Reference Intervals for Blood Biomarkers in Farmed Atlantic Salmon, Coho Salmon and Rainbow Trout in Chile: Promoting a Preventive Approach in Aquamedicine
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Pre-Analytical Stage
2.1.1. Fish Selection and Catching
2.1.2. Anesthetic Procedure
2.1.3. Blood Sampling Procedure
2.1.4. Procedure for Transport, Preparation and Storage of Samples at the Laboratory
2.2. Analytical Stage
2.2.1. Hemotological and Blood Gasometry Biomarkers
2.2.2. Blood Biochemistry and Hormones Biomarkers
2.3. Post-Analytical Stage
2.3.1. Differences between Salmon Species and Age Ranges
2.3.2. Reference Intervals (RIs) and Confidence Intervals (CIs)
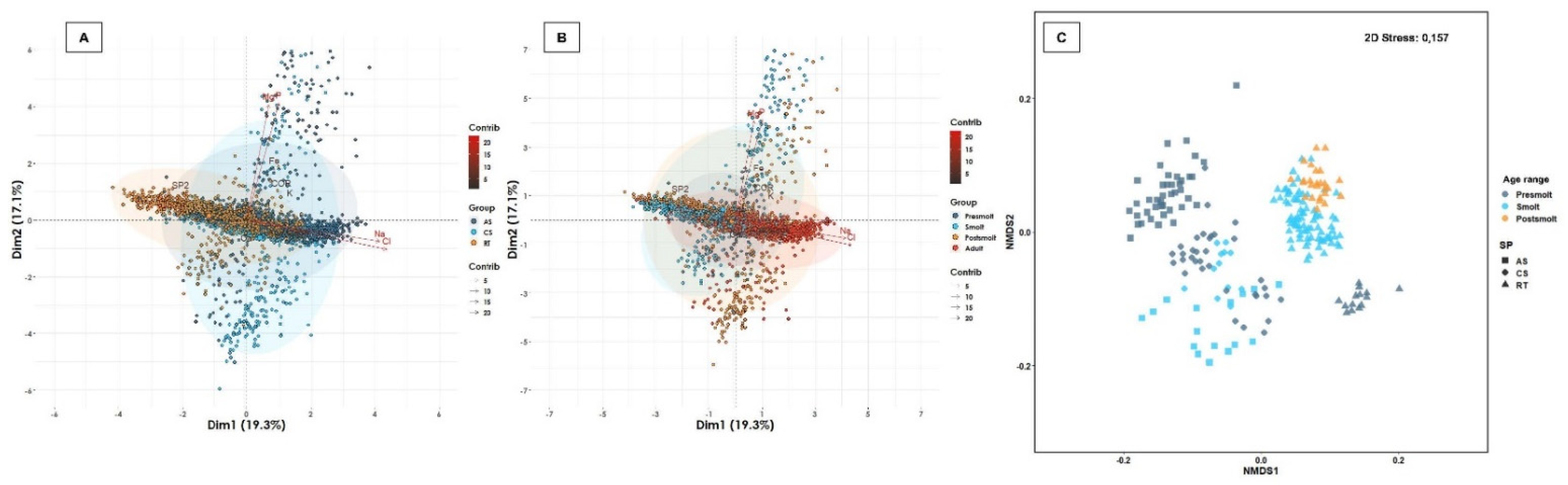
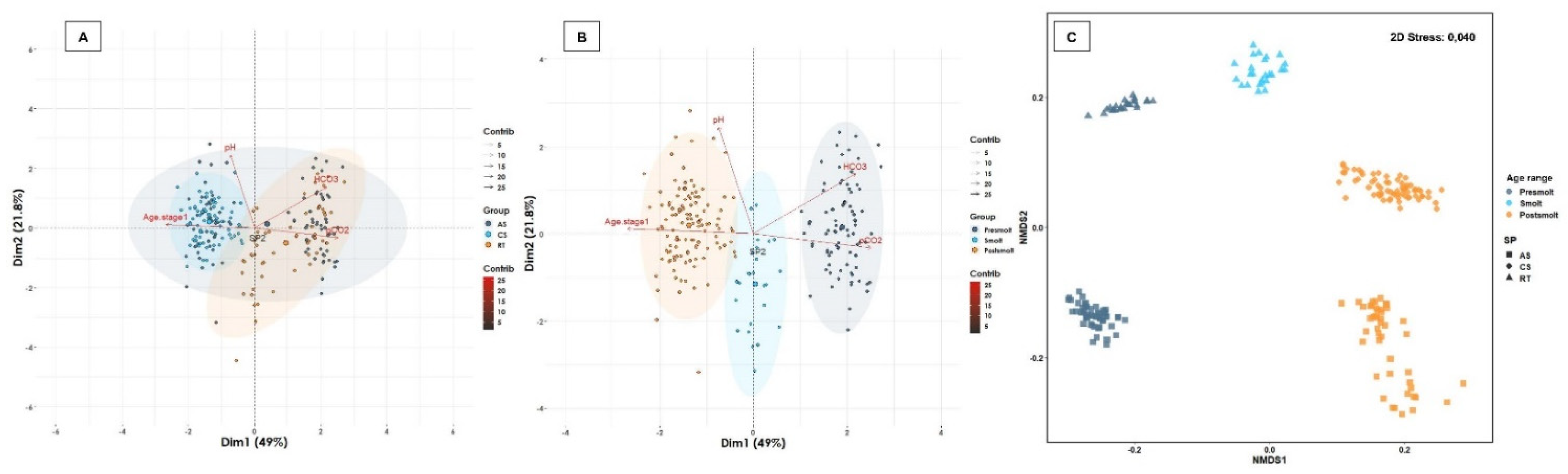
2.3.3. Multivariate Analysis
3. Results
3.1. Erythrogram
3.2. Leukogram
3.3. Plasma Substrates
3.4. Plasma Enzymes
3.5. Plasma Electrolytes and Minerals, Cortisol and Blood Gases
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Subsecretaría de Pesca y Acuicultura de Chile, S. Informe Sectorial de Pesca Y Acuicultura Consolidado 2020–2021; Subpesca: Valparaíso, Chile, 2022; p. 11. [Google Scholar]
- Burgos-Aceves, A.M.; Lionetti, L.; Faggio, C. Multidisciplinary haematology as prognostic device in environmental and xenobiotic stress-induced response in fish. Sci. Total Environ. 2019, 670, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- Parrino, V.; Cappello, T.; Costa, G.; Cannavà, C.; Sanfilippo, M.; Fazio, F.; Fasulo, S. Comparative study of haematology of two teleost fish (Mugil cephalus and Carassius auratus) from different environments and feeding habits. Eur. Zool. J. 2018, 85, 193–199. [Google Scholar] [CrossRef]
- Wade, N.M.; Clark, T.D.; Maynard, B.T.; Atherton, S.; Wilkinson, R.J.; Smullen, R.P.; Taylor, R.S. Effects of an unprecedented summer heatwave on the growth performance, flesh colour and plasma biochemistry of marine cage-farmed Atlantic salmon (Salmo salar). J. Therm. Biol. 2019, 80, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Seibel, H.; Bassmann, B.; Rebl, A. Blood Will Tell: What Hematological Analyses Can Reveal About Fish Welfare. Front. Vet. Sci. 2021, 8, 616955. [Google Scholar] [CrossRef]
- Witeska, M.; Kondera, E.; Ługowska, K.; Bojarski, B. Hematological methods in fish–Not only for beginners. Aquaculture 2022, 547, 737498. [Google Scholar] [CrossRef]
- Smith, L.S. Blood Volumes of Three Salmonids. J. Fish. Res. Board Can. 1966, 23, 1439–1446. [Google Scholar] [CrossRef]
- Lamas, J.; Ellis, A.E. Atlantic salmon (Salmo salar) neutrophil responses to Aeromonas salmonicida. Fish Shellfish. Immunol. 1994, 4, 201–219. [Google Scholar] [CrossRef]
- Claver, J.A.; Quaglia, A.I.E. Comparative Morphology, Development, and Function of Blood Cells in Nonmammalian Vertebrates. J. Exot. Pet Med. 2009, 18, 87–97. [Google Scholar] [CrossRef]
- Saunders, D.C. Differential blood cell counts of 121 species of marine fishes of Puerto Rico. Trans. Am. Microsc. Soc. 1966, 85, 427–449. [Google Scholar] [CrossRef]
- Tavares-Dias, M.; Moraes, F.R. Morphological, cytochemical, and ultrastructural study of thrombocytes and leukocytes in neotropical fish, Brycon orbignyanus Valenciennes, 1850 (Characidae, Bryconinae). J. Submicrosc. Cytol. Pathol. 2006, 38, 209–215. [Google Scholar]
- Fazio, F.; Filiciotto, F.; Marafioti, S.; Di Stefano, V.; Assenza, A.; Placenti, F.; Buscaino, G.; Piccione, G.; Mazzola, S. Automatic analysis to assess haematological parameters in farmed gilthead sea bream (Sparus aurata Linnaeus, 1758). Mar. Freshw. Behav. Physiol. 2012, 45, 63–73. [Google Scholar] [CrossRef]
- Fazio, F.; Saoca, C.; Costa, G.; Zumbo, A.; Piccione, G.; Parrino, V. Flow cytometry and automatic blood cell analysis in striped bass Morone saxatilis (Walbaum, 1792): A new hematological approach. Aquaculture 2019, 513, 734398. [Google Scholar] [CrossRef]
- Shouval, R.; Fein, J.A.; Savani, B.; Mohty, M.; Nagler, A. Machine learning and artificial intelligence in haematology. Br. J. Haematol. 2021, 192, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Gültepe, Y.; Gültepe, N. Preliminary Study for the Evaluation of the Hematological Blood Parameters of Seabream with Machine Learning Classification Methods. Mar. Freshw. Behav. Physiol. 2020, 42, 63–73. [Google Scholar]
- Mani, S.R.; Kandhasamy, P.; Vijayan, K.; Palanichamy, M.; Jacob, J.P.; Kandhasamy, S. Haematological parameters of Cyprinus carpio with reference to probiotic feed: A machine learning approach. Isr. J. Aquac. Bamidgeh 2021, 73, 1–10. [Google Scholar] [CrossRef]
