A New Horizon in Reproductive Research with Pluripotent Stem Cells: Successful In Vitro Gametogenesis in Rodents, Its Application to Large Animals, and Future In Vitro Reconstitution of Reproductive Organs Such as “Uteroid” and “Oviductoid”
Abstract
Simple Summary
Abstract
1. Introduction
2. Development of Germ Cells In Vivo
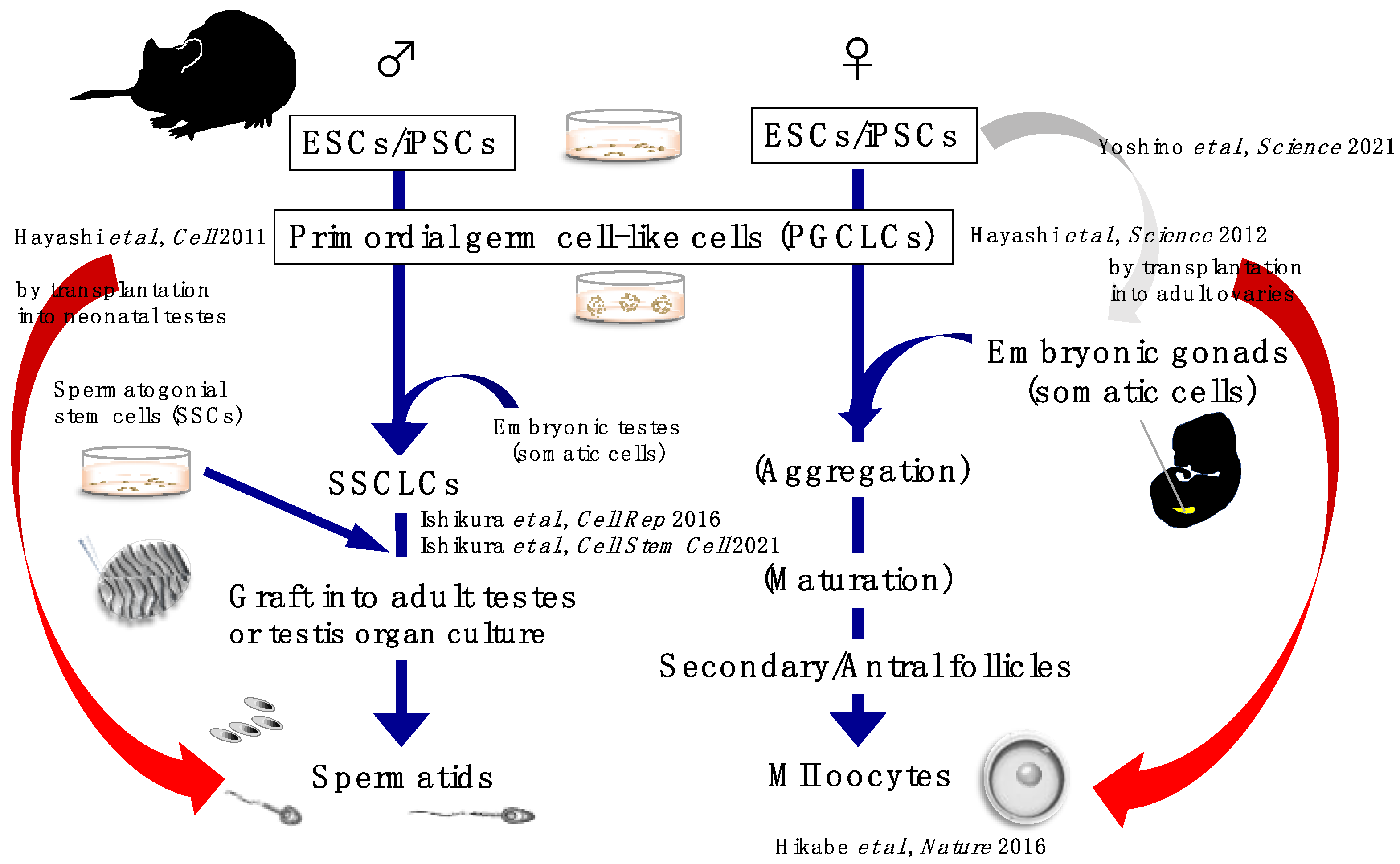
3. Successful In Vitro Reconstitution of Mouse Gametes
4. Attempts of In Vitro Gametogenesis in Large Animals and Humans
5. Female Reproductive Organs Required for Full-Term Development of an Embryo
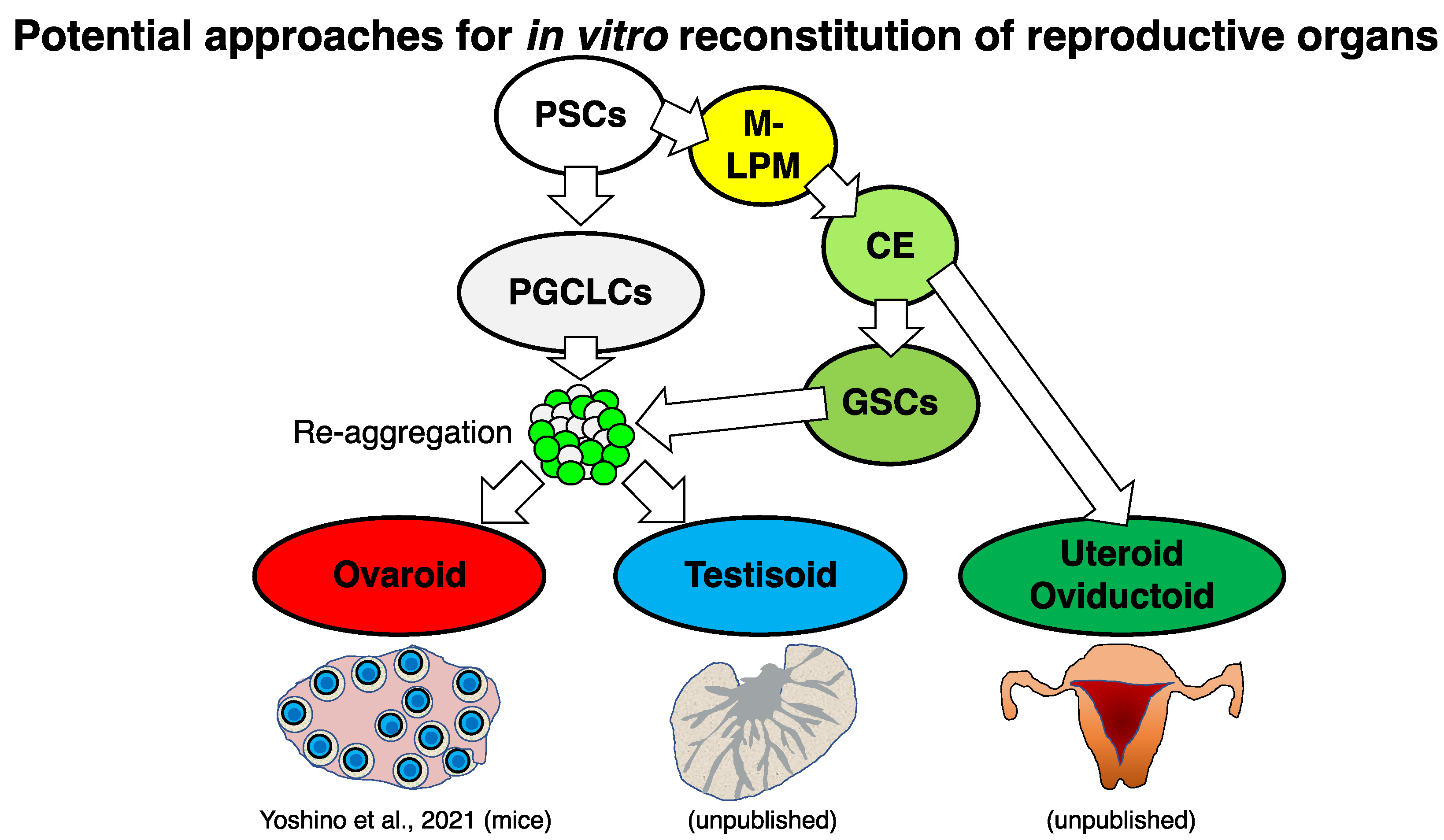
6. Potential Approaches for In Vitro Reconstitution of Female Reproductive Organs
7. Ethical Concerns
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Surani, M.A. Reprogramming of genome function through epigenetic inheritance. Nature 2001, 414, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.W.; Kobayashi, T.; Irie, N.; Dietmann, S.; Surani, M.A. Specification and epigenetic programming of the human germ line. Nat. Rev. Genet. 2016, 17, 585–600. [Google Scholar] [CrossRef] [PubMed]
- Saitou, M.; Payer, B.; Lange, U.C.; Erhardt, S.; Barton, S.C.; Surani, M.A. Specification of germ cell fate in mice. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Nakamura, T.; Okamoto, I.; Yabuta, Y.; Iwatani, C.; Tsuchiya, H.; Seita, Y.; Nakamura, S.; Shiraki, N.; Takakuwa, T.; et al. The Germ Cell Fate of Cynomolgus Monkeys Is Specified in the Nascent Amnion. Dev. Cell 2016, 39, 169–185. [Google Scholar] [CrossRef]
- Zhu, Q.; Sang, F.; Withey, S.; Tang, W.; Dietmann, S.; Klisch, D.; Ramos-Ibeas, P.; Zhang, H.; Requena, C.E.; Hajkova, P.; et al. Specification and epigenomic resetting of the pig germline exhibit conservation with the human lineage. Cell Rep. 2021, 34, 108735. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Castillo-Venzor, A.; Penfold, C.A.; Morgan, M.; Mizuno, N.; Tang, W.W.C.; Osada, Y.; Hirao, M.; Yoshida, F.; Sato, H.; et al. Tracing the emergence of primordial germ cells from bilaminar disc rabbit embryos and pluripotent stem cells. Cell Rep. 2021, 37, 109812. [Google Scholar] [CrossRef] [PubMed]
- Noce, T.; Okamoto-Ito, S.; Tsunekawa, N. Vasa homolog genes in mammalian germ cell development. Cell Struct. Funct. 2001, 26, 131–136. [Google Scholar] [CrossRef]
- Hayashi, K.; Shimamoto, S.; Nagamatsu, G. Environmental factors for establishment of the dormant state in oocytes. Dev. Growth Differ. 2020, 62, 150–157. [Google Scholar] [CrossRef]
- Bradley, A.; Evans, M.; Kaufman, M.H.; Robertson, E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature 1984, 309, 255–256. [Google Scholar] [CrossRef]
