Alterations in Gut Microbiota and Upregulations of VPAC2 and Intestinal Tight Junctions Correlate with Anti-Inflammatory Effects of Electroacupuncture in Colitis Mice with Sleep Fragmentation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
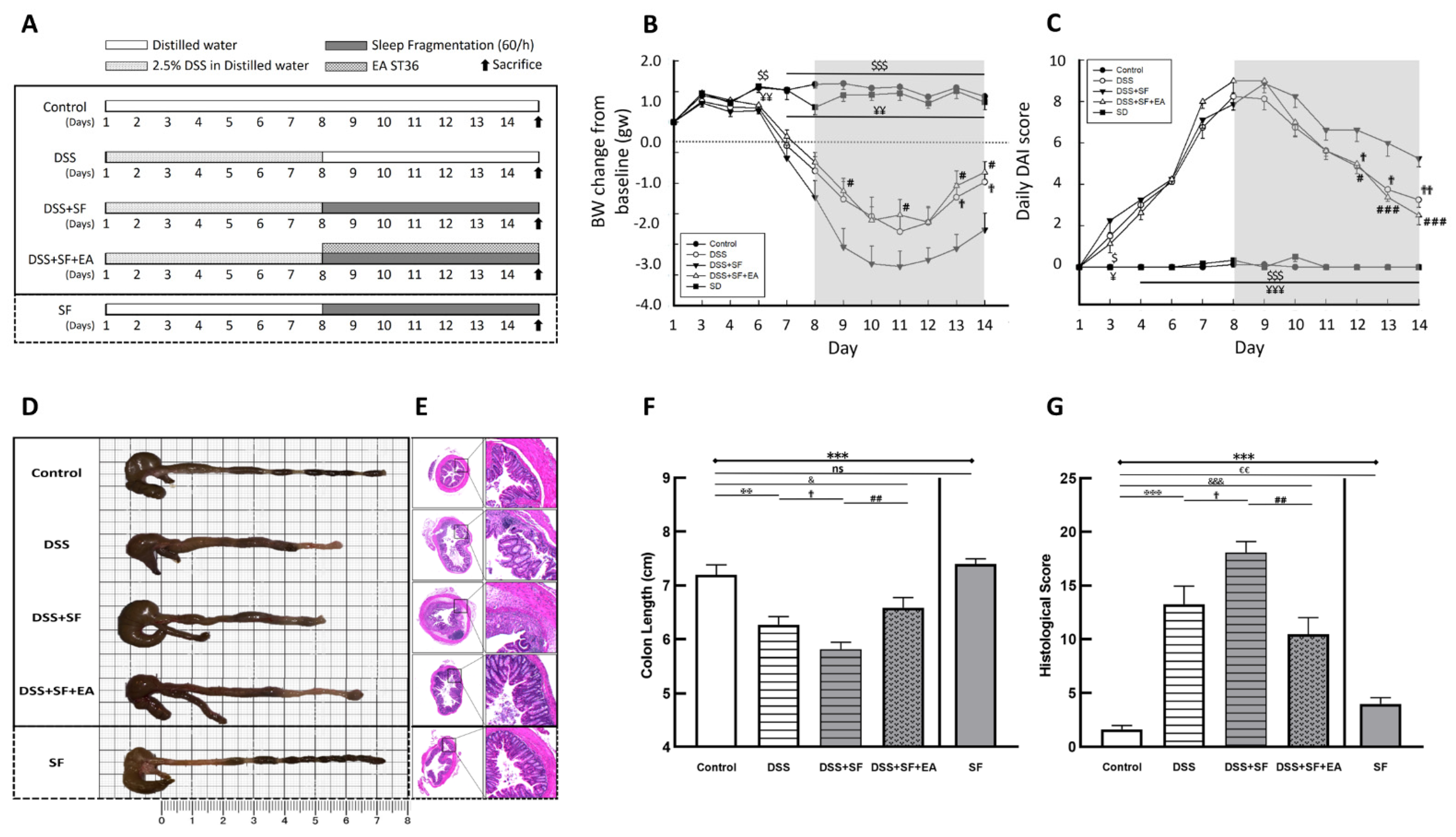
2.2. Experimental Groups and Protocol
2.3. Induction of Experimental Colitis
2.4. Sleep Fragmentation Animal Model
2.5. EA Intervention Procedure
2.6. Assessment of Colitis Severity
- (1)
- Clinical indicators
- (2)
- Macroscopic evidence of colitis
- (3)
- Microscopic evidence of colitis
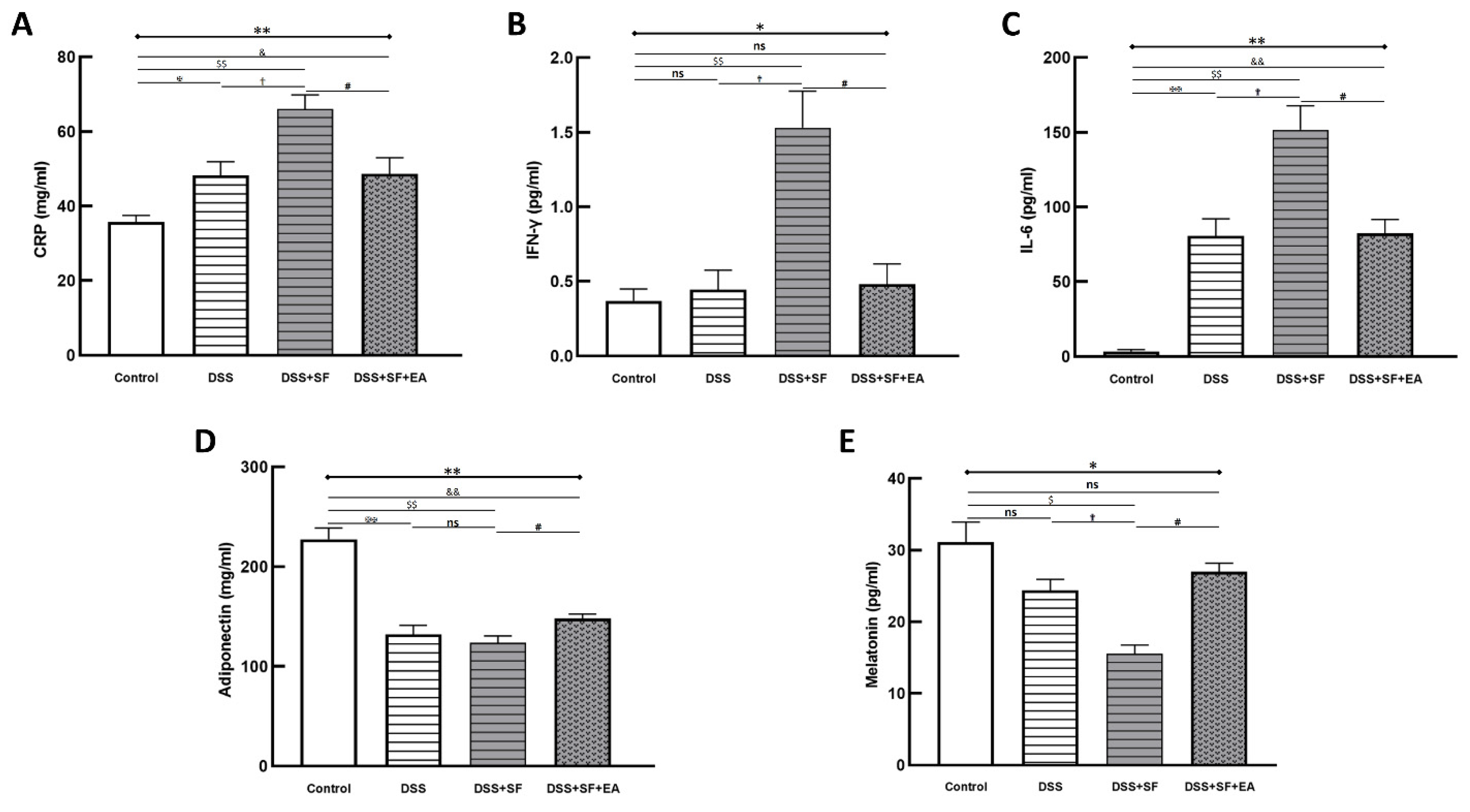
2.7. Plasma Biomarker Analysis
2.8. Stool Collection, Gut Microbiota Processing, and 16S Metagenomics Analysis
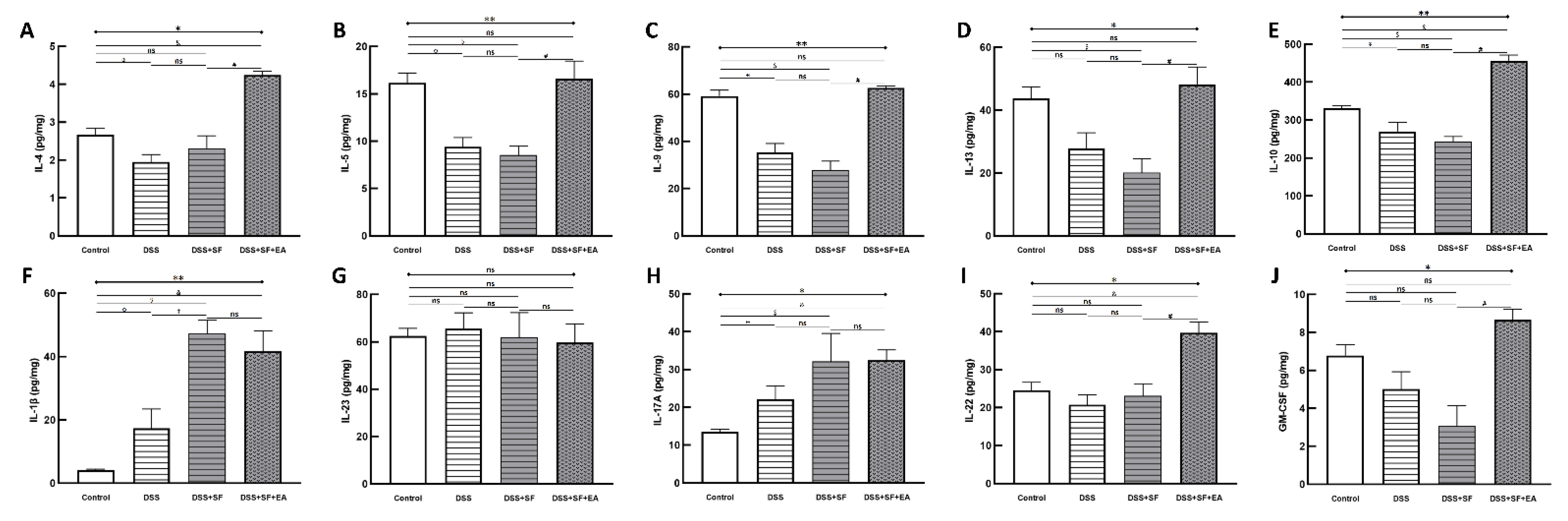
2.9. Multiplex Immunoassay of Colon Tissue
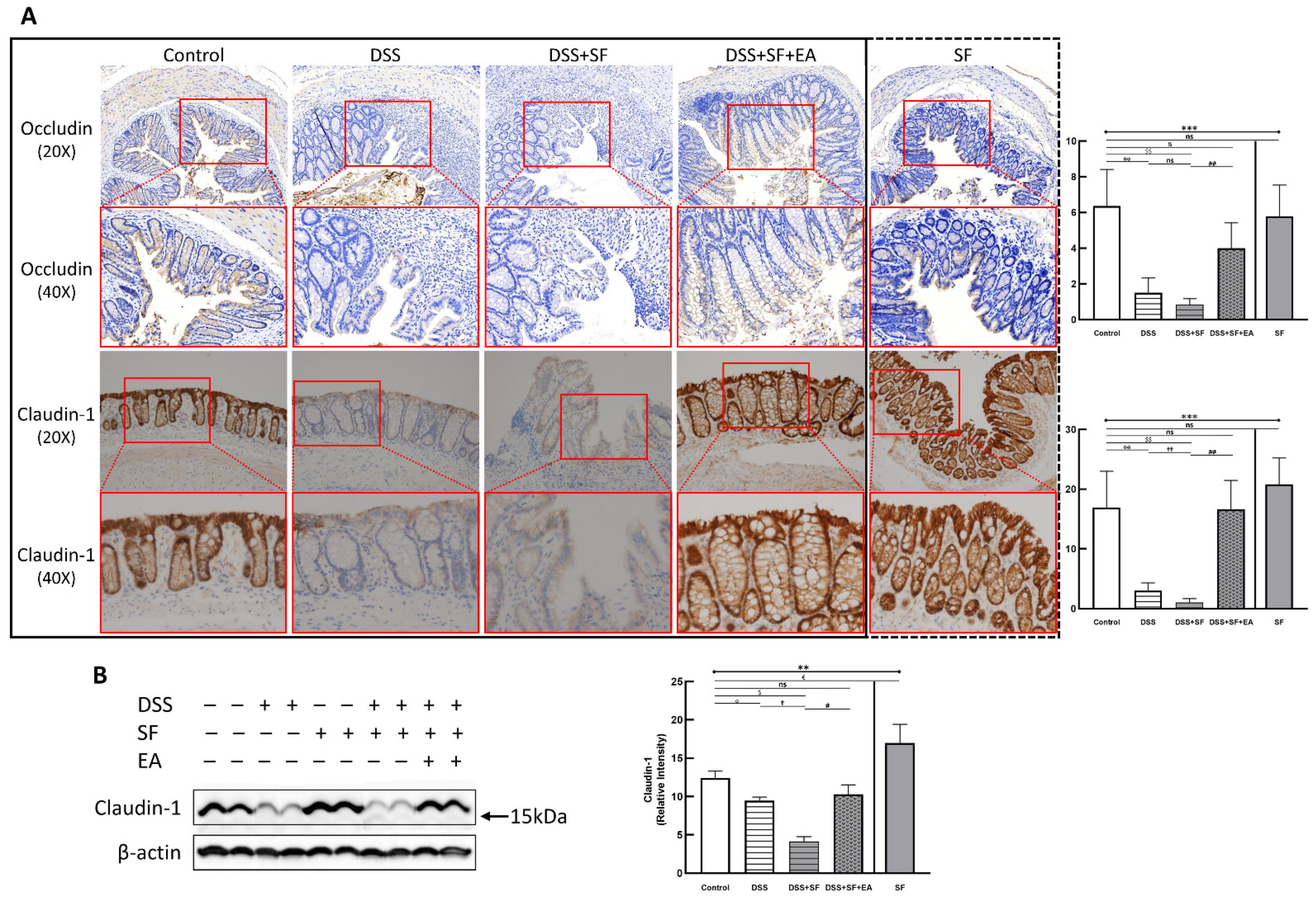
2.10. Immunohistochemistry Staining
2.11. Western Blot
2.12. Statistical Analysis
3. Results
3.1. Severity of DSS Colitis and Intestinal Barrier Damage Was Aggravated during Sleep Fragmentation and Was Ameliorated after EA
3.2. Sleep Fragmentation Disturbs Immunology in Colonic Layers in the Normal State Rather than the Inflammatory Condition
3.3. EA Modulated Inflammatory and Immune Reaction in DSS-Mice with Sleep Fragmentation
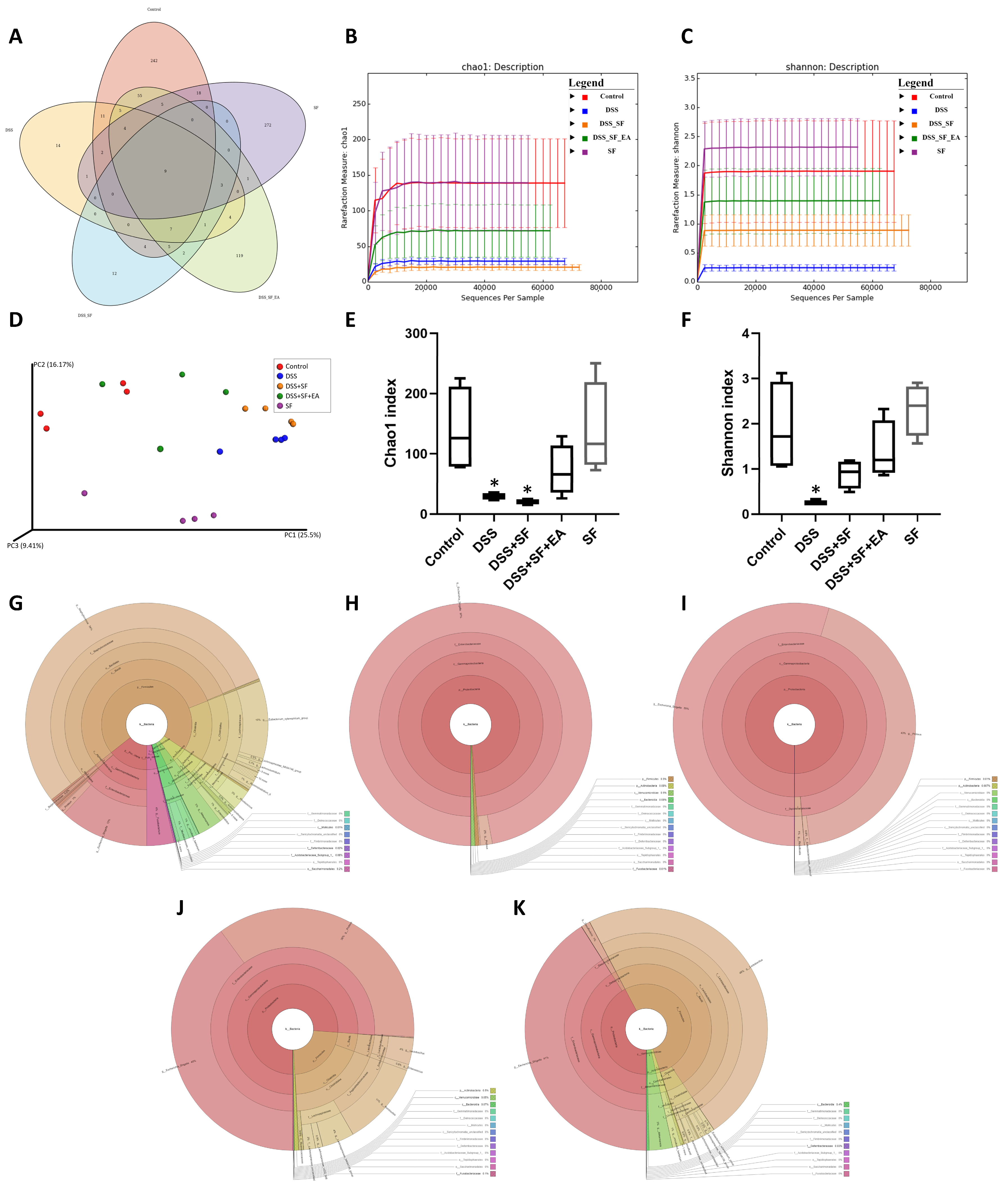
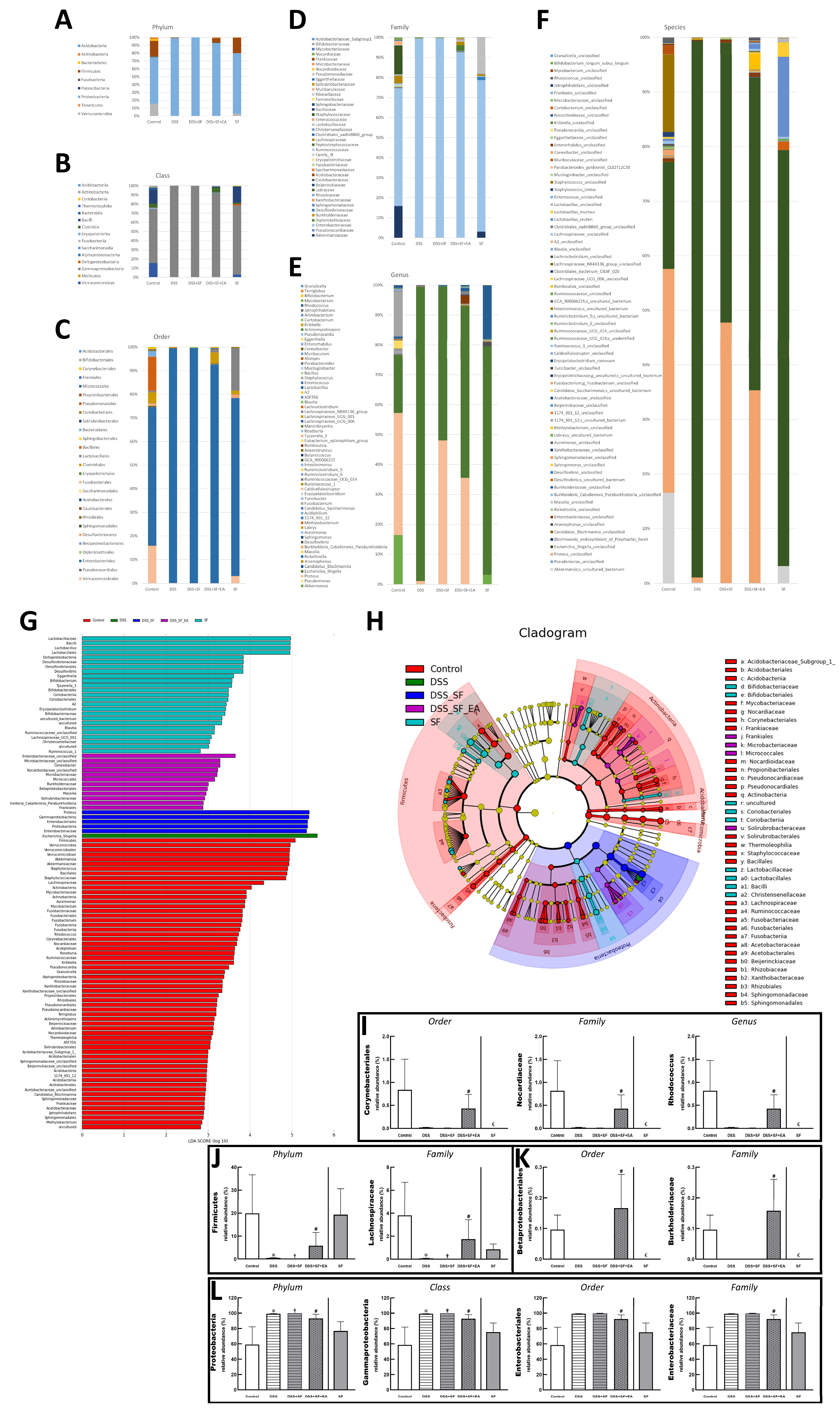
3.4. Dysbiosis Was Improved with EA Intervention in Colitis Mice with Sleep Fragmentation
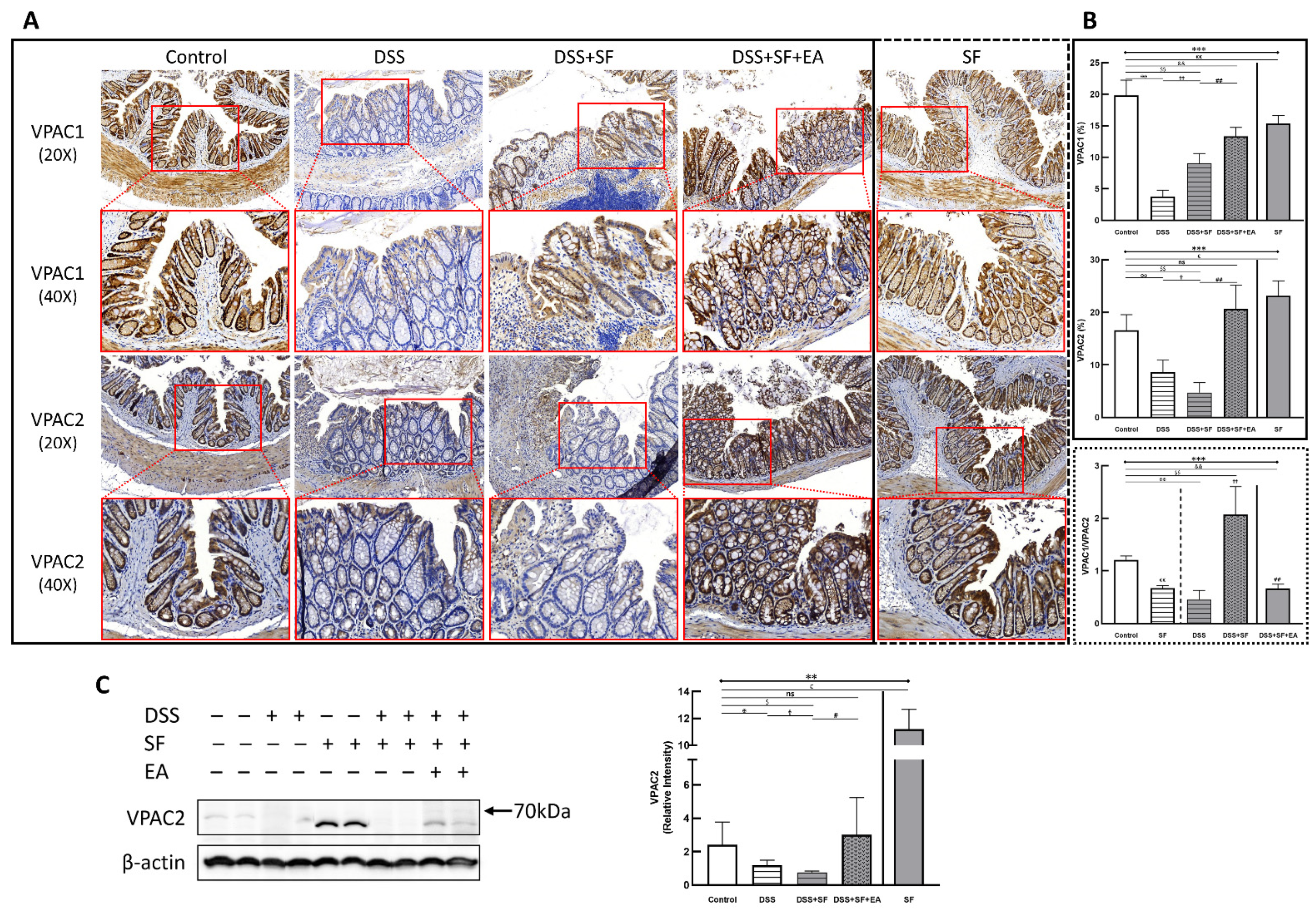
3.5. Vasoactive Intestinal Peptide (VIP) Performance on Intestinal Homeostasis with EA Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, T.; Orr, W.C. Sleep disturbances and inflammatory bowel disease. Inflamm. Bowel Dis. 2014, 20, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Ranjbaran, Z.; Keefer, L.; Farhadi, A.; Stepanski, E.; Sedghi, S.; Keshavarzian, A. Impact of sleep disturbances in inflammatory bowel disease. J. Gastroenterol. Hepatol. 2007, 22, 1748–1753. [Google Scholar] [CrossRef]
- Ali, T.; Madhoun, M.F.; Orr, W.C.; Rubin, D.T. Assessment of the relationship between quality of sleep and disease activity in inflammatory bowel disease patients. Inflamm. Bowel Dis. 2013, 19, 2440–2443. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Long, M.D.; Martin, C.F.; Sandler, R.S.; Kappelman, M.D. Sleep disturbance and risk of active disease in patients with Crohn’s disease and ulcerative colitis. Clin. Gastroenterol. Hepatol. 2013, 11, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Desai, D.; Faubion, W.A.; Sandborn, W.J. Review article: Biological activity markers in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2007, 25, 247–255. [Google Scholar] [CrossRef]
- Spiegel, K.; Leproult, R.; Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 1999, 354, 1435–1439. [Google Scholar] [CrossRef]
- Parekh, P.J.; Oldfield Iv, E.C.; Challapallisri, V.; Ware, J.C.; Johnson, D.A. Sleep disorders and inflammatory disease activity: Chicken or the egg? Am. J. Gastroenterol. 2015, 110, 484–488. [Google Scholar] [CrossRef]
