Structure and Strength of Bovine and Equine Amniotic Membrane
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
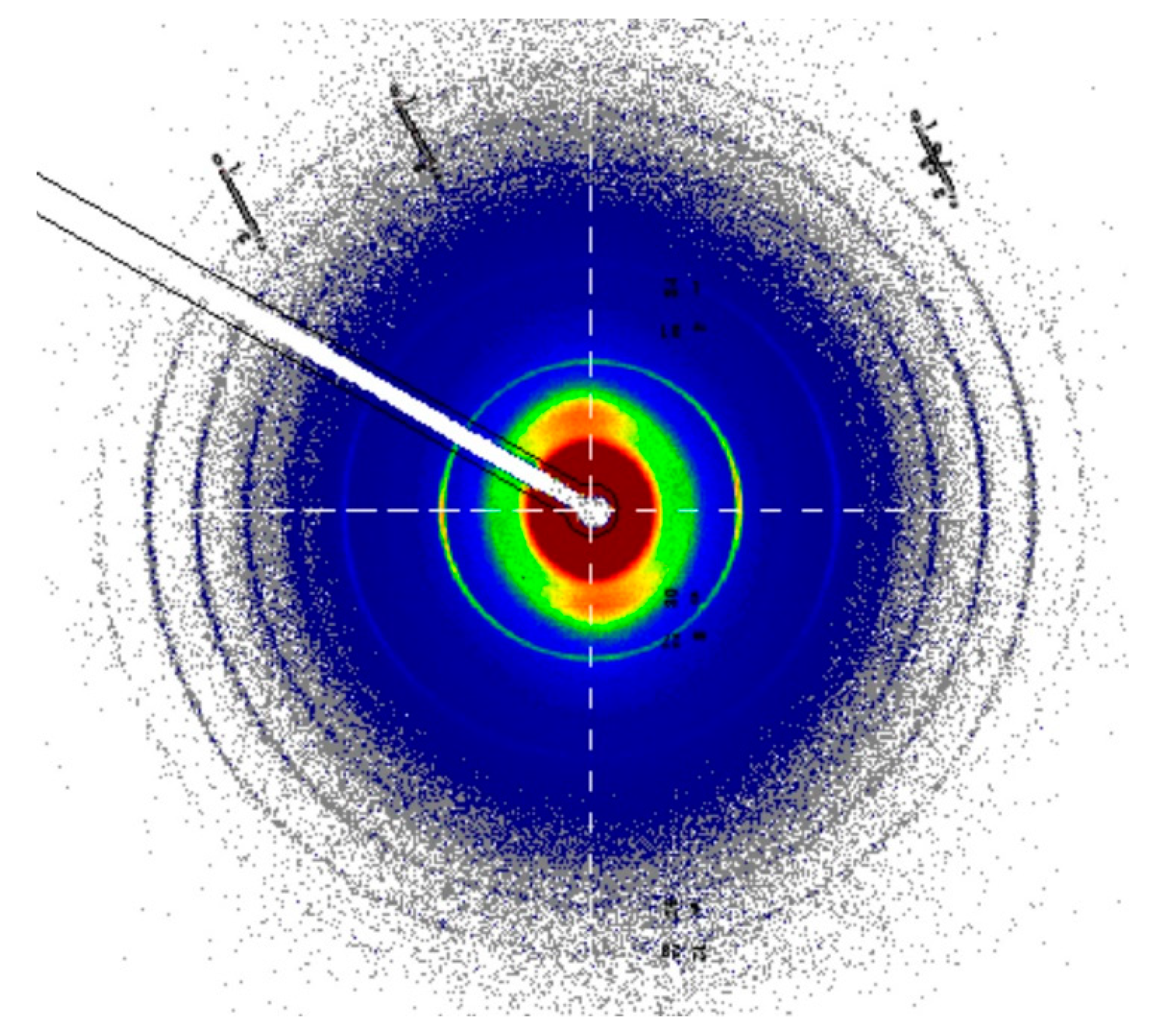
2.2. SAXS Measurement
2.3. Differential Interference Contrast Microscopy (DIC)
2.4. Atomic Force Microscopy (AFM)
2.5. Ultrasonics
2.6. Tear and Tensile Test
3. Results
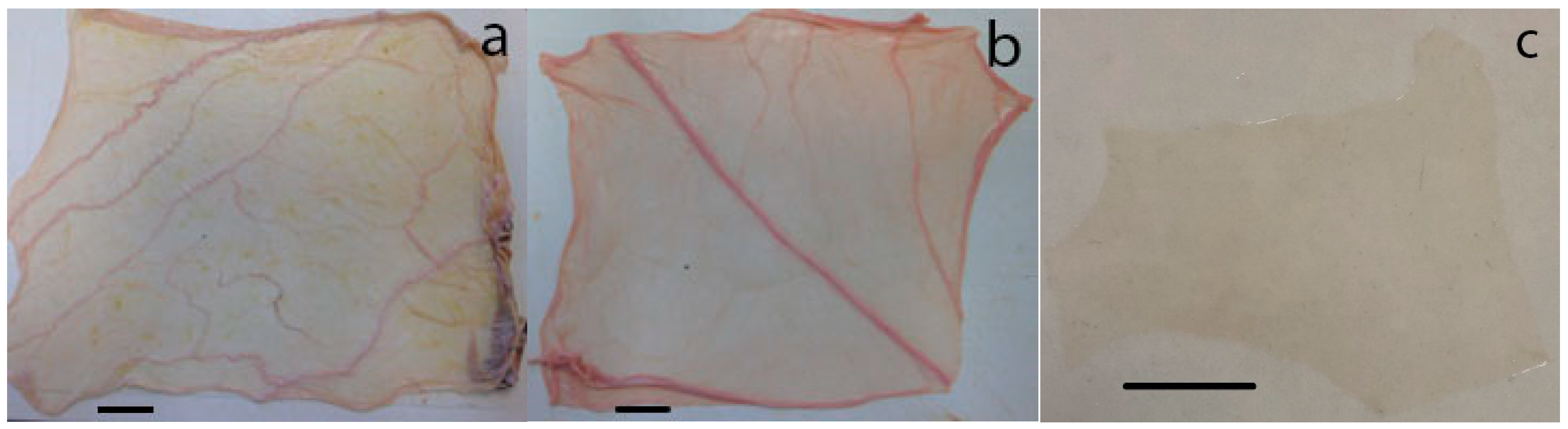
3.1. Membrane Material
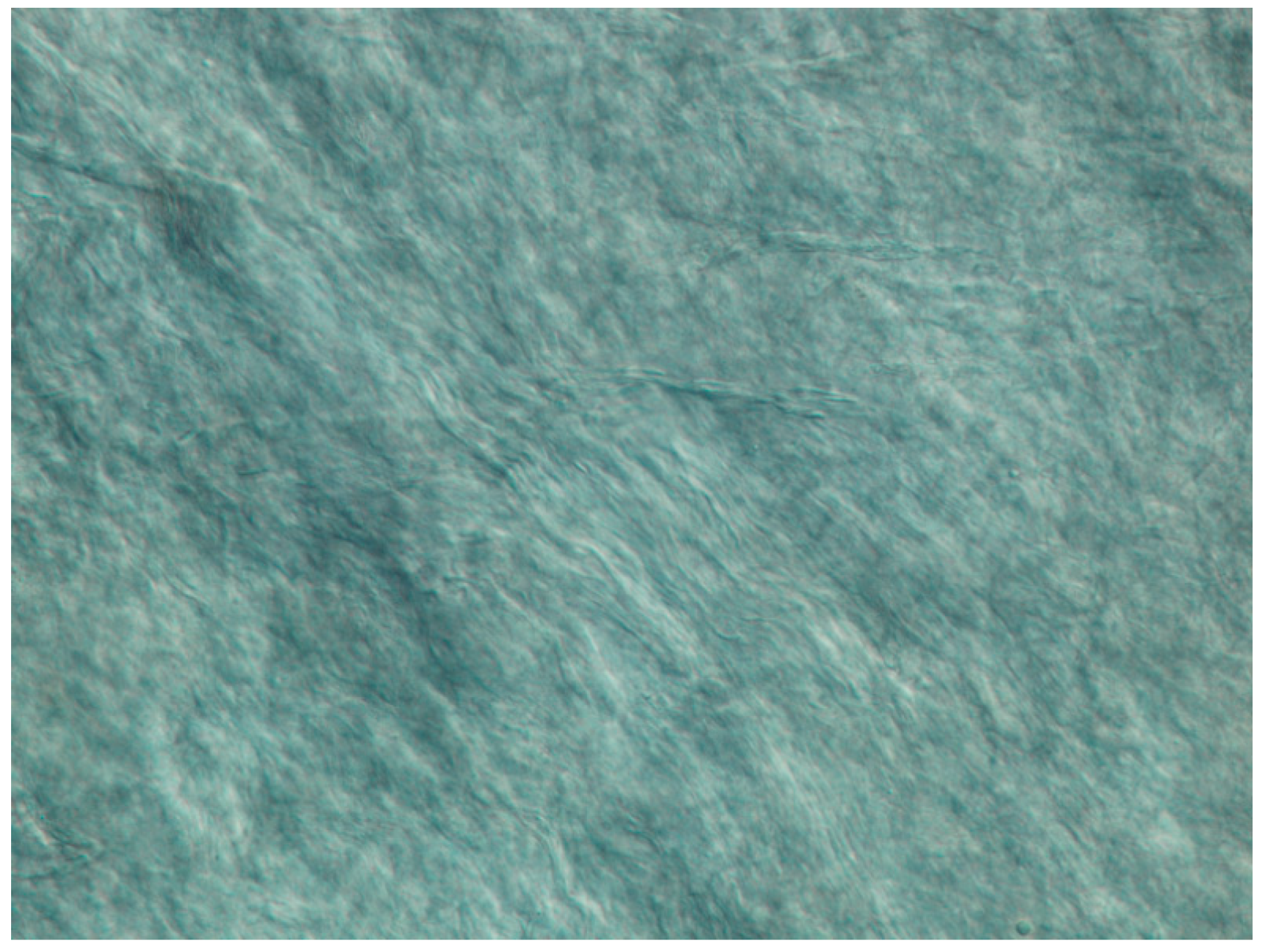
3.2. Differential Interference Contrast Microscopy
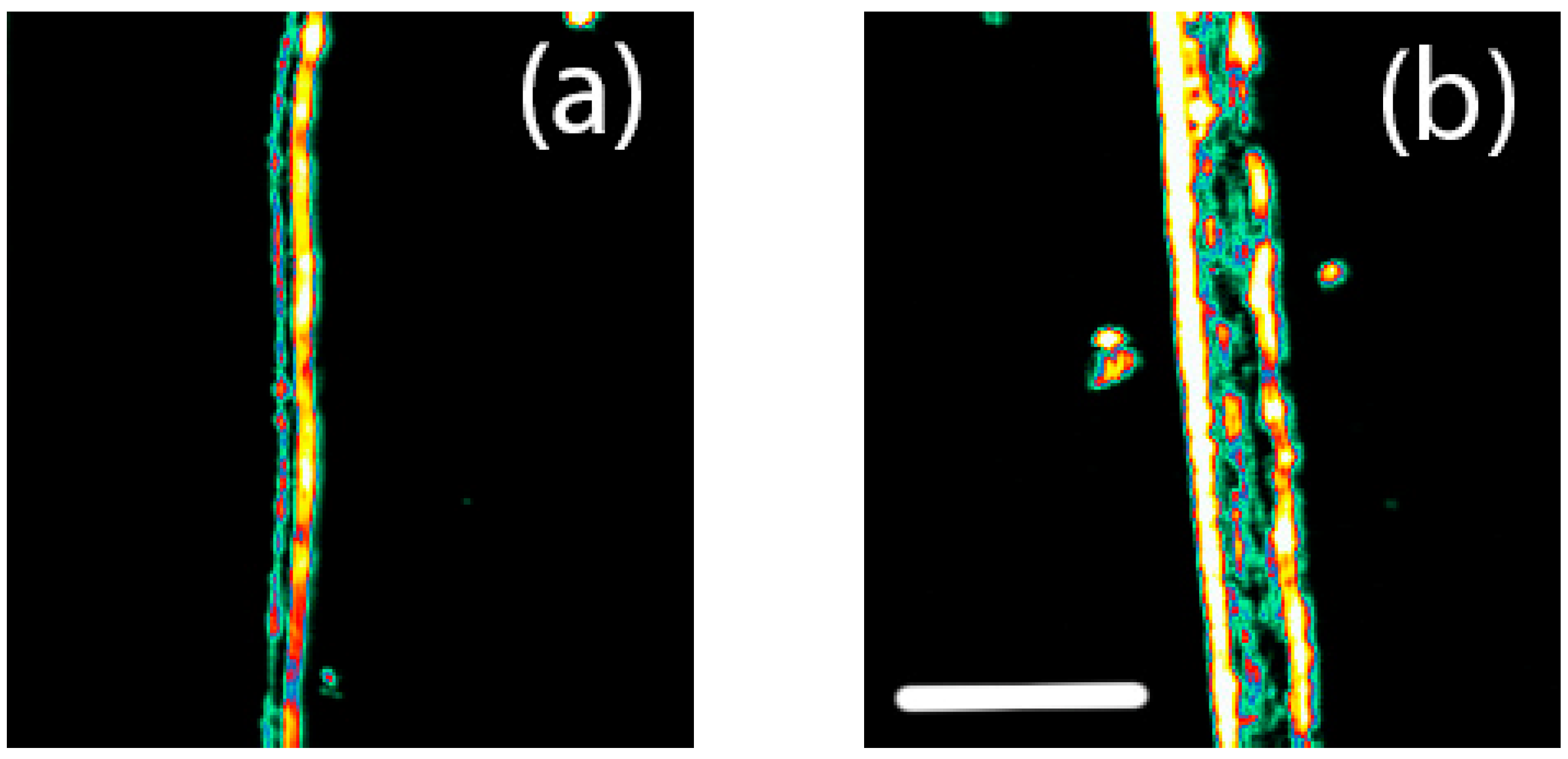
3.3. Ultrasonics
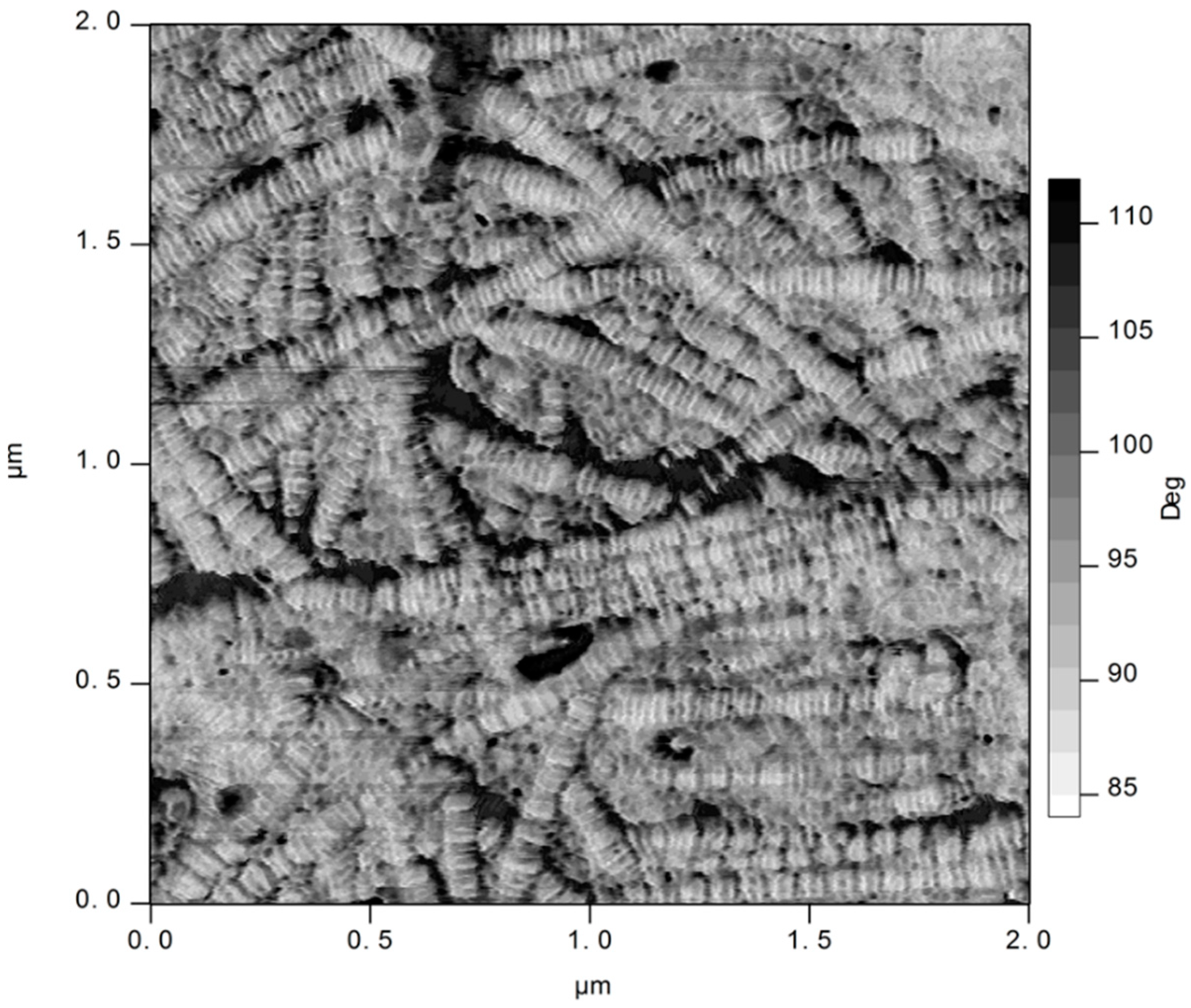
3.4. Atomic Force Microscopy
3.5. Tear Strength
3.6. Tensile Strength
3.7. SAXS of Collagen Structure
4. Discussion
4.1. Strength Compared with Human Amnion and Other Materials
4.2. Structural Arrangement
4.3. Application
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calvin, S.E.; Oyen, M.L. Microstructure and mechanics of the chorioamnion membrane with an emphasis on fracture properties. Ann. N. Y. Acad. Sci. 2007, 1101, 166–185. [Google Scholar] [CrossRef] [PubMed]
- Koob, T.J.; Lim, J.J.; Massee, M.; Zabek, N.; Denozière, G. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J. Biomed. Mater. Res. B 2014, 102, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- Leal-Marin, S.; Kern, T.; Hofmann, N.; Pogozhykh, O.; Framme, C.; Börgel, M.; Figueiredo, C.; Glasmacher, B.; Gryshkov, O. Human Amniotic Membrane: A review on tissue engineering, application, and storage. J. Biomed. Mater. Res. B 2021, 109, 1198–1215. [Google Scholar] [CrossRef] [PubMed]
- Arrizabalaga, J.H.; Nollert, M.U. Human Amniotic Membrane: A Versatile Scaffold for Tissue Engineering. ACS Biomater. Sci. Eng. 2018, 4, 2226–2236. [Google Scholar] [CrossRef]
