Rodents Prefer Going Downhill All the Way (Gravitaxis) Instead of Taking an Uphill Task
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethical Statement
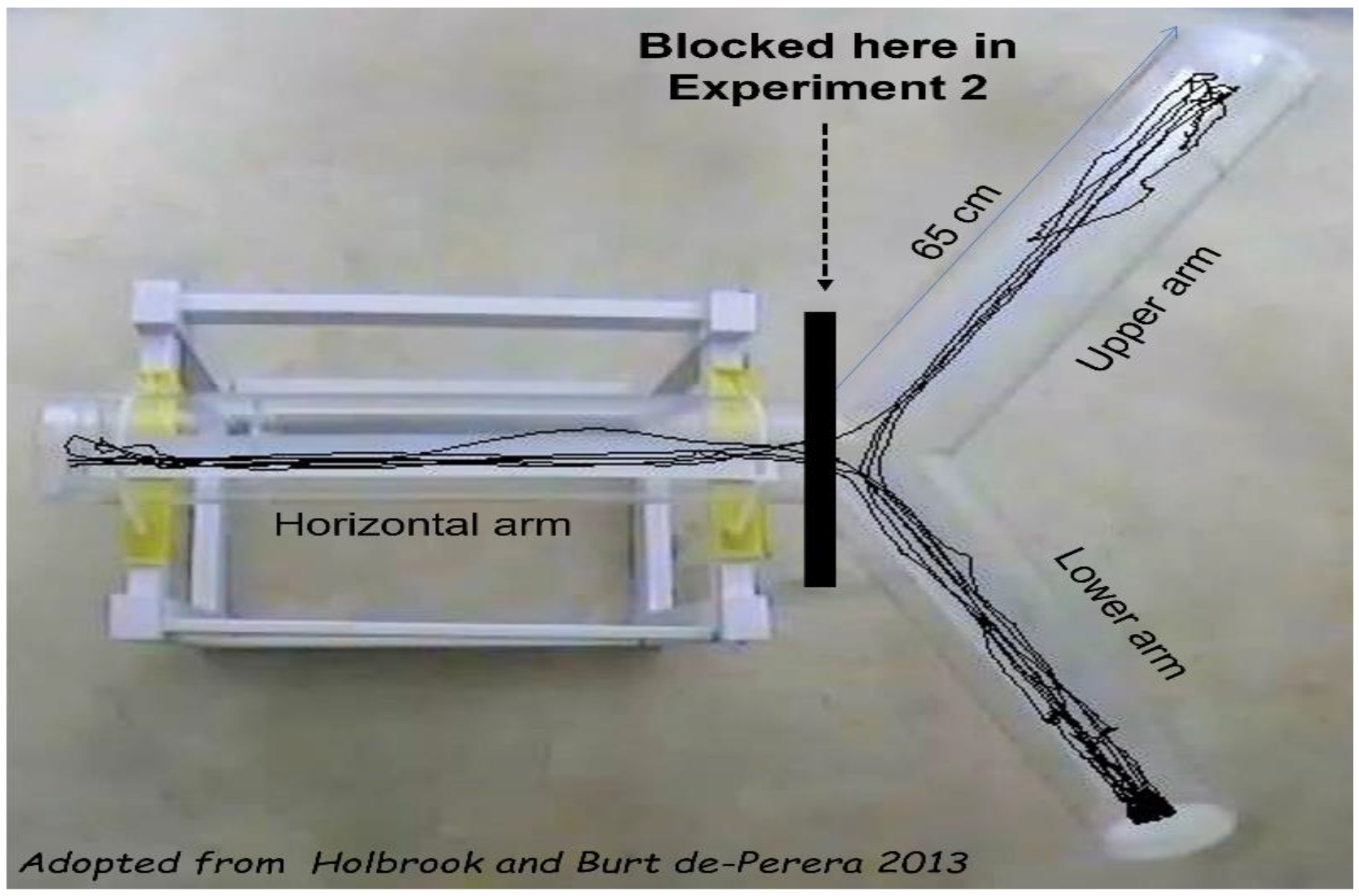
2.3. Apparatus
2.4. Procedure
2.4.1. Data Acquisition and Analysis
2.4.2. Total Distance Traveled
2.4.3. Home-Base Location
2.4.4. First Arm Entered
