Simple Summary
This study used a nutrition-based approach to examine the effects of foods supplemented with fish oil and a polyphenol blend (citrus pulp, carrot, and spinach) with or without added tomato pomace on anxiety-related biomarkers in dogs. First, all dogs consumed the same initial food, then either the control or test (with tomato pomace) foods, then the washout food, then switched over to the test or control foods, each for 30-day periods. Many more changes in plasma and fecal metabolites were observed when comparing the washout food with the control or test foods than when the control and test foods were compared. Plasma levels of several metabolites that were previously associated with anxiety disorders, including 4-ethylphenyl sulfate, were decreased with the control or test foods compared with the washout food. In addition, bacterial genera that are decreased in the feces of those with anxiety-like disorders were increased following the consumption of the control or test foods. Overall, these data indicate that foods supplemented with omega-3 fatty acids and selected fiber and polyphenol sources lead to beneficial changes in anxiety-related metabolites and gut bacteria.
Abstract
A nutrition-based approach was utilized to examine the effects of fish oil and a polyphenol blend (with or without tomato pomace) on the fecal microbiota and plasma/fecal metabolomes. Forty dogs, aged 5–14 years, were fed a washout food, then randomized to consume a control (fish oil and polyphenol blend without tomato pomace) or test (fish oil and polyphenol blend with tomato pomace) food, then the washout food, and crossed over to consume the test or control food; each for 30 days. Several metabolites differed when comparing consumption of the washout with either the control or test foods, but few changed significantly between the test and control foods. Plasma levels of 4-ethylphenyl sulfate (4-EPS), a metabolite associated with anxiety disorders, demonstrated the largest decrease between the washout food and the control/test foods. Plasma 4-EPS levels were also significantly lower after dogs ate the test food compared with the control food. Other plasma metabolites linked with anxiety disorders were decreased following consumption of the control/test foods. Significant increases in Blautia, Parabacteroides, and Odoribacter in the fecal microbiota correlated with decreases in 4-EPS when dogs ate the control/test foods. These data indicate that foods supplemented with polyphenols and omega-3 fatty acids can modulate the gut microbiota to improve the profile of anxiety-linked metabolites.
1. Introduction
The gut-brain axis involves a communication loop among the brain, gut, and gut microbiota [1,2]. Gut microbiota can metabolize food from the host into a variety of metabolites that then may enter host circulation and influence the central nervous system, while the brain may affect the gut microbiota by influencing gut motility, secretion, and permeability [1,2].
Anxiety disorders are quite prevalent in human beings as well as in dogs [3,4], with about 26–29% of dogs exhibiting fearfulness, an anxiety-related disorder that increases with age [5,6]. In addition, some dogs may exhibit behavioral phenotypes (canine dysfunctional behavior) similar to autism spectrum disorders (ASD) in human beings, including repetitive motions such as tail chasing as well as trance-like behavior [7,8]. Some work has been done to characterize the microbiome in people and dogs with anxiety or ASD [9,10,11,12]. Several studies have shown that transplantation of fecal microbiota from patients with a variety of psychiatric conditions into germ-free mice appears to result in the development of these conditions in the mice as measured by behavioral and physiological parameters. A systematic review and meta-analysis found that several psychiatric disorders, including anxiety, are characterized by an enrichment of pro-inflammatory gut microbiota and a reduction of anti-inflammatory genera [9]. Further, microbial metabolic pathways appear to be altered in several psychiatric and neurological disorders [13].
In addition to defining the gut microbiota in anxiety-related disorders, several studies have attempted to identify biomarkers to differentiate between subjects with anxiety and healthy controls in mice, dogs, and human beings [14]. A variety of biomarkers have been identified in anxiety disorders, including neurotransmitters, neuropeptides, neurotrophic factors, and immunological factors, but none were specific or sufficient for diagnosis [15]. One study proposed a panel of four urinary biomarkers that could distinguish patients with depression or anxiety from healthy controls, but this was based on a relatively small sample size of 16 people per group [16]. In dogs that exhibit fearfulness, greater plasma levels of glutamine and gamma-glutamylglutamine were observed compared with non-fearful dogs [3]. More recently, administration of 4-ethylphenyl sulfate (4-EPS), a metabolite produced by the gut microbiota [17], to wild-type mice induced anxiety-like behavior [18]. 4-EPS was also shown to enter the brain and impair oligodendrocyte maturation in mice and to decrease interactions between oligodendrocytes and neurons in ex vivo brain cultures, indicating a direct role in anxiety [18]. Notably, 4-EPS was 46-fold higher in serum from a mouse model of ASD compared with wild-type mice [17]. Levels of 4-EPS were restored following administration of Bacillus fragilis, establishing a gut-brain connection [17]. 4-EPS was also identified as one of several plasma metabolites that could most distinguish between children with ASD and those that are typically developing [19].
Part of the ability of gut microbiota to produce certain metabolites depends on the food source from the host. The influence of food on the gut microbiota and behavior has been reported in mouse studies [20,21]. However, some of the findings in rodent models have not translated well to human beings [22], though preliminary work indicates that there are links among the diet, gut microbiota, and depression/anxiety disorders [23] and mood [24] in people. Furthermore, the gut microbiota are similar between human beings and dogs [25], so studies in dogs are likely to be relevant to human beings.
Dietary polyphenols are known to confer many beneficial health effects, including amelioration of inflammation, oxidative stress, and neurodegeneration [26,27]. In particular, citrus fruits [28,29], spinach [30,31], carrots [32], and tomatoes [33,34] serve as excellent sources of polyphenols and prebiotics. In addition, tomato juice was shown to reduce anxiolytic activity in mice [35], and tomato consumption confers numerous other beneficial health effects [36]. Thus, it was hypothesized that a nutrition-based approach including polyphenols could affect the gut-brain axis via modulation of the canine gut microbiome and/or by affecting the levels of anxiety-related metabolites. While fish oil and its omega-3 fatty acids are known to confer health benefits, there are conflicting reports on their effects on anxiety [37,38]. This study aimed to evaluate effects of polyphenols and fish oil on plasma and fecal levels of metabolites and on the fecal microbiota in healthy older dogs, and to examine if any were related to anxiety.
2. Materials and Methods
2.1. Study Foods
Three food formulations were used in this study: a washout, control, and test food, which were all manufactured at the Hill’s experimental food laboratory and met the maintenance nutrition requirements of the Association of American Feed Control Officials (2021). The washout food (to allow for standardization of all dogs on the same food) contained >10-fold more corn compared with the control and test foods. It also contained chicken meal, rice, pork, corn gluten, soybean oil, carnitine, and about half the amount of flax seed in the control or test foods. The control and test foods had rice, pea flour, chicken, barley, oat groats, egg, fish oil, lipoic acid, and a polyphenol blend of citrus pulp, carrot, and spinach. The control and test foods both contained fish oil (0.5%) and were predicted to contain about 106 mg/g more polyphenols than the washout food. The test food also contained tomato pomace in the blend as a source of polyphenols, with lycopene estimated at 0.054 ppm.