- Ahmed, I.; Reshi, Q.M.; Fazio, F. The influence of the endogenous and exogenous factors on hematological parameters in different fish species: A review. Aquac. Int. 2020, 28, 869–899. [Google Scholar] [CrossRef]
- Petri, D.; Glover, C.N.; Ylving, S.; Kolås, K.; Fremmersvik, G.; Waagbø, R.; Berntssen, M.H. Sensitivity of Atlantic salmon (Salmo salar) to dietary endosulfan as assessed by haematology, blood biochemistry, and growth parameters. Aquat. Toxicol. 2006, 80, 207–216. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, I.; Dar, N.A. Effects of dietary leucine levels on growth performance, hematobiochemical parameters, liver profile, intestinal enzyme activities and target of rapamycin signalling pathway related gene expression in rainbow trout, Oncorhynchus mykiss fingerlings. Aquac. Nutr. 2021, 27, 1837–1852. [Google Scholar] [CrossRef]
- Ahmed, I.; Ahmad, I. Effect of dietary protein levels on growth performance, hematological profile and biochemical composition of fingerlings rainbow trout, Oncorhynchus mykiss reared in Indian himalayan region. Aquac. Rep. 2020, 16, 100268. [Google Scholar] [CrossRef]
- Fazio, F.; Saoca, C.; Vazzana, I.; Piccione, G. Influence of body size on blood hemogram in rainbow trout Oncorhynchus mykiss (Walbaum, 1792). Vet. Med. Open J. 2017, 2, 91–94. [Google Scholar] [CrossRef]
- Jobling, M.; Koskela, J.; Savolainen, R. Influence of dietary fat level and increased adiposity on growth and fat deposition in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Res. 1998, 29, 601–607. [Google Scholar] [CrossRef]
- Manera, M.; Britti, D. Assessment of blood chemistry normal ranges in rainbow trout. J. Fish Biol. 2006, 69, 1427–1434. [Google Scholar] [CrossRef]
- Congleton, J.; Wagner, T. Blood-chemistry indicators of nutritional status in juvenile salmonids. J. Fish Biol. 2006, 69, 473–490. [Google Scholar] [CrossRef]
- Guzmán, V.J.; Badilla, X.; Meneses, C.C. Determinación de rangos hematológicos en salmón del Atlántico (Salmo salar) en dos etapas productivas. REDVET. Rev. Electrónica De Vet. 2016, 17, 1–10. [Google Scholar]
- Meng, Y.; Han, B.; Li, C.; Qian, K.; Liu, X.; Hu, X.; Yang, X.; Tian, H.; Ma, R. Digestive characteristics and blood chemistry profile of triploid rainbow trout Oncorhynchus mykiss: Influence of body size and seasonal variation. Fish. Sci. 2019, 85, 1001–1010. [Google Scholar] [CrossRef]
- Shahsavani, D.; Mohri, M.; Gholipour Kanani, H. Determination of normal values of some blood serum enzymes in Acipenser stellatus Pallas. Fish Physiol. Biochem. 2010, 36, 39–43. [Google Scholar] [CrossRef]
- Edsall, C.C. A blood chemistry profile for lake trout. J. Aquat. Anim. Health 1999, 11, 81–86. [Google Scholar] [CrossRef]
- Nabi, N.; Ahmed, I.; Wani, G.B. Hematological and serum biochemical reference intervals of rainbow trout, Oncorhynchus mykiss cultured in Himalayan aquaculture: Morphology, morphometrics and quantification of peripheral blood cells. Saudi J. Biol. Sci. 2022, 29, 2942–2957. [Google Scholar] [CrossRef]
- Casanovas, P.; Walker, S.P.; Johnston, H.; Johnston, C.; Symonds, J.E. Comparative assessment of blood biochemistry and haematology normal ranges between Chinook salmon (Oncorhynchus tshawytscha) from seawater and freshwater farms. Aquaculture 2021, 537, 736464. [Google Scholar] [CrossRef]
- Bastardo, H.; Scorza, C.; Sofía, S. Variables hematológicas y bioquímicas en la trucha arcoíris, relacionadas con la condición hepática y la edad. Zootec. Trop. 2006, 24, 1–15. [Google Scholar]
- Chen, C.-Y.; Wooster, G.A.; Getchell, R.G.; Bowser, P.R.; Timmons, M.B. Blood chemistry of healthy, nephrocalcinosis-affected and ozone-treated tilapia in a recirculation system, with application of discriminant analysis. Aquaculture 2003, 218, 89–102. [Google Scholar] [CrossRef]
- Denson, M.R.; Stuart, K.R.; Smith, T.I.; Weirlch, C.R.; Segars, A. Effects of salinity on growth, survival, and selected hematological parameters of juvenile cobia Rachycentron canadum. J. World Aquac. Soc. 2003, 34, 496–504. [Google Scholar] [CrossRef]
- Fazio, F.; Marafioti, S.; Arfuso, F.; Piccione, G.; Faggio, C. Influence of different salinity on haematological and biochemical parameters of the widely cultured mullet, Mugil cephalus. Mar. Freshw. Behav. Physiol. 2013, 46, 211–218. [Google Scholar] [CrossRef]
- McCormick, S.D.; Farrell, A.P.; Brauner, C.J. Fish Physiology: Euryhaline Fishes; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Damsgaard, C.; McGrath, M.; Wood, C.M.; Richards, J.G.; Brauner, C.J. Ion-regulation, acid/base-balance, kidney function, and effects of hypoxia in coho salmon, Oncorhynchus kisutch, after long-term acclimation to different salinities. Aquaculture 2020, 528, 735571. [Google Scholar] [CrossRef]