- von Versen-Hoynck, F.; Hesselbarth, U.; Moller, D.E. Application of sterilised human amnion for reconstruction of the ocular surface. Cell Tissue Bank 2004, 5, 57–65. [Google Scholar] [CrossRef]
- Jirsova, K.; Jones, G.L.A. Amniotic membrane in ophthalmology: Properties, preparation, storage and indications for grafting—A review. Cell Tissue Bank. 2017, 18, 193–204. [Google Scholar] [CrossRef]
- Riordan, N.H.; George, B.A.; Chandler, T.B.; McKenna, R.W. Case report of non-healing surgical wound treated with dehydrated human amniotic membrane. J. Transl. Med. 2015, 13, 242. [Google Scholar] [CrossRef]
- Monavarian, M.; Kader, S.; Moeinzadeh, S.; Jabbari, E. Regenerative Scar-Free Skin Wound Healing. Tissue Eng. Part B Rev. 2019, 25, 294–311. [Google Scholar] [CrossRef]
- Castellanos, G.; Bernabé-García, Á.; Moraleda, J.M.; Nicolás, F.J. Amniotic membrane application for the healing of chronic wounds and ulcers. Placenta 2017, 59, 146–153. [Google Scholar] [CrossRef]
- Li, W.; Ma, G.; Brazile, B.; Li, N.; Dai, W.; Butler, J.R.; Claude, A.A.; Wertheim, J.A.; Liao, J.; Wang, B. Investigating the Potential of Amnion-Based Scaffolds as a Barrier Membrane for Guided Bone Regeneration. Langmuir 2015, 31, 8642–8653. [Google Scholar] [CrossRef]
- Elahi, A.; Taib, H.; Berahim, Z.; Ahmad, A.; Hamid, S.S.A.; Mocktar, N.A. Amniotic membrane as a scaffold for periodontal tissue engineering. J. Health Sci. Med. Res. 2021, 39, 169–180. [Google Scholar] [CrossRef]
- Gulameabasse, S.; Gindraux, F.; Catros, S.; Fricain, J.C.; Fenelon, M. Chorion and amnion/chorion membranes in oral and periodontal surgery: A systematic review. J. Biomed. Mater. Res. B 2021, 109, 1216–1229. [Google Scholar] [CrossRef]