- Swanson, G.R.; Burgess, H.J.; Keshavarzian, A. Sleep disturbances and inflammatory bowel disease: A potential trigger for disease flare? Expert Rev. Clin. Immunol. 2011, 7, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Chakradeo, P.S.; Keshavarzian, A.; Singh, S.; Dera, A.E.; Esteban, J.P.G.; Lee, A.A.; Burgess, H.J.; Fogg, L.; Swanson, G.R. Chronotype, social jet lag, sleep debt and food timing in inflammatory bowel disease. Sleep Med. 2018, 52, 188–195. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Zeevi, D.; Levy, M.; Zilberman-Schapira, G.; Suez, J.; Tengeler, A.C.; Abramson, L.; Katz, M.N.; Korem, T.; Zmora, N.; et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell 2014, 159, 514–529. [Google Scholar] [CrossRef] [Green Version]
- Mukherji, A.; Kobiita, A.; Ye, T.; Chambon, P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell 2013, 153, 812–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voigt, R.M.; Forsyth, C.B.; Green, S.J.; Mutlu, E.; Engen, P.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. Circadian disorganization alters intestinal microbiota. PLoS ONE 2014, 9, e97500. [Google Scholar] [CrossRef] [PubMed]
- Serban, D.E. Microbiota in Inflammatory Bowel Disease Pathogenesis and Therapy: Is It All About Diet? Nutr. Clin. Pract. 2015, 30, 760–779. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zou, J.; Xu, H.; Huang, W.; Zhang, X.; Wei, Z.; Li, X.; Liu, Y.; Zou, J.; Liu, F.; et al. Effects of Chronic Intermittent Hypoxia and Chronic Sleep Fragmentation on Gut Microbiome, Serum Metabolome, Liver and Adipose Tissue Morphology. Front. Endocrinol. 2022, 13, 820939. [Google Scholar] [CrossRef]
- Ohkusa, T.; Koido, S. Intestinal microbiota and ulcerative colitis. J. Infect. Chemother. 2015, 21, 761–768. [Google Scholar] [CrossRef]
- Sanford, L.D.; Wellman, L.L.; Ciavarra, R.P.; Oldfield, E.C.t.; Shams, R.; Copare, J.L.; Johnson, D.A. Differential Effect of Light and Dark Period Sleep Fragmentation on Composition of Gut Microbiome and Inflammation in Mice. Life 2021, 11, 1283. [Google Scholar] [CrossRef]
- Horta, D.; Lira, A.; Sanchez-Lloansi, M.; Villoria, A.; Teggiachi, M.; García-Rojo, D.; García-Molina, S.; Figuerola, A.; Esteve, M.; Calvet, X. A Prospective Pilot Randomized Study: Electroacupuncture vs. Sham Procedure for the Treatment of Fatigue in Patients With Quiescent Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2020, 26, 484–492. [Google Scholar] [CrossRef]
- Song, G.; Fiocchi, C.; Achkar, J.P. Acupuncture in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1129–1139. [Google Scholar] [CrossRef]
- Zijlstra, F.J.; van den Berg-de Lange, I.; Huygen, F.J.; Klein, J. Anti-inflammatory actions of acupuncture. Mediat. Inflamm. 2003, 12, 59–69. [Google Scholar] [CrossRef]
- Kavoussi, B.; Ross, B.E. The neuroimmune basis of anti-inflammatory acupuncture. Integr. Cancer Ther. 2007, 6, 251–257. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Y.; Liu, Z. Acupuncture for restless legs syndrome. Cochrane Database Syst. Rev. 2008, 4, CD006457. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wang, M.; Li, M.; Wang, Q.; Kwak, S.; Jiang, W.; Yamamoto, Y. Actigraph evaluation of acupuncture for treating restless legs syndrome. Evid. Based Complement. Alter. Med. 2015, 2015, 343201. [Google Scholar] [CrossRef]
- Kalavapalli, R.; Singareddy, R. Role of acupuncture in the treatment of insomnia: A comprehensive review. Complement. Ther. Clin. Pract. 2007, 13, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Yeung, W.-F.; Chung, K.-F.; Leung, Y.-K.; Zhang, S.-P.; Law, A.C.K. Traditional needle acupuncture treatment for insomnia: A systematic review of randomized controlled trials. Sleep Med. 2009, 10, 694–704. [Google Scholar] [CrossRef]