- Holan, A.B.; Good, C.; Powell, M.D. Health management in recirculating aquaculture systems (RAS). In Aquaculture Health Management; Elsevier: Amsterdam, The Netherlands, 2020; pp. 281–318. [Google Scholar]
- Good, C.; Davidson, J.; Terjesen, B.F.; Takle, H.; Kolarevic, J.; Bæverfjord, G.; Summerfelt, S. The effects of long-term 20 mg/L carbon dioxide exposure on the health and performance of Atlantic salmon Salmo salar post-smolts in water recirculation aquaculture systems. Aquac. Eng. 2018, 81, 1–9. [Google Scholar] [CrossRef]
- Good, C.; Davidson, J.; Welsh, C.; Snekvik, K.; Summerfelt, S. The effects of ozonation on performance, health and welfare of rainbow trout Oncorhynchus mykiss in low-exchange water recirculation aquaculture systems. Aquac. Eng. 2011, 44, 97–102. [Google Scholar] [CrossRef]
- Fivelstad, S.; Olsen, A.B.; Kløften, H.; Ski, H.; Stefansson, S. Effects of carbon dioxide on Atlantic salmon (Salmo salar L.) smolts at constant pH in bicarbonate rich freshwater. Aquaculture 1999, 178, 171–187. [Google Scholar] [CrossRef]
- Fivelstad, S.; Olsen, A.B.; Åsgård, T.; Baeverfjord, G.; Rasmussen, T.; Vindheim, T.; Stefansson, S. Long-term sublethal effects of carbon dioxide on Atlantic salmon smolts (Salmo salar L.): Ion regulation, haematology, element composition, nephrocalcinosis and growth parameters. Aquaculture 2003, 215, 301–319. [Google Scholar] [CrossRef]
- Wittwer, F. Hematología Clínica. In Manual de Patología Clínica Veterinaria; Ediciones Universidad Austral de Chile: Valdivia, Chile, 2012; Volume 2. [Google Scholar]
- Miller, W.R., III; Hendricks, A.C.; Cairns, J., Jr. Normal ranges for diagnostically important hematological and blood chemistry characteristics of rainbow trout (Salmo gairdneri). Can. J. Fish. Aquat. Sci. 1983, 40, 420–425. [Google Scholar] [CrossRef]
- Waagbø, R.; Sandnes, K.; Espelid, S.; Lie, Ø. Haematological and biochemical analyses of Atlantic salmon, Salmo solar L., suffering from coldwater vibriosis (‘Hitra disease’). J. Fish Dis. 1988, 11, 417–423. [Google Scholar] [CrossRef]
- Treasurer, J.W.; Laidler, L.A. Temporal and Spatial Variation of Plasma Biochemistry in Farmed Atlantic Salmon Salmo salar L. and Determination of Normal Ranges. Bull. Eur. Ass. Fish Pathol. 2001, 21, 178–185. [Google Scholar]
- Braceland, M.; Houston, K.; Ashby, A.; Matthews, C.; Haining, H.; Rodger, H.; Eckersall, P.D. Technical pre-analytical effects on the clinical biochemistry of Atlantic salmon (Salmo salar L.). J. Fish Dis. 2017, 40, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Wintrobe, M. Anemia: Classification and treatment on the basis of differences in the average volume and hemoglobin content of the red corpuscles. Arch. Intern. Med. 1934, 54, 256–280. [Google Scholar] [CrossRef]
- Friedrichs, K.R.; Harr, K.E.; Freeman, K.P.; Szladovits, B.; Walton, R.M.; Barnhart, K.F.; Blanco-Chavez, J. ASVCP reference interval guidelines: Determination of de novo reference intervals in veterinary species and other related topics. Vet. Clin. Pathol. 2012, 41, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Horn, P.S.; Feng, L.; Li, Y.; Pesce, A.J. Effect of outliers and nonhealthy individuals on reference interval estimation. Clin. Chem. 2001, 47, 2137–2145. [Google Scholar] [CrossRef]
- Congleton, J.L.; LaVoie, W.J. Comparison of Blood Chemistry Values for Samples Collected from Juvenile Chinook Salmon by Three Methods. J. Aquat. Anim. Health 2001, 13, 168–172. [Google Scholar] [CrossRef]
- Mazur, C.F.; Iwama, G.K. Effect of handling and stocking density on hematocrit, plasma cortisol, and survival in wild and hatchery-reared chinook salmon (Oncorhynchus tshawytscha). Aquaculture 1993, 112, 291–299. [Google Scholar] [CrossRef]
- Bepgheim, A.; Kroglund, F.; Vatne, D.; Rosseland, B. Blood plasma parameters in farmed Atlantic salmon (Salmo salar L.) transferred to sea cages at age eight to ten months. Aquaculture 1990, 84, 159–165. [Google Scholar] [CrossRef]
- Sandnes, K.; Lie, Ø.; Waagbø, R. Normal ranges of some blood chemistry parameters in adult farmed Atlantic salmon, Salmo salar. J. Fish Biol. 1988, 32, 129–136. [Google Scholar] [CrossRef]
- Ferguson, H.; Rice, D.; Lynas, J. Clinical pathology of myodegeneration (pancreas disease) in Atlantic salmon (Salmo salar). Vet. Rec. 1986, 119, 297–299. [Google Scholar] [CrossRef]
- Grant, A.; Brown, A.; Laidler, L. Plasma lipase concentration as an aid to the early detection of pancreas disease in farmed Atlantic salmon (Salmo salar). Vet. Rec. 1994, 135, 107–108. [Google Scholar] [CrossRef] [PubMed]