- Ramuta, T.Ž.; Kreft, M.E. Human Amniotic Membrane and Amniotic Membrane–Derived Cells: How Far Are We from Their Use in Regenerative and Reconstructive Urology? Cell Transplant. 2018, 27, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Maan, Z.N.; Rennert, R.C.; Koob, T.J.; Januszyk, M.; Li, W.W.; Gurtner, G.C. Cell recruitment by amnion chorion grafts promotes neovascularization. J. Surg. Res. 2015, 193, 953–962. [Google Scholar] [CrossRef]
- Song, M.; Wang, W.; Ye, Q.; Bu, S.; Shen, Z.; Zhu, Y. The repairing of full-thickness skin deficiency and its biological mechanism using decellularized human amniotic membrane as the wound dressing. Mater. Sci. Eng. C 2017, 77, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Chai, Y.; Yu, Y. Progress in developing decellularized bioscaffolds for enhancing skin construction. J. Biomed. Mater. Res. A 2019, 107, 1849–1859. [Google Scholar] [CrossRef]
- Heath, D.E. A Review of Decellularized Extracellular Matrix Biomaterials for Regenerative Engineering Applications. Regen. Eng. Transl. Med. 2019, 5, 155–166. [Google Scholar] [CrossRef]
- Wells, H.C.; Sizeland, K.H.; Kirby, N.; Hawley, A.; Mudie, S.; Haverkamp, R.G. Collagen fibril structure and strength in acellular dermal matrix materials of bovine, porcine and human origin. ACS Biomater. Sci. Eng. 2015, 1, 1026–1038. [Google Scholar] [CrossRef]