Proximate analyses were performed using Association of Official Analytical Chemists methods as previously described [39]. Digestibility analyses were carried out as previously described [40,41], using the following equations:
True protein digestibility = [(protein intake − (fecal protein − endogenous metabolic protein))/protein intake]
Apparent protein digestibility = [(protein intake − fecal protein)/protein intake]
2.2. Animals and Experimental Design
Forty older healthy beagle dogs, all spayed or neutered, between 5–14 years of age, were included in the study. Dogs with compromised health conditions such as kidney disease, inflammatory bowel disease, colitis, or food allergy, and dogs that received antibiotics within the month before study start were excluded from this study. Dogs were to be removed from the study if it was to their benefit in the opinion of the colony veterinarian. All dogs were owned by Hill’s Pet Nutrition and were housed in pairs at the Pet Nutrition Center, where they had regular access to natural light, socialization with other dogs, and daily exercise.
The study protocol was approved by the Hill’s Institutional Animal Care and Use Committee (CP798.0.0.0-A-C-D-ADH-MULTI-120-LFS) and Animal Welfare Committee. Dogs were fed the washout food for the initial 30 days of the study and were then randomized to consume the control or test foods for the next 30 days (Figure 1). Dogs were then fed the washout food for 30 days prior to a cross-over to consume the control or test food for 30 days. Feeding was administered once daily from an electronic feeder, with the offered amount calculated for body weight maintenance.
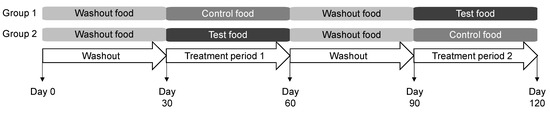
Figure 1.
Study design in which dogs consumed the washout, control, and test foods. Each group contained 20 dogs.
2.3. Sample Collection, Scoring, Processing, and Metabolite Analysis
Blood and fecal samples were collected at the end of each 30-day feeding period. Stools were collected within 30 min of defecation and scored from 1 to 5 as previously described, where a score of 1 indicates >75% liquid and 5 is >80% firm [41]. Feces were then homogenized in a Thinky Mixer (Thinky USA, Inc., Laguna Hills, CA, USA), aliquoted into vials, and frozen at −80 °C until further processed.
Blood chemistry parameters were analyzed using enzymatic colorimetric methods as in a prior report [42]. Global plasma and fecal metabolite analyses were performed by Metabolon (Morrisville, NC, USA) as previously described [43,44]. Briefly, gas chromatography (for hydrophobic molecules) and liquid chromatography mass spectrometer (for hydrophilic molecules) platforms were utilized to identify and provide relative quantification of metabolites.
2.4. Microbiota and Bioinfomatics Processing
Total DNA was extracted from thawed fecal samples using the Qiagen MagAttract Power Microbiome DNA/RNA EP DNA isolation kit (Qiagen, Germantown, MD, USA) with the Eppendorf epMotion 5075 TMX platform (Eppendorf AG, Hamburg, Germany). As previously described [45], PCR amplification spanned the V3-V4 hypervariable regions of the 16S rRNA gene, amplicon sequencing was performed using the Illumina (San Diego, CA, USA) library preparation protocol (15044223 Rev. A), sequences were de-multiplexed to obtain FASTQ Files, and bacterial taxa were classified using the GreenGenes reference taxonomy at the genus level. Copy numbers of the 16S genes in microbial taxa were corrected and numerical values were centered log ratio (CLR) transformed for statistical analysis.
2.5. Statistical Analysis
Data analyses utilized JMP Pro software (JMP, Cary, NC, USA). Relative levels of metabolomics data were log-transformed prior to statistical analysis, and mean units for metabolites were scaled to a median of 1 in order to compare fold changes between samples. Treatment effect during the treatment period, using animal ID as random designate, was compared using linear mixed modeling. Metabolites that were significantly different between foods after 30 days are shown.
Fecal microbiome data were processed as previously reported, with CLR-transformed data compared via linear mixed modeling with animal ID as a random variable [39]. Pearson’s correlation coefficient (r) regression analyses were used for correlations between 4-EPS, metabolites, and operational taxonomic units (OTUs). Statistical significance was set at p ≤ 0.05.
3. Results
3.1. Food, Study Design, and Animals
The control and test foods were similar as seen via proximate analyses, with the exception of slightly higher moisture levels in the test food (Table 1). The washout food contained higher levels of crude fiber and neutral detergent fiber compared with the control and test foods, with lower levels of omega-3 fatty acids (constituents of fish oil).

Table 1.
Proximate analysis of foods used in this study.
The apparent dry matter digestibility was similar for each food (Table 1). True protein digestibilities were also similar.
Fecal analysis parameters of pH and ash were not significantly different among the study foods (Table 2). Moisture was significantly lower and ammonia significantly higher in dogs after consuming the washout food compared with either the control or test foods (Table 2).

Table 2.
Food intake and fecal analyses following consumption of the indicated foods.
Forty healthy beagle dogs (24 spayed females, 16 neutered males) were included in the study (Table S1). The mean ± standard deviation age at baseline was 10.2 ± 2.4 years (range 5–13.7) and mean body weight at baseline was 10.3 ± 2.0 kg (range 5.7–16.1). On average, dogs consumed 163.7, 170.7, and 166.1 g of the washout, control, and test foods per day (Table 2). There were no differences in body weight following consumption of the three foods. Stool scores were not significantly different after consumption of each food (Table 2). There were no protocol deviations or adverse events encountered during the study period.
3.2. Effect of the Study Foods on Blood Chemistry and Plasma Metabolites
Blood chemistry parameters were similar with no significant differences between the control and test foods (Table 3). However, albumin, BUN, cholesterol, and calcium were significantly lower following consumption of the washout food compared with the control or test foods. In addition, chloride was significantly lower after dogs consumed the test food compared with the washout food, and potassium was significantly lower after dogs consumed the control and test foods compared with the washout food. Of note, all values were within normal colony ranges despite the significant differences.

Table 3.
Blood chemistry parameters following consumption of the control and test foods.
In plasma, the levels of a number of metabolites differed when comparing the washout food with either the control or test foods (Table 4). In contrast, few metabolites changed significantly when comparing the test and control foods. 4-EPS, several uremic toxins, and other metabolites implicated in anxiety disorders, were significantly lower with either the control food and/or test food compared with the washout food. 4-EPS and sphingomyelin (d18:2/23:1) were both significantly decreased with the test food compared with the control food.

Table 4.
Plasma metabolites of interest.
Examination of the levels of serum 4-EPS (Figure 2) show that consumption of the washout food in both time periods led to increases, while both the control and test foods resulted in decreases, indicating that there was no time effect.
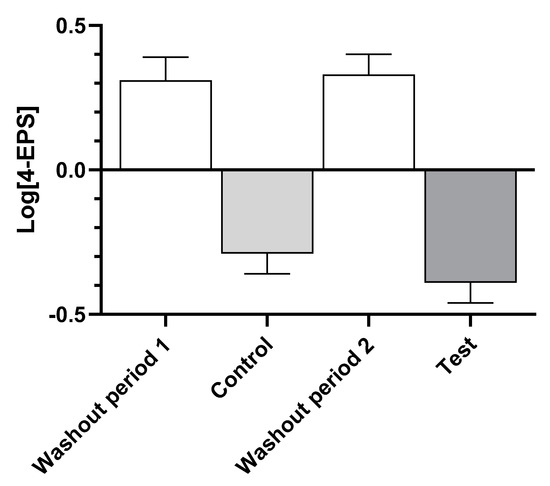
Figure 2.