- Amend, D.F.; Smith, L. Pathophysiology of infectious hematopoietic necrosis virus disease in rainbow trout: Hematological and blood chemical changes in moribund fish. Infect. Immun. 1975, 11, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Bruno, D.; Munro, A. Haematological assessment of rainbow trout, Salmo gairdneri Richardson, and Atlantic salmon, Salmo salar L., infected with Renibacterium salmoninarum. J. Fish Dis. 1986, 9, 195–204. [Google Scholar] [CrossRef]
- Falk, K.; Namork, E.; Rimstad, E.; Mjaaland, S.; Dannevig, B.H. Characterization of infectious salmon anemia virus, an orthomyxo-like virus isolated from Atlantic salmon (Salmo salar L.). J. Virol. 1997, 71, 9016–9023. [Google Scholar] [CrossRef]
- McCoy, M.; McLoughlin, M.; Rice, D.; Kennedy, D. Pancreas disease in Atlantic salmon (Salmo salar) and vitamin E supplementation. Comp. Biochem. Physiol. Part A Physiol. 1994, 109, 905–912. [Google Scholar] [CrossRef]
- Rodger, H.D. Summer lesion syndrome in salmon: A retrospective study. Vet. Rec. 1991, 129, 237–239. [Google Scholar] [CrossRef]
- Rodger, H.; Murphy, T.; Drinan, E.; Rice, D. Acute skeletal myopathy in farmed Atlantic salmon Salmo salar. Dis. Aquat. Org. 1991, 12, 17–23. [Google Scholar] [CrossRef]
- Rehulka, J. Haematological analyses in rainbow trout Oncorhynchus mykiss affected by viral haemorrhagic septicaemia (VHS). Dis. Aquat. Organ. 2003, 56, 185–193. [Google Scholar] [CrossRef]
- Rehulka, J.; Kubatova, A.; Hubka, V. Cephalotheca sulfurea (Ascomycota, Sordariomycetes), a new fungal pathogen of the farmed rainbow trout Oncorhynchus mykiss. J. Fish Dis. 2016, 39, 1413–1419. [Google Scholar] [CrossRef]
- Rozas-Serri, M.; Ildefonso, R.; Pena, A.; Enriquez, R.; Barrientos, S.; Maldonado, L. Comparative pathogenesis of piscirickettsiosis in Atlantic salmon (Salmo salar L.) post-smolt experimentally challenged with LF-89-like and EM-90-like Piscirickettsia salmonis isolates. J. Fish Dis. 2017, 40, 1451–1472. [Google Scholar] [CrossRef]
- Rozas-Serri, M.; Lobos, C.; Correa, R.; Ildefonso, R.; Vasquez, J.; Munoz, A.; Maldonado, L.; Jaramillo, V.; Conuecar, D.; Oyarzun, C.; et al. Atlantic Salmon Pre-smolt Survivors of Renibacterium salmoninarum Infection Show Inhibited Cell-Mediated Adaptive Immune Response and a Higher Risk of Death During the Late Stage of Infection at Lower Water Temperatures. Front. Immunol. 2020, 11, 1378. [Google Scholar] [CrossRef]
- Yousaf, M.N.; Koppang, E.O.; Skjodt, K.; Kollner, B.; Hordvik, I.; Zou, J.; Secombes, C.; Powell, M.D. Cardiac pathological changes of Atlantic salmon (Salmo salar L.) affected with heart and skeletal muscle inflammation (HSMI). Fish Shellfish. Immunol. 2012, 33, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Rehulka, J.; Kolarik, M.; Hubka, V. Clinical and histopathological changes in rainbow trout Oncorhynchus mykiss experimentally infected with fungus Bradymyces oncorhynchi. Folia Microbiol. 2020, 65, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Rehulka, J.; Minarik, B. Effect of polychlorinated biphenyls (Delor 103) on haematological and enzyme parameters of the rainbow trout Oncorhynchus mykiss. Dis. Aquat. Organ. 2004, 62, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Řehulka, J. The blood indices of the rainbow trout, Oncorhynchus mykiss (Walbaum) in Aeromonas-induced ulcerous dermatitis. Acta Vet. Brno 1998, 67, 317–322. [Google Scholar] [CrossRef][Green Version]
- Espelid, S.; Løkken, G.B.; Steiro, K.; Bøgwald, J. Effects of cortisol and stress on the immune system in Atlantic Salmon (Salmo salar L.). Fish Shellfish Immunol. 1996, 6, 95–110. [Google Scholar] [CrossRef]
- Haney, D.; Hursh, D.; Mix, M.; Winton, J. Physiological and hematological changes in chum salmon artificially infected with erythrocytic necrosis virus. J. Aquat. Anim. Health 1992, 4, 48–57. [Google Scholar] [CrossRef]
- Harbell, S.; Hodgins, H.O.; Schiewe, M.H. Studies on the pathogenesis of vibriosis in coho salmon Oncorhynchus kisutch (Walbaum). J. Fish Dis. 1979, 2, 391–404. [Google Scholar] [CrossRef]
- Carrizo, V.; Valenzuela, C.A.; Zuloaga, R.; Aros, C.; Altamirano, C.; Valdés, J.A.; Molina, A. Effect of cortisol on the immune-like response of rainbow trout (Oncorhynchus mykiss) myotubes challenged with Piscirickettsia salmonis. Vet. Immunol. Immunopathol. 2021, 237, 110240. [Google Scholar] [CrossRef]
- Zuloaga, R.; Dettleff, P.; Bastias-Molina, M.; Meneses, C.; Altamirano, C.; Valdés, J.A.; Molina, A. RNA-Seq-Based Analysis of Cortisol-Induced Differential Gene Expression Associated with Piscirickettsia salmonis Infection in Rainbow Trout (Oncorhynchus mykiss) Myotubes. Animals 2021, 11, 2399. [Google Scholar] [CrossRef]