- Patel, S.; Ziai, K.; Lighthall, J.G.; Walen, S.G. Biologics and acellular dermal matrices in head and neck reconstruction: A comprehensive review. Am. J. Otolaryngol. 2022, 43, 103233. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, Y.M.; Jeong, S.W.; Williams, D.L. Effect of bovine freeze-dried amniotic membrane (Amnisite-BA™) on uncomplicated canine corneal erosion. Vet. Ophthalmol. 2009, 12, 36–42. [Google Scholar] [CrossRef]
- Ollivier, F.J.; Kallberg, M.E.; Plummer, C.E.; Barrie, K.P.; O’Reilly, S.; Taylor, D.P.; Gelatt, K.N.; Brooks, D.E. Amniotic membrane transplantation for corneal surface reconstruction after excision of corneolimbal squamous cell carcinomas in nine horses. Vet. Ophthalmol. 2006, 9, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, K.; Yamashita, K.; Izumisawa, Y.; Kotani, T. Microstructure and glycosaminoglycan ratio of canine cornea after reconstructive transplantation with glycerin-preserved porcine amniotic membranes. Vet. Ophthalmol. 2008, 11, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Barros, P.S.M.; Safatle, A.M.V.; Godoy, C.A.; Souza, M.S.B.; Barros, L.F.M.; Brooks, D.E. Amniotic membrane transplantation for the reconstruction of the ocular surface in three cases. Vet. Ophthalmol. 2005, 8, 189–192. [Google Scholar] [CrossRef] [PubMed]
- Withavatpongtorn, N.; Tuntivanich, N. Characterization of cryopreserved canine amniotic membrane. Membranes 2021, 11, 824. [Google Scholar] [CrossRef]
- Oxlund, H.; Helmig, R.; Halaburt, J.T.; Uldbjerg, N. Biomechanical analysis of human chorioamniotic membranes. Eur. J. Obstet. Gynecol. 1990, 34, 247–255. [Google Scholar] [CrossRef]
- Moore, R.M.; Mansour, J.M.; Redline, R.W.; Mercer, B.M.; Moore, J.J. The Physiology of Fetal Membrane Rupture: Insight Gained from the Determination of Physical Properties. Placenta 2006, 27, 1037–1051. [Google Scholar] [CrossRef]
