Application of Micro-Computed Tomography for the Estimation of the Post-Mortem Interval of Human Skeletal Remains
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Ethical Considerations
2.2. Micro-CT
2.3. Segmentation and Quantitative Analysis
2.4. Statistical Analysis and Machine Learning
3. Results
3.1. Statistical Analysis and Classification of Post-Mortem Interval
3.2. Deep Learning-Based Classification
4. Discussion
- Further development of prospective technical and scientific protocols.
- Creating a more extensive data pool of bones with different PMIs, stages of autolysis and putrefaction, where temperature, moisture, insects, depth of burial, and scavenging should be essential factors.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vavpotic, M.; Turk, T.; Martincic, D.S.; Balazic, J. Characteristics of the number of odontoblasts in human dental pulp post-mortem. Forensic Sci. Int. 2009, 193, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, N.; Cetin, S.; Bilecenoglu, B.; Altan, A.; Akbulut, S.; Ocak, M.; Orhan, K. The micro-CT evaluation of enamel-cement thickness, abrasion, and mineral density in teeth in the postmortem interval (PMI): New parameters for the determination of PMI. Int. J. Leg. Med. 2020, 134, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Madea, B. Methods for determining time of death. Forensic Sci. Med. Pathol. 2016, 12, 451–485. [Google Scholar] [CrossRef]
- Johnson, L.A.; Ferris, J.A. Analysis of postmortem DNA degradation by single-cell gel electrophoresis. Forensic Sci. Int. 2002, 126, 43–47. [Google Scholar] [CrossRef]
- Pittner, S.; Ehrenfellner, B.; Monticelli, F.C.; Zissler, A.; Sanger, A.M.; Stoiber, W.; Steinbacher, P. Postmortem muscle protein degradation in humans as a tool for PMI delimitation. Int. J. Leg. Med. 2016, 130, 1547–1555. [Google Scholar] [CrossRef]
- Dollerup, E. Chemical analyses and microradiographic investigations on bone biopsies from cases of osteoporosis and osteomalacia as compared with normal. Part I. In Calcium, Phophorus and Nitrogen Content of Normal and Osteoporotic Human Bone; Bone Booth; Macmillan Co.: New York, NY, USA, 1964; pp. 399–404. [Google Scholar]
- Schultz, M. Microscopic investigation of excavated skeletal remains: A contribution to paleopathology and forensic medicine. In Forensic Taphonomy: The Postmortem Fate of Human Remains; CRC Press: Boca Raton, FL, USA, 1997; pp. 201–222. [Google Scholar]
- Yoshino, M.; Kimijima, T.; Miyasaka, S.; Sato, H.; Seta, S. Microscopical study on estimation of time since death in skeletal remains. Forensic Sci. Int. 1991, 49, 143–158. [Google Scholar] [CrossRef]