2.4.5. Traveling along Each Arm at Each Inclination
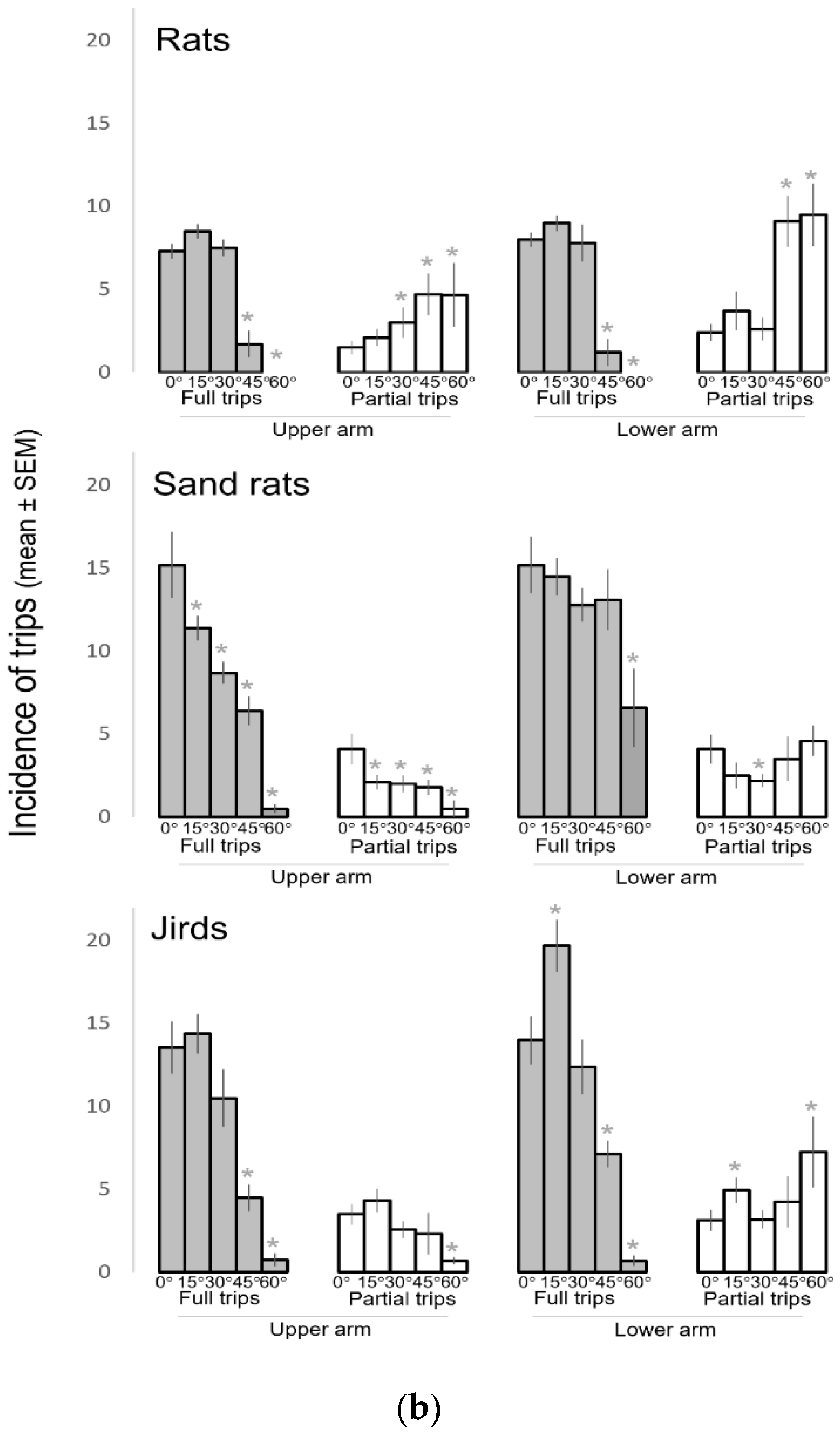
2.4.6. Type of Trips Inside the Inclined Arms
2.5. Statistics
3. Results
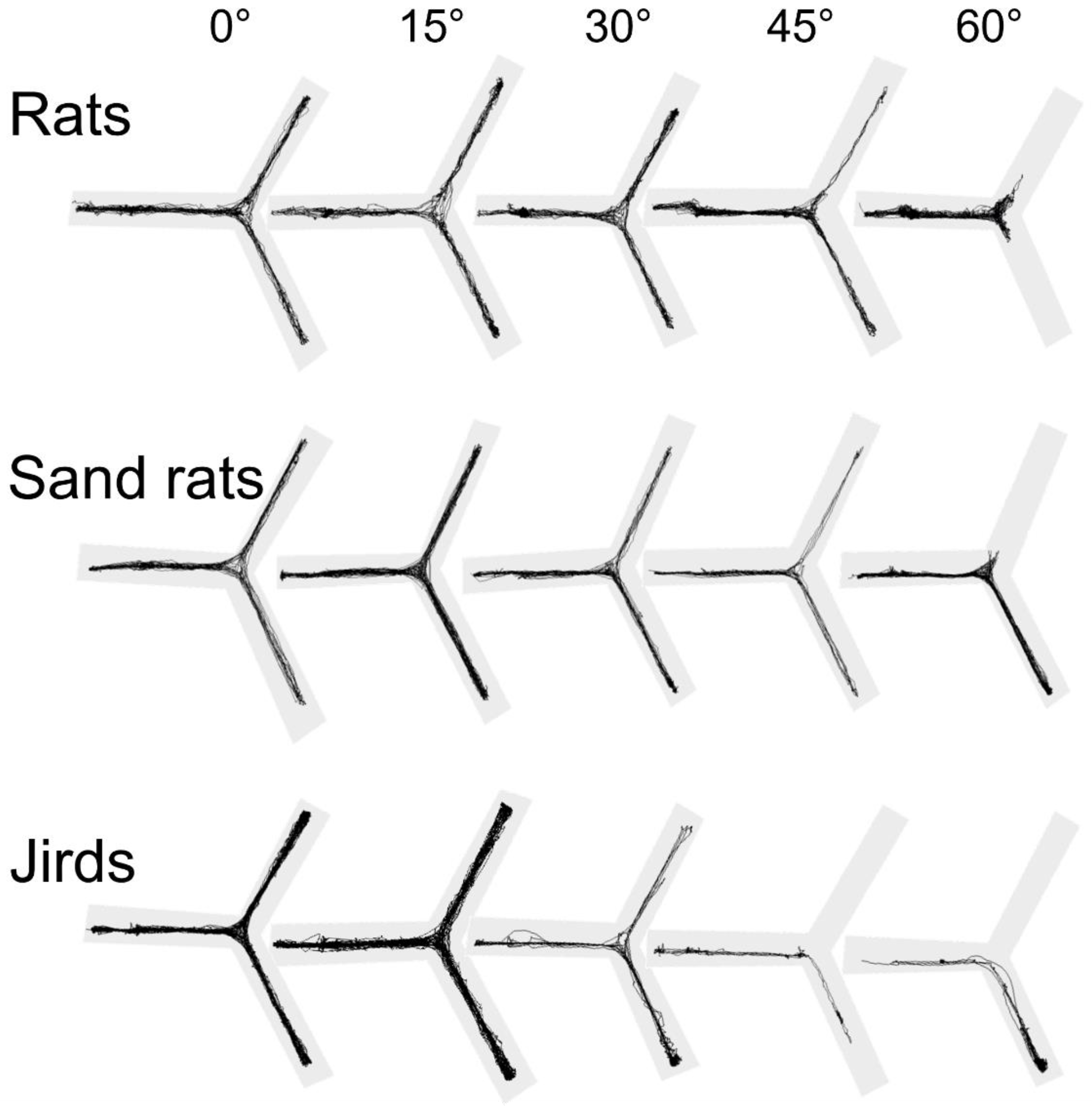
3.1. Activity Decreased with the Increase in Inclination
3.2. Rodents Tended to Establish a Home-Base in the Horizontal Arm