- Bircher, K.; Ehret, A.E.; Spiess, D.; Ehrbar, M.; Simões-Wüst, A.P.; Ochsenbein-Kölble, N.; Zimmermann, R.; Mazza, E. On the defect tolerance of fetal membranes. Interface Focus 2019, 9, 20190010. [Google Scholar] [CrossRef]
- Pressman, E.K.; Cavanaugh, J.L.; Woods, J.R. Physical properties of the chorioamnion throughout gestation. Am. J. Obstet. Gynecol. 2002, 187, 672–675. [Google Scholar] [CrossRef]
- Helmig, R.; Oxlund, H.; Petersen, L.K.; Uldbjerg, N. Different biomechanical properties of human fetal membranes obtained before and after delivery. Eur. J. Obstet. Gynecol. 1993, 48, 183–189. [Google Scholar] [CrossRef]
- Benson-Martin, J.; Zammaretti, P.; Bilic, G.; Schweizer, T.; Portmann-Lanz, B.; Burkhardt, T.; Zimmermann, R.; Ochsenbein-Kölble, N. The Young’s modulus of fetal preterm and term amniotic membranes. Eur. J. Obstet. Gynecol. 2006, 128, 103–107. [Google Scholar] [CrossRef]
- Baird, P.N.; Machin, H.; Brown, K.D. Corneal supply and the use of technology to reduce its demand: A review. Clin. Exp. Ophthalmol. 2021, 49, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Sacks, M.S.; Smith, D.B.; Hiester, E.D. A small angle light scattering device for planar connective tissue microstructural analysis. Ann. Biomed. Eng. 1997, 25, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Cookson, D.; Kirby, N.; Knott, R.; Lee, M.; Schultz, D. Strategies for data collection and calibration with a pinhole-geometry SAXS instrument on a synchrotron beamline. J. Synchrotron Radiat. 2006, 13, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Chua, W.K.; Oyen, M.L. Do we know the strength of the chorioamnion? A critical review and analysis. Eur. J. Obstet. Gynecol. 2009, 144, S128–S133. [Google Scholar] [CrossRef]