- Chibnall, A.; Rees, M.; Williams, E. The total nitrogen content of egg albumin and other proteins. Biochem. J. 1943, 37, 354. [Google Scholar] [CrossRef]
- Camps, F.E.; Cameron, J.M.; Lanham, D.J. Practical Forensic Medicine; Hutchinson: Paris, France, 1971. [Google Scholar]
- Cattaneo, C.; Gelsthorpe, K.; Phillips, P.; Sokol, R.J. Reliable identification of human albumin in ancient bone using ELISA and monoclonal antibodies. Am. J. Phys. Anthr. 1992, 87, 365–372. [Google Scholar] [CrossRef]
- Procopio, N.; Williams, A.; Chamberlain, A.T.; Buckley, M. Forensic proteomics for the evaluation of the post-mortem decay in bones. J. Proteom. 2018, 177, 21–30. [Google Scholar] [CrossRef]
- Prieto-Bonete, G.; Pérez-Cárceles, M.D.; Maurandi-López, A.; Pérez-Martínez, C.; Luna, A. Association between protein profile and postmortem interval in human bone remains. J. Proteom. 2019, 192, 54–63. [Google Scholar] [CrossRef]
- Castellano, M.A.; Villanueva, E.C.; von Frenckel, R. Estimating the date of bone remains: A multivariate study. J. Forensic Sci. 1984, 29, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Scrivano, S.; Sanavio, M.; Tozzo, P.; Caenazzo, L. Analysis of RNA in the estimation of post-mortem interval: A review of current evidence. Int. J. Leg. Med. 2019, 133, 1629–1640. [Google Scholar] [CrossRef] [PubMed]
- Locci, E.; Stocchero, M.; Gottardo, R.; De-Giorgio, F.; Demontis, R.; Nioi, M.; Chighine, A.; Tagliaro, F.; d’Aloja, E. Comparative use of aqueous humour 1H NMR metabolomics and potassium concentration for PMI estimation in an animal model. Int. J. Leg. Med. 2021, 135, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Martínez, C.; Pérez-Cárceles, M.D.; Legaz, I.; Prieto-Bonete, G.; Luna, A. Quantification of nitrogenous bases, DNA and Collagen type I for the estimation of the postmortem interval in bone remains. Forensic Sci. Int. 2017, 281, 106–112. [Google Scholar] [CrossRef]
- Taylor, R.E.; Suchey, J.M.; Payen, L.A.; Slota, P.J., Jr. The use of radiocarbon (14C) to identify human skeletal materials of forensic science interest. J. Forensic Sci. 1989, 34, 1196–1205. [Google Scholar] [CrossRef]
- Boaks, A.; Siwek, D.; Mortazavi, F. The temporal degradation of bone collagen: A histochemical approach. Forensic Sci. Int. 2014, 240, 104–110. [Google Scholar] [CrossRef]
- Kanz, F.; Reiter, C.; Risser, D.U. Citrate Content of Bone for Time Since Death Estimation: Results from Burials with Different Physical Characteristics and Known PMI. J. Forensic Sci. 2014, 59, 613–620. [Google Scholar] [CrossRef]
