Development of Long-Acting Human Adrenomedullin Fc-Fusion Proteins
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cloning and Production of Recombinant Proteins
2.2. Biological Activity
2.3. Pharmacokinetics
2.4. Sample Preparation for Enzyme-Linked Immuno Sorbent Assay (ELISA)
2.5. Effect on Blood Pressure
2.6. Statistical Analysis
3. Results
3.1. Properties of Fc-AM
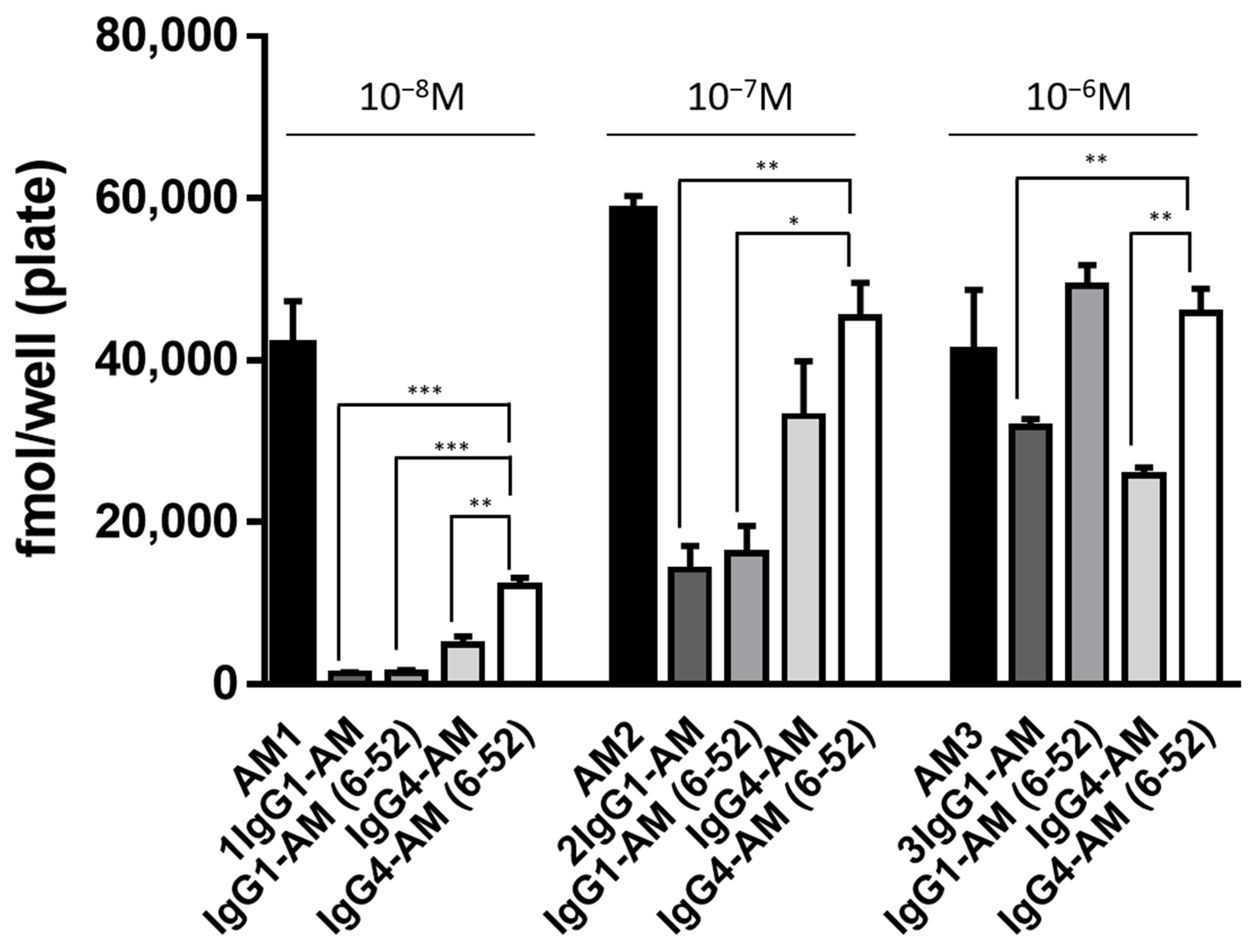
3.2. Intracellular cAMP Accumulation Induced by Fc-Fusion Proteins
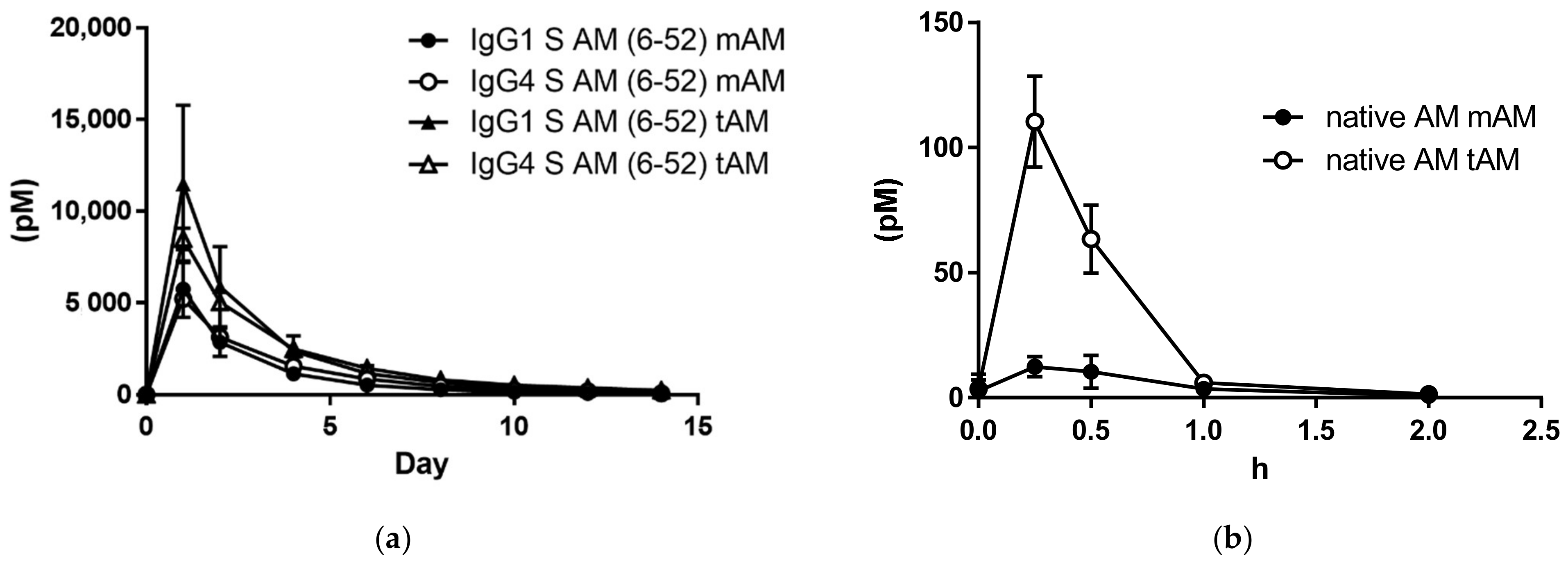
3.3. Plasma Concentrations after a Single Subcutaneous Administration of IgG1-AM (6-52) or IgG4-AM (6-52) to Rats
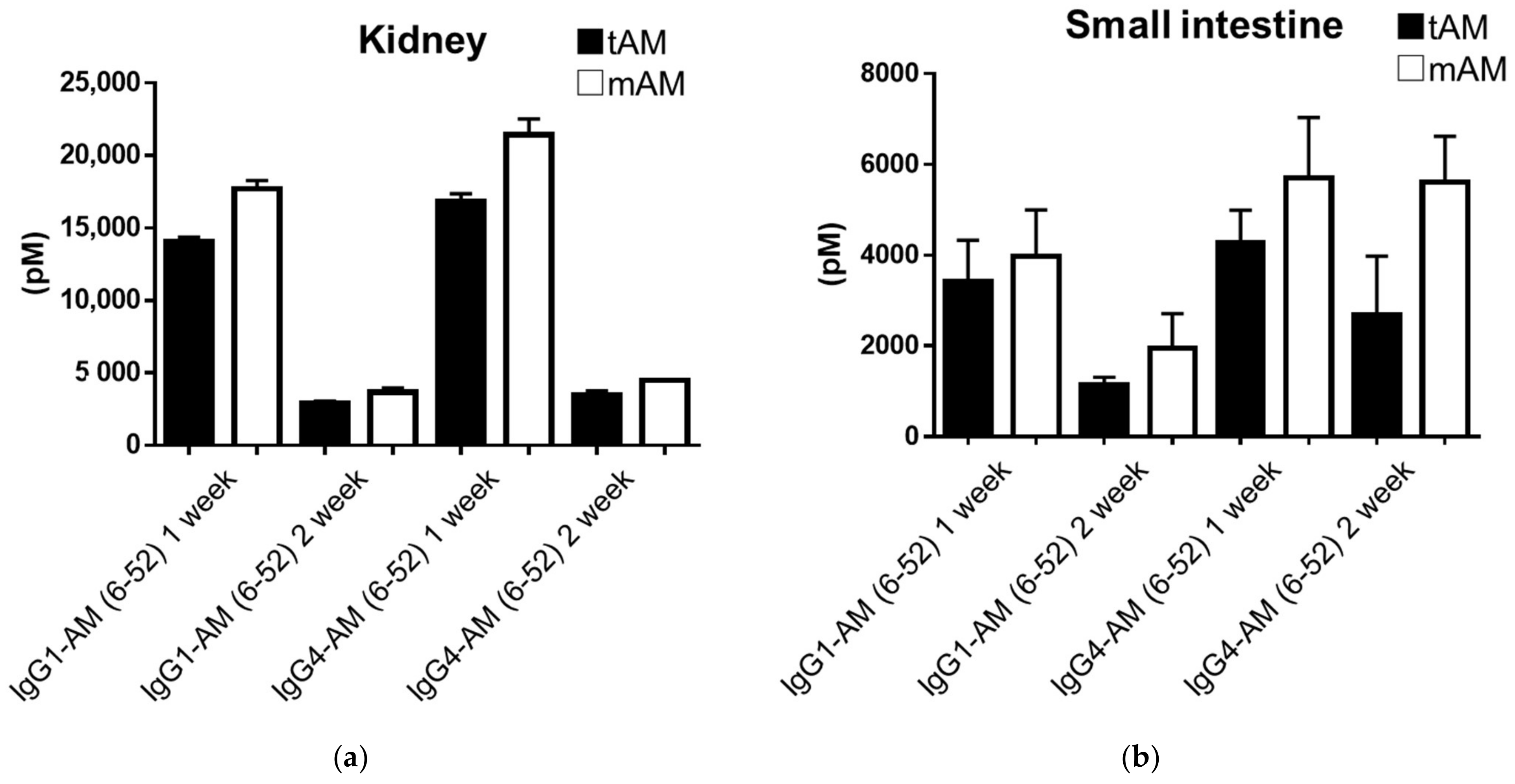
3.4. Concentrations of IgG1-AM (6-52) and IgG4-AM (6-52) in Rat Tissues
3.5. Hypotensive Effect of IgG4-AM (6-52) on SHRs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kitamura, K.; Kangawa, K.; Kawamoto, M.; Ichiki, Y.; Nakamura, S.; Matsuo, H.; Eto, T. Adrenomedullin: A novel hypotensive peptide isolated from human pheochromocytoma. Biochem. Biophys. Res. Commun. 1993, 192, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Hikosaka, T.; Kitamura, K. Effect of adrenomedullin administration in two rat models of experimental inflammatory bowel disease. Am. J. Life Sci. 2015, 3, 39–42. [Google Scholar] [CrossRef][Green Version]
- Nakamura, R.; Kato, J.; Kitamura, K.; Onitsuka, H.; Imamura, T.; Cao, Y.; Marutsuka, K.; Asada, Y.; Kangawa, K.; Eto, T. Adrenomedullin administration immediately after myocardial infarction ameliorates progression of heart failure in rats. Circulation 2004, 110, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Pugin, J. Adrenomedullin: A vasodilator to treat sepsis? Crit. Care 2014, 18, 152. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ponz, M.; Rodrigo-Gasque, C.; Siles, E.; Martinez-Lara, E.; Ochoa-Callejero, L.; Martinez, A. Temporal profiles of blood pressure, circulating nitric oxide, and adrenomedullin as predictors of clinical outcome in acute ischemic stroke patients. Mol. Med. Rep. 2016, 13, 3724–3734. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Nagata, S.; Akashi, E.; Yamasaki, M.; Kitamura, K. Thrombin rapidly digests adrenomedullin: Synthesis of adrenomedullin analogs resistant to thrombin. Biochem. Biophys. Res. Commun. 2020, 529, 778–783. [Google Scholar] [CrossRef]
- Nagata, S.; Yamasaki, M.; Kitamura, K. Anti-Inflammatory Effects of PEGylated Human Adrenomedullin in a Mouse DSS-Induced Colitis Model. Drug Dev. Res. 2017, 78, 129–134. [Google Scholar] [CrossRef]
- Kuroishi, N.; Nagata, S.; Akashi, E.; Ashizuka, S.; Kato, J.; Yamasaki, M.; Kitamura, K. Development of a novel human adrenomedullin derivative: Human serum albumin-conjugated adrenomedullin. J. Biochem. 2021, 170, 445–451. [Google Scholar] [CrossRef]
- Geven, C.; Peters, E.; Schroedter, M.; Struck, J.; Bergmann, A.; McCook, O.; Radermacher, P.; Kox, M.; Pickkers, P. Effects of the Humanized Anti-Adrenomedullin Antibody Adrecizumab (HAM8101) on Vascular Barrier Function and Survival in Rodent Models of Systemic Inflammation and Sepsis. Shock 2018, 50, 648–654. [Google Scholar] [CrossRef]
- Blet, A.; Deniau, B.; Geven, C.; Sadoune, M.; Caillard, A.; Kounde, P.R.; Polidano, E.; Pickkers, P.; Samuel, J.L.; Mebazaa, A. Adrecizumab, a non-neutralizing anti-adrenomedullin antibody, improves haemodynamics and attenuates myocardial oxidative stress in septic rats. Intensive Care Med. Exp. 2019, 7, 25. [Google Scholar] [CrossRef]
- Laterre, P.F.; Pickkers, P.; Marx, G.; Wittebole, X.; Meziani, F.; Dugernier, T.; Huberlant, V.; Schuerholz, T.; Francois, B.; Lascarrou, J.B.; et al. Safety and tolerability of non-neutralizing adrenomedullin antibody adrecizumab (HAM8101) in septic shock patients: The AdrenOSS-2 phase 2a biomarker-guided trial. Intensive Care Med. 2021, 47, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Nagata, S.; Yamasaki, M.; Kawano, A.; Kitamura, K. Developments of human adrenomedullin-IgG1 Fc fusion proteins. J. Biochem. 2019, 166, 157–162. [Google Scholar] [CrossRef]