- Mesa, M.G.; Maule, A.G.; Schreck, C.B. Interaction of infection with Renibacterium salmoninarum and physical stress in juvenile chinook salmon: Physiological responses, disease progression, and mortality. Trans. Am. Fish. Soc. 2000, 129, 158–173. [Google Scholar] [CrossRef]
- Maule, A.G.; Tripp, R.A.; Kaattari, S.L.; Schreck, C.B. Stress alters immune function and disease resistance in chinook salmon (Oncorhynchus tshawytscha). J. Endocrinol. 1989, 120, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Gadan, K.; Marjara, I.S.; Sundh, H.; Sundell, K.; Evensen, Ø. Slow release cortisol implants result in impaired innate immune responses and higher infection prevalence following experimental challenge with infectious pancreatic necrosis virus in Atlantic salmon (Salmo salar) parr. Fish Shellfish Immunol. 2012, 32, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Niklasson, L.; Sundh, H.; Olsen, R.E.; Jutfelt, F.; Skjødt, K.; Nilsen, T.O.; Sundell, K.S. Effects of cortisol on the intestinal mucosal immune response during cohabitant challenge with IPNV in Atlantic salmon (Salmo salar). PLoS ONE 2014, 9, e94288. [Google Scholar] [CrossRef]
- Finstad, O.W.; Dahle, M.K.; Lindholm, T.H.; Nyman, I.B.; Løvoll, M.; Wallace, C.; Olsen, C.M.; Storset, A.K.; Rimstad, E. Piscine orthoreovirus (PRV) infects Atlantic salmon erythrocytes. Vet. Res. 2014, 45, 35. [Google Scholar] [CrossRef]
- Olsen, Y.A.; Falk, K.; Reite, O.B. Cortisol and lactate levels in Atlantic salmon Salmo salar developing infectious anaemia (ISA). Dis. Aquat. Org. 1992, 14, 99. [Google Scholar] [CrossRef]
- Schreck, C.B.; Tort, L. The concept of stress in fish. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 35, pp. 1–34. [Google Scholar]
- Conte, F. Stress and the welfare of cultured fish. Appl. Anim. Behav. Sci. 2004, 86, 205–223. [Google Scholar] [CrossRef]
- Uren Webster, T.M.; Rodriguez-Barreto, D.; Consuegra, S.; Garcia de Leaniz, C. Cortisol-Related Signatures of Stress in the Fish Microbiome. Front. Microbiol. 2020, 11, 1621. [Google Scholar] [CrossRef]
- Selvam, C.; Philip, A.J.P.; Lutfi, E.; Sigholt, T.; Norberg, B.; Bæverfjord, G.; Rosenlund, G.; Ruyter, B.; Sissener, N.H. Long-term feeding of Atlantic salmon with varying levels of dietary EPA + DHA alters the mineral status but does not affect the stress responses after mechanical delousing stress. Br. J. Nutr. 2022, 1–17. [Google Scholar] [CrossRef]
- Fast, M.D.; Hosoya, S.; Johnson, S.C.; Afonso, L.O.B. Cortisol response and immune-related effects of Atlantic salmon (Salmo salar Linnaeus) subjected to short- and long-term stress. Fish Shellfish Immunol. 2008, 24, 194–204. [Google Scholar] [CrossRef]
- Culbert, B.M.; Regish, A.M.; Hall, D.J.; McCormick, S.D.; Bernier, N.J. Neuroendocrine Regulation of Plasma Cortisol Levels During Smoltification and Seawater Acclimation of Atlantic Salmon. Front. Endocrinol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- González, M.P.; Vargas-Chacoff, L.; Marín, S.L. Stress response of Salmo salar (Linnaeus 1758) when heavily infested by Caligus rogercresseyi (Boxshall & Bravo 2000) copepodids. Fish Physiol. Biochem. 2016, 42, 263–274. [Google Scholar] [PubMed]
- Handeland, S.; Arnesen, A.; Stefansson, S. Seawater adaptation and growth of post-smolt Atlantic salmon (Salmo salar) of wild and farmed strains. Aquaculture 2003, 220, 367–384. [Google Scholar] [CrossRef]
- Mota, V.C.; Nilsen, T.O.; Gerwins, J.; Gallo, M.; Kolarevic, J.; Krasnov, A.; Terjesen, B.F. Molecular and physiological responses to long-term carbon dioxide exposure in Atlantic salmon (Salmo salar). Aquaculture 2020, 519, 734715. [Google Scholar] [CrossRef]
- Mommsen, T.P.; Vijayan, M.M.; Moon, T.W. Cortisol in teleosts: Dynamics, mechanisms of action, and metabolic regulation. Rev. Fish Biol. Fish. 1999, 9, 211–268. [Google Scholar] [CrossRef]
- Cornelius, F. Modulation of Na,K-ATPase and Na-ATPase activity by phospholipids and cholesterol. I. Steady-state kinetics. Biochemistry 2001, 40, 8842–8851. [Google Scholar] [CrossRef]
- van Rijn, C.A.; Jones, P.L.; Evans, B.S.; Afonso, L.O.B. Physiological and growth responses of juvenile Atlantic salmon (Salmo salar) transferred to seawater during different stages of smolt development. Aquaculture 2021, 538, 736527. [Google Scholar] [CrossRef]
- Fazio, F.; Saoca, C.; Piccione, G.; Kesbiç, O.S.; Acar, Ü. Comparative study of some hematological and biochemical parameters of Italian and Turkish farmed rainbow trout Oncorhynchus mykiss (Walbaum, 1792). Turk. J. Fish. Aquat. Sci. 2016, 16, 715–721. [Google Scholar] [CrossRef]