- Manabe, Y.; Himeno, N.; Fukumoto, M. Tensile strength and collagen content of amniotic membrane do not change after the second trimester or during delivery. Obstet. Gynecol. 1991, 78, 24–27. [Google Scholar]
- Sizeland, K.H.; Wells, H.C.; Higgins, J.J.; Cunanan, C.M.; Kirby, N.; Hawley, A.; Haverkamp, R.G. Age dependant differences in collagen fibril alignment of glutaraldehyde fixed bovine pericardium. BioMed. Res. Int. 2014, 2014, 189197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kayed, H.R.; Sizeland, K.H.; Wells, H.C.; Kirby, N.; Hawley, A.; Mudie, S.; Haverkamp, R.G. Age differences with glutaraldehyde treatment in collagen fibril orientation of bovine pericardium. J. Biomater. Tissue Eng. 2016, 6, 992–997. [Google Scholar] [CrossRef]
- Kayed, H.R.; Sizeland, K.H.; Kirby, N.; Hawley, A.; Mudie, S.T.; Haverkamp, R.G. Collagen cross linking and fibril alignment in pericardium. RSC Adv. 2015, 5, 3611–3618. [Google Scholar] [CrossRef]
- Seto, J.; Gupta, H.S.; Zaslansky, P.; Wagner, H.D.; Fratzl, P. Tough lessons from bone: Extreme mechanical anisotropy at the mesoscale. Adv. Funct. Mater. 2008, 18, 1905–1911. [Google Scholar] [CrossRef]
- Wells, H.C.; Sizeland, K.H.; Kayed, H.R.; Kirby, N.; Hawley, A.; Mudie, S.T.; Haverkamp, R.G. Poisson’s ratio of collagen fibrils measured by SAXS of strained bovine pericardium. J. Appl. Phys. 2015, 117, 044701. [Google Scholar] [CrossRef]