Log-transformed mean ± standard error of levels of serum 4-ethylphenyl sulfate (4-EPS) after dogs consumed the washout, control, and test foods.
3.3. Effect of the Study Foods on Fecal Metabolites
Similar to the results with plasma, the levels of few fecal metabolites were significantly different between the control and test foods (Table 5). Levels of azelate and choline were significantly greater after dogs consumed the test food compared with the control food. Of note, 2-aminobutyrate, 4-EPS, citrate, gamma-glutamylglutamate, and sphingomyelin (d18:2/23:1) were not detected in feces.

Table 5.
Fecal metabolites of interest.
3.4. Effect of the Study Foods on Fecal Microbiota
As with the plasma and fecal metabolites, many more significant differences in OTUs were observed in feces from dogs following consumption of the washout food compared to the control or test foods (Table 6). OTUs from the genera Blautia, Parabacteroides, and Odoribacter were among those most greatly increased following consumption of the control or test foods compared with the washout food.

Table 6.
Center-log ratio means ± standard error of operational taxonomic units (OTUs) that were significantly different between groups.
Significantly higher levels of OTUs of Eubacterium biforme, the genus Acholeplasma, and the family Coxiellaceae were seen in fecal samples from dogs fed the test food compared with the control food. Conversely, significantly higher levels of OTUs in Mitsuaria chitosanitabida, the genera Flexispira and Anaerovorax, the family Gemellaceae, and the class Gammaproteobacteria were seen in fecal samples from dogs fed the control food compared with the test food.
3.5. Correlations of 4-Ethylphenyl Sulfate with Plasma and Fecal Metabolites and OTUs
Because 4-EPS is the metabolite that showed the greatest differences in plasma levels between dogs fed the control and washout foods as well as between the test and washout foods, and it was recently shown to induce anxiety-like behavior in mice [18], correlation analyses were carried out with metabolites and OTUs. Plasma levels of 4-EPS positively correlated with levels of several known renal metabolites in plasma such as indolelactate, kynurenine, N-acetylkynurenine, and phenol sulfate (Table 7). Levels of 4-EPS in plasma showed negative correlations with levels of several metabolites known to decrease in anxiety-like disorders or depression, such as 2-aminobutyrate, DHA, hippurate, and sphingomyelin (d18:1/24:1, d18:2/24:0). In contrast to the plasma metabolites, levels of plasma 4-EPS negatively correlated with levels of several renal metabolites in feces.

Table 7.
Significant correlations between levels of plasma 4-ethylphenyl sulfate and levels of plasma and fecal metabolites of interest.
Several OTUs were significantly correlated with levels of plasma 4-EPS (Table 8). Odoribacter, Blautia, and Parabacteroides were all negatively correlated with plasma 4-EPS levels.

Table 8.
Correlations between plasma levels of 4-ethylphenyl sulfate and OTUs.
4. Discussion
This study aimed to investigate changes in the plasma and fecal metabolomes and fecal microbiota in senior dogs following consumption of a control food without tomato pomace or a test food containing tomato pomace. However, a limited effect of addition of the tomato pomace was observed compared with the control food, with significant changes only in the metabolites 4-EPS and sphingomyelin (d18:2/23:1) in plasma, and azelate and choline in feces. In contrast, many changes in microbiota and metabolites were observed when comparing metabolites following consumption of the washout food compared with the control or test foods. These effects are likely due to the presence of polyphenols in the fiber blend and increased omega-3 fatty acids from the fish oil in the control and test foods compared with the washout food [46].
Levels of 4-EPS demonstrated the largest decrease in plasma and feces following consumption of the control or test foods. A metabolite of the gut microbiome [17], 4-EPS is associated with anxiety disorders and ASD. As mentioned above, 4-EPS induced anxiety-like behavior in mice and impaired oligodendrocyte-neuron interactions [18] was at much higher levels in serum in a mouse model of ASD than wild-type mice [17], and was one of the plasma metabolites that could reliably distinguish between children with ASD and those that are typically developing [19]. Interestingly, ASD is often accompanied by gastrointestinal comorbidities including constipation [47].
A number of metabolites that changed in response to the foods tested in this study are also implicated in anxiety disorders. In general, metabolites related to oxidative stress, glutamine metabolism, and neurotransmission pathways appear to be involved in anxiety disorders [14]. High anxiety in mice is associated with high levels of intracellular reactive oxygen species, and oxidative stress has been shown to lead to anxious behavior in mice [4,48]. 2-aminobutyrate, which was significantly higher in plasma following consumption of the control or test foods in this study, appears to protect against oxidative stress by increasing cellular levels of reduced glutathione [49]. In addition to oxidative stress, 2-aminobutyrate plays a role in decreasing reductive stress, as the attack of a hydrogen atom on a sulfur atom in methionine leads to formation of 2-aminobutyrate [50]. Thus, the increased plasma levels of 2-aminobutyrate observed here following consumption of the control or test foods may indicate an increased ability to withstand oxidative and reductive stress. From a clinical standpoint, levels of 2-aminobutyrate were significantly lower in plasma from older adults in Japan who exhibited depressive symptoms compared with those in the non-depressive group [51].
The lipid components of neural membranes can influence signaling and have been implicated in psychiatric disorders [52,53,54]. Plasma levels of the polyunsaturated fatty acid DHA were significantly higher following consumption of the control or test foods in this study. This result is expected given that the control and test foods contained fish oil and showed increased level of omega-3 fatty acids compared with the washout food. Previous work showed that dietary supplementation with DHA in mice reduced anxiety-related behaviors, though only in males [52]. In human beings, those with anxiety or depressive disorders exhibit lower levels of the omega-3 polyunsaturated fatty acids DHA and eicosapentaenoic acid in erythrocyte membranes and plasma [54]. In addition, two forms of sphingomyelin (d18:2/23:1 and d18:1/24:1, d18:2/24:0) were at higher levels following consumption of the control or test foods compared with the washout food in the present study. A Dutch family-based lipidomics study of 742 people found inverse correlations between the ratio of sphingomyelin 23:1/sphingomyelin 16:0 and depression/anxiety symptoms, indicating a role for sphingomyelins in these disorders [53].
Another metabolite that exhibited increased levels in plasma following consumption of the control or test foods in this study was 1-methylnicotinamide, which is known to exert anti-inflammatory, anti-thrombotic, and vasoprotective effects [55]. Both 1-methylnicotinamide and serotonin are downstream metabolites of tryptophan, and increases in nicotinamide have been shown to increase plasma levels of serotonin [56]. Indeed, plasma levels of serotonin, which can inhibit the “fight or flight” response [15], were increased after consumption of the control food compared with the washout food in this study. While about 95% of serotonin is produced by the host via enterochromaffin cells in the gut, tryptophan metabolism by the gut microbiota regulates the availability of serotonin precursors [13]. Serotonin is also thought to play a role in ASD since a gain of function mutation in the gene encoding the serotonin reuptake transporter is found in some individuals with ASD [47].