- Huang, W.; Kutner, N.; Bliwise, D.L. A systematic review of the effects of acupuncture in treating insomnia. Sleep Med. Rev. 2009, 13, 73–104. [Google Scholar] [CrossRef]
- Huang, W.; Kutner, N.; Bliwise, D.L. Autonomic Activation in Insomnia: The Case for Acupuncture. J. Clin. Sleep Med. 2011, 7, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Yi, P.L.; Tsai, C.H.; Lin, J.G.; Liu, H.J.; Chang, F.C. Effects of electroacupuncture at ‘Anmian (Extra)’ acupoints on sleep activities in rats: The implication of the caud al nucleus tractus solitarius. J. Biomed. Sci. 2004, 11, 579–590. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Yi, P.-L.; Lin, J.-G.; Chang, F.-C. Endogenous opiates in the nucleus tractus solitarius mediate electroacupuncture-induced sleep activities in rats. Evid. Based. Complement. Alter. Med. 2011, 2011, 159209. [Google Scholar] [CrossRef]
- Chung, S.H.; Park, Y.S.; Kim, O.S.; Kim, J.H.; Baik, H.W.; Hong, Y.O.; Kim, S.S.; Shin, J.H.; Jun, J.H.; Jo, Y.; et al. Melatonin attenuates dextran sodium sulfate induced colitis with sleep deprivation: Possible mechanism by microarray analysis. Dig. Dis. Sci. 2014, 59, 1134–1141. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef]
- Tang, Y.; Preuss, F.; Turek, F.W.; Jakate, S.; Keshavarzian, A. Sleep deprivation worsens inflammation and delays recovery in a mouse model of colitis. Sleep Med. 2009, 10, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.-Y.; Chen, W.-H.; Hsieh, C.-L.; Lin, Y.-W. Abundant expression and functional participation of TRPV1 at Zusanli acupoint (ST36) in mice: Mechanosensitive TRPV1 as an “acupuncture-responding channel”. BMC Complement. Altern. Med. 2014, 14, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.J.; Shajib, M.S.; Manocha, M.M.; Khan, W.I. Investigating intestinal inflammation in DSS-induced model of IBD. J. Vis. Exp. 2012, 60, e3678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, D.J.; Künzli, B.M.; YI, A.R.; Sevigny, J.; Berberat, P.O.; Enjyoji, K.; Csizmadia, E.; Friess, H.; Robson, S.C. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2009, 106, 16788–16793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Sluis, M.; De Koning, B.A.; De Bruijn, A.C.; Velcich, A.; Meijerink, J.P.; Van Goudoever, J.B.; Büller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Rath, H.C.; Herfarth, H.H.; Ikeda, J.S.; Grenther, W.B.; Hamm, T.E., Jr.; Balish, E.; Taurog, J.D.; Hammer, R.E.; Wilson, K.H.; Sartor, R.B. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J. Clin. Investig. 1996, 98, 945–953. [Google Scholar] [CrossRef] [Green Version]
- Munyaka, P.M.; Eissa, N.; Bernstein, C.N.; Khafipour, E.; Ghia, J.E. Antepartum Antibiotic Treatment Increases Offspring Susceptibility to Experimental Colitis: A Role of the Gut Microbiota. PLoS ONE 2015, 10, e0142536. [Google Scholar] [CrossRef]
- Mullington, J.M.; Simpson, N.S.; Meier-Ewert, H.K.; Haack, M. Sleep loss and inflammation. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Gao, T.; Wang, Z.; Dong, Y.; Cao, J.; Lin, R.; Wang, X.; Yu, Z.; Chen, Y. Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J. Pineal Res. 2019, 67, e12574. [Google Scholar] [CrossRef]