- Meyer, M.; de Angelis, M.H.; Wurst, W.; Kuhn, R. Gene targeting by homologous recombination in mouse zygotes mediated by zinc-finger nucleases. Proc. Natl. Acad. Sci. USA 2010, 107, 15022–15026. [Google Scholar] [CrossRef]
- Wang, H.; Yang, H.; Shivalila, C.S.; Dawlaty, M.M.; Cheng, A.W.; Zhang, F.; Jaenisch, R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 2013, 153, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, M.; Ochiya, T. Generation of genetically modified rats from embryonic stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 14223–14228. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Sparman, M.; Ramsey, C.; Ma, H.; Lee, H.S.; Penedo, M.C.; Mitalipov, S. Generation of chimeric rhesus monkeys. Cell 2012, 148, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; Grieshammer, U. Chimeric primates: Embryonic stem cells need not apply. Cell 2012, 148, 19–21. [Google Scholar] [CrossRef][Green Version]
- Izpisua Belmonte, J.C.; Callaway, E.M.; Caddick, S.J.; Churchland, P.; Feng, G.; Homanics, G.E.; Lee, K.F.; Leopold, D.A.; Miller, C.T.; Mitchell, J.F.; et al. Brains, genes, and primates. Neuron 2015, 86, 617–631. [Google Scholar] [CrossRef]
- Nichols, J.; Smith, A. Naive and primed pluripotent states. Cell Stem Cell 2009, 4, 487–492. [Google Scholar] [CrossRef]
- Hubner, K.; Fuhrmann, G.; Christenson, L.K.; Kehler, J.; Reinbold, R.; De La Fuente, R.; Wood, J.; Strauss, J.F., 3rd; Boiani, M.; Scholer, H.R. Derivation of oocytes from mouse embryonic stem cells. Science 2003, 300, 1251–1256. [Google Scholar] [CrossRef]
- Toyooka, Y.; Tsunekawa, N.; Akasu, R.; Noce, T. Embryonic stem cells can form germ cells in vitro. Proc. Natl. Acad. Sci. USA 2003, 100, 11457–11462. [Google Scholar] [CrossRef]
- Ohinata, Y.; Payer, B.; O’Carroll, D.; Ancelin, K.; Ono, Y.; Sano, M.; Barton, S.C.; Obukhanych, T.; Nussenzweig, M.; Tarakhovsky, A.; et al. Blimp1 is a critical determinant of the germ cell lineage in mice. Nature 2005, 436, 207–213. [Google Scholar] [CrossRef]
- Ohinata, Y.; Ohta, H.; Shigeta, M.; Yamanaka, K.; Wakayama, T.; Saitou, M. A signaling principle for the specification of the germ cell lineage in mice. Cell 2009, 137, 571–584. [Google Scholar] [CrossRef]
- Aramaki, S.; Hayashi, K.; Kurimoto, K.; Ohta, H.; Yabuta, Y.; Iwanari, H.; Mochizuki, Y.; Hamakubo, T.; Kato, Y.; Shirahige, K.; et al. A mesodermal factor, T, specifies mouse germ cell fate by directly activating germline determinants. Dev. Cell 2013, 27, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, T.; Isotani, A.; Matsumura, T.; Nakanishi, K.; Nonaka, S.; Shibata, S.; Ikawa, M.; Okano, H. Developmental analyses of mouse embryos and adults using a non-overlapping tracing system for all three germ layers. Development 2019, 146, dev174938. [Google Scholar] [CrossRef]