- Currie, A.; Cockerill, D.; Diez-Padrisa, M.; Haining, H.; Henriquez, F.; Quinn, B. Anemia in salmon aquaculture: Scotland as a case study. Aquaculture 2022, 546, 737313. [Google Scholar] [CrossRef]
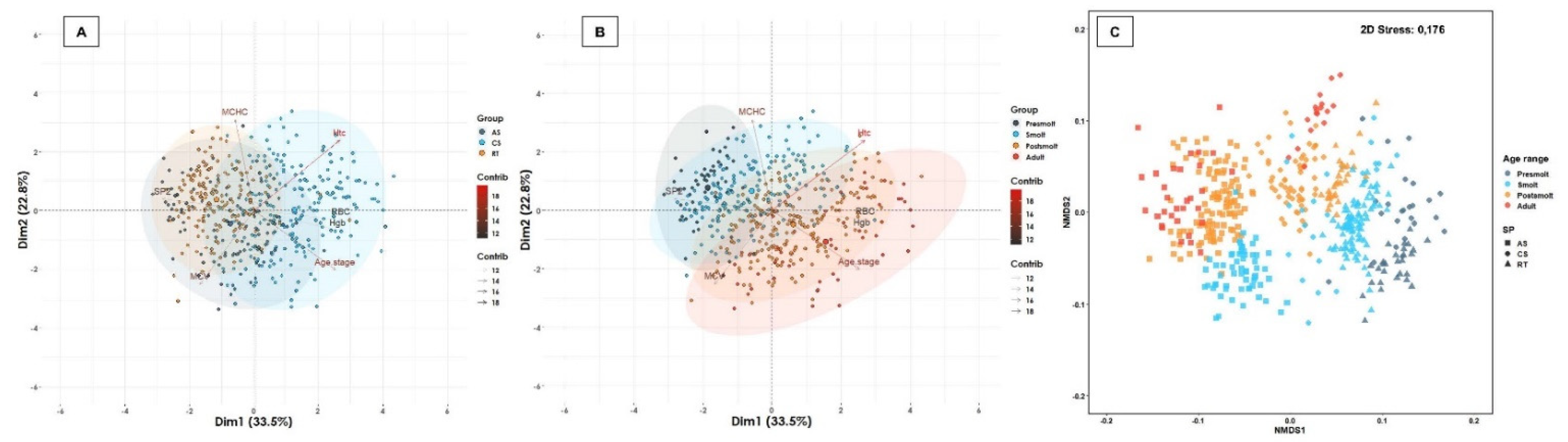
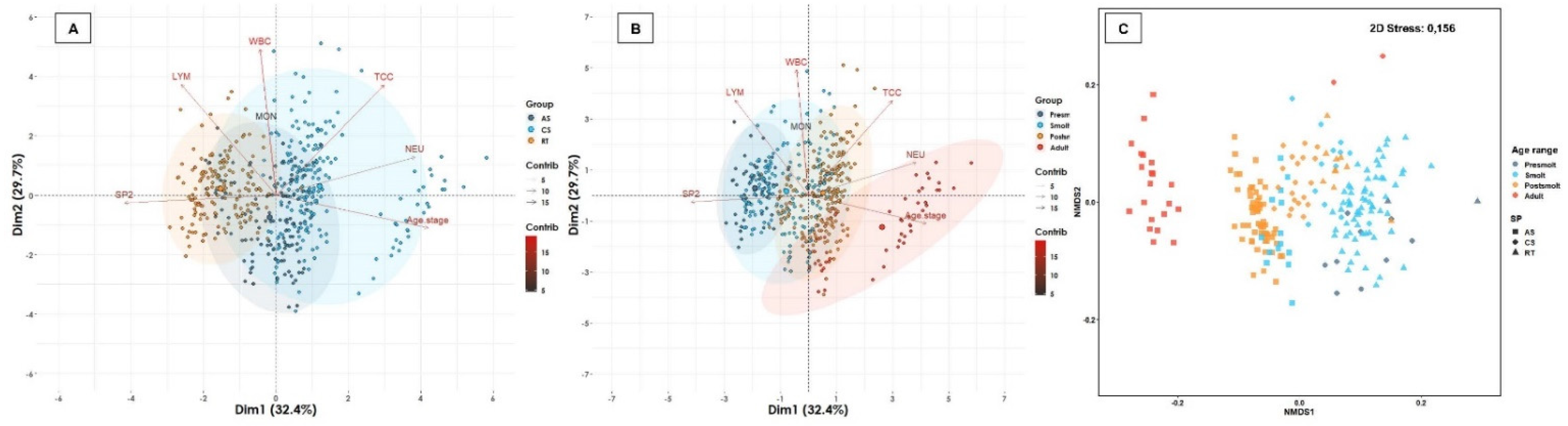
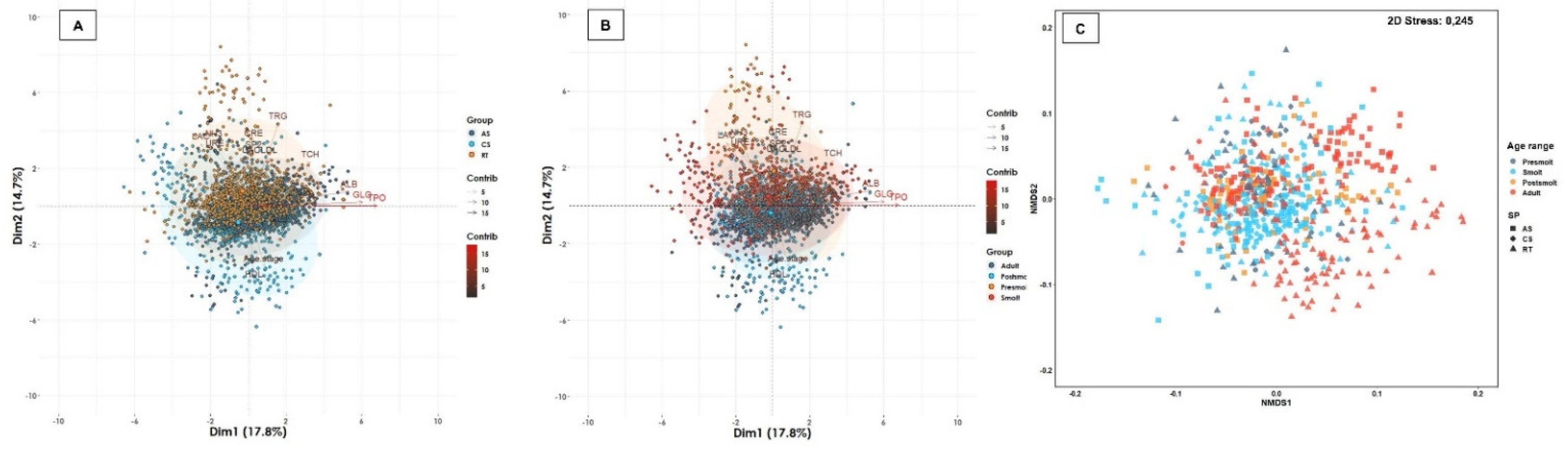
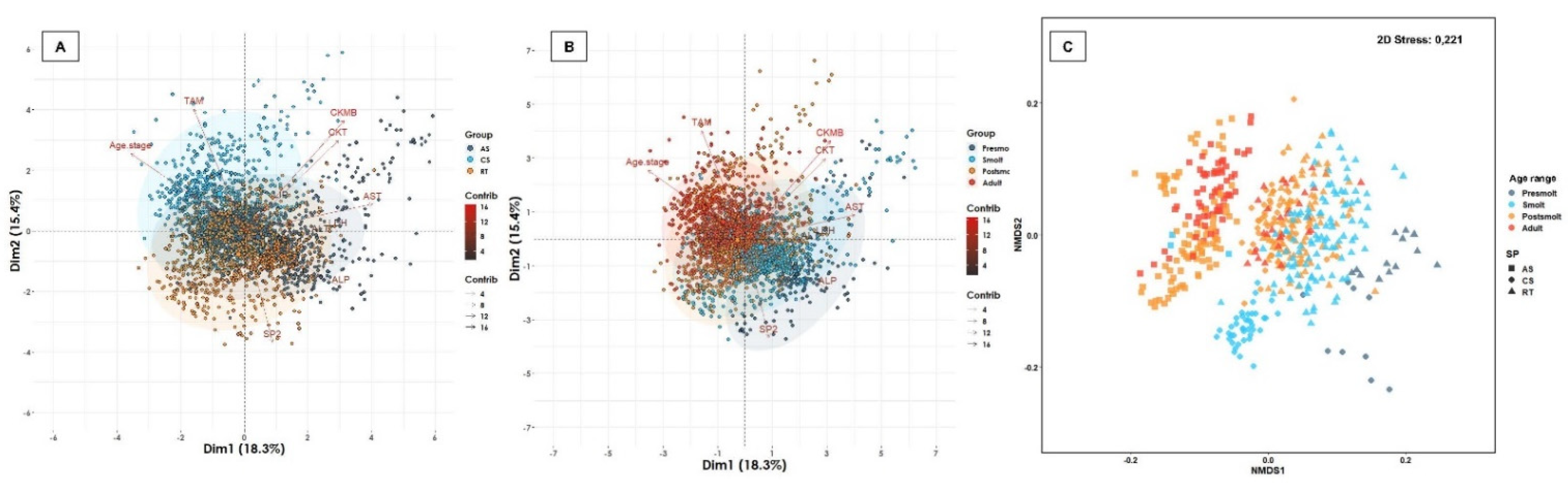
| Water Type | Age Range | Atlantic Salmon | Coho Salmon | Rainbow Trout | Total of Fish | Total (%) | Total of Farms | Total (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Healthy Fish | Number of Farms | Number of Healthy Fish | Number of Farms | Number of Healthy Fish | Number of Farms | ||||||
| Freshwater | Presmolt (<50 g) | 230 | 9 | 202 | 5 | 432 | 14.1% | 14 | 17.9% | ||
| Smolt (50 to 150 g) | 380 | 9 | 259 | 7 | 120 | 4 | 759 | 24.8% | 20 | 25.6% | |
| Seawater | Postsmolt (150 to 800 g) | 560 | 12 | 150 | 5 | 242 | 6 | 952 | 31.1% | 23 | 29.5% |
| Adult (>800 g) | 380 | 8 | 306 | 7 | 230 | 6 | 916 | 29.9% | 21 | 26.9% | |
| Total (N) | 1550 | 38 | 715 | 19 | 794 | 21 | 3059 | 100.0% | 78 | 100.0% | |
| Total (%) | 50.7% | 48.7% | 23.4% | 24.4% | 26.0% | 26.9% | 100.0% | 100.0% | |||
| Parameter | Abbreviation | Unit of Measure | Species | Age Stage | Median | Mean | S. D | S. E | Difference between Means | 95% Reference Interval | Confidence Interval (95%) | Test | Data Processing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hematocrit | Htc | % | Coho salmon | Smolt | 53.00 | 53.20 | 7.46 | 0.97 | a | 38.31–68.49 | 35.58–71.19 | ANOVA, Tukey | Normal |
| Postsmolt | 51.00 | 51.17 | 9.94 | 0.95 | a | 31.14–70.79 | 28.65–73.38 | ANOVA, Tukey | Normal | ||||
| Adult | 55.00 | 53.42 | 10.21 | 1.83 | a | 32.43–75.49 | 26.16–79.85 | ANOVA, Tukey | Normal | ||||
| Atlantic salmon | Presmolt | 37.50 | 40.46 | 7.02 | 1.43 | b | 22.41–54.60 | 17.27–60.64 | ANOVA, Tukey | Normal | |||
| Smolt | 39.00 | 41.23 | 7.43 | 1.46 | b | 23.97–56.60 | 19.80–62.83 | ANOVA, Tukey | Normal | ||||
| Postsmolt | 47.00 | 46.59 | 6.99 | 0.91 | a | 32.62–60.94 | 29.72–63.28 | ANOVA, Tukey | Normal | ||||
| Adult | 40.00 | 41.24 | 5.25 | 1.27 | b | 29.15–52.80 | 25.27–57.75 | ANOVA, Tukey | Normal | ||||
| Rainbow trout | Presmolt | 46.00 | 46.83 | 4.39 | 0.80 | a | 36.76–55.31 | 34.05–58.02 | ANOVA, Tukey | Normal | |||
| Smolt | 47.00 | 47.42 | 6.90 | 0.73 | a | 33.04–60.67 | 30.57–63.30 | ANOVA, Tukey | Normal | ||||
| Postsmolt | 49.00 | 48.72 | 7.27 | 1.29 | a | 34.25–64.35 | 29.65–69.06 | ANOVA, Tukey | Normal | ||||
| Red Blood Cell Count | RBC | 107/μL | Coho salmon | Smolt | 9832.40 | 9389.25 | 3388.90 | 441.19 | c | 2225.89–16,017.98 | 962.34–17,318.70 | ANOVA, Tukey | log(x + 1) |
| Postsmolt | 10,762.60 | 11,354.00 | 4526.60 | 414.95 | b | 1641.65–19,781.52 | 1012.74–21,512.40 | ANOVA, Tukey | log(x + 1) | ||||
| Adult | 15,384.60 | 16,389.70 | 6885.50 | 1236.70 | a | 4995.0–32,447.52 | 4995.0–32,447.52 | ANOVA, Tukey | log(x + 1) | ||||
| Atlantic salmon | Presmolt | 3629.70 | 4479.40 | 1663.50 | 339.56 | a | 2419.8–8436.0 | 2419.80–8436.0 | ANOVA, Tukey | log(x + 1) | |||
| Smolt | 6371.40 | 6840.67 | 3620.80 | 710.11 | a | 2131.2–13,066.92 | 131.20–13,066.92 | ANOVA, Tukey | log(x + 1) | ||||
| Postsmolt | 5423.50 | 6344.49 | 3309.90 | 434.61 | a | 1968.25–16,083.4 | 1740.48–16,643.34 | ANOVA, Tukey | log(x + 1) | ||||
| Adult | 1953.60 | 2181.08 | 1543.90 | 374.44 | b | 319.68–6233.76 | 319.68–6233.76 | ANOVA, Tukey | log(x + 1) | ||||
| Rainbow trout | Presmolt | 3747.40 | 4620.12 | 2412.60 | 440.48 | a | 1678.32–10,966.80 | 1678.32–10,966.8 | ANOVA, Tukey | log(x + 1) | |||
| Smolt | 4955.00 | 5078.64 | 1900.10 | 201.41 | ab | 1071.69–8683.46 | 508.05–9387.03 | ANOVA, Tukey | log(x + 1) | ||||
| Postsmolt | 3636.40 | 3768.45 | 1483.80 | 262.30 | b | 952.38–7965.36 | 952.38–7965.36 | ANOVA, Tukey | log(x + 1) | ||||
| Hemoglobin | Hgb | g/L | Coho salmon | Smolt | 509.30 | 525.51 | 110.50 | 14.39 | b | 299.15–749.86 | 254.73–801.51 | ANOVA, Tukey | Normal |
| Postsmolt | 607.50 | 599.16 | 107.07 | 10.26 | a | 391.02–819.18 | 358.59–843.14 | ANOVA, Tukey | Normal | ||||
| Adult | 640.80 | 628.51 | 87.76 | 15.76 | a | 454.66–823.04 | 394.08–861.15 | ANOVA, Tukey | Normal | ||||
| Atlantic salmon | Presmolt | 430.90 | 477.24 | 132.27 | 27.00 | bc | 144.71–738.62 | 56.0–844.63 | ANOVA, Tukey | log(x + 1) | |||
| Smolt | 383.60 | 409.10 | 111.94 | 21.95 | c | 154.32–636.29 | 84.93–723.91 | ANOVA, Tukey | log(x + 1) | ||||
| Postsmolt | 545.50 | 566.49 | 110.73 | 14.42 | a | 326.56–780.92 | 268.60–844.32 | ANOVA, Tukey | log(x + 1) | ||||
| Adult | 555.60 | 545.63 | 62.70 | 15.21 | ab | 407.50–687.30 | 355.78–728.24 | ANOVA, Tukey | log(x + 1) | ||||