Another tryptophan metabolite, kynurenine, was found at significantly lower levels in plasma following consumption of the test food (and numerically but not significantly lower with the control food) compared with the washout food. Plasma kynurenine levels were found to positively correlate with anxiety scores in people [57]. In addition, kynurenine, its metabolites, and dysregulated signaling were increased in the brain and gut in a mouse model of chronic restraint stress in which mice display depression and anxiety-like behaviors [58].
Indolelactate, also a product of bacterial-mediated tryptophan metabolism [59], was increased in plasma following consumption of the control or test foods in this study. Along with 4-EPS (at lower plasma levels in typically developing children), indolelactate (at higher plasma levels in typically developing children) was the other top metabolite that allowed for discrimination between children with autism and typically developing children [19].
The changes in the levels of tryptophan metabolites in response to the foods in this study is of interest in relation to prior work on the supplementation of dog food with tryptophan. In dogs with dominance or territorial aggression, dietary supplementation with tryptophan for one week reduced these aggressive behaviors [60]. Sled dogs fed supplemental tryptophan displayed a decrease in agonistic behaviors, including teeth baring, snapping, biting, and nosing [61]. In contrast, tryptophan supplementation did not result in changes in the behavior of dogs in response to the approach of familiar or unfamiliar individuals [62].
Lactate and citrate, both of which were lower in plasma following consumption of the control or test foods, were some of the urinary metabolites that were higher in people with depression and anxiety disorders that enabled discrimination from healthy controls [16]. Further, lactate derived from the gut microbiome promoted anxiety-like behaviors in a mouse model [63]. Thus, decreased levels of these metabolites may indicate benefits in anxiety.
In addition to its well-known role as a building block of proteins, glutamine is a precursor to the neurotransmitters glutamate and GABA [64] and can enhance intestinal barrier function, of key importance in the gut-brain axis [65]. Glutamate is also a precursor to the antioxidant glutathione [66]. Impairment of the glutamine/glutamate cycle is implicated in the pathology of several conditions involving the brain [64]. In this study, levels of glutamine and glutamate were both significantly lower in plasma and feces following consumption of the control or test foods compared with the washout food. Several prior studies have demonstrated that glutamine and glutamate are implicated in anxiety-like disorders and in ASD. Increased plasma glutamine, along with gamma-glutamyl glutamine, was identified as a metabolic feature in dogs that exhibited fearfulness, an anxiety-related behavioral problem [3]. That study also found that SDMA was associated with fearfulness in one of the studied breeds, Great Danes, but not in the other (German shepherds). In addition to 4-EPS, levels of glutamine were higher in serum from a mouse model of ASD compared with untreated mice [17]. Glutamine was correlated with neuroticism and trait anxiety, with increased glutamine concentrations in people with anxiety disorders [67]. Both glutamate and glutamine levels were significantly higher in plasma from children with ASD compared with healthy controls [68]. Similarly, serum glutamate levels were significantly higher in adults with autism compared with healthy controls [69].
Intriguingly, 4-EPS is a derivative of tyrosine with a very similar structure to that of the uremic toxin p-cresol sulfate [17]. 4-EPS was identified as a uremic solute in a mouse model of chronic renal failure [70] and was about 17-fold higher in plasma from patients on hemodialysis compared with healthy controls [71]. In addition to 4-EPS, several of the other metabolites whose levels showed differential effects with the control/test foods and washout food are known to be uremic toxins. Carboxyethyl-GABA, choline, dimethylarginine (ADMA + SDMA), kynurenine, and phenol sulfate were all significantly lower in plasma following consumption of the control or test foods compared with the washout food, and all were found to be higher in plasma from patients on hemodialysis [71] and/or patients with end-stage renal disease [72]. Serum SDMA can be used as a biomarker for early kidney dysfunction in dogs [73]. Levels of several of these metabolites (carboxyethyl-GABA, dimethylarginine [ADMA + SDMA], and kynurenine) are higher in feces following consumption of the control or test foods compared with the washout food, indicating that this may be a mode for their elimination.
In addition to characterizing the plasma and fecal metabolomes in response to the study foods, the fecal microbiota was also evaluated in this study. While fecal bacterial profiles did not match those of greater abundance in human beings with end-stage renal disease [74], anxiety/depression [75,76], or ASD [77], this may be due to the absence of these conditions in the dogs in this study. Other studies have observed greater changes in microbiota in disease states than in nutritional interventions, some with notable metabolomic changes but little to no change in the microbiota [78,79].
The three genera that increased the most after dogs were fed the control or test food compared to the washout food were Blautia, Parabacteroides, and Odoribacter. The abundances of all of these negatively correlated with 4-EPS. Significantly lower abundances of Blautia were found in the gut microbiota of people with high trait anxiety compared with healthy controls [10]. Because Blautia were significantly increased when dogs consumed the control/test foods in this study, they may shift the gut microbiome toward one associated with a less anxious state. In addition, Blautia was of lower relative abundance in the gut microbiota of children with ASD compared with controls in a systematic review [80] and meta-analysis [81]. There is some conflicting evidence on Parabacteroides in the gut microbiota of children with ASD, as one systematic review and meta-analysis found lower percentages of Parabacteroides (as well as other genera) compared with controls [82], while another found higher relative abundances [81], even though the analyses utilized some of the same studies. Nevertheless, significantly reduced relative abundances of Parabacteroides were seen in a mouse model of chronic restraint stress, fecal abundance of Parabacteroides positively correlated with serotonin levels and negatively correlated with kynurenine levels in the prefrontal cortex, and administration of Parabacteroides distasonis partially mitigated the depression and anxiety-like behaviors in these mice [58]. Regarding Odoribacter, a lower relative abundance was observed in the gut microbiota of people with obsessive compulsive disorder compared with healthy controls [83], and a potential anti-inflammatory effect of Odoribacter splanchnicus on the gut epithelium was recently identified [84].
In contrast to the above genera, an OTU in the family Pseudomonadaceae was significantly reduced after dogs consumed the test and the control foods and positively correlated with 4-EPS levels. The abundance of bacteria in this family were reported to increase with aging in the bladder and seminal microbiota of human beings [85]. Future research should investigate the association of these bacteria with behavioral disorders like anxiety.
In addition to their differential abundances in individuals with anxiety/ASD compared with healthy controls, there may be a role for these genera in short-chain fatty acid (SCFA)-mediated effects in these disorders. Digestion of tomato pomace in an in vitro model with gut bacteria led to an increase in the concentrations of SCFAs [34]. Both Blautia and Odoribacter produce SCFAs via fermentation of dietary fiber [84,86], while the abundance of Parabacteroides was correlated with SCFA concentrations in the quail intestinal tract [87]. Future work could examine the role of SCFAs and these gut bacteria in anxiety and other disorders such as ASD. Indeed, SCFAs are decreased in patients with major depressive disorder [86], have been shown to ameliorate stress-induced alterations in the brain-gut axis in a mouse model [88], and along with butyrate-producing bacteria, are at lower levels in feces of children with ASD [89].