- Nakaki, F.; Hayashi, K.; Ohta, H.; Kurimoto, K.; Yabuta, Y.; Saitou, M. Induction of mouse germ-cell fate by transcription factors in vitro. Nature 2013, 501, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011, 146, 519–532. [Google Scholar] [CrossRef]
- Hayashi, K.; Ogushi, S.; Kurimoto, K.; Shimamoto, S.; Ohta, H.; Saitou, M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science 2012, 338, 971–975. [Google Scholar] [CrossRef]
- Hayashi, K.; Saitou, M. Generation of eggs from mouse embryonic stem cells and induced pluripotent stem cells. Nat. Protoc. 2013, 8, 1513–1524. [Google Scholar] [CrossRef]
- Hikabe, O.; Hamazaki, N.; Nagamatsu, G.; Obata, Y.; Hirao, Y.; Hamada, N.; Shimamoto, S.; Imamura, T.; Nakashima, K.; Saitou, M.; et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 2016, 539, 299–303. [Google Scholar] [CrossRef]
- Ishikura, Y.; Yabuta, Y.; Ohta, H.; Hayashi, K.; Nakamura, T.; Okamoto, I.; Yamamoto, T.; Kurimoto, K.; Shirane, K.; Sasaki, H.; et al. In Vitro Derivation and Propagation of Spermatogonial Stem Cell Activity from Mouse Pluripotent Stem Cells. Cell Rep. 2016, 17, 2789–2804. [Google Scholar] [CrossRef]
- Sato, T.; Katagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y.; Ogawa, T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011, 471, 504–507. [Google Scholar] [CrossRef]
- Ishikura, Y.; Ohta, H.; Sato, T.; Murase, Y.; Yabuta, Y.; Kojima, Y.; Yamashiro, C.; Nakamura, T.; Yamamoto, T.; Ogawa, T.; et al. In vitro reconstitution of the whole male germ-cell development from mouse pluripotent stem cells. Cell Stem Cell 2021, 28, 2167–2179. [Google Scholar] [CrossRef]
- Arey, L.B. Developmental Anatomy: A Textbook and Laboratory Manual of Embryology, 7th ed.; Saunders: Philadelphia, PA, USA, 1965; pp. 295–341. [Google Scholar]
- Yoshino, T.; Murai, H.; Saito, D. Hedgehog-BMP signalling establishes dorsoventral patterning in lateral plate mesoderm to trigger gonadogenesis in chicken embryos. Nat. Commun. 2016, 7, 12561. [Google Scholar] [CrossRef]
- Yoshino, T.; Saito, D. Epithelial-to-mesenchymal transition-based morphogenesis of dorsal mesentery and gonad. Semin. Cell Dev. Biol. 2019, 92, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Suzuki, T.; Nagamatsu, G.; Yabukami, H.; Ikegaya, M.; Kishima, M.; Kita, H.; Imamura, T.; Nakashima, K.; Nishinakamura, R.; et al. Generation of ovarian follicles from mouse pluripotent stem cells. Science 2021, 373, eabe0237. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, M.; Kobayashi, H.; Sanbo, M.; Mizuno, N.; Iwatsuki, K.; Takashima, T.; Yamauchi, K.; Yoshida, F.; Yamamoto, T.; Shinohara, T.; et al. Functional primordial germ cell-like cells from pluripotent stem cells in rats. Science 2022, 376, 176–179. [Google Scholar] [CrossRef] [PubMed]