- Schwarcz, H.P.; Agur, K.; Jantz, L.M. A New Method for Determination of Postmortem Interval: Citrate Content of Bone*. J. Forensic Sci. 2010, 55, 1516–1522. [Google Scholar] [CrossRef]
- Sterzik, V.; Jung, T.; Jellinghaus, K.; Bohnert, M. Estimating the postmortem interval of human skeletal remains by analyzing their optical behavior. Int. J. Leg. Med. 2016, 130, 1557–1566. [Google Scholar] [CrossRef]
- Swift, B. Dating human skeletal remains:: Investigating the viability of measuring the equilibrium between 210Po and 210Pb as a means of estimating the post-mortem interval. Forensic Sci. Int. 1998, 98, 119–126. [Google Scholar] [CrossRef]
- Cappella, A.; Gibelli, D.; Muccino, E.; Scarpulla, V.; Cerutti, E.; Caruso, V.; Sguazza, E.; Mazzarelli, D.; Cattaneo, C. The comparative performance of PMI estimation in skeletal remains by three methods (C-14, luminol test and OHI): Analysis of 20 cases. Int. J. Leg. Med. 2018, 132, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Szelecz, I.; Lösch, S.; Seppey, C.V.W.; Lara, E.; Singer, D.; Sorge, F.; Tschui, J.; Perotti, M.A.; Mitchell, E.A.D. Comparative analysis of bones, mites, soil chemistry, nematodes and soil micro-eukaryotes from a suspected homicide to estimate the post-mortem interval. Sci. Rep. 2018, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Maclaughlin-Black, S.M.; Herd, R.J.; Willson, K.; Myers, M.; West, I.E. Strontium-90 as an indicator of time since death: A pilot investigation. Forensic Sci. Int 1992, 57, 51–56. [Google Scholar] [CrossRef]
- Introna, F., Jr.; Di Vella, G.; Campobasso, C.P. Determination of postmortem interval from old skeletal remains by image analysis of luminol test results. J. Forensic Sci. 1999, 44, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Ramsthaler, F.; Kreutz, K.; Zipp, K.; Verhoff, M.A. Dating skeletal remains with luminol-chemiluminescence. Validity, intra- and interobserver error. Forensic Sci. Int. 2009, 187, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Sarabia, J.; Pérez-Martínez, C.; Hernández del Rincón, J.P.; Luna, A. Study of chemiluminescence measured by luminometry and its application in the estimation of postmortem interval of bone remains. Leg. Med. 2018, 33, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Ramsthaler, F.; Ebach, S.C.; Birngruber, C.G.; Verhoff, M.A. Postmortem interval of skeletal remains through the detection of intraosseal hemin traces. A comparison of UV-fluorescence, luminol, Hexagon-OBTI®, and Combur® tests. Forensic Sci. Int. 2011, 209, 59–63. [Google Scholar] [CrossRef]