- Hakim, R.; Benhar, I. “Inclonals”: IgGs and IgG-enzyme fusion proteins produced in an E. coli expression-refolding system. MAbs 2009, 1, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Mammalian cell protein expression for biopharmaceutical production. Biotechnol. Adv. 2012, 30, 1158–1170. [Google Scholar] [CrossRef]
- Kuwasako, K.; Shimekake, Y.; Masuda, M.; Nakahara, K.; Yoshida, T.; Kitaura, M.; Kitamura, K.; Eto, T.; Sakata, T. Visualization of the calcitonin receptor-like receptor and its receptor activity-modifying proteins during internalization and recycling. J. Biol. Chem. 2000, 275, 29602–29609. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Tsuji, T.; Asai, S.; Sasakura, K.; Teraoka, H.; Kitamura, K.; Kangawa, K. One-step direct assay for mature-type adrenomedullin with monoclonal antibodies. Clin. Chem. 1999, 45, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Tsuji, T.; Asai, S.; Tanizaki, S.; Sasakura, K.; Teraoka, H.; Kitamura, K.; Kangawa, K. A simple immunoradiometric assay for measuring the entire molecules of adrenomedullin in human plasma. Clin. Chim. Acta. 1999, 287, 131–143. [Google Scholar] [CrossRef]
- Beck, A.; Reichert, J.M. Therapeutic Fc-fusion proteins and peptides as successful alternatives to antibodies. MAbs 2011, 3, 415–416. [Google Scholar] [CrossRef]
- Glaesner, W.; Vick, A.M.; Millican, R.; Ellis, B.; Tschang, S.H.; Tian, Y.; Bokvist, K.; Brenner, M.; Koester, A.; Porksen, N.; et al. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab. Res. Rev. 2010, 26, 287–296. [Google Scholar] [CrossRef]
- McLatchie, L.M.; Fraser, N.J.; Main, M.J.; Wise, A.; Brown, J.; Thompson, N.; Solari, R.; Lee, M.G.; Foord, S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 1998, 393, 333–339. [Google Scholar] [CrossRef]