- Triplett, J.; Ellis, D.; Braddock, A.; Roberts, E.; Ingram, K.; Perez, E.; Short, A.; Brown, D.; Hutzley, V.; Webb, C.; et al. Temporal and region-specific effects of sleep fragmentation on gut microbiota and intestinal morphology in Sprague Dawley rats. Gut Microbes 2020, 11, 706–720. [Google Scholar] [CrossRef]
- Park, Y.S.; Chung, S.H.; Lee, S.K.; Kim, J.H.; Kim, J.B.; Kim, T.K.; Kim, D.S.; Baik, H.W. Melatonin improves experimental colitis with sleep deprivation. Int. J. Mol. Med. 2015, 35, 979–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opp, M.R. Cytokines and sleep. Sleep Med. Rev. 2005, 9, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Chang, Z.Y.; Liu, H.M.; Leu, Y.L.; Hsu, C.H.; Lee, T.Y. Modulation of Gut Microbiota Combined with Upregulation of Intestinal Tight Junction Explains Anti-Inflammatory Effect of Corylin on Colitis-Associated Cancer in Mice. Int. J. Mol. Sci. 2022, 23, 2667. [Google Scholar] [CrossRef] [PubMed]
- Poritz, L.S.; Garver, K.I.; Green, C.; Fitzpatrick, L.; Ruggiero, F.; Koltun, W.A. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J. Surg. Res. 2007, 140, 12–19. [Google Scholar] [CrossRef]
- Okumura, R.; Takeda, K. Maintenance of intestinal homeostasis by mucosal barriers. Inflamm. Regen. 2018, 38, 5. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.H.; Liu, H.M.; Chen, Y.S.; Lee, T.Y. Effect of Electroacupuncture in Mice with Dextran Sulfate Sodium-Induced Colitis and the Influence of Gut Microbiota. Evid. Based Complement. Altern. Med. 2020, 2020, 2087903. [Google Scholar] [CrossRef]
- Ott, S.J.; Musfeldt, M.; Wenderoth, D.F.; Hampe, J.; Brant, O.; Fölsch, U.R.; Timmis, K.N.; Schreiber, S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 2004, 53, 685–693. [Google Scholar] [CrossRef] [Green Version]
- Manichanh, C.; Borruel, N.; Casellas, F.; Guarner, F. The gut microbiota in IBD. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 599–608. [Google Scholar] [CrossRef]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [Green Version]
- Munyaka, P.M.; Rabbi, M.F.; Khafipour, E.; Ghia, J.E. Acute dextran sulfate sodium (DSS)-induced colitis promotes gut microbial dysbiosis in mice. J. Basic Microbiol. 2016, 56, 986–998. [Google Scholar] [CrossRef]
- Wei, D.; Xie, L.; Zhuang, Z.; Zhao, N.; Huang, B.; Tang, Y.; Yu, S.; Zhou, Q.; Wu, Q. Gut Microbiota: A New Strategy to Study the Mechanism of Electroacupuncture and Moxibustion in Treating Ulcerative Colitis. Evid. Based Complement. Altern. Med. 2019, 2019, 9730176. [Google Scholar] [CrossRef] [PubMed]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The Role of Enterobacteriaceae in Gut Microbiota Dysbiosis in Inflammatory Bowel Diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.M.; Majde, J.A. Sleep and the Immune Response. In Sleep: A Comprehensive Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 767–772. [Google Scholar]
- Smith, R.P.; Easson, C.; Lyle, S.M.; Kapoor, R.; Donnelly, C.P.; Davidson, E.J.; Parikh, E.; Lopez, J.V.; Tartar, J.L. Gut microbiome diversity is associated with sleep physiology in humans. PLoS ONE 2019, 14, e0222394. [Google Scholar] [CrossRef] [PubMed]
- Peloquin, J.M.; Nguyen, D.D. The microbiota and inflammatory bowel disease: Insights from animal models. Anaerobe 2013, 24, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Collins, S.M.; Bercik, P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 2009, 136, 2003–2014. [Google Scholar] [CrossRef] [Green Version]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Fung, T.C. The microbiota-immune axis as a central mediator of gut-brain communication. Neurobiol. Dis. 2020, 136, 104714. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [Green Version]