- Piga, G.; Malgosa, A.; Thompson, T.; Enzo, S. A new calibration of the XRD technique for the study of archaeological burned human remains. J. Archaeol. Sci. 2008, 35, 2171–2178. [Google Scholar] [CrossRef]
- Prieto-Castelló, M.J.; Hernández del Rincón, J.P.; Pérez-Sirvent, C.; Álvarez-Jiménez, P.; Pérez-Cárceles, M.D.; Osuna, E.; Luna, A. Application of biochemical and X-ray diffraction analyses to establish the postmortem interval. Forensic Sci. Int. 2007, 172, 112–118. [Google Scholar] [CrossRef]
- Longato, S.; Woss, C.; Hatzer-Grubwieser, P.; Bauer, C.; Parson, W.; Unterberger, S.H.; Kuhn, V.; Pemberger, N.; Pallua, A.K.; Recheis, W.; et al. Post-mortem interval estimation of human skeletal remains by micro-computed tomography, mid-infrared microscopic imaging and energy dispersive X-ray mapping. Anal. Methods 2015, 7, 2917–2927. [Google Scholar] [CrossRef]
- Surovell, T.A.; Stiner, M.C. Standardizing infra-red measures of bone mineral crystallinity: An experimental approach. J. Archaeol. Sci. 2001, 28, 633–642. [Google Scholar] [CrossRef]
- Thompson, T.; Islam, M.; Piduru, K.; Marcel, A. An investigation into the internal and external variables acting on crystallinity index using Fourier Transform Infrared Spectroscopy on unaltered and burned bone. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 299, 168–174. [Google Scholar] [CrossRef]
- Gourion-Arsiquaud, S.; Faibish, D.; Myers, E.; Spevak, L.; Compston, J.; Hodsman, A.; Shane, E.; Recker, R.R.; Boskey, E.R.; Boskey, A.L. Use of FTIR spectroscopic imaging to identify parameters associated with fragility fracture. J. Bone Miner. Res. 2009, 24, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.; Gauthier, M.; Islam, M. The application of a new method of Fourier Transform Infrared Spectroscopy to the analysis of burned bone. J. Archaeol. Sci. 2009, 36, 910–914. [Google Scholar] [CrossRef]
- Pucéat, E.; Reynard, B.; Lécuyer, C. Can crystallinity be used to determine the degree of chemical alteration of biogenic apatites? Chem. Geol. 2004, 205, 83–97. [Google Scholar] [CrossRef]
- Patonai, Z.; Maasz, G.; Avar, P.; Schmidt, J.; Lorand, T.; Bajnoczky, I.; Mark, L. Novel dating method to distinguish between forensic and archeological human skeletal remains by bone mineralization indexes. Int. J. Leg. Med. 2013, 127, 529–533. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, G.; Lednev, I.K. Potential application of Raman spectroscopy for determining burial duration of skeletal remains. Anal. Bioanal. Chem. 2011, 401, 2511–2518. [Google Scholar] [CrossRef]