3.3. There Was an Overall Preference to Enter the Lower Arm First
3.4. Traveling along the Inclined Arms Decreased with the Increase in Inclination
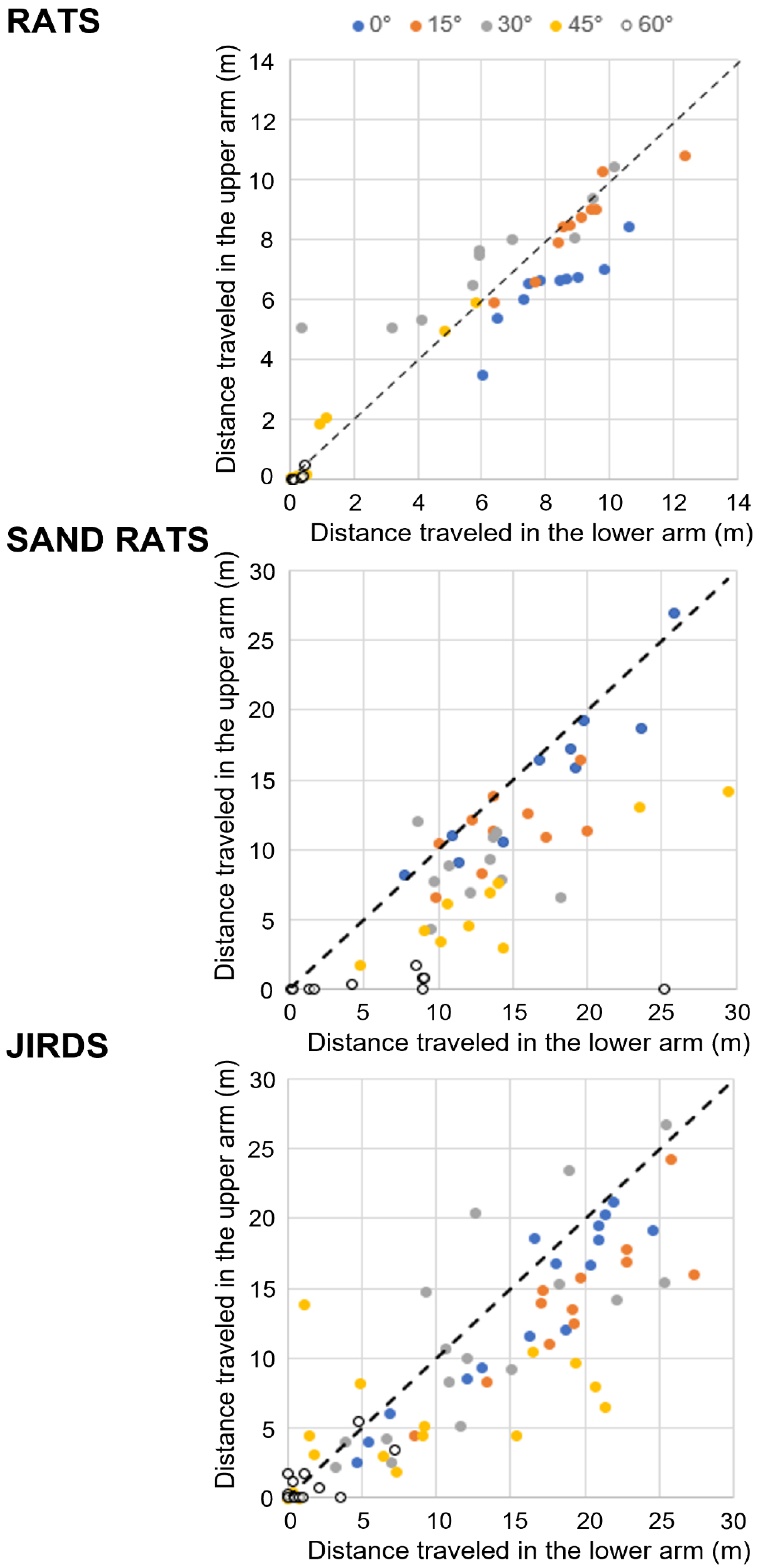
3.5. Sand Rats and Jirds but Not Rats Traveled More along the Lower Arm Than the Upper Arm
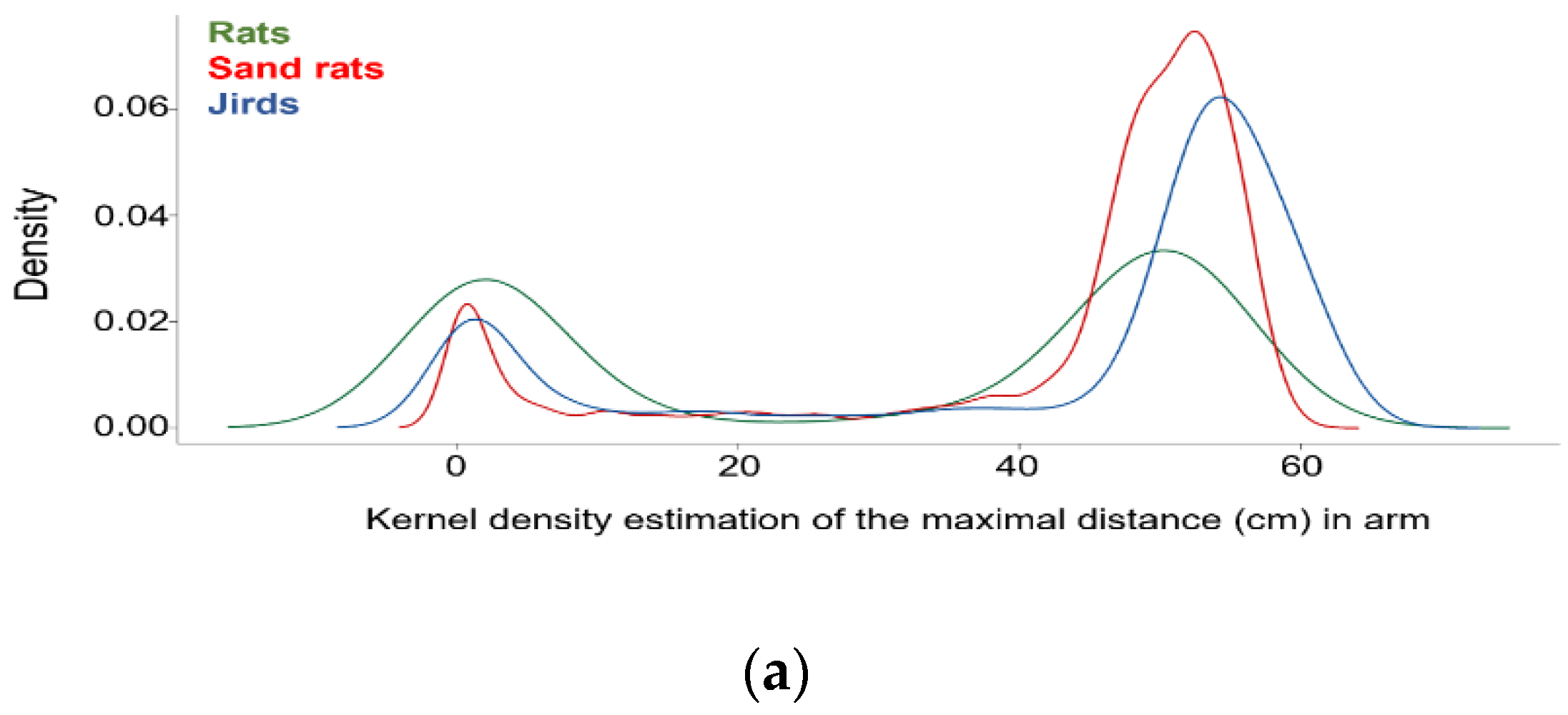
