S.; Massoud, O.I. Fibrosis improvement in patients with HCV treated with direct-acting antivirals. Eur. J. Gastroenterol. Hepatol. 2021, 33, 996–1000. [Google Scholar] [CrossRef] [PubMed]
- Knop, V.; Hoppe, D.; Vermehren, J.; Troetschler, S.; Herrmann, E.; Vermehren, A.; Friedrich-Rust, M.; Sarrazin, C.; Trebicka, J.; Zeuzem, S.; et al. Non-invasive assessment of fibrosis regression and portal hypertension in patients with advanced chronic hepatitis C virus (HCV)-associated liver disease and sustained virologic response (SVR): 3 years follow-up of a prospective longitudinal study. J. Viral Hepat. 2021, 28, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Lens, S.; Baiges, A.; Alvarado-Tapias, E.; LLop, E.; Martinez, J.; Fortea, J.J.; Ibáñez-Samaniego, L.; Mariño, Z.; Rodríguez-Tajes, S.; Gallego, A.; et al. Clinical outcome and hemodynamic changes following HCV eradication with oral antiviral therapy in patients with clinically significant portal hypertension. J. Hepatol. 2020, 73, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- de Franchis, R.; On behalf of the Baveno VI Faculty. Expanding consensus in portal hypertension Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amico, G.; Garcia-Tsao, G.; Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J. Hepatol. 2006, 44, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Child, C.G.; Turcotte, J.G. Surgery and portal hypertension. Major. Probl. Clin. Surg. 1964, 1, 1–85. [Google Scholar] [PubMed]
- Calvaruso, V.; Cacciola, I.; Licata, A.; Madonia, S.; Benigno, R.; Petta, S.; Rosa, B.; Elisabetta, C.; Antonio, D.; Pietro, C.; et al. Is Transient Elastography Needed for Noninvasive Assessment of High-Risk Varices? The REAL Experience. Off. J. Am. Coll. Gastroenterol. ACG 2019, 114, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Agarwal, S.; Gunjan, D.; Kaushal, K.; Anand, A.; Saraya, A. Deciding among Noninvasive Tools for Predicting Varices Needing Treatment in Chronic Liver Disease: An Analysis of Asian Cohort. Off. J. Am. Coll. Gastroenterol. ACG 2020, 115, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Verna, E.C.; Morelli, G.; Terrault, N.A.; Lok, A.S.; Lim, J.K.; Di Bisceglie, A.M.; Zeuzem, S.; Landis, C.S.; Kwo, P.; Hassan, M.; et al. DAA therapy and long-term hepatic function in advanced/decompensated cirrhosis: Real-world experience from HCV-TARGET cohort. J. Hepatol. 2020, 73, 540–548. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, O.; Jiang, Z.J.; Tapper, E.B.; Huang, K.C.; Zhong, A.; Osinusi, A.; Charlton, M.; Manns, M.; Afdhal, N.H.; Mukamal, K.; et al. Baseline Factors Associated with Improvements in Decompensated Cirrhosis After Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection. Gastroenterology 2018, 154, 2111–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maan, R.; van Tilborg, M.; Deterding, K.; Ramji, A.; van der Meer, A.J.; Wong, F.; Fung, S.; Sherman, M.; Manns, M.P.; Cornberg, M.; et al. Safety and Effectiveness of Direct-Acting Antiviral Agents for Treatment of Patients with Chronic Hepatitis C Virus Infection and Cirrhosis. Clin. Gastroenterol. Hepatol. 2016, 14, 1821–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Franchis, R.; Bosch, J.; Garcia-Tsao, G.; Reiberger, T.; Ripoll, C.; Baveno VII Faculty. Baveno VII—Renewing consensus in portal hypertension. J. Hepatol. 2022, 76, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Pons, M.; Santos, B.; Simón-Talero, M.; Ventura-Cots, M.; Riveiro-Barciela, M.; Esteban, R.; Augustin, S.; Genescà, J. Rapid liver and spleen stiffness improvement in compensated advanced chronic liver disease patients treated with oral antivirals. Ther. Adv. Gastroenterol. 