- Verhoff, M.A.; Kreutz, K.; Jopp, E.; Kettner, M. Forensische Anthropologie im 21. Jahrhundert. Rechtsmedizin 2013, 23, 79–84. [Google Scholar] [CrossRef]
- Nagy, G.; Lorand, T.; Patonai, Z.; Montsko, G.; Bajnoczky, I.; Marcsik, A.; Mark, L. Analysis of pathological and non-pathological human skeletal remains by FT-IR spectroscopy. Forensic Sci. Int. 2008, 175, 55–60. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Lin, H.; Zha, S.; Fang, R.; Wei, X.; Fan, S.; Wang, Z. Estimation of the late postmortem interval using FTIR spectroscopy and chemometrics in human skeletal remains. Forensic Sci. Int. 2017, 281, 113–120. [Google Scholar] [CrossRef]
- Creagh, D.; Cameron, A. Estimating the Post-Mortem Interval of skeletonized remains: The use of Infrared spectroscopy and Raman spectro-microscopy. Radiat. Phys. Chem. 2017, 137, 225–229. [Google Scholar] [CrossRef]
- Woess, C.; Unterberger, S.H.; Roider, C.; Ritsch-Marte, M.; Pemberger, N.; Cemper-Kiesslich, J.; Hatzer-Grubwieser, P.; Parson, W.; Pallua, J.D. Assessing various Infrared (IR) microscopic imaging techniques for post-mortem interval evaluation of human skeletal remains. PLoS ONE 2017, 12, e0174552. [Google Scholar] [CrossRef]
- Ortiz-Herrero, L.; Uribe, B.; Armas, L.H.; Alonso, M.L.; Sarmiento, A.; Irurita, J.; Alonso, R.M.; Maguregui, M.I.; Etxeberria, F.; Bartolome, L. Estimation of the post-mortem interval of human skeletal remains using Raman spectroscopy and chemometrics. Forensic Sci. Int. 2021, 329, 111087. [Google Scholar] [CrossRef]
- De-Giorgio, F.; Ciasca, G.; Fecondo, G.; Mazzini, A.; De Spirito, M.; Pascali, V.L. Estimation of the time of death by measuring the variation of lateral cerebral ventricle volume and cerebrospinal fluid radiodensity using postmortem computed tomography. Int. J. Leg. Med. 2021, 135, 2615–2623. [Google Scholar] [CrossRef]
- Wilk, L.S.; Edelman, G.J.; Roos, M.; Clerkx, M.; Dijkman, I.; Melgar, J.V.; Oostra, R.J.; Aalders, M.C.G. Individualised and non-contact post-mortem interval determination of human bodies using visible and thermal 3D imaging. Nat. Commun. 2021, 12, 5997. [Google Scholar] [CrossRef]
- Wells, J.D.; Lecheta, M.C.; Moura, M.O.; LaMotte, L.R. An evaluation of sampling methods used to produce insect growth models for postmortem interval estimation. Int. J. Leg. Med. 2015, 129, 405–410. [Google Scholar] [CrossRef]
- Higgins, D.; Rohrlach, A.B.; Kaidonis, J.; Townsend, G.; Austin, J.J. Differential nuclear and mitochondrial DNA preservation in post-mortem teeth with implications for forensic and ancient DNA studies. PLoS ONE 2015, 10, e0126935. [Google Scholar] [CrossRef]