- Hayama, T.; Yamaguchi, T.; Kato-Itoh, M.; Hamanaka, S.; Kawarai, M.; Sanbo, M.; Tamura, C.; Lee, Y.S.; Yanagida, A.; Murayama, H.; et al. Generation of mouse functional oocytes in rat by xeno-ectopic transplantation of primordial germ cells. Biol. Reprod. 2014, 91, 89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sosa, E.; Chen, D.; Rojas, E.J.; Hennebold, J.D.; Peters, K.A.; Wu, Z.; Lam, T.N.; Mitchell, J.M.; Sukhwani, M.; Tailor, R.C.; et al. Differentiation of primate primordial germ cell-like cells following transplantation into the adult gonadal niche. Nat. Commun. 2018, 9, 5339. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Yokobayashi, S.; Nakamura, T.; Okamoto, I.; Yabuta, Y.; Kurimoto, K.; Ohta, H.; Moritoki, Y.; Iwatani, C.; Tsuchiya, H.; et al. Robust In Vitro Induction of Human Germ Cell Fate from Pluripotent Stem Cells. Cell Stem Cell 2015, 17, 178–194. [Google Scholar] [CrossRef]
- Sakai, Y.; Nakamura, T.; Okamoto, I.; Gyobu-Motani, S.; Ohta, H.; Yabuta, Y.; Tsukiyama, T.; Iwatani, C.; Tsuchiya, H.; Ema, M.; et al. Induction of the germ cell fate from pluripotent stem cells in cynomolgus monkeysdagger. Biol. Reprod. 2020, 102, 620–638. [Google Scholar] [CrossRef]
- Yoshimatsu, S.; Nakajima, M.; Iguchi, A.; Sanosaka, T.; Sato, T.; Nakamura, M.; Nakajima, R.; Arai, E.; Ishikawa, M.; Imaizumi, K.; et al. Non-viral Induction of Transgene-free iPSCs from Somatic Fibroblasts of Multiple Mammalian Species. Stem Cell Rep. 2021, 16, 754–770. [Google Scholar] [CrossRef]
- Gao, X.; Nowak-Imialek, M.; Chen, X.; Chen, D.; Herrmann, D.; Ruan, D.; Chen, A.C.H.; Eckersley-Maslin, M.A.; Ahmad, S.; Lee, Y.L.; et al. Establishment of porcine and human expanded potential stem cells. Nat. Cell Biol. 2019, 21, 687–699. [Google Scholar] [CrossRef]
- Irie, N.; Weinberger, L.; Tang, W.W.; Kobayashi, T.; Viukov, S.; Manor, Y.S.; Dietmann, S.; Hanna, J.H.; Surani, M.A. SOX17 is a critical specifier of human primordial germ cell fate. Cell 2015, 160, 253–268. [Google Scholar] [CrossRef]
- Yamashiro, C.; Sasaki, K.; Yabuta, Y.; Kojima, Y.; Nakamura, T.; Okamoto, I.; Yokobayashi, S.; Murase, Y.; Ishikura, Y.; Shirane, K.; et al. Generation of human oogonia from induced pluripotent stem cells in vitro. Science 2018, 362, 356–360. [Google Scholar] [CrossRef]