2017, 10, 619–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krassenburg, L.A.P.; Maan, R.; Ramji, R.; Manns, M.P.; Cornberg, M.; Wedemeyer, H.; de Knegt, R.J.; Hansen, B.E.; Janssen, H.L.A.; Feld, J.J.; et al. Clinical outcomes following DAA therapy in patients with HCV-related cirrhosis depend on disease severity. J. Hepatol. 2021, 74, 1053–1063. [Google Scholar] [CrossRef] [PubMed]
- Vutien, P.; Kim, N.J.; Moon, A.M.; Pearson, M.; Su, F.; Berry, K.; Gelman, H.; Ioannou, G.N. Fibroscan liver stiffness after anti-viral treatment for hepatitis C is independently associated with adverse outcomes. Aliment. Pharmacol. Ther. 2020, 52, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | Baseline | Follow-Up | p-Value |
|---|---|---|---|
| Age (years) | 64 (57–77) | - | - |
| Male n (%) | 267 (41.5) | - | - |
| Female n (%) | 377 (58.5) | - | - |
| AST (IU/L) | 69 (45–101) | 23 (19–29) | <0.001 |
| ALT (IU/L) | 67 (43–111) | 20 (16–27) | <0.001 |
| GGT (IU/L) | 66 (40–109) | 24 (16–37) | <0.001 |
| Albumin (g/dL) | 4.1 (3.7–4.4) | 4.4 (4.0–4.6) | <0.001 |
| Platelet count × 109/L | 119 (86–158) | 127 (90–170) | <0.001 |
| “Low-risk” RESIST-HCV score n (%) | 170 (45.6%) | 194 (52%) | 0.004 |
| CHILD n (%) | |||
| A | 345 (94.4%) | 357 (95.7%) | <0.001 |
| B/C | 28 (37.5%) | 16 (4.3%) | |
| Liver stiffness (kPa) | 19.3 (14.7–27) | 11.6 (7.7–16.8) | <0.001 |
| Baseline PLT < 150 (×109/L) | Baseline PLT ≥ 150(×109/L) | Baseline Alb < 3.5 (g/dL) | Baseline Alb ≥ 3.5 (g/dL) | ||
|---|---|---|---|---|---|
| Follow-up PLT < 150 (×109/L) | 204 (80.6%) | 21 (17.5%) | Follow-up Alb < 3.5 (g/dL) | 18 (34%) | 1 (0.3%) |
| Follow-up PLT ≥ 150 (×109/L) | 49 (19.4%) | 99 (82.5%) | Follow-up Alb ≥ 3.5 (g/dL) | 35 (66%) | 319 (99.7%) |
| Tot | 253 | 120 | 53 | 320 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armandi, A.; Rosso, C.; Troshina, G.; Pérez Diaz Del Campo, N.; Marinoni, C.; Nicolosi, A.; Caviglia, G.P.; Saracco, G.M.; Bugianesi, E.; Ciancio, A. Changes in Liver Stiffness and Markers of Liver Synthesis and Portal Hypertension following Hepatitis C Virus Eradication in Cirrhotic Individuals. Biology 2022, 11, 1160. https://doi.org/10.3390/biology11081160
Armandi A, Rosso C, Troshina G, Pérez Diaz Del Campo N, Marinoni C, Nicolosi A, Caviglia GP, Saracco GM, Bugianesi E, Ciancio A. Changes in Liver Stiffness and Markers of Liver Synthesis and Portal Hypertension following Hepatitis C Virus Eradication in Cirrhotic Individuals. Biology. 2022; 11(8):1160. https://doi.org/10.3390/biology11081160
Chicago/Turabian StyleArmandi, Angelo, Chiara Rosso, Giulia Troshina, Nuria Pérez Diaz Del Campo, Chiara Marinoni, Aurora Nicolosi, Gian Paolo Caviglia, Giorgio Maria Saracco, Elisabetta Bugianesi, and Alessia Ciancio. 2022. "Changes in Liver Stiffness and Markers of Liver Synthesis and Portal Hypertension following Hepatitis C Virus Eradication in Cirrhotic Individuals" Biology 11, no. 8: 1160. https://doi.org/10.3390/biology11081160
APA StyleArmandi, A., Rosso, C., Troshina, G., Pérez Diaz Del Campo, N., Marinoni, C., Nicolosi, A., Caviglia, G. P., Saracco, G. M., Bugianesi, E., & Ciancio, A. (2022). Changes in Liver Stiffness and Markers of Liver Synthesis and Portal Hypertension following Hepatitis C Virus Eradication in Cirrhotic Individuals. Biology, 11(8), 1160. https://doi.org/10.3390/biology11081160