- Klima, M.; Altenburger, M.J.; Kempf, J.; Auwarter, V.; Neukamm, M.A. Determination of medicinal and illicit drugs in post mortem dental hard tissues and comparison with analytical results for body fluids and hair samples. Forensic Sci. Int. 2016, 265, 166–171. [Google Scholar] [CrossRef]
- Swain, M.V.; Xue, J. State of the art of Micro-CT applications in dental research. Int. J. Oral. Sci. 2009, 1, 177–188. [Google Scholar] [CrossRef]
- Engelke, K.; Karolczak, M.; Lutz, A.; Seibert, U.; Schaller, S.; Kalender, W. Micro-CT. Technology and application for assessing bone structure. Radiologe 1999, 39, 203–212. [Google Scholar] [CrossRef]
- Cavanaugh, D.; Johnson, E.; Price, R.E.; Kurie, J.; Travis, E.L.; Cody, D.D. In vivo respiratory-gated micro-CT imaging in small-animal oncology models. Mol. Imaging 2004, 3, 55–62. [Google Scholar] [CrossRef]
- Kalender, W.A.; Deak, P.; Kellermeier, M.; van Straten, M.; Vollmar, S.V. Application- and patient size-dependent optimization of x-ray spectra for CT. Med. Phys. 2009, 36, 993–1007. [Google Scholar] [CrossRef]
- Brouwers, J.E.; van Rietbergen, B.; Huiskes, R. No effects of in vivo micro-CT radiation on structural parameters and bone marrow cells in proximal tibia of wistar rats detected after eight weekly scans. J. Orthop. Res. 2007, 25, 1325–1332. [Google Scholar] [CrossRef]
- Brouwers, J.E.; Lambers, F.M.; Gasser, J.A.; van Rietbergen, B.; Huiskes, R. Bone degeneration and recovery after early and late bisphosphonate treatment of ovariectomized wistar rats assessed by in vivo micro-computed tomography. Calcif. Tissue Int. 2008, 82, 202–211. [Google Scholar] [CrossRef][Green Version]
- Macdonald, H.M.; Nishiyama, K.K.; Kang, J.; Hanley, D.A.; Boyd, S.K. Age-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: A population-based HR-pQCT study. J. Bone Miner. Res. 2011, 26, 50–62. [Google Scholar] [CrossRef]
- Hildebrand, T.; Rüegsegger, P. A new method for the model-independent assessment of thickness in three-dimensional images. J. Microsc. 1997, 185, 67–75. [Google Scholar] [CrossRef]
- Ulrich, D.; van Rietbergen, B.; Laib, A.; Ruegsegger, P. The ability of three-dimensional structural indices to reflect mechanical aspects of trabecular bone. Bone 1999, 25, 55–60. [Google Scholar] [CrossRef]
- Ulrich, D.; Rietbergen, B.; Laib, A.; Ruegsegger, P. Mechanical analysis of bone and its microarchitecture based on in vivo voxel images. Technol. Health Care 1998, 6, 421–427. [Google Scholar] [CrossRef]