- Kita, T.; Kaji, Y.; Kitamura, K. Safety, Tolerability, and Pharmacokinetics of Adrenomedullin in Healthy Males: A Randomized, Double-Blind, Phase 1 Clinical Trial. Drug Des. Dev. Ther. 2020, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kita, T.; Ashizuka, S.; Ohmiya, N.; Yamamoto, T.; Kanai, T.; Motoya, S.; Hirai, F.; Nakase, H.; Moriyama, T.; Nakamura, M.; et al. Adrenomedullin for steroid-resistant ulcerative colitis: A randomized, double-blind, placebo-controlled phase-2a clinical trial. J. Gastroenterol. 2021, 56, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Akashi, E.; Nagata, S.; Yamasaki, M.; Kitamura, K. Activation of Calcitonin Gene-Related Peptide and Adrenomedullin Receptors by PEGylated Adrenomedullin. Biol. Pharm. Bull. 2020, 43, 1799–1803. [Google Scholar] [CrossRef] [PubMed]
- Miki, G.; Kuroishi, N.; Tokashiki, M.; Nagata, S.; Tamura, M.; Yoshiya, T.; Yoshizawa-Kumagaye, K.; Ashizuka, S.; Kato, J.; Yamasaki, M.; et al. 20 kDa PEGylated Adrenomedullin as a New Therapeutic Candidate for Inflammatory Bowel Disease. Gastrointest. Disord. 2020, 2, 366–377. [Google Scholar] [CrossRef]
- Kubo, K.; Tokashiki, M.; Kuwasako, K.; Tamura, M.; Tsuda, S.; Kubo, S.; Yoshizawa-Kumagaye, K.; Kato, J.; Kitamura, K. Biological properties of adrenomedullin conjugated with polyethylene glycol. Peptides 2014, 57, 118–121. [Google Scholar] [CrossRef]
- Sockolosky, J.T.; Szoka, F.C. The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy. Adv. Drug Deliv. Rev. 2015, 91, 109–124. [Google Scholar] [CrossRef]
- Alonso Martinez, L.M.; Harel, F.; Nguyen, Q.T.; Letourneau, M.; D’Oliviera-Sousa, C.; Meloche, B.; Finnerty, V.; Fournier, A.; Dupuis, J.; DaSilva, J.N. Al[(18)F]F-complexation of DFH17, a NOTA-conjugated adrenomedullin analog, for PET imaging of pulmonary circulation. Nucl. Med. Biol. 2018, 67, 36–42. [Google Scholar] [CrossRef]