Although the greatest number of differences in metabolites were observed when the control or test foods were compared with the washout food, there were a few key differences between the control and test foods. Notably, the only ingredient that differed between the control and test foods was tomato pomace in the test food. While 4-EPS was lower in plasma following consumption of either the control or test foods compared with the washout food, its levels were lowest following the test food. Azelate was higher in feces following consumption of the test food compared with the control food. It has anti-inflammatory properties and as such, its conjugate acid form, azelaic acid, is a first-line treatment for acne vulgaris [90]. Azelaic acid was also found to be one of four urinary metabolites that could distinguish between patients with depression or anxiety disorders from healthy controls [16]. Choline has many essential functions, from serving as a component of cell membranes as well as a precursor to the neurotransmitter acetylcholine and to the universal methyl donor S-adenosylmethionine. In this study, fecal choline levels in feces were higher following consumption of the test food compared with the control food. Dietary supplementation with choline has been shown to decrease anxiety-like behavior [91] and autism-like repetitive behavior and anxiety [92] in mouse models.
Some of the microbiota also differed significantly following consumption of the control and test foods. Eubacterium biforme (also known as Holdemanella biformis), which was of higher abundance following the test food, produces the long-chain fatty acid 3-hydroxyoctadecaenoic acid that has anti-inflammatory effects in colitis [93]. Flexispira and an OTU of the class Gammaproteobacteria were both of lower abundance following consumption of the test food compared with the control food, and both positively correlated with 4-EPS levels. In contrast, Coxiellaceae was at higher abundance after consumption of the test food and negatively correlated with 4-EPS levels.
A limitation of this study is that only healthy dogs were included. Future work could focus on studying food-based interventions in dogs exhibiting anxiety-like behaviors. Further, although most of the markers were previously reported to be associated with anxiety-like behaviors and senior dogs are more prone to anxiety [6], changes in the behaviors of the dogs were not assessed in relation to the changes in metabolites and microbiota.
In summary, this study examined a nutrition-based approach in which the control and test foods were supplemented with polyphenols and fish oil to observe changes in the levels of plasma and fecal metabolites and the gut microbiota in senior dogs. A number of differences between the control/test foods and the washout food were observed in metabolites related to anxiety-like disorders, ASD, and uremia. Overall, consumption of polyphenols and omega-3 fatty acids appeared to shift metabolites toward a profile beneficial for the gut-brain axis and kidney health.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/biology11070976/s1, Table S1: Animal signalment.
Author Contributions
Conceptualization, E.E., J.A.B. and D.E.J.; methodology, E.E. and D.E.J.; formal analysis, E.E.; investigation, E.E.; resources, E.E. and D.E.J.; data curation, E.E.; writing—review and editing, E.E., J.A.B. and D.E.J.; visualization, E.E.; supervision, E.E. and D.E.J.; project administration, E.E. and D.E.J.; funding acquisition, E.E. and D.E.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hill’s Pet Nutrition, Inc.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Hill’s Institutional Animal Care and Use Committee and Animal Welfare Committee and was approved by both on August 16, 2017 (CP798.0.0.0-A-C-D-ADH-MULTI-120-LFS).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Jennifer L. Giel assisted with the writing and development of the manuscript.
Conflicts of Interest
Although the funder (Hill’s Pet Nutrition, Inc.) provided support in the form of salaries for the authors, the funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The research was performed at the Pet Nutrition Center, Topeka, KS, where the authors currently work (E.E. and J.A.B.) or formerly worked (D.E.J.).
References
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The brain-gut-microbiome axis. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Puurunen, J.; Tiira, K.; Vapalahti, K.; Lehtonen, M.; Hanhineva, K.; Lohi, H. Fearful dogs have increased plasma glutamine and gamma-glutamyl glutamine. Sci. Rep. 2018, 8, 15976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouayed, J.; Rammal, H.; Soulimani, R. Oxidative stress and anxiety: Relationship and cellular pathways. Oxid. Med. Cell Longev. 2009, 2, 63–67. [Google Scholar] [CrossRef]
- Tiira, K.; Sulkama, S.; Lohi, H. Prevalence, comorbidity, and behavioral variation in canine anxiety. J. Vet. Behav. 2016, 16, 36–44. [Google Scholar] [CrossRef]
- Salonen, M.; Sulkama, S.; Mikkola, S.; Puurunen, J.; Hakanen, E.; Tiira, K.; Araujo, C.; Lohi, H. Prevalence, comorbidity, and breed differences in canine anxiety in 13,700 Finnish pet dogs. Sci. Rep. 2020, 10, 2962. [Google Scholar] [CrossRef] [Green Version]
- Tsilioni, I.; Dodman, N.; Petra, A.I.; Taliou, A.; Francis, K.; Moon-Fanelli, A.; Shuster, L.; Theoharides, T.C. Elevated serum neurotensin and CRH levels in children with autistic spectrum disorders and tail-chasing Bull Terriers with a phenotype similar to autism. Transl. Psychiatry 2014, 4, e466. [Google Scholar] [CrossRef] [Green Version]
- Moon-Fanelli, A.A.; Dodman, N.H.; Famula, T.R.; Cottam, N. Characteristics of compulsive tail chasing and associated risk factors in Bull Terriers. J. Am. Vet. Med. Assoc. 2011, 238, 883–889. [Google Scholar] [CrossRef]
- Nikolova, V.L.; Hall, M.R.B.; Hall, L.J.; Cleare, A.J.; Stone, J.M.; Young, A.H. Perturbations in gut microbiota composition in psychiatric disorders: A review and meta-analysis. JAMA Psychiatry 2021, 78, 1343–1354. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Xu, X.; Xiao, Y.; Yang, M.; Zhao, X.; Jin, C.; Hu, F.; Yang, S.; Tang, B.; et al. Gut microbiota associated with effectiveness and responsiveness to mindfulness-based cognitive therapy in improving trait anxiety. Front. Cell Infect. Microbiol. 2022, 12, 719829. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Vargason, T.; Santiago, M.; Hahn, J.; Krajmalnik-Brown, R. Distinct Fecal and plasma metabolites in children with autism spectrum disorders and their modulation after microbiota transfer therapy. mSphere 2020, 5, e00314-20. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Ilhan, Z.E.; Isern, N.G.; Hoyt, D.W.; Howsmon, D.P.; Shaffer, M.; Lozupone, C.A.; Hahn, J.; Adams, J.B.; Krajmalnik-Brown, R. Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe 2018, 49, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Spichak, S.; Bastiaanssen, T.F.S.; Berding, K.; Vlckova, K.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Mining microbes for mental health: Determining the role of microbial metabolic pathways in human brain health and disease. Neurosci. Biobehav. Rev. 2021, 125, 698–761. [Google Scholar] [CrossRef] [PubMed]