- Prot, M.; Saletti, D.; Pattofatto, S.; Bousson, V.; Laporte, S. Links between mechanical behavior of cancellous bone and its microstructural properties under dynamic loading. J. Biomech. 2015, 48, 498–503. [Google Scholar] [CrossRef]
- Ding, M.; Odgaard, A.; Danielsen, C.C.; Hvid, I. Mutual associations among microstructural, physical and mechanical properties of human cancellous bone. J. Bone Jt. Surg. Br. 2002, 84, 900–907. [Google Scholar] [CrossRef]
- Muller, R.; Ruegsegger, P. Analysis of mechanical properties of cancellous bone under conditions of simulated bone atrophy. J. Biomech. 1996, 29, 1053–1060. [Google Scholar] [CrossRef]
- Prot, M.; Saletti, D.; Pattofatto, S.; Bousson, V.; Laporte, S. Links between microstructural properties of cancellous bone and its mechanical response to different strain rates. Comput. Methods Biomech. Biomed. Engin. 2012, 15 (Suppl. S1), 291–292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Laib, A.; Hildebrand, T.; Hauselmann, H.J.; Ruegsegger, P. Ridge number density: A new parameter for in vivo bone structure analysis. Bone 1997, 21, 541–546. [Google Scholar] [CrossRef]
- Cascant, M.M.; Rubio, S.; Gallello, G.; Pastor, A.; Garrigues, S.; Guardia, M.d.l. Burned bones forensic investigations employing near infrared spectroscopy. Vib. Spectrosc. 2017, 90, 21–30. [Google Scholar] [CrossRef]
- Power, A.C.; Chapman, J.; Chandra, S.; Roberts, J.J.; Cozzolino, D. Illuminating the flesh of bone identification—An application of near infrared spectroscopy. Vib. Spectrosc. 2018, 98, 64–68. [Google Scholar] [CrossRef]
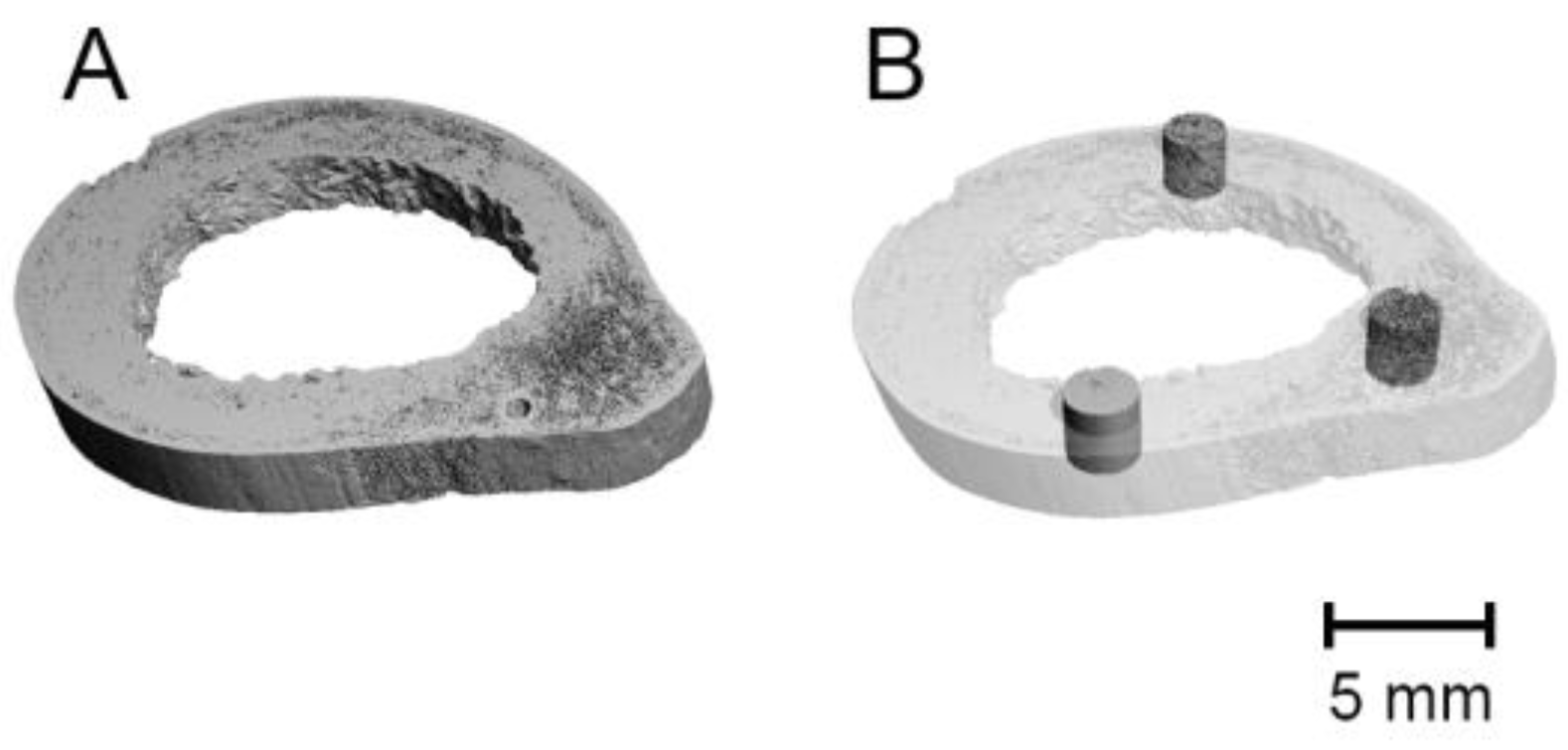


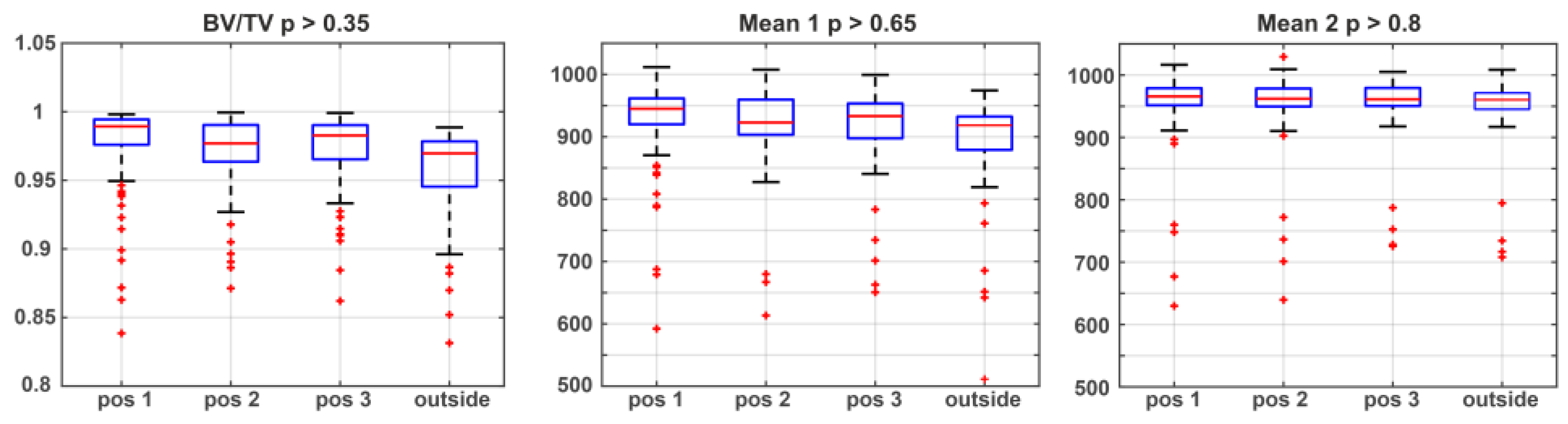
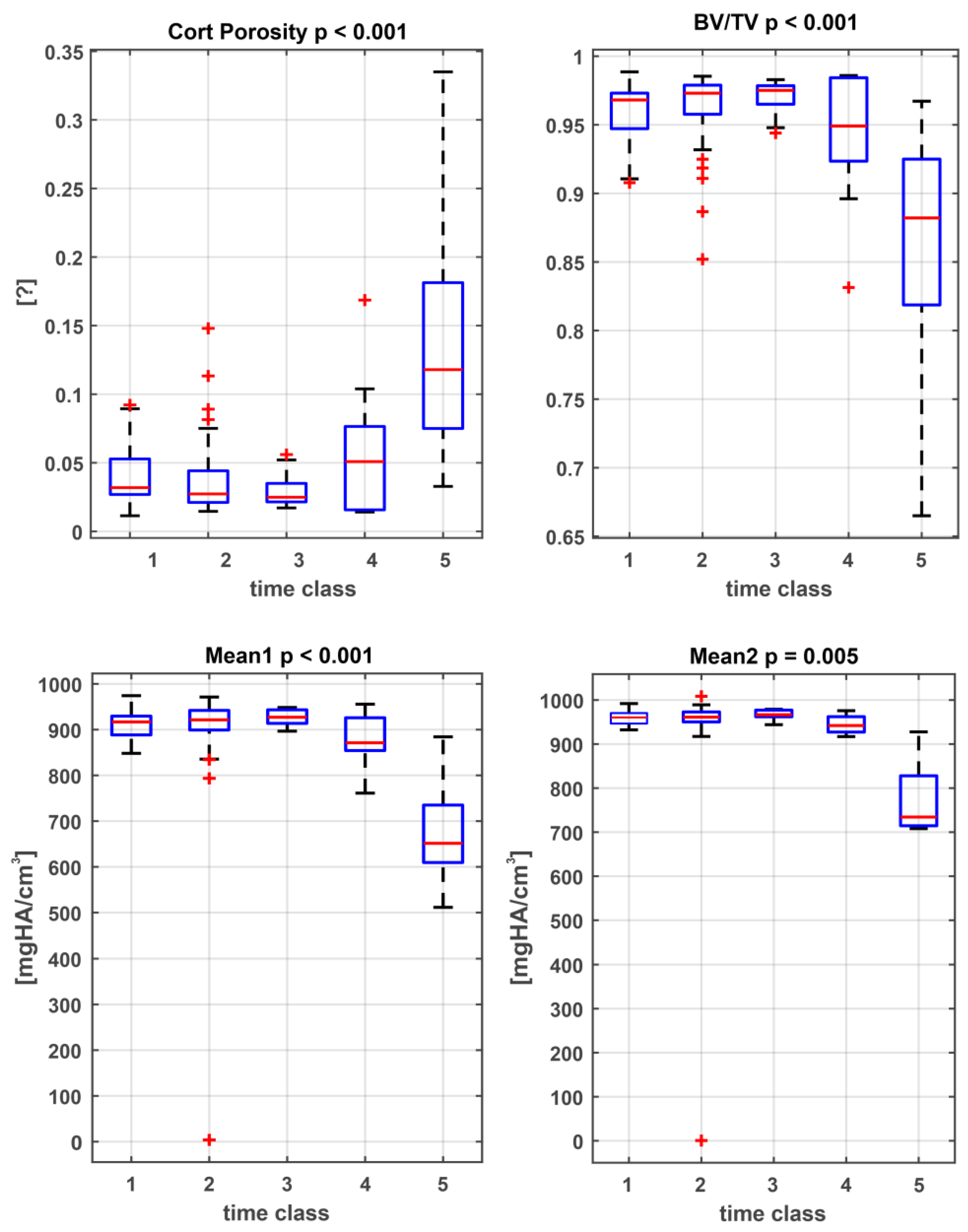
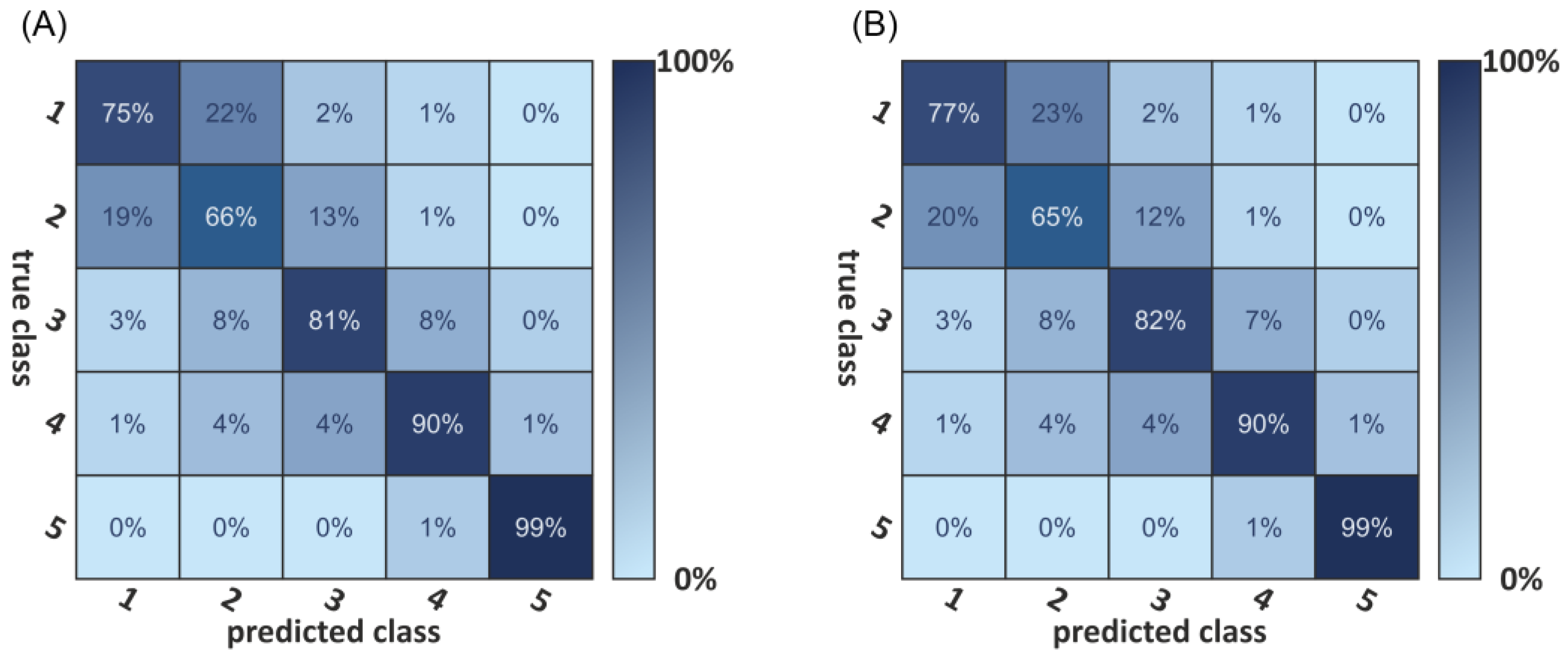
| Metric Measures | Abbreviation | Description | Standard Unit |
|---|---|---|---|
| Bone volume ratio | BV/TV | Ratio of bone volume to total volume in the ROI | % |
| Cortical Porosity | Cort Porosity | cortical volume | % |
| Trabecular number | Tb.N | Mean number of trabeculae per unit length | mm−1 |
| Trabecular thickness | Tb.Th | Mean thickness of the trabeculae | mm |
| Trabecular separation | Tb.Sp | Mean distance between trabeculae | Mm |
| Apparent density | Mean1 | Mean density of the ROI | mgHA/mm3 |
| Material density | Mean2 | Mean density of the bone fraction of the ROI | mgHA/mm3 |
| Age Class | PMI | Analyzed Areas | BV/TV [%] | Cort Porosity | Tb.N [mm] | Tb.Th [mm] | Tb.Sp [mm] | Mean1 [mgHA/cm3] | Mean2 [mgHA/cm3] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0–2 wk | whole cortical bone | 0.96 ± 0.10 | 0.041 ± 0.022 | 1.8 ± 0.2 | 1.40 ± 0.65 | 2.5 ± 1.2 | 928 ± 39 | 950± 121 |