3.6. Rodents Traveled Either All the Way or about Half-Way along the Inclined Arms
3.7. Path Visualization
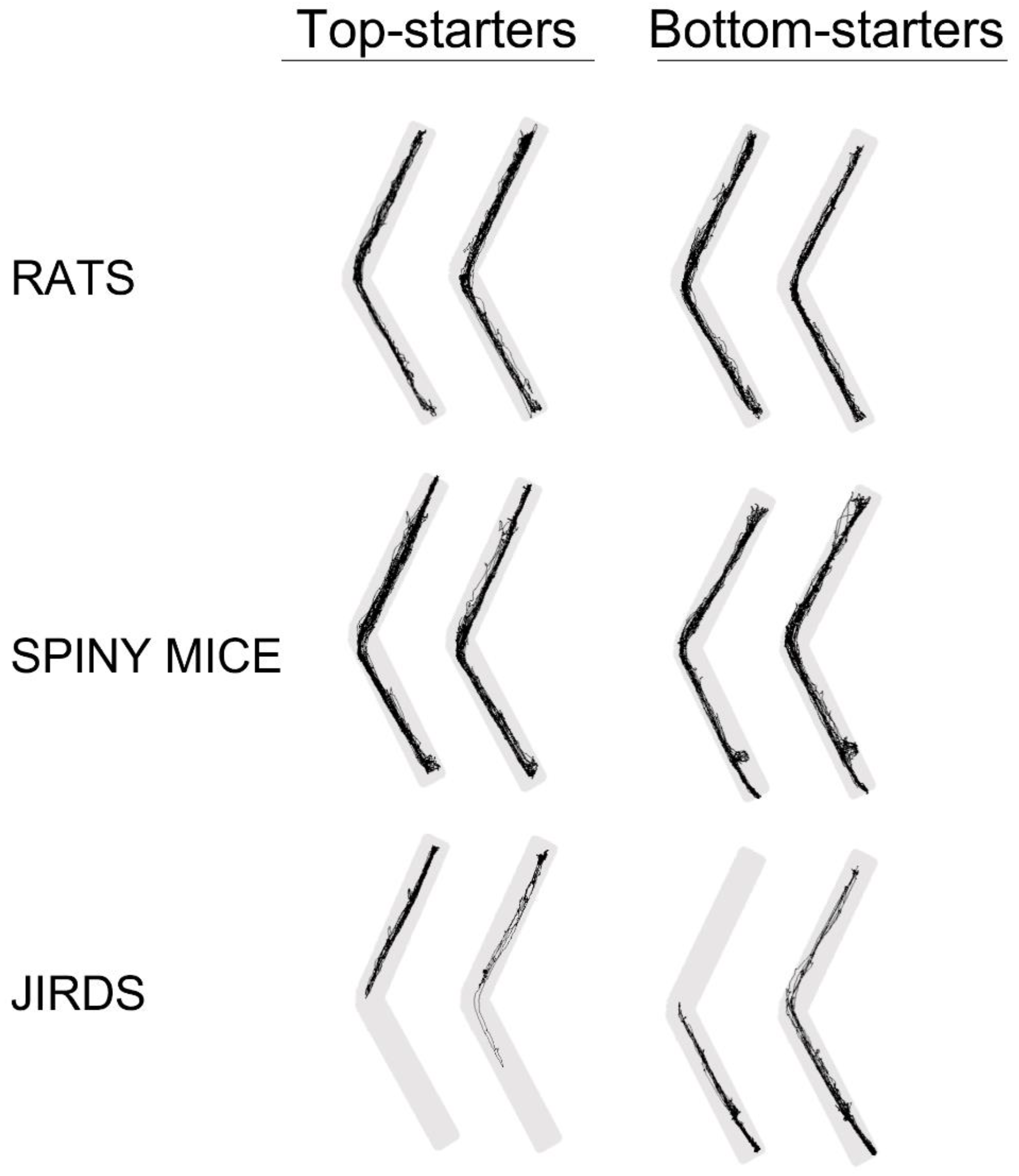
3.8. Experiment 2—The Impact of Start Points
- A.
- In rats, the distance traveled by top-starters and bottom-starters scattered near the line of equivalence, indicating little difference between top- and bottom-starters. This was also the case with spiny mice which are used to ascending and descending in their natural habitat. In contrast, in jirds, which inhabit flatlands, all but one bottom-starter traveled a greater distance along the lower arm while all but one top-starter traveled solely along the upper arm and did not enter the lower arm.
- B.