- Humer, E.; Pieh, C.; Probst, T. Metabolomic biomarkers in anxiety disorders. Int. J. Mol. Sci. 2020, 21, 4784. [Google Scholar] [CrossRef]
- Bandelow, B.; Baldwin, D.; Abelli, M.; Bolea-Alamanac, B.; Bourin, M.; Chamberlain, S.R.; Cinosi, E.; Davies, S.; Domschke, K.; Fineberg, N.; et al. Biological markers for anxiety disorders, OCD and PTSD: A consensus statement. Part II: Neurochemistry, neurophysiology and neurocognition. World J. Biol. Psychiatry 2017, 18, 162–214. [Google Scholar] [CrossRef]
- Chen, J.J.; Bai, S.J.; Li, W.W.; Zhou, C.J.; Zheng, P.; Fang, L.; Wang, H.Y.; Liu, Y.Y.; Xie, P. Urinary biomarker panel for diagnosing patients with depression and anxiety disorders. Transl. Psychiatry 2018, 8, 192. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [Green Version]
- Needham, B.D.; Funabashi, M.; Adame, M.D.; Wang, Z.; Boktor, J.C.; Haney, J.; Wu, W.L.; Rabut, C.; Ladinsky, M.S.; Hwang, S.J.; et al. A gut-derived metabolite alters brain activity and anxiety behaviour in mice. Nature 2022, 602, 647–653. [Google Scholar] [CrossRef]
- Needham, B.D.; Adame, M.D.; Serena, G.; Rose, D.R.; Preston, G.M.; Conrad, M.C.; Campbell, A.S.; Donabedian, D.H.; Fasano, A.; Ashwood, P.; et al. Plasma and fecal metabolite profiles in autism spectrum disorder. Biol. Psychiatry 2021, 89, 451–462. [Google Scholar] [CrossRef]
- Li, W.; Dowd, S.E.; Scurlock, B.; Acosta-Martinez, V.; Lyte, M. Memory and learning behavior in mice is temporally associated with diet-induced alterations in gut bacteria. Physiol. Behav. 2009, 96, 557–567. [Google Scholar] [CrossRef]
- Pyndt Jørgensen, B.; Hansen, J.T.; Krych, L.; Larsen, C.; Klein, A.B.; Nielsen, D.S.; Josefsen, K.; Hansen, A.K.; Sørensen, D.B. A possible link between food and mood: Dietary impact on gut microbiota and behavior in BALB/c mice. PLoS ONE 2014, 9, e103398. [Google Scholar] [CrossRef] [PubMed]
- Forssten, S.D.; Ouwehand, A.C.; Griffin, S.M.; Patterson, E. One giant leap from mouse to man: The microbiota-gut-brain axis in mood disorders and translational challenges moving towards human clinical trials. Nutrients 2022, 14, 568. [Google Scholar] [CrossRef] [PubMed]
- Bear, T.L.K.; Dalziel, J.E.; Coad, J.; Roy, N.C.; Butts, C.A.; Gopal, P.K. The role of the gut microbiota in dietary interventions for depression and anxiety. Adv. Nutr. 2020, 11, 890–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, A.M.; Thompson, S.V.; Edwards, C.G.; Musaad, S.M.A.; Khan, N.A.; Holscher, H.D. Associations among diet, the gastrointestinal microbiota, and negative emotional states in adults. Nutr. Neurosci. 2020, 23, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Coelho, L.P.; Kultima, J.R.; Costea, P.I.; Fournier, C.; Pan, Y.; Czarnecki-Maulden, G.; Hayward, M.R.; Forslund, S.K.; Schmidt, T.S.B.; Descombes, P.; et al. Similarity of the dog and human gut microbiomes in gene content and response to diet. Microbiome 2018, 6, 72. [Google Scholar] [CrossRef]
- Serra, D.; Almeida, L.M.; Dinis, T.C.P. Polyphenols in the management of brain disorders: Modulation of the microbiota-gut-brain axis. Adv. Food Nutr. Res. 2020, 91, 1–27. [Google Scholar] [CrossRef]
- Panickar, K.S.; Jewell, D.E. The beneficial role of anti-inflammatory dietary ingredients in attenuating markers of chronic low-grade inflammation in aging. Horm. Mol. Biol. Clin. Investig. 2015, 23, 59–70. [Google Scholar] [CrossRef]
- Pontifex, M.G.; Malik, M.M.A.H.; Connell, E.; Muller, M.; Vauzour, D. Citrus polyphenols in brain health and disease: Current perspectives. Front. Neurosci. 2021, 15, 640648. [Google Scholar] [CrossRef]
- Foti, P.; Ballistreri, G.; Timpanaro, N.; Rapisarda, P.; Romeo, F.V. Prebiotic effects of citrus pectic oligosaccharides. Nat. Prod. Res. 2021, 36, 3173–3176. [Google Scholar] [CrossRef]
- Ligor, M.; Trziszka, T. Study of antioxidant activity of biologically active compounds isolated from green vegetables by coupled analytical techniques. Food Anal. Methods 2013, 6, 630–636. [Google Scholar] [CrossRef] [Green Version]
- Ko, S.H.; Park, J.H.; Kim, S.Y.; Lee, S.W.; Chun, S.S.; Park, E. Antioxidant effects of spinach (Spinacia oleracea L.) supplementation in hyperlipidemic rats. Prev. Nutr. Food Sci. 2014, 19, 19–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva Dias, J.C. Nutritional and health benefits of carrots and their seed extracts. Food Nutr. Sci. 2014, 5, 2147–2156. [Google Scholar] [CrossRef] [Green Version]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An extensive review of the associated health impacts of tomatoes and factors that can affect their cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, K.; Górecka, D.; Szwengiel, A.; Michniewicz, J.; Drożdżyńska, A.; Walkowiak, J. Interactions between fecal bacteria, bile acids and components of tomato pomace. Food Sci. Biotechnol. 2019, 28, 649–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarfraz, S.; Khan, S.; Augustine, Z.; Raees, H.; Arif, R.; Saeed, K. Anxiolytic activity of tomato juice on animal model. J. Anal. Pharm. Res. 2018, 7, 249–252. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial effects and potential risks of tomato consumption for human health: An overview. Nutrition 2019, 62, 201–208. [Google Scholar] [CrossRef]
- Su, K.P.; Tseng, P.T.; Lin, P.Y.; Okubo, R.; Chen, T.Y.; Chen, Y.W.; Matsuoka, Y.J. Association of use of omega-3 polyunsaturated fatty acids with changes in severity of anxiety symptoms: A systematic review and meta-analysis. JAMA Netw. Open 2018, 1, e182327. [Google Scholar] [CrossRef]
- Van de Rest, O.; Geleijnse, J.M.; Kok, F.J.; van Staveren, W.A.; Hoefnagels, W.H.; Beekman, A.T.; de Groot, L.C. Effect of fish-oil supplementation on mental well-being in older subjects: A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2008, 88, 706–713. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.A.; Jackson, M.I.; Jewell, D.E.; Ephraim, E. Chronic kidney disease in cats alters response of the plasma metabolome and fecal microbiome to dietary fiber. PLoS ONE 2020, 15, e0235480. [Google Scholar] [CrossRef]