- Ganal-Vonarburg, S.C.; Duerr, C.U. The interaction of intestinal microbiota and innate lymphoid cells in health and disease throughout life. Immunology 2020, 159, 39–51. [Google Scholar] [CrossRef]
- Robinette, M.L.; Colonna, M. Immune modules shared by innate lymphoid cells and T cells. J. Allergy Clin. Immunol. 2016, 138, 1243–1251. [Google Scholar] [CrossRef] [Green Version]
- Domingues, R.G.; Hepworth, M.R. Immunoregulatory Sensory Circuits in Group 3 Innate Lymphoid Cell (ILC3) Function and Tissue Homeostasis. Front. Immunol. 2020, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Saez, A.; Gomez-Bris, R.; Herrero-Fernandez, B.; Mingorance, C.; Rius, C.; Gonzalez-Granado, J.M. Innate Lymphoid Cells in Intestinal Homeostasis and Inflammatory Bowel Disease. Int. J. Mol. Sci. 2021, 22, 7618. [Google Scholar] [CrossRef] [PubMed]
- Neill, D.R.; Wong, S.H.; Bellosi, A.; Flynn, R.J.; Daly, M.; Langford, T.K.; Bucks, C.; Kane, C.M.; Fallon, P.G.; Pannell, R.; et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 2010, 464, 1367–1370. [Google Scholar] [CrossRef] [Green Version]
- Monticelli, L.A.; Sonnenberg, G.F.; Abt, M.C.; Alenghat, T.; Ziegler, C.G.; Doering, T.A.; Angelosanto, J.M.; Laidlaw, B.J.; Yang, C.Y.; Sathaliyawala, T.; et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat. Immunol. 2011, 12, 1045–1054. [Google Scholar] [CrossRef]
- Nussbaum, J.C.; Van Dyken, S.J.; von Moltke, J.; Cheng, L.E.; Mohapatra, A.; Molofsky, A.B.; Thornton, E.E.; Krummel, M.F.; Chawla, A.; Liang, H.E.; et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013, 502, 245–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein Wolterink, R.G.; Kleinjan, A.; van Nimwegen, M.; Bergen, I.; de Bruijn, M.; Levani, Y.; Hendriks, R.W. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur. J. Immunol. 2012, 42, 1106–1116. [Google Scholar] [CrossRef] [Green Version]
- Seehus, C.R.; Kadavallore, A.; Torre, B.; Yeckes, A.R.; Wang, Y.; Tang, J.; Kaye, J. Alternative activation generates IL-10 producing type 2 innate lymphoid cells. Nat. Commun. 2017, 8, 1900. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Imaeda, H.; Fujimoto, T.; Ban, H.; Bamba, S.; Tsujikawa, T.; Sasaki, M.; Fujiyama, Y.; Andoh, A. Regulation of eotaxin-3/CC chemokine ligand 26 expression by T helper type 2 cytokines in human colonic myofibroblasts. Clin. Exp. Immunol. 2013, 173, 323–331. [Google Scholar] [CrossRef]
- Shajib, M.S.; Wang, H.; Kim, J.J.; Sunjic, I.; Ghia, J.E.; Denou, E.; Collins, M.; Denburg, J.A.; Khan, W.I. Interleukin 13 and serotonin: Linking the immune and endocrine systems in murine models of intestinal inflammation. PLoS ONE 2013, 8, e72774. [Google Scholar] [CrossRef] [Green Version]
- Strober, W.; Fuss, I.J.; Blumberg, R.S. The immunology of mucosal models of inflammation. Annu. Rev. Immunol. 2002, 20, 495–549. [Google Scholar] [CrossRef]