- Hay, D.L.; Smith, D.M. Adrenomedullin receptors: Molecular identity and function. Peptides 2001, 22, 1753–1763. [Google Scholar] [CrossRef]
- Pio, R.; Martinez, A.; Unsworth, E.J.; Kowalak, J.A.; Bengoechea, J.A.; Zipfel, P.F.; Elsasser, T.H.; Cuttitta, F. Complement factor H is a serum-binding protein for adrenomedullin, and the resulting complex modulates the bioactivities of both partners. J. Biol. Chem. 2001, 276, 12292–12300. [Google Scholar] [CrossRef]
- Petrie, M.C.; McDonald, J.E.; Hillier, C.; Morton, J.J.; McMurray, J.J. Effects of adrenomedullin on angiotensin II stimulated atrial natriuretic peptide and arginine vasopressin secretion in healthy humans. Br. J. Clin. Pharmacol. 2001, 52, 165–168. [Google Scholar] [CrossRef]
- Hay, D.L.; Garelja, M.L.; Poyner, D.R.; Walker, C.S. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review 25. Br. J. Pharmacol. 2018, 175, 3–17. [Google Scholar] [CrossRef] [PubMed]

| mAM | tAM | ||||
|---|---|---|---|---|---|
| IgG1 S AM (6-52) | IgG4 S AM (6-52) | IgG1 S AM (6-52) | IgG4 S AM (6-52) | ||
| t1/2 (days) | 2.110 | 2.370 | t1/2 (days) | 2.885 | 3.235 |
| Elimination rate constant (K) (1/days) | 0.332 | 0.296 | Elimination rate constant (K) (1/days) | 0.240 | 0.216 |
| Vz/F (L/kg) | 6.640 | 6.065 | Vz/F (L/kg) | 4.360 | 5.370 |
| CL/F (L/day/kg) | 2.135 | 1.775 | CL/F (L/day/kg) | 1.047 | 1.165 |
| Day | Raw data (pM) | Day | Raw data (pM) | ||
| 0 | 31.2 | 0.1 | 0 | 35.9 | 0.5 |
| 1 | 5757.4 | 5220.1 | 1 | 11,499.8 | 8486.3 |
| 2 | 2865.0 | 3135.1 | 2 | 5892.1 | 5040.4 |
| 4 | 1145.6 | 1561.8 | 4 | 2376.1 | 2484.5 |
| 6 | 530.1 | 857.3 | 6 | 1132.8 | 1433.5 |
| 8 | 281.1 | 452.0 | 8 | 663.7 | 813.2 |
| 10 | 141.0 | 260.0 | 10 | 391.3 | 517.0 |
| 12 | 81.5 | 185.5 | 12 | 245.1 | 380.9 |
| 14 | 34.0 | 81.3 | 14 | 147.8 | 235.6 |
| tAM | IgG1-AM (6-52) | IgG4-AM (6-52) | ||
|---|---|---|---|---|