- Golder, C.; Weemhoff, J.L.; Jewell, D.E. Cats have increased protein digestibility as compared to dogs and improve their ability to absorb protein as dietary protein intake shifts from animal to plant sources. Animals 2020, 10, 541. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.A.; Melendez, L.D.; Jewell, D.E. Using gross energy improves metabolizable energy predictive equations for pet foods whereas undigested protein and fiber content predict stool quality. PLoS ONE 2013, 8, e54405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, J.A.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Jewell, D.E. Comparison of serum concentrations of symmetric dimethylarginine and creatinine as kidney function biomarkers in cats with chronic kidney disease. J. Vet. Intern. Med. 2014, 28, 1676–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floerchinger, A.M.; Jackson, M.I.; Jewell, D.E.; MacLeay, J.M.; Paetau-Robinson, I.; Hahn, K.A. Effect of feeding a weight loss food beyond a caloric restriction period on body composition and resistance to weight gain in dogs. J. Am. Vet. Med. Assoc. 2015, 247, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Jackson, M.I.; Vondran, J.C.; Vanchina, M.A.; Jewell, D.E. Comparison of circulating metabolite concentrations in dogs and cats when allowed to freely choose macronutrient intake. Biol. Open 2018, 7, bio036228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, M.I.; Jewell, D.E. Balance of saccharolysis and proteolysis underpins improvements in stool quality induced by adding a fiber bundle containing bound polyphenols to either hydrolyzed meat or grain-rich foods. Gut Microbes 2019, 10, 298–320. [Google Scholar] [CrossRef] [Green Version]
- Panickar, K.S.; Jewell, D.E. The benefit of anti-inflammatory and renal-protective dietary ingredients on the biological processes of aging in the kidney. Biology 2018, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Israelyan, N.; Margolis, K.G. Reprint of: Serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharm. Res. 2019, 140, 115–120. [Google Scholar] [CrossRef]
- Fedoce, A.D.G.; Ferreira, F.; Bota, R.G.; Bonet-Costa, V.; Sun, P.Y.; Davies, K.J.A. The role of oxidative stress in anxiety disorder: Cause or consequence? Free. Radic. Res. 2018, 52, 737–750. [Google Scholar] [CrossRef]
- Irino, Y.; Toh, R.; Nagao, M.; Mori, T.; Honjo, T.; Shinohara, M.; Tsuda, S.; Nakajima, H.; Satomi-Kobayashi, S.; Shinke, T.; et al. 2-Aminobutyric acid modulates glutathione homeostasis in the myocardium. Sci. Rep. 2016, 6, 36749. [Google Scholar] [CrossRef] [Green Version]
- Chatgilialoglu, C.; Ferreri, C. Reductive stress of sulfur-containing amino acids within proteins and implication of tandem protein-lipid damage. Int. J. Mol. Sci. 2021, 22, 12863. [Google Scholar] [CrossRef]
- Adachi, Y.; Toyoshima, K.; Nishimoto, R.; Ueno, S.; Tanaka, T.; Imaizumi, A.; Arashida, N.; Nakamura, M.; Abe, Y.; Hakamada, T.; et al. Association between plasma α-aminobutyric acid and depressive symptoms in older community-dwelling adults in Japan. Geriatr. Gerontol. Int. 2019, 19, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.J.; Hecht, P.M.; Jasarevic, E.; Beversdorf, D.Q.; Will, M.J.; Fritsche, K.; Gillespie, C.H. Sex-specific effects of docosahexaenoic acid (DHA) on the microbiome and behavior of socially-isolated mice. Brain Behav. Immun. 2017, 59, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Demirkan, A.; Isaacs, A.; Ugocsai, P.; Liebisch, G.; Struchalin, M.; Rudan, I.; Wilson, J.F.; Pramstaller, P.P.; Gyllensten, U.; Campbell, H.; et al. Plasma phosphatidylcholine and sphingomyelin concentrations are associated with depression and anxiety symptoms in a Dutch family-based lipidomics study. J. Psychiatr. Res. 2013, 47, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, T.; Layé, S. Food for mood: Relevance of nutritional omega-3 fatty acids for depression and anxiety. Front. Physiol. 2018, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Nejabati, H.R.; Mihanfar, A.; Pezeshkian, M.; Fattahi, A.; Latifi, Z.; Safaie, N.; Valiloo, M.; Jodati, A.R.; Nouri, M. N1-methylnicotinamide (MNAM) as a guardian of cardiovascular system. J. Cell Physiol. 2018, 233, 6386–6394. [Google Scholar] [CrossRef]
- Tian, Y.J.; Li, D.; Ma, Q.; Gu, X.Y.; Guo, M.; Lun, Y.Z.; Sun, W.P.; Wang, X.Y.; Cao, Y.; Zhou, S.S. Excess nicotinamide increases plasma serotonin and histamine levels. Sheng Li Xue Bao 2013, 65, 33–38. [Google Scholar]
- Evrensel, A.; Unsalver, B.O.; Ceylan, M.E. Immune-kynurenine pathways and the gut microbiota-brain axis in anxiety disorders. In Anxiety Disorders; Kim, Y.-K., Ed.; Advances in Experimental Medicine and Biology 1191; Springer Nature: Singapore, 2020; pp. 155–167. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.; Wang, J.; Gao, R. Involvement of the microbiota-gut-brain axis in chronic restraint stress: Disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 2021, 13, 1–16. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef] [Green Version]
- DeNapoli, J.S.; Dodman, N.H.; Shuster, L.; Rand, W.M.; Gross, K.L. Effect of dietary protein content and tryptophan supplementation on dominance aggression, territorial aggr.ression, and hyperactivity in dogs. J. Am. Vet. Med. Assoc. 2000, 217, 504–508. [Google Scholar] [CrossRef]
- Robinson, E.; Templeman, J.R.; Thornton, E.; Croney, C.C.; Niel, L.; Shoveller, A.K. Investigating the effects of incremental conditioning and supplemental dietary tryptophan on the voluntary activity and behaviour of mid-distance training sled dogs. PLoS ONE 2020, 15, e0232643. [Google Scholar] [CrossRef]
- Templeman, J.R.; Davenport, G.M.; Cant, J.P.; Osborne, V.R.; Shoveller, A.K. The effect of graded concentrations of dietary tryptophan on canine behavior in response to the approach of a familiar or unfamiliar individual. Can. J. Vet. Res. 2018, 82, 294–305. [Google Scholar] [PubMed]
- Shan, B.; Ai, Z.; Zeng, S.; Song, Y.; Song, J.; Zeng, Q.; Liao, Z.; Wang, T.; Huang, C.; Su, D. Gut microbiome-derived lactate promotes to anxiety-like behaviors through GPR81 receptor-mediated lipid metabolism pathway. Psychoneuroendocrinology 2020, 117, 104699. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, J.; Sidoryk-Węgrzynowicz, M.; Zielińska, M.; Aschner, M. Roles of glutamine in neurotransmission. Neuron Glia Biol. 2010, 6, 263–276. [Google Scholar] [CrossRef]