- In rats and spiny mice, there was a slight trend of top-starters to spend more time in the upper arm, and of bottom starters to spend more time in the lower arm. This became very distinctive in jirds, with all but one bottom-starter spending more time in the lower arm while all but one of the 10 top-starters spent all the time in the upper arm and never entered the lower arm. Moreover, these latter jirds remained at the far top end of the upper arm, where they had been introduced into the apparatus, and remained there for the entire test duration.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tolman, E.C. Purposive Behavior in Animal and Man; University of California Press: Berkley, CA, USA, 1932. [Google Scholar]
- Tolman, E.C. Cognitiva maps in rats and men. Psychobiol. Rev. 1948, 55, 189–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkelstein, A.; Derdikman, D.; Rubin, A.; Foerster, J.N.; Las, L.; Ulanovsky, N. Three-dimensional head-direction coding in the bat brain. Nature 2015, 517, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, A.; Las, L.; Ulanovsky, N. 3-D Maps and Compasses in the Brain. Annu. Rev. Neurosci. 2016, 39, 171–196. [Google Scholar] [CrossRef]
- Voigt, C.C.; Frick, W.F.; Holderied, M.W.; Holland, R.; Kerth, G.; Mello, M.A.; Plowright, R.K.; Swartz, S.; Yovel, Y. Principles and patterns of bat movements: From aerodynamics to ecology. Q. Rev. Biol. 2017, 92, 267–287. [Google Scholar] [CrossRef] [Green Version]
- Holbrook, R.I.; Burt de Perera, T. Fish navigation in the verticaldimension: Can fish use hydrostatic pressure to determine depth? Fish Fish. 2011, 12, 370–379. [Google Scholar] [CrossRef]
- Jedidi-Ayoub, S.; Mishchanchuk, K.; Liu, A.; Renaudineau, S.; Duvelle, É.; Grieves, R.M. Volumetric spatial behaviour in rats reveals the anisotropic organisation of navigation. Anim. Cogn. 2021, 24, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Hagbi, Z.; Dorfman, A.; Blumenfeld-Lieberthal, E.; Eilam, D. “It’s all in their head”: Hierarchical exploration of a three-dimensional layered pyramid in rats. Anim. Cogn. 2020, 23, 277–288. [Google Scholar] [CrossRef]
- Hagbi, Z.; Eilam, D. On heights and plains: How rodents from different habitats cope with three-dimensional environments? PLoS ONE 2022, 17, e0265176. [Google Scholar] [CrossRef]
- Gielman, S.; Hagbi, Z.; Dulitzky, Y.; Blumenfeld-Lieberthal, E.; Eilam, D. How do rodents explore a three-dimensional environment? Habitat-dependent and direction-dependent differences. Behav. Processes 2020, 178, 104183. [Google Scholar] [CrossRef]
- Jovalekic, A.; Hayman, R.; Becares, N.; Reid, H.; Thomas, G.; Wilson, J.; Jeffery, K. Horizontal biases in rats’ use of three-dimensional space. Behav. Brain Res. 2011, 222, 279–288. [Google Scholar] [CrossRef]
- Huck, U.W.; Price, E.O. Effect of the post-weaning environment on the climbing behaviour of wild and domestic Norway rats. Anim. Behav. 1976, 24, 364–371. [Google Scholar] [CrossRef]
- Barnett, S.A. The Rat: A Study in Behavior; Routledge: London, UK, 2017. [Google Scholar]
- Ewer, R.F. The biology and behaviour of a free-living population of black rats (Rattus rattus). Anim. Behav. Monog. 1971, 4, 127–174. [Google Scholar] [CrossRef] [Green Version]
- Makowska, I.J.; Weary, D.M. The importance of burrowing, climbing and standing upright for laboratory rats. R. Soc. Open Sci. 2016, 3, 160136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, A.; Jenner, P.; Marsden, C.D. Differential ability of selective and non-selective dopamine agonists to induce climbing in the rat indicates the involvement of both D-1 and D-2 receptors in this behaviour. Psychopharmacology 1990, 100, 19–26. [Google Scholar] [CrossRef]
- Denenberg, V.H. Open field behavior in the rat: What does it mean? Ann. N. Y. Acad. Sci. 1969, 159, 852–859. [Google Scholar] [CrossRef]
- Hall, C.S.; Ballachey, E.L. A study of the rat’s behaviour in a field: A contribution to methods in comparative psychology. Univ. Calif. Publ. Psychol. 1932, 6, 1–12. [Google Scholar]
- Stanford, S.C. The open field test: Reinventing the wheel. J. Psychopharmacol. 2007, 21, 134–136. [Google Scholar] [CrossRef]
- Walsh, R.N.; Cummins, R.A. The open field test: A critical review. Psychol. Bull. 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Eilam, D.; Golani, I. Home base behavior of rats (Rattus norvegicus) exploring a novel environment. Behav. Brain Res. 1989, 34, 199–211. [Google Scholar] [CrossRef]