| (pmol/g protein) | Day 7 | Day 14 | Day 7 | Day 14 |
| Brain | 2.49 | 1.23 | 2.10 | 0.90 |
| Lung | 7.66 | 3.73 | 10.37 | 2.13 |
| Heart | 4.22 | 1.57 | 4.21 | 1.37 |
| Kidney | 53.53 | 10.93 | 42.05 | 8.63 |
| Adrenal gland | 2.68 | 1.52 | 2.83 | 1.10 |
| Liver | 0.46 | 0.12 | 0.31 | 0.13 |
| Pancreas | 1.79 | 0.48 | 0.59 | 0.21 |
| Spleen | 1.42 | 0.40 | 1.37 | 0.48 |
| Large intestine | 4.74 | 1.27 | 4.20 | 0.92 |
| Small intestine | 12.95 | 4.30 | 10.69 | 6.73 |
| Stomach | 3.38 | 0.59 | 2.24 | 0.98 |
| mAM | IgG1-AM (6-52) | IgG4-AM (6-52) | ||
|---|---|---|---|---|
| (pmol/g protein) | Day 7 | Day 14 | Day 7 | Day 14 |
| Brain | 2.07 | 1.14 | 1.67 | 0.75 |
| Lung | 5.82 | 3.46 | 8.30 | 1.67 |
| Heart | 3.55 | 1.58 | 3.29 | 1.10 |
| Kidney | 76.05 | 15.86 | 57.86 | 12.06 |
| Adrenal gland | 3.63 | 2.29 | 2.54 | 1.29 |
| Liver | 0.30 | 0.11 | 0.22 | 0.11 |
| Pancreas | 1.55 | 0.51 | 0.68 | 0.34 |
| Spleen | 0.98 | 0.37 | 1.00 | 0.36 |
| Large intestine | 26.79 | 1.48 | 6.00 | 1.48 |
| Small intestine | 17.09 | 8.40 | 15.41 | 15.15 |
| Stomach | 4.75 | 0.64 | 2.61 | 1.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagata, S.; Yamasaki, M.; Kuroishi, N.; Kitamura, K. Development of Long-Acting Human Adrenomedullin Fc-Fusion Proteins. Biology 2022, 11, 1074. https://doi.org/10.3390/biology11071074
Nagata S, Yamasaki M, Kuroishi N, Kitamura K. Development of Long-Acting Human Adrenomedullin Fc-Fusion Proteins. Biology. 2022; 11(7):1074. https://doi.org/10.3390/biology11071074
Chicago/Turabian StyleNagata, Sayaka, Motoo Yamasaki, Nobuko Kuroishi, and Kazuo Kitamura. 2022. "Development of Long-Acting Human Adrenomedullin Fc-Fusion Proteins" Biology 11, no. 7: 1074. https://doi.org/10.3390/biology11071074
APA StyleNagata, S., Yamasaki, M., Kuroishi, N., & Kitamura, K. (2022). Development of Long-Acting Human Adrenomedullin Fc-Fusion Proteins. Biology, 11(7), 1074. https://doi.org/10.3390/biology11071074