| Rainbow trout | Presmolt | 483.90 | 480.50 | 55.60 | 10.15 | b | 395.16–570.93 | 361.38–599.16 | ANOVA, Tukey | BOX COX | |||
| Smolt | 520.30 | 548.31 | 154.52 | 16.29 | a | 213.48–836.16 | 78.77–976.65 | ANOVA, Tukey | BOX COX | ||||
| Postsmolt | 579.00 | 595.76 | 110.84 | 19.91 | a | 344.94–805.38 | 239.48–909.49 | ANOVA, Tukey | BOX COX | ||||
| Mean Corpuscular Volume | MCV | fL | Coho salmon | Smolt | 110.9 | 119.74 | 33.12 | 4.31 | b | 40.12–177.76 | 22.08–196.44 | ANOVA, Tukey | log(x + 1) |
| Postsmolt | 145.70 | 146.24 | 33.77 | 3.23 | a | 77.89–212.55 | 69.63–222.13 | ANOVA, Tukey | log(x + 1) | ||||
| Adult | 133.30 | 131.62 | 22.40 | 4.02 | ab | 84.83–178.64 | 73.21–189.43 | ANOVA, Tukey | log(x + 1) | ||||
| Atlantic salmon | Presmolt | 165.90 | 169.84 | 24.46 | 4.99 | a | 115.81–220.94 | 102.93–240.33 | ANOVA, Tukey | log(x + 1) | |||
| Smolt | 158.60 | 156.26 | 22.58 | 4.43 | ab | 110.72–205.97 | 96.41–220.43 | ANOVA, Tukey | log(x + 1) | ||||
| Postsmolt | 138.10 | 145.97 | 24.91 | 3.24 | b | 88.55–194.02 | 78.10–207.21 | ANOVA, Tukey | log(x + 1) | ||||
| Adult | 155.60 | 159.71 | 18.37 | 4.45 | ab | 116.36–198.56 | 102.10–216.07 | ANOVA, Tukey | log(x + 1) | ||||
| Rainbow trout | Presmolt | 118.10 | 119.58 | 15.27 | 2.79 | c | 86.37–149.92 | 75.23–160.49 | ANOVA, Tukey | log(x + 1) | |||
| Smolt | 137.20 | 140.97 | 26.41 | 2.78 | b | 86.01–192.72 | 79.36–202.20 | ANOVA, Tukey | log(x + 1) | ||||
| Postsmolt | 158.50 | 158.52 | 16.68 | 2.95 | a | 122.4–191.51 | 112.81–201.41 | ANOVA, Tukey | log(x + 1) | ||||
| Mean Corpuscular Hemoglobin Concentration | MCHC | g/L | Coho salmon | Smolt | 1.00 | 1.02 | 0.17 | 0.02 | a | 0.69–1.36 | 0.62–1.42 | ANOVA, Tukey | Normal |
| Postsmolt | 0.90 | 0.87 | 0.14 | 0.01 | b | 0.59–1.13 | 0.55–1.18 | ANOVA, Tukey | Normal | ||||
| Adult | 0.90 | 0.86 | 0.16 | 0.03 | b | 0.53–1.19 | 0.45–1.28 | ANOVA, Tukey | Normal | ||||
| Atlantic salmon | Presmolt | 0.90 | 0.89 | 0.22 | 0.05 | b | 0.42–1.36 | 0.28–1.50 | ANOVA, Tukey | Normal | |||
| Smolt | 1.00 | 1.04 | 0.18 | 0.03 | a | 0.68–1.42 | 0.56–1.49 | ANOVA, Tukey | Normal | ||||
| Postsmolt | 0.80 | 0.84 | 0.15 | 0.02 | b | 0.52–1.14 | 0.46–1.21 | ANOVA, Tukey | Normal | ||||
| Adult | 0.80 | 0.76 | 0.10 | 0.03 | b | 0.52–0.98 | 0.44–1.08 | ANOVA, Tukey | Normal | ||||
| Rainbow trout | Presmolt | 0.90 | 0.99 | 0.19 | 0.04 | a | 0.68–1.15 | 0.60–1.23 | ANOVA, Tukey | log(x + 1) | |||
| Smolt | 0.90 | 0.89 | 0.15 | 0.02 | b | 0.65–1.13 | 0.61–1.17 | ANOVA, Tukey | log(x + 1) | ||||
| Postsmolt | 0.80 | 0.81 | 0.16 | 0.03 | b | 0.62–1.06 | 0.55–1.13 | ANOVA, Tukey | log(x + 1) |
| Parameter | Abbreviation | Unit of Measure | Species | Age Stage | Median | Mean | S. D | S. E | Difference between Means | 95% Reference Interval | Confidence Interval (95%) | Test | Data Processing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White Blood Cell Count | WBC | N°/μL | Coho salmon | Smolt | 13,906.08 | 14,026.11 | 5075.93 | 660.83 | ab | 3662.20–24,199.37 | 2007.55–25,976.80 | ANOVA, Tukey | Normal |
| Postsmolt | 14,385.60 | 14,517.42 | 4396.45 | 403.02 | ab | 5820.81–23,316.12 | 4626.99–24,548.04 | ANOVA, Tukey | Normal | ||||
| Adult | 12,387.60 | 11,976.18 | 3658.25 | 657.04 | b | 4325.58–19,625.58 | 2457.66–21,488.70 | ANOVA, Tukey | Normal | ||||
| Atlantic salmon | Presmolt | 15,631.02 | 14,927.09 | 3543.82 | 723.38 | a | 7426.32–22,727.96 | 5492.03–24,359.88 | ANOVA, Tukey | log(x + 1) | |||
| Smolt | 8531.46 | 9510.48 | 3604.02 | 706.81 | b | 4528.80–16,472.40 | 4528.80–16,472.40 | ANOVA, Tukey | log(x + 1) | ||||
| Postsmolt | 12,170.04 | 12,797.27 | 3794.08 | 498.19 | a | 4816.03–20,387.69 | 3378.23–21,844.69 | ANOVA, Tukey | log(x + 1) | ||||
| Adult | 7192.80 | 8670.80 | 4206.19 | 1020.15 | b | 3247.86–18,115.20 | 3247.86–18,115.20 | ANOVA, Tukey | log(x + 1) | ||||
| Rainbow trout | Presmolt | 15,717.60 | 15,130.48 | 6015.99 | 1098.36 | a | 6493.50–29,989.98 | 6493.50–29,989.98 | ANOVA, Tukey | Normal | |||
| Smolt | 15,984.0 | 15,834.26 | 4328.83 | 458.85 | a | 7172.65–24,508.47 | 5998.39–25,667.40 | ANOVA, Tukey | Normal | ||||
| Postsmolt | 14,635.35 | 15,087.67 | 4069.13 | 719.33 | a | 10,657.80–18,645.87 | 9740.24–19,738.03 | ANOVA, Tukey | Normal | ||||
| Lymphocytes | LYM | N°/μL | Coho salmon | Smolt | 10,842.48 | 11,034.92 | 4160.62 | 541.67 | a | 2417.39–19,259.23 | 1149.24–20,820.34 | ANOVA, Tukey | Normal |
| Postsmolt | 11,487.17 | 11,272.84 | 3470.47 | 318.14 | a | 4472.30–18,300.84 | 3498.96–19,228.08 | ANOVA, Tukey | Normal | ||||
| Adult | 2511.93 | 2543.75 | 1176.67 | 211.34 | b | 426.20–5388.60 | 426.20–5388.60 | ANOVA, Tukey | Normal | ||||