- Weiss, O.; Segev, E.; Eilam, D. “Shall two walk together except they be agreed?” Spatial behavior in rat dyads. Anim. Cogn. 2015, 18, 39–51. [Google Scholar] [CrossRef]
- Hagbi, Z.; Segev, E.; Eilam, D. Keep a level head to know the way ahead: How rodents travel on inclined surfaces? iScience 2022, 25, 104424. [Google Scholar] [CrossRef] [PubMed]
- Nemati, F.; Whishaw, I. The point of entry contributes to the organization of exploratory behavior of rats on an open field: An example of spontaneous episodic memory. Behav. Brain Res. 2007, 182, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, K.J.; Jovalekic, A.; Verriotis, M.; Hayman, R. Navigating in a three-dimensional world. Behav. Brain Sci. 2013, 36, 523–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, V.A.; Holbrook, R.I.; Burt de Perera, T. The influence of locomotory style on three-dimensional spatial learning. Anim. Behav. 2018, 142, 39–47. [Google Scholar] [CrossRef]
- Brunjes, P.C. The precocial mouse, Acomys cahirinus. Psychobiol. Rev. 1990, 18, 339–350. [Google Scholar] [CrossRef]
- Greenberg, G. Depth perception in in mogolian gerbils (Meriones unguiculatus) and spiny mice (Acomys russatus and A. cahirinus). J. Comp. Psychol. 1986, 100, 81–84. [Google Scholar] [CrossRef]
- Goldman, M.; Skolnick, A.J.; Hernandez, T.P.; Tobach, E. Distance perception in the spiny mouse Acomys cahirinus: Vertical jumping. Percept. Mot. Skills 1992, 75, 883–895. [Google Scholar] [CrossRef]
- Birke, L.I.A.; D’Udine, B.; Albonetti, M.E. Exploratory behavior of two species of murid rodents, Acomys cahirinus and Mus musculus: A comparative study. Behav. Neural Biol. 1985, 43, 143–161. [Google Scholar] [CrossRef]
- Birke, L.I.A.; Sadler, D. Patterns of exploratory behavior in the spiny mouse, Acomys cahirinus. Behav. Neural Biol. 1986, 45, 88–106. [Google Scholar] [CrossRef]
- Eilam, D. Locomotor activity in common spiny mice (Acomys cahirinuse): The effect of light and environmental complexity. BMC Ecol. 2004, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Marona-Lewicka, D.; Michaluk, J.; Antkiewicz-Michaluk, L.; Vetulani, J. A comparison of locomotor responses to some psychotropic drugs and cerebral receptors in the Acomys cahirinus and the laboratory mouse. Pol. J. Pharmacol. Pharm. 1987, 39, 293–302. [Google Scholar] [PubMed]
- Yerkes, R.M. Space perception of tortoises. J. Comp. Neurol. Psychol. 1904, 14, 17–26. [Google Scholar] [CrossRef] [Green Version]
- McLannahan, H.M. Some aspects of the ontogeny of cliff nesting behavior in the kittiwake (Rissa tridactyla) and the Herring gull (Larus argentatus). Behaviour 1973, 1, 336–388. [Google Scholar]
- Glickman, S.E.; Hartz, K.E. Exploratory behavior in several species of rodents. J. Comp. Physiol. Psychol. 1964, 58, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Cullen, E. Adaptations in the kittiwake to cliff-nesting. IBIS 1957, 99, 275–302. [Google Scholar] [CrossRef]
- Mendelssohn, H.; Yom-Tov, Y. Fauna Palaestina: Mammalia of Israel; Keterpress Enterprises: Jerusalem, Israel, 1999. [Google Scholar]
- Holbrook, R.I.; de Perera, T.B. Three-dimensional spatial cognition: Freely swimming fish accurately learn and remember metric information in a volume. Anim. Behav. 2013, 86, 1077–1083. [Google Scholar] [CrossRef]
- Ellard, C.G.; Goodale, M.A.; Timney, B. Distance estimation in the Mongolian gerbil: The role of dynamic depth cues. Behav. Brain Res. 1984, 14, 29–39. [Google Scholar] [CrossRef]
- Goodale, M.A.; Milner, D.A. Separate visual pathways for perception and action. Trends Neurosci. 1992, 15, 20–25. [Google Scholar] [CrossRef]
- Goodale, M.A.; MIlner, D.A.; Jakobson, L.S.; Carey, D.P. A neurological dissociation between perceiving objects and grasping them. Nature 1991, 349, 154–156. [Google Scholar] [CrossRef]
- Porter, B.S.; Hillman, K.L.; Bilkey, D.K. Anterior cingulate cortex encoding of effortful behavior. J. Neurophysiol. 2019, 121, 701–714. [Google Scholar] [CrossRef]