- Sizeland, K.H.; Basil-Jones, M.M.; Edmonds, R.L.; Cooper, S.M.; Kirby, N.; Hawley, A.; Haverkamp, R.G. Collagen orientation and leather strength for selected mammals. J. Agric. Food Chem. 2013, 61, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Basil-Jones, M.M.; Edmonds, R.L.; Norris, G.E.; Haverkamp, R.G. Collagen fibril alignment and deformation during tensile strain of leather: A SAXS study. J. Agric. Food Chem. 2012, 60, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sherman, V.R.; Gludovatz, B.; Schaible, E.; Stewart, P.; Ritchie, R.O.; Meyers, M.A. On the tear resistance of skin. Nat. Commun. 2015, 6, 6649. [Google Scholar] [CrossRef] [PubMed]
- Haverkamp, R.G.; Williams, M.A.K.; Scott, J.E. Stretching single molecules of connective tissue glycans to characterize their shape-maintaining elasticity. Biomacromolecules 2005, 6, 1816–1818. [Google Scholar] [CrossRef]
- Haugh, M.G.; Jaasma, M.J.; O’Brien, F.J. The effect of dehydrothermal treatment on the mechanical and structural properties of collagen-GAG scaffolds. J. Biomed. Mater. Res. A 2009, 89, 363–369. [Google Scholar] [CrossRef]
- Oxlund, H.; Manschot, J.; Viidik, A. The role of elastin in the mechanical properties of skin. J. Biomech. 1988, 21, 213–218. [Google Scholar] [CrossRef]
| Amnion Type | Average Max Tear Force (N) | Average Material Thickness (mm) | Thickness-Normalized Tear Strength (N/mm) |
|---|---|---|---|
| Equine | 2.95 (1.03) | 0.20 (0.06) | 14.8 (5.3) |
| Bovine | 2.685 (0.92) | 0.21 (0.03) | 12.6 (3.8) |
| Amnionic Membrane Type | Average Max. Tensile Force (N) | Average Material Thickness (mm) | Thickness-Normalized Tensile Strength (MPa) |
|---|---|---|---|
| Equine | 4.46 (1.73) | 0.20 (0.07) | 2.46 (1.23) |
| Bovine | 5.85 (0.42) | 0.21 (0.03) | 2.79 (2.05) |
| Amnion Type | Average Collagen OI (σ) | Average Collagen d-Spacing (nm) (σ) |
|---|---|---|