- Saba, E.; Lee, Y.Y.; Rhee, M.H.; Kim, S.D. Alleviation of Ulcerative Colitis Potentially through th1/th2 Cytokine Balance by a Mixture of Rg3-enriched Korean Red Ginseng Extract and Persicaria tinctoria. Molecules 2020, 25, 5230. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, X.M.; Di, S.; Gao, Z.Z.; Li, Q.W.; Wu, H.R.; Wang, Q.; Zhao, L.H.; Tong, X.L. Innate Lymphoid Cells: A Link between the Nervous System and Microbiota in Intestinal Networks. Mediat. Inflamm. 2019, 2019, 1978094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Alli, R.; Vogel, P.; Geiger, T.L. IL-10 modulates DSS-induced colitis through a macrophage-ROS-NO axis. Mucosal Immunol. 2014, 7, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Klose, C.S.; Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016, 17, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Hamnett, R.; Crosby, P.; Chesham, J.E.; Hastings, M.H. Vasoactive intestinal peptide controls the suprachiasmatic circadian clock network via ERK1/2 and DUSP4 signalling. Nat. Commun. 2019, 10, 542. [Google Scholar] [CrossRef] [Green Version]
- Mill, J.E.; Granados-Fuentes, D.; Wang, T.; Marpegan, L.; Holy, T.E.; Herzog, E.D. Vasoactive intestinal polypeptide mediates circadian rhythms in mammalian olfactory bulb and olfaction. J. Neurosci. 2014, 34, 6040–6046. [Google Scholar] [CrossRef] [Green Version]
- Seillet, C.; Luong, K.; Tellier, J.; Jacquelot, N.; Shen, R.D.; Hickey, P.; Wimmer, V.C.; Whitehead, L.; Rogers, K.; Smyth, G.K.; et al. The neuropeptide VIP confers anticipatory mucosal immunity by regulating ILC3 activity. Nat. Immunol. 2020, 21, 168–177. [Google Scholar] [CrossRef]
- Yu, H.B.; Yang, H.; Allaire, J.M.; Ma, C.; Graef, F.A.; Mortha, A.; Liang, Q.; Bosman, E.S.; Reid, G.S.; Waschek, J.A.; et al. Vasoactive intestinal peptide promotes host defense against enteric pathogens by modulating the recruitment of group 3 innate lymphoid cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2106634118. [Google Scholar] [CrossRef]
- Talbot, J.; Hahn, P.; Kroehling, L.; Nguyen, H.; Li, D.; Littman, D.R. Feeding-dependent VIP neuron-ILC3 circuit regulates the intestinal barrier. Nature 2020, 579, 575–580. [Google Scholar] [CrossRef]
- Pascal, M.; Kazakov, A.; Chevalier, G.; Dubrule, L.; Deyrat, J.; Dupin, A.; Saha, S.; Jagot, F.; Sailor, K.; Dulauroy, S.; et al. The neuropeptide VIP potentiates intestinal innate type 2 and type 3 immunity in response to feeding. Mucosal Immunol. 2022, 1–13. [Google Scholar] [CrossRef]
- Martínez, C.; Juarranz, Y.; Gutiérrez-Cañas, I.; Carrión, M.; Pérez-García, S.; Villanueva-Romero, R.; Castro, D.; Lamana, A.; Mellado, M.; González-Álvaro, I.; et al. A Clinical Approach for the Use of VIP Axis in Inflammatory and Autoimmune Diseases. Int. J. Mol. Sci. 2019, 21, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bains, M.; Laney, C.; Wolfe, A.E.; Orr, M.; Waschek, J.A.; Ericsson, A.C.; Dorsam, G.P. Vasoactive Intestinal Peptide Deficiency is Associated with Altered Gut Microbiota Communities in Male and Female C57BL/6 Mice. Front. Microbiol. 2019, 10, 2689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messing, M.; Jan-Abu, S.C.; McNagny, K. Group 2 Innate Lymphoid Cells: Central Players in a Recurring Theme of Repair and Regeneration. Int. J. Mol. Sci. 2020, 21, 1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Jonge, W.J. Chapter 46—Neuronal Regulation of Mucosal Immune Responses. In Mucosal Immunology, 4th ed.; Mestecky, J., Strober, W., Russell, M.W., Kelsall, B.L., Cheroutre, H., Lambrecht, B.N., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 929–942. [Google Scholar]