- Yamashiro, C.; Sasaki, K.; Yokobayashi, S.; Kojima, Y.; Saitou, M. Generation of human oogonia from induced pluripotent stem cells in culture. Nat. Protoc. 2020, 15, 1560–1583. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Suzuki, S.; Seita, Y.; Ito, J.; Sakata, Y.; Aso, H.; Sato, K.; Hermann, B.P.; Sasaki, K. Reconstitution of prospermatogonial specification in vitro from human induced pluripotent stem cells. Nat. Commun. 2020, 11, 5656. [Google Scholar] [CrossRef]
- Kojima, Y.; Sasaki, K.; Yokobayashi, S.; Sakai, Y.; Nakamura, T.; Yabuta, Y.; Nakaki, F.; Nagaoka, S.; Woltjen, K.; Hotta, A.; et al. Evolutionarily Distinctive Transcriptional and Signaling Programs Drive Human Germ Cell Lineage Specification from Pluripotent Stem Cells. Cell Stem Cell 2017, 21, 517–532. [Google Scholar] [CrossRef]
- Sybirna, A.; Tang, W.W.C.; Pierson Smela, M.; Dietmann, S.; Gruhn, W.H.; Brosh, R.; Surani, M.A. A critical role of PRDM14 in human primordial germ cell fate revealed by inducible degrons. Nat. Commun. 2020, 11, 1282. [Google Scholar] [CrossRef]
- Perrett, R.M.; Turnpenny, L.; Eckert, J.J.; O’Shea, M.; Sonne, S.B.; Cameron, I.T.; Wilson, D.I.; Rajpert-De Meyts, E.; Hanley, N.A. The early human germ cell lineage does not express SOX2 during in vivo development or upon in vitro culture. Biol. Reprod. 2008, 78, 852–858. [Google Scholar] [CrossRef]
- Sasaki, K.; Oguchi, A.; Cheng, K.; Murakawa, Y.; Okamoto, I.; Ohta, H.; Yabuta, Y.; Iwatani, C.; Tsuchiya, H.; Yamamoto, T.; et al. The embryonic ontogeny of the gonadal somatic cells in mice and monkeys. Cell Rep. 2021, 35, 109075. [Google Scholar] [CrossRef]
- Shami, A.N.; Zheng, X.; Munyoki, S.K.; Ma, Q.; Manske, G.L.; Green, C.D.; Sukhwani, M.; Orwig, K.E.; Li, J.Z.; Hammoud, S.S. Single-Cell RNA Sequencing of Human, Macaque, and Mouse Testes Uncovers Conserved and Divergent Features of Mammalian Spermatogenesis. Dev. Cell 2020, 54, 529–547. [Google Scholar] [CrossRef]
- Bazer, F.W.; Spencer, T.E.; Johnson, G.A.; Burghardt, R.C.; Wu, G. Comparative aspects of implantation. Reproduction 2009, 138, 195–209. [Google Scholar] [CrossRef]
- Aguilera-Castrejon, A.; Oldak, B.; Shani, T.; Ghanem, N.; Itzkovich, C.; Slomovich, S.; Tarazi, S.; Bayerl, J.; Chugaeva, V.; Ayyash, M.; et al. Ex utero mouse embryogenesis from pre-gastrulation to late organogenesis. Nature 2021, 593, 119–124. [Google Scholar] [CrossRef]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.E.; Stephenson, E.; Polanski, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal-fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef]
- Bedzhov, I.; Leung, C.Y.; Bialecka, M.; Zernicka-Goetz, M. In vitro culture of mouse blastocysts beyond the implantation stages. Nat. Protoc. 2014, 9, 2732–2739. [Google Scholar] [CrossRef]