- Jacobs, L.F.; Schenk, F. Unpacking the cognitive map: The parallel map theory of hippocampal function. Psychol. Rev. 2003, 110, 285–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whishaw, I.Q.; Gharbawie, O.M.; Clark, B.J.; Lehmann, H. The exploratory behavior of rats in an open environment optimizes security. Behav. Brain Res. 2006, 171, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Halliday, M. Exploratory behavior in elevated and enclosed mazes. Q. J. Exp. Psychol. Sect. B Comp. Physiol. Psychol. 1967, 19, 254–263. [Google Scholar]
- Lester, D. Effects of fear upon exploratory behavior. Psychon. Sci. 1967, 9, 117–118. [Google Scholar] [CrossRef]
- Russell, P.A. Relationships between exploratory behaviour and fear: A review. Br. J. Psychol. 1973, 64, 417–433. [Google Scholar] [CrossRef]

| Distance Traveled (m) | |||
|---|---|---|---|
| Inclination | Rats | Sand Rats | Jirds |
| 0° | 30.1 ± 1.9 | 59.9 ± 6.5 | 58.1 ±5.6 |
| 15° | 31.0 ± 1.7 | 47.3 ± 3.4 | 69.9 ± 5.4 |
| 30° | 26.4 ± 1.9 | 40.4 ± 2.1 * | 47.5 ± 5.8 |
| 45° | 19.3 ± 1.9 * | 44.8 ± 6.6 | 33.7 ± 5.5 * |
| 60° | 15.0 ± 1.4 * | 33.2 ± 3.0 * | 26.9 ± 4.1 * |
| Effect of Species | F2,161 = 31.1 | p < 0.0001 | |
| Effect of Inclination | F4,161 = 13.6 | p < 0.0001 | |
| Interaction of Inclination × Species | F8,161 = 2.7 | p = 0.0084 | |
| Inclination | Horizontal Arm (%) | Lower Arm (%) | Upper Arm (%) | |
|---|---|---|---|---|
| Rats (n = 10, except for 60° where n = 6) | 0° | 90 | 10 | 0 |
| 15° | 60 | 10 | 30 | |
| 30° | 70 | 10 | 20 | |
| 45° | 100 | 0 | 0 | |
| 60° | 100 | 0 | 0 | |
| Sand rats (n = 10) | 0° | 70 | 10 | 20 |
| 15° | 50 | 30 | 20 | |
| 30° | 70 | 30 | 0 | |
| 45° | 90 | 10 | 0 | |
| 60° | 90 | 10 | 0 | |
| Jirds (n = 16) | 0° | 44 | 25 | 31 |
| 15° | 25 | 56 | 19 | |
| 30° | 56 | 31 | 13 | |
| 45° | 75 | 19 | 6 | |
| 60° | 88 | 12 | 0 |
| Species | Inclination | Entries to the Lower Arm (%) | Entries to the Upper Arm (%) |
|---|---|---|---|
| Rats (n = 10; except for 60° where n = 6) | 0° | 70 | 30 |
| 15° | 60 | 40 | |
| 30° | 80 | 20 | |
| 45° | 10 | 90 | |
| 60° | 100 | 0 | |
| Sand rats (n = 10) | 0° | 60 | 40 |
| 15° | 50 | 50 | |
| 30° | 50 | 50 | |
| 45° | 100 | 0 | |
| 60° | 100 | 0 | |
| Jirds (n = 16) | 0° | 56 | 44 |
| 15° | 69 | 31 | |
| 30° | 75 | 25 | |
| 45° | 75 | 25 | |
| 60° | 100 | 0 |
| Distance Traveled in Each Arm (m) | ||||
|---|---|---|---|---|
| Inclination | Horizontal | Lower | Upper | |
| Rats | 0° | 15.5 ± 1.2 | 8.1 ± 0.5 | 6.4 ± 0.4 |
| 15° | 13.4 ± 0.9 | 9.0 ± 0.5 | 8.5 ± 0.5 | |
| 30° | 13.1 ± 0.7 * | 6.0 ± 1.0 | 7.3 ± 0.6 | |
| 45° | 16.4 ± 1.2 * | 1.4 ± 0.7 | 1.5 ± 0.7 | |
| 60° | 14.6 ± 1.3 * | 0.2 ± 0.1 | 0.1 ± 0.1 | |
| Sand rats | 0° | 27.8 ± 2.9 | 16.8 ± 1.9 | 15.3 ± 1.9 |
| 15° | 21.4 ± 1.6 | 14.5 ± 1.2 | 11.4 ± 0.9 | |
| 30° | 19.4 ± 1.1 * | 12.4 ± 1.0 | 8.6 ± 0.8 | |
| 45° | 24.2 ± 3.1 * | 14.1 ± 2.4 | 6.5 ± 1.4 | |
| 60° | 26.0 ± 1.2 * | 6.8 ± 2.5 | 0.4 ± 0.2 | |
| Jirds | 0° | 26.6 ± 2.3 | 17.0 ± 1.9 | 14.6 ± 1.9 |
| 15° | 31.1 ± 2.0 | 22.6 ± 1.9 | 16.1 ± 1.4 | |
| 30° | 22.5 ± 2.6 * | 13.3 ± 1.9 | 11.7 ± 2.1 | |
| 45° | 20.0 ± 3.3 * | 8.5 ± 2.1 | 5.3 ± 1.0 | |
| 60° | 24.5 ± 3.9 * | 1.5 ± 0.6 | 0.9 ± 0.4 | |
| Effect of Species | F1,161 = 31.1 | p < 0.0001 | ||
| Effect of Inclination | F1,161 = 13.6 | p < 0.0001 | ||
| Interaction of Inclination × Species | F1,161 = 2.7 | p = 0.0084 | ||
| Effect of Arms | F2,322 = 513.6 | p < 0.0001 | ||
| Interaction of Arm × Species | F4,322 = 8.3 | p < 0.0001 | ||
| Interaction of Arm × Inclination | F8,322 = 15.4 | p < 0.0001 | ||
| Interaction of Arm × Species × Inclination | F16,322 = 2.0 | p = 0.0142 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-Shaul, Y.; Hagbi, Z.; Dorfman, A.; Zadicario, P.; Eilam, D. Rodents Prefer Going Downhill All the Way (Gravitaxis) Instead of Taking an Uphill Task. Biology 2022, 11, 1090. https://doi.org/10.3390/biology11071090
Ben-Shaul Y, Hagbi Z, Dorfman A, Zadicario P, Eilam D. Rodents Prefer Going Downhill All the Way (Gravitaxis) Instead of Taking an Uphill Task. Biology. 2022; 11(7):1090. https://doi.org/10.3390/biology11071090
Chicago/Turabian StyleBen-Shaul, Yehonatan, Zohar Hagbi, Alex Dorfman, Pazit Zadicario, and David Eilam. 2022. "Rodents Prefer Going Downhill All the Way (Gravitaxis) Instead of Taking an Uphill Task" Biology 11, no. 7: 1090. https://doi.org/10.3390/biology11071090
APA StyleBen-Shaul, Y., Hagbi, Z., Dorfman, A., Zadicario, P., & Eilam, D. (2022). Rodents Prefer Going Downhill All the Way (Gravitaxis) Instead of Taking an Uphill Task. Biology, 11(7), 1090. https://doi.org/10.3390/biology11071090