- Tan, Y.V.; Abad, C.; Wang, Y.; Lopez, R.; Waschek, J. VPAC2 (vasoactive intestinal peptide receptor type 2) receptor deficient mice develop exacerbated experimental autoimmune encephalomyelitis with increased Th1/Th17 and reduced Th2/Treg responses. Brain Behav. Immun. 2015, 44, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.; Zhang, H.; Huang, L.; Liu, X.; Chen, J. Anti-hyperglycemic, antioxidant and anti-inflammatory effects of VIP and a VPAC1 agonist on streptozotocin-induced diabetic mice. Peptides 2011, 32, 216–222. [Google Scholar] [CrossRef]
- Casado-Bedmar, M.; Heil, S.D.S.; Myrelid, P.; Söderholm, J.D.; Keita, Å.V. Upregulation of intestinal mucosal mast cells expressing VPAC1 in close proximity to vasoactive intestinal polypeptide in inflammatory bowel disease and murine colitis. Neurogastroenterol. Motil. 2019, 31, e13503. [Google Scholar] [CrossRef]
- Yadav, M.; Huang, M.C.; Goetzl, E.J. VPAC1 (vasoactive intestinal peptide (VIP) receptor type 1) G protein-coupled receptor mediation of VIP enhancement of murine experimental colitis. Cell. Immunol. 2011, 267, 124–132. [Google Scholar] [CrossRef]
- Nishihara, T.; Matsuda, M.; Araki, H.; Oshima, K.; Kihara, S.; Funahashi, T.; Shimomura, I. Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology 2006, 131, 853–861. [Google Scholar] [CrossRef] [Green Version]
- Obeid, S.; Wankell, M.; Charrez, B.; Sternberg, J.; Kreuter, R.; Esmaili, S.; Ramezani-Moghadam, M.; Devine, C.; Read, S.; Bhathal, P.; et al. Adiponectin confers protection from acute colitis and restricts a B cell immune response. J. Biol. Chem. 2017, 292, 6569–6582. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.K.; Park, Y.S.; Baik, H.W.; Jun, J.H.; Kim, E.K.; Sull, J.W.; Sung, H.J.; Choi, J.W.; Chung, S.H.; Gye, M.C.; et al. Melatonin modulates adiponectin expression on murine colitis with sleep deprivation. World J. Gastroenterol. 2016, 22, 7559–7568. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, S.; Son, M.; Cheon, J.H.; Park, Y.S. Melatonin controls microbiota in colitis by goblet cell differentiation and antimicrobial peptide production through Toll-like receptor 4 signalling. Sci. Rep. 2020, 10, 2232. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, J.; Rehman, J.U.; Akerstedt, T.; Ekman, R.; Miller, G.E.; Höglund, C.O.; Lekander, M. Effects of sustained sleep restriction on mitogen-stimulated cytokines, chemokines and T helper 1/T helper 2 balance in humans. PLoS ONE 2013, 8, e82291. [Google Scholar] [CrossRef] [PubMed]
- Said, E.A.; Al-Abri, M.A.; Al-Saidi, I.; Al-Balushi, M.S.; Al-Busaidi, J.Z.; Al-Reesi, I.; Koh, C.Y.; Idris, M.A.; Al-Jabri, A.A.; Habbal, O. Sleep deprivation alters neutrophil functions and levels of Th1-related chemokines and CD4(+) T cells in the blood. Sleep Breath 2019, 23, 1331–1339. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, M.R.; Opp, M.R. Sleep Health: Reciprocal Regulation of Sleep and Innate Immunity. Neuropsychopharmacology 2017, 42, 129–155. [Google Scholar] [CrossRef] [Green Version]