- Bedzhov, I.; Zernicka-Goetz, M. Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell 2014, 156, 1032–1044. [Google Scholar] [CrossRef]
- Ichikawa, T.; Zhang, H.T.; Panavaite, L.; Erzberger, A.; Fabreges, D.; Snajder, R.; Wolny, A.; Korotkevich, E.; Tsuchida-Straeten, N.; Hufnagel, L.; et al. An ex vivo system to study cellular dynamics underlying mouse peri-implantation development. Dev. Cell 2022, 57, 373–386.e9. [Google Scholar] [CrossRef]
- Ma, H.; Zhai, J.; Wan, H.; Jiang, X.; Wang, X.; Wang, L.; Xiang, Y.; He, X.; Zhao, Z.A.; Zhao, B.; et al. In vitro culture of cynomolgus monkey embryos beyond early gastrulation. Science 2019, 366, eaax7890. [Google Scholar] [CrossRef]
- Niu, Y.; Sun, N.; Li, C.; Lei, Y.; Huang, Z.; Wu, J.; Si, C.; Dai, X.; Liu, C.; Wei, J.; et al. Dissecting primate early post-implantation development using long-term in vitro embryo culture. Science 2019, 366, eaaw5754. [Google Scholar] [CrossRef]
- Ramos-Ibeas, P.; Gonzalez-Brusi, L.; Used, M.T.; Cocero, M.J.; Marigorta, P.; Alberio, R.; Bermejo-Alvarez, P. In vitro culture of ovine embryos up to early gastrulating stages. Development 2022, 149, dev.99743. [Google Scholar] [CrossRef]
- Garcia-Alonso, L.; Handfield, L.F.; Roberts, K.; Nikolakopoulou, K.; Fernando, R.C.; Gardner, L.; Woodhams, B.; Arutyunyan, A.; Polanski, K.; Hoo, R.; et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat. Genet. 2021, 53, 1698–1711. [Google Scholar] [CrossRef]
- Santana Gonzalez, L.; Rota, I.A.; Artibani, M.; Morotti, M.; Hu, Z.; Wietek, N.; Alsaadi, A.; Albukhari, A.; Sauka-Spengler, T.; Ahmed, A.A. Mechanistic Drivers of Mullerian Duct Development and Differentiation into the Oviduct. Front. Cell Dev. Biol. 2021, 9, 605301. [Google Scholar] [CrossRef]
- Guioli, S.; Sekido, R.; Lovell-Badge, R. The origin of the Mullerian duct in chick and mouse. Dev. Biol. 2007, 302, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, R. Development of the human Mullerian duct in the sexually undifferentiated stage. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 2003, 272, 514–519. [Google Scholar] [CrossRef]
- Nakajima, T.; Sato, T.; Iguchi, T.; Takasugi, N. Retinoic acid signaling determines the fate of the uterus from the mouse Mullerian duct. Reprod. Toxicol. 2019, 86, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Iguchi, T.; Sato, T. Retinoic acid signaling determines the fate of uterine stroma in the mouse Mullerian duct. Proc. Natl. Acad. Sci. USA 2016, 113, 14354–14359. [Google Scholar] [CrossRef]