| Equine | 0.681 (0.05) | 64.7 (0.25) |
| Bovine | 0.587 (0.06) | 64.5 (0.25) |
| Material Type | Species | Average OI Edge on | Average d-Spacing | Thickness of Material (mm) | Thickness-Normalized Tear Strength (N/mm) |
|---|---|---|---|---|---|
| Surgical scaffolds (ADM) | Human ADM [18] | 0.23 | 64.60 | 1.06 | 79.0 |
| Porcine ADM [18] | 0.40 | 64.20 | 1.63 | 43.2 | |
| Bovine Neonatal ADM [18] | 0.34 | 64.08 | 2.69 | 90.2 | |
| Bovine Fetal ADM [18] | 0.43 | 64.00 | 0.98 | 78.0 | |
| Native Pericardium | Bovine adult [36] | 0.58–0.67 | 0.36 | ||
| Bovine neonatal [36] | 0.76–0.80 | 0.12 | |||
| Bovine adult [37] | 0.47 | ||||
| Bovine neonatal [37] | 0.61 | ||||
| Native amniotic membrane | Bovine amniotic membrane | 0.59 | 64.56 | 0.21 | 12.6 |
| (This work) | Equine amniotic membrane | 0.68 | 64.70 | 0.20 | 14.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wells, H.C.; Sizeland, K.H.; Kirby, N.; Haverkamp, R.G. Structure and Strength of Bovine and Equine Amniotic Membrane. Biology 2022, 11, 1096. https://doi.org/10.3390/biology11081096
Wells HC, Sizeland KH, Kirby N, Haverkamp RG. Structure and Strength of Bovine and Equine Amniotic Membrane. Biology. 2022; 11(8):1096. https://doi.org/10.3390/biology11081096
Chicago/Turabian StyleWells, Hannah C., Katie H. Sizeland, Nigel Kirby, and Richard G. Haverkamp. 2022. "Structure and Strength of Bovine and Equine Amniotic Membrane" Biology 11, no. 8: 1096. https://doi.org/10.3390/biology11081096
APA StyleWells, H. C., Sizeland, K. H., Kirby, N., & Haverkamp, R. G. (2022). Structure and Strength of Bovine and Equine Amniotic Membrane. Biology, 11(8), 1096. https://doi.org/10.3390/biology11081096