- Haack, M.; Sanchez, E.; Mullington, J.M. Elevated inflammatory markers in response to prolonged sleep restriction are associated with increased pain experience in healthy volunteers. Sleep 2007, 30, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, W.M.; Lehto, M.; Karisola, P.; Lindholm, H.; Luukkonen, R.; Sallinen, M.; Härmä, M.; Porkka-Heiskanen, T.; Alenius, H. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting proinflammatory responses through IL-17 and CRP. PLoS ONE 2009, 4, e4589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chopra, S.; Polotsky, V.Y.; Jun, J.C. Sleep Apnea Research in Animals. Past, Present, and Future. Am. J. Respir. Cell Mol. Biol. 2016, 54, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Volynets, V.; Reichold, A.; Bárdos, G.; Rings, A.; Bleich, A.; Bischoff, S.C. Assessment of the Intestinal Barrier with Five Different Permeability Tests in Healthy C57BL/6J and BALB/cJ Mice. Dig. Dis. Sci. 2016, 61, 737–746. [Google Scholar] [CrossRef]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef]
| Cytokines (pg/mg) | Control | SF | DSS | DSS + SF |
| IFN-γ | 2.71 ± 0.17 | 5.26 ± 0.39 € | 3.83 ± 0.77 | 3.23 ± 0.75 |
| TNF-α | 38.49 ± 1.46 | 66.29 ± 7.03 € | 44.42 ± 6.51 | 46.44 ± 6.92 |
| IL-1β | 4.09 ± 0.31 | 7.52 ± 0.61 € | 17.30 ± 6.14 ✠ | 47.26 ± 4.20 ✟ |
| IL-6 | 46.19 ± 3.13 | 76.63 ± 4.70 € | 72.91 ± 15.70 | 104.41 ± 13.48 |
| IL-10 | 331.56 ± 6.09 | 566.06 ± 41.86 € | 270.49 ± 24.03 ✠ | 243.02 ± 13.45 |
| IL-23 | 62.47 ± 3.20 | 111.85 ± 11.10 € | 65.54 ± 6.73 | 61.88 ± 10.44 |
| IL-17A | 13.50 ± 0.72 | 27.63 ± 2.49 € | 22.12 ± 3.54 ✠ | 32.13 ± 7.39 |
| IL-22 | 24.59 ± 2.09 | 42.29 ± 3.11 € | 20.76 ± 2.64 | 23.10 ± 3.21 |
| GM-CSF | 6.79 ± 0.56 | 12.27 ± 1.40 € | 5.02 ± 0.91 | 3.06 ± 1.08 |
| IL-4 | 2.67 ± 0.17 | 5.24 ± 0.59 € | 1.95 ± 0.20 ✠ | 2.31 ± 0.34 |
| IL-5 | 16.15 ± 1.05 | 28.58 ± 2.02 € | 9.40 ± 0.98 ✠ | 8.55 ± 0.92 |
| IL-9 | 59.19 ± 2.43 | 105.32 ± 9.90 € | 35.26 ± 3.81 ✠ | 27.80 ± 3.83 |
| IL-13 | 43.62 ± 3.77 | 83.38 ± 7.56 € | 27.82 ± 4.96 ✠ | 20.10 ± 4.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.-H.; Zhuo, X.-C.; Huang, Y.-H.; Liu, H.-M.; Wu, R.-C.; Kuo, C.-J.; Chen, N.-H.; Chuang, L.-P.; Lin, S.-W.; Chen, Y.-L.; et al. Alterations in Gut Microbiota and Upregulations of VPAC2 and Intestinal Tight Junctions Correlate with Anti-Inflammatory Effects of Electroacupuncture in Colitis Mice with Sleep Fragmentation. Biology 2022, 11, 962. https://doi.org/10.3390/biology11070962
Liu G-H, Zhuo X-C, Huang Y-H, Liu H-M, Wu R-C, Kuo C-J, Chen N-H, Chuang L-P, Lin S-W, Chen Y-L, et al. Alterations in Gut Microbiota and Upregulations of VPAC2 and Intestinal Tight Junctions Correlate with Anti-Inflammatory Effects of Electroacupuncture in Colitis Mice with Sleep Fragmentation. Biology. 2022; 11(7):962. https://doi.org/10.3390/biology11070962
Chicago/Turabian StyleLiu, Geng-Hao, Xin-Cheng Zhuo, Yueh-Hsiang Huang, Hsuan-Miao Liu, Ren-Chin Wu, Chia-Jung Kuo, Ning-Hung Chen, Li-Pang Chuang, Shih-Wei Lin, Yen-Lung Chen, and et al. 2022. "Alterations in Gut Microbiota and Upregulations of VPAC2 and Intestinal Tight Junctions Correlate with Anti-Inflammatory Effects of Electroacupuncture in Colitis Mice with Sleep Fragmentation" Biology 11, no. 7: 962. https://doi.org/10.3390/biology11070962
APA StyleLiu, G.-H., Zhuo, X.-C., Huang, Y.-H., Liu, H.-M., Wu, R.-C., Kuo, C.-J., Chen, N.-H., Chuang, L.-P., Lin, S.-W., Chen, Y.-L., Yang, H.-Y., & Lee, T.-Y. (2022). Alterations in Gut Microbiota and Upregulations of VPAC2 and Intestinal Tight Junctions Correlate with Anti-Inflammatory Effects of Electroacupuncture in Colitis Mice with Sleep Fragmentation. Biology, 11(7), 962. https://doi.org/10.3390/biology11070962