- Du, H.; Taylor, H.S. The Role of Hox Genes in Female Reproductive Tract Development, Adult Function, and Fertility. Cold Spring Harb. Perspect. Med. 2015, 6, a023002. [Google Scholar] [CrossRef]
- Turco, M.Y.; Gardner, L.; Hughes, J.; Cindrova-Davies, T.; Gomez, M.J.; Farrell, L.; Hollinshead, M.; Marsh, S.G.E.; Brosens, J.J.; Critchley, H.O.; et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat. Cell Biol. 2017, 19, 568–577. [Google Scholar] [CrossRef]
- Syed, S.M.; Kumar, M.; Ghosh, A.; Tomasetig, F.; Ali, A.; Whan, R.M.; Alterman, D.; Tanwar, P.S. Endometrial Axin2(+) Cells Drive Epithelial Homeostasis, Regeneration, and Cancer following Oncogenic Transformation. Cell Stem Cell 2020, 26, 64–80.e13. [Google Scholar] [CrossRef]
- Boretto, M.; Cox, B.; Noben, M.; Hendriks, N.; Fassbender, A.; Roose, H.; Amant, F.; Timmerman, D.; Tomassetti, C.; Vanhie, A.; et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 2017, 144, 1775–1786. [Google Scholar] [CrossRef]
- Fitzgerald, H.C.; Dhakal, P.; Behura, S.K.; Schust, D.J.; Spencer, T.E. Self-renewing endometrial epithelial organoids of the human uterus. Proc. Natl. Acad. Sci. USA 2019, 116, 23132–23142. [Google Scholar] [CrossRef]
- Rawlings, T.M.; Makwana, K.; Taylor, D.M.; Mole, M.A.; Fishwick, K.J.; Tryfonos, M.; Odendaal, J.; Hawkes, A.; Zernicka-Goetz, M.; Hartshorne, G.M.; et al. Modelling the impact of decidual senescence on embryo implantation in human endometrial assembloids. eLife 2021, 10, e69603. [Google Scholar] [CrossRef]
- Miyazaki, K.; Dyson, M.T.; Coon, V.J.; Furukawa, Y.; Yilmaz, B.D.; Maruyama, T.; Bulun, S.E. Generation of Progesterone-Responsive Endometrial Stromal Fibroblasts from Human Induced Pluripotent Stem Cells: Role of the WNT/CTNNB1 Pathway. Stem Cell Rep. 2018, 11, 1136–1155. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhao, X.; Sun, H.; Li, X.; Lin, N.; Ding, L.; Dai, J.; Hu, Y. Regeneration of uterine horns in rats using collagen scaffolds loaded with human embryonic stem cell-derived endometrium-like cells. Tissue Eng. Part A 2015, 21, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Magalhaes, R.S.; Williams, J.K.; Yoo, K.W.; Yoo, J.J.; Atala, A. A tissue-engineered uterus supports live births in rabbits. Nat. Biotechnol. 2020, 38, 1280–1287. [Google Scholar] [CrossRef]
- Shaw, J.L.; Dey, S.K.; Critchley, H.O.; Horne, A.W. Current knowledge of the aetiology of human tubal ectopic pregnancy. Hum. Reprod. Update 2010, 16, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Brannstrom, M.; Belfort, M.A.; Ayoubi, J.M. Uterus transplantation worldwide: Clinical activities and outcomes. Curr. Opin. Organ Transpl. 2021, 26, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Kisu, I.; Kato, Y.; Obara, H.; Matsubara, K.; Matoba, Y.; Banno, K.; Aoki, D. Emerging problems in uterus transplantation. BJOG 2018, 125, 1352–1356. [Google Scholar] [CrossRef]