- Camilleri, M. Human Intestinal Barrier: Effects of Stressors, Diet, Prebiotics, and Probiotics. Clin. Transl. Gastroenterol. 2021, 12, e00308. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Pérez-Gómez, C.; Núñez de Castro, I.; Asenjo, M.; Márquez, J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. Int. J. Biochem. Cell Biol. 2002, 34, 439–458. [Google Scholar] [CrossRef]
- Hasler, G.; Buchmann, A.; Haynes, M.; Muller, S.T.; Ghisleni, C.; Brechbuhl, S.; Tuura, R. Association between prefrontal glutamine levels and neuroticism determined using proton magnetic resonance spectroscopy. Transl. Psychiatry 2019, 9, 170. [Google Scholar] [CrossRef]
- Anwar, A.; Abruzzo, P.M.; Pasha, S.; Rajpoot, K.; Bolotta, A.; Ghezzo, A.; Marini, M.; Posar, A.; Visconti, P.; Thornalley, P.J.; et al. Advanced glycation endproducts, dityrosine and arginine transporter dysfunction in autism—A source of biomarkers for clinical diagnosis. Mol. Autism 2018, 9, 3. [Google Scholar] [CrossRef]
- Shinohe, A.; Hashimoto, K.; Nakamura, K.; Tsujii, M.; Iwata, Y.; Tsuchiya, K.J.; Sekine, Y.; Suda, S.; Suzuki, K.; Sugihara, G.; et al. Increased serum levels of glutamate in adult patients with autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 1472–1477. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, K.; Itoh, Y.; Tateoka, R.; Ezawa, A.; Murakami, K.; Niwa, T. Metabolomic search for uremic toxins as indicators of the effect of an oral sorbent AST-120 by liquid chromatography/tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 2997–3002. [Google Scholar] [CrossRef]
- Mair, R.D.; Sirich, T.L.; Plummer, N.S.; Meyer, T.W. Characteristics of colon-derived uremic solutes. Clin. J. Am. Soc. Nephrol. 2018, 13, 1398–1404. [Google Scholar] [CrossRef] [Green Version]
- Rhee, E.P.; Souza, A.; Farrell, L.; Pollak, M.R.; Lewis, G.D.; Steele, D.J.; Thadhani, R.; Clish, C.B.; Greka, A.; Gerszten, R.E. Metabolite profiling identifies markers of uremia. J. Am. Soc. Nephrol. 2010, 21, 1041–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, J.A.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Almes, K.; Jewell, D.E. Serum concentrations of symmetric dimethylarginine and creatinine in dogs with naturally occurring chronic kidney disease. J. Vet. Intern. Med. 2016, 30, 794–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaziri, N.D.; Wong, J.; Pahl, M.; Piceno, Y.M.; Yuan, J.; DeSantis, T.Z.; Ni, Z.; Nguyen, T.H.; Andersen, G.L. Chronic kidney disease alters intestinal microbial flora. Kidney Int. 2013, 83, 308–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered composition of gut microbiota in depression: A systematic review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef]
- Simpson, C.A.; Diaz-Arteche, C.; Eliby, D.; Schwartz, O.S.; Simmons, J.G.; Cowan, C.S.M. The gut microbiota in anxiety and depression—A systematic review. Clin. Psychol. Rev. 2021, 83, 101943. [Google Scholar] [CrossRef]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef] [Green Version]
- Jewell, D.E.; Jackson, M.I.; Cochrane, C.Y.; Badri, D.V. Feeding fiber-bound polyphenol ingredients at different levels modulates colonic postbiotics to improve gut health in dogs. Animals 2022, 12, 627. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J.S. The gut microbiome of dogs and cats, and the influence of diet. Vet. Clin. N. Am. Small Anim. Pract. 2021, 51, 605–621. [Google Scholar] [CrossRef]
- Liu, F.; Li, J.; Wu, F.; Zheng, H.; Peng, Q.; Zhou, H. Altered composition and function of intestinal microbiota in autism spectrum disorders: A systematic review. Transl. Psychiatry 2019, 9, 43. [Google Scholar] [CrossRef]
- Iglesias-Vázquez, L.; Van Ginkel Riba, G.; Arija, V.; Canals, J. Composition of gut microbiota in children with autism spectrum disorder: A systematic review and meta-analysis. Nutrients 2020, 12, 792. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Xu, X.; Li, J.; Li, F. Association between gut microbiota and autism spectrum disorder: A systematic review and meta-analysis. Front. Psychiatry 2019, 10, 473. [Google Scholar] [CrossRef] [PubMed]
- Turna, J.; Grosman Kaplan, K.; Anglin, R.; Patterson, B.; Soreni, N.; Bercik, P.; Surette, M.G.; Van Ameringen, M. The gut microbiome and inflammation in obsessive-compulsive disorder patients compared to age- and sex-matched controls: A pilot study. Acta Psychiatr. Scand. 2020, 142, 337–347. [Google Scholar] [CrossRef]
- Hiippala, K.; Barreto, G.; Burrello, C.; Diaz-Basabe, A.; Suutarinen, M.; Kainulainen, V.; Bowers, J.R.; Lemmer, D.; Engelthaler, D.M.; Eklund, K.K.; et al. Novel Odoribacter splanchnicus strain and its outer membrane vesicles exert immunoregulatory effects in vitro. Front. Microbiol. 2020, 11, 575455. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Zhao, J.; Wu, L.; Carru, C.; Biagi, E.; Franceschi, C. Microbiomes other than the gut: Inflammaging and age-related diseases. Semin. Immunopathol. 2020, 42, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Caspani, G.; Kennedy, S.; Foster, J.A.; Swann, J. Gut microbial metabolites in depression: Understanding the biochemical mechanisms. Microb. Cell 2019, 6, 454–481. [Google Scholar] [CrossRef]
- Du, X.; Xiang, Y.; Lou, F.; Tu, P.; Zhang, X.; Hu, X.; Lyu, W.; Xiao, Y. Microbial community and short-chain fatty acid mapping in the intestinal tract of quail. Animals 2020, 10, 1006. [Google Scholar] [CrossRef]
- Van de Wouw, M.; Boehme, M.; Lyte, J.M.; Wiley, N.; Strain, C.; O’Sullivan, O.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Short-chain fatty acids: Microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018, 596, 4923–4944. [Google Scholar] [CrossRef]
- Liu, S.; Li, E.; Sun, Z.; Fu, D.; Duan, G.; Jiang, M.; Yu, Y.; Mei, L.; Yang, P.; Tang, Y.; et al. Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 2019, 9, 287. [Google Scholar] [CrossRef]
- Eichenfield, D.Z.; Sprague, J.; Eichenfield, L.F. Management of acne vulgaris: A review. JAMA 2021, 326, 2055–2067. [Google Scholar] [CrossRef]
- Gámiz, F.; Gallo, M. A systematic review of the dietary choline impact on cognition from a psychobiological approach: Insights from animal studies. Nutrients 2021, 13, 1966. [Google Scholar] [CrossRef]
- Agam, G.; Taylor, Z.; Vainer, E.; Golan, H.M. The influence of choline treatment on behavioral and neurochemical autistic-like phenotype in Mthfr-deficient mice. Transl. Psychiatry 2020, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Pujo, J.; Petitfils, C.; Le Faouder, P.; Eeckhaut, V.; Payros, G.; Maurel, S.; Perez-Berezo, T.; Van Hul, M.; Barreau, F.; Blanpied, C.; et al. Bacteria-derived long chain fatty acid exhibits anti-inflammatory properties in colitis. Gut 2021, 70, 1088–1097. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).