| 1 | 0–2 wk | 3 x cylinders aligned centrally | 0.95 ± 0.02 | n.a | 3.1 ± 1.0 | 0.65 ± 0.22 | 2.2 ± 1.4 | 909 ± 31 | 959 ± 15 |
| 2 | 2 wk–6 mth. | whole cortical bone | 0.97 ± 0.027 | 0.038 ± 0.028 | 1.8 ± 0.3 | 1.54 ± 0.60 | 2.7 ± 1.2 | 934 ± 41 | 967 ± 22 |
| 2 | 2 wk–6 mth. | 3 x cylinders aligned centrally | 0.96 ± 0.02 | n.a | 3.6 ± 1.4 | 0.75 ± 0.21 | 2.9 ± 1.9 | 894 ± 139 | 940 ± 142 |
| 3 | 6 mth.–1 yr. | whole cortical bone | 0.97 ± 0.02 | 0.030 ±0.013 | 1.6 ± 0.2 | 1.48 ± 0.47 | 2.5 ± 0.9 | 939 ± 31 | 972 ± 12 |
| 3 | 6 mth.–1 yr. | 3 x cylinders aligned centrally | 0.97 ± 0.01 | n.a | 2.9 ± 1.2 | 0.71 ± 0.20 | 2.3 ± 1.5 | 926 ± 18 | 966 ± 11 |
| 4 | 1 yr.–10 yr. | whole cortical bone | 0.97 ± 0.02 | 0.059 ± 0.049 | 1.8 ± 0.2 | 1.40 ± 0.78 | 2.6 ± 1.6 | 923 ± 37 | 956 ± 24 |
| 4 | 1 yr.–10 yr. | 3 x cylinders aligned centrally | 0.94 ± 0.05 | n.a | 3.6 ± 1.8 | 0.76 ± 0.20 | 3.0 ± 2.4 | 877 ± 59 | 944 ± 19 |
| 5 | >100 yr. | whole cortical bone | 0.84± 0.23 | 0.141 ± 0.115 | 1.4 ± 0.2 | 0.52 ± 0.40 | 0.76 ± 0.65 | 663 ± 162 | 758 ± 89 |
| 5 | >100 yr. | 3 x cylinders aligned centrally | 0.85 ± 0.11 | n.a | n.a. | n.a. | n.a. | 674 ± 134 | 776 ± 91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmidt, V.-M.; Zelger, P.; Woess, C.; Pallua, A.K.; Arora, R.; Degenhart, G.; Brunner, A.; Zelger, B.; Schirmer, M.; Rabl, W.; et al. Application of Micro-Computed Tomography for the Estimation of the Post-Mortem Interval of Human Skeletal Remains. Biology 2022, 11, 1105. https://doi.org/10.3390/biology11081105
Schmidt V-M, Zelger P, Woess C, Pallua AK, Arora R, Degenhart G, Brunner A, Zelger B, Schirmer M, Rabl W, et al. Application of Micro-Computed Tomography for the Estimation of the Post-Mortem Interval of Human Skeletal Remains. Biology. 2022; 11(8):1105. https://doi.org/10.3390/biology11081105
Chicago/Turabian StyleSchmidt, Verena-Maria, Philipp Zelger, Claudia Woess, Anton K. Pallua, Rohit Arora, Gerald Degenhart, Andrea Brunner, Bettina Zelger, Michael Schirmer, Walter Rabl, and et al. 2022. "Application of Micro-Computed Tomography for the Estimation of the Post-Mortem Interval of Human Skeletal Remains" Biology 11, no. 8: 1105. https://doi.org/10.3390/biology11081105
APA StyleSchmidt, V.-M., Zelger, P., Woess, C., Pallua, A. K., Arora, R., Degenhart, G., Brunner, A., Zelger, B., Schirmer, M., Rabl, W., & Pallua, J. D. (2022). Application of Micro-Computed Tomography for the Estimation of the Post-Mortem Interval of Human Skeletal Remains. Biology, 11(8), 1105. https://doi.org/10.3390/biology11081105