- Kisu, I.; Banno, K.; Aoki, D. Considerations in performing hysterectomy secondary to uterus transplantation. Acta Obstet. Gynecol. Scand. 2022. [Google Scholar] [CrossRef]
- Lovell-Badge, R.; Anthony, E.; Barker, R.A.; Bubela, T.; Brivanlou, A.H.; Carpenter, M.; Charo, R.A.; Clark, A.; Clayton, E.; Cong, Y.; et al. ISSCR Guidelines for Stem Cell Research and Clinical Translation: The 2021 update. Stem Cell Rep. 2021, 16, 1398–1408. [Google Scholar] [CrossRef]
- Yoshimatsu, S.; Okahara, J.; Sone, T.; Takeda, Y.; Nakamura, M.; Sasaki, E.; Kishi, N.; Shiozawa, S.; Okano, H. Robust and efficient knock-in in embryonic stem cells and early-stage embryos of the common marmoset using the CRISPR-Cas9 system. Sci. Rep. 2019, 9, 1528. [Google Scholar] [CrossRef]
- Matlashov, M.E.; Shcherbakova, D.M.; Alvelid, J.; Baloban, M.; Pennacchietti, F.; Shemetov, A.A.; Testa, I.; Verkhusha, V.V. A set of monomeric near-infrared fluorescent proteins for multicolor imaging across scales. Nat. Commun. 2020, 11, 239. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimatsu, S.; Kisu, I.; Qian, E.; Noce, T. A New Horizon in Reproductive Research with Pluripotent Stem Cells: Successful In Vitro Gametogenesis in Rodents, Its Application to Large Animals, and Future In Vitro Reconstitution of Reproductive Organs Such as “Uteroid” and “Oviductoid”. Biology 2022, 11, 987. https://doi.org/10.3390/biology11070987
Yoshimatsu S, Kisu I, Qian E, Noce T. A New Horizon in Reproductive Research with Pluripotent Stem Cells: Successful In Vitro Gametogenesis in Rodents, Its Application to Large Animals, and Future In Vitro Reconstitution of Reproductive Organs Such as “Uteroid” and “Oviductoid”. Biology. 2022; 11(7):987. https://doi.org/10.3390/biology11070987
Chicago/Turabian StyleYoshimatsu, Sho, Iori Kisu, Emi Qian, and Toshiaki Noce. 2022. "A New Horizon in Reproductive Research with Pluripotent Stem Cells: Successful In Vitro Gametogenesis in Rodents, Its Application to Large Animals, and Future In Vitro Reconstitution of Reproductive Organs Such as “Uteroid” and “Oviductoid”" Biology 11, no. 7: 987. https://doi.org/10.3390/biology11070987
APA StyleYoshimatsu, S., Kisu, I., Qian, E., & Noce, T. (2022). A New Horizon in Reproductive Research with Pluripotent Stem Cells: Successful In Vitro Gametogenesis in Rodents, Its Application to Large Animals, and Future In Vitro Reconstitution of Reproductive Organs Such as “Uteroid” and “Oviductoid”. Biology, 11(7), 987. https://doi.org/10.3390/biology11070987