| Atlantic salmon | Presmolt | 11,979.79 | 12,302.29 | 2736.12 | 583.34 | a | 6543.81–18,283.54 | 4835.86–19,814.92 | ANOVA, Tukey | Normal | |||
| Smolt | 6852.27 | 7427.00 | 2756.76 | 540.64 | c | 3849.50–13,013.20 | 3849.50–13,013.20 | ANOVA, Tukey | Normal | ||||
| Postsmolt | 9131.13 | 9394.85 | 3050.20 | 404.01 | b | 3076.28–15,484.43 | 2041.53–16,610.05 | ANOVA, Tukey | Normal | ||||
| Adult | 5665.26 | 6035.71 | 2542.45 | 616.63 | c | 2435.90–12,774.40 | 2435.90–12,774.40 | ANOVA, Tukey | Normal | ||||
| Rainbow trout | Presmolt | 13,661.82 | 12,758.34 | 4590.63 | 838.13 | a | 9704.06–17,824.74 | 8145.22–19,054.68 | ANOVA, Tukey | BOX COX | |||
| Smolt | 12,221.97 | 12,004.36 | 1903.40 | 200.64 | b | 9113.07–15,677.21 | 8434.18–16,138.72 | ANOVA, Tukey | BOX COX | ||||
| Postsmolt | 11,489.03 | 11,889.42 | 3299.63 | 623.57 | b | 8021.64–15,157.85 | 7146.13–16,124.68 | ANOVA, Tukey | BOX COX | ||||
| Neutrophils | NEU | N°/μL | Coho salmon | Smolt | 2850.48 | 2924.59 | 1180.06 | 153.63 | b | 512.42–5301.31 | 181.04–5723.28 | ANOVA, Tukey | POWER BOX COX |
| Postsmolt | 2815.28 | 3023.69 | 1217.21 | 112.05 | b | 442.96–5350.66 | 32.73–5766.80 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 9292.92 | 9214.03 | 3073.02 | 551.93 | a | 2618.39–15,383.08 | 1111.62–17,089.10 | ANOVA, Tukey | POWER BOX COX | ||||
| Atlantic salmon | Presmolt | 3201.53 | 3039.47 | 930.72 | 198.43 | a | 1071.70–5108.78 | 554.10–5569.84 | ANOVA, Tukey | BOX COX | |||
| Smolt | 1657.21 | 1844.93 | 699.71 | 137.22 | b | 679.32–3459.20 | 679.32–3459.20 | ANOVA, Tukey | BOX COX | ||||
| Postsmolt | 3020.98 | 3366.32 | 1100.68 | 145.79 | a | 1612.89–6397.74 | 1551.51–6764.83 | ANOVA, Tukey | BOX COX | ||||
| Adult | 1948.05 | 2068.82 | 1057.98 | 256.60 | b | 772.03–4967.83 | 772.03–4967.83 | ANOVA, Tukey | BOX COX | ||||
| Rainbow trout | Presmolt | 1627.37 | 1781.97 | 1047.13 | 191.18 | b | 417.72–4523.81 | 417.72–4523.81 | ANOVA, Tukey | BOX COX | |||
| Smolt | 1914.28 | 2215.56 | 1438.91 | 154.27 | b | 323.96–5860.65 | 294.51–6184.61 | ANOVA, Tukey | BOX COX | ||||
| Postsmolt | 2826.50 | 3028.72 | 1364.58 | 257.88 | a | 956.04–7970.69 | 956.04–7970.69 | ANOVA, Tukey | BOX COX | ||||
| Monocytes | MON | N°/μL | Coho salmon | Smolt | 174.58 | 280.68 | 247.41 | 66.12 | a | 59.19–978.22 | 59.19–978.22 | ANOVA, Tukey | log(x + 1) |
| Postsmolt | 251.75 | 304.65 | 187.17 | 23.96 | a | 84.95–834.96 | 78.59–991.01 | ANOVA, Tukey | log(x + 1) | ||||
| Adult | 245.75 | 307.76 | 204.13 | 43.52 | a | 83.12–796.54 | 83.12–796.54 | ANOVA, Tukey | log(x + 1) | ||||
| Atlantic salmon | Presmolt | 350.76 | 351.87 | 148.66 | 49.55 | a | 163.61–600.07 | 163.61–600.07 | ANOVA, Tukey | log(x + 1) | |||
| Smolt | 144.52 | 182.02 | 123.89 | 34.36 | b | 51.77–445.24 | 51.77–445.24 | ANOVA, Tukey | log(x + 1) | ||||
| Postsmolt | 256.41 | 258.11 | 110.56 | 24.13 | ab | 86.58–496.30 | 86.58–496.30 | ANOVA, Tukey | log(x + 1) | ||||
| Smolt | 84.18 | 162.21 | 173.82 | 86.91 | b | 58.50–421.98 | 58.50–421.98 | ANOVA, Tukey | log(x + 1) | ||||
| Rainbow trout | Postsmolt | 139.24 | 188.44 | 145.54 | 48.51 | b | 65.36–447.55 | 65.36–447.55 | Kruskal-Wallis, Dunn | ||||
| Adult | 294.51 | 446.15 | 387.88 | 47.39 | a | 147.25–1472.53 | 250.05–1472.53 | Kruskal-Wallis, Dunn | |||||
| Presmolt | 208.86 | 269.41 | 190.49 | 54.99 | ab | 121.57–703.30 | 121.57–703.30 | Kruskal-Wallis, Dunn | |||||
| Thrombocyte count | TCC | N°/μL | Coho salmon | Smolt | 5688.88 | 5550.22 | 1898.31 | 247.14 | a | 1632.27–9319.54 | 930.44–10,048.76 | ANOVA, Tukey | BOX COX |
| Postsmolt | 5967.36 | 6003.87 | 1623.96 | 148.87 | a | 2705.02–9166.50 | 2275.34–9644.43 | ANOVA, Tukey | BOX COX | ||||
| Adult | 6433.56 | 6252.88 | 1364.83 | 245.13 | a | 3479.77–9212.12 | 2739.57–9871.98 | ANOVA, Tukey | BOX COX | ||||
| Atlantic salmon | Presmolt | 3184.88 | 3468.78 | 1386.72 | 283.06 | a | 1606.39–6600.73 | 1606.39–6600.73 | ANOVA, Tukey | Normal | |||
| Smolt | 3491.17 | 3854.07 | 1852.63 | 363.33 | a | 1594.14–7948.71 | 1594.14–7948.71 | ANOVA, Tukey | Normal | ||||
| Postsmolt | 3562.63 | 4173.17 | 1757.30 | 234.83 | a | 1763,70–8826.51 | 1759.04–9224.77 | ANOVA, Tukey | Normal | ||||
| Adult | 1566.52 | 1660.49 | 963.82 | 233.76 | b | 357.26–3985.34 | 357.26–3985.34 | ANOVA, Tukey | Normal | ||||
| Rainbow trout | Postsmolt | 3449.88 | 3533.37 | 1629.58 | 302.61 | a | 1438.56–7384.61 | 1438.56–7384.61 | ANOVA, Tukey | log(x + 1) | |||
| Adult | 3866.66 | 3779.87 | 1198.54 | 127.05 | ab | 1308.01–6113.98 | 961.13–6498.79 | ANOVA, Tukey | log(x + 1) | ||||
| Presmolt | 2781.35 | 2935.75 | 971.37 | 177.35 | b | 797.91–4886.41 | 213.08–5488.10 | ANOVA, Tukey | log(x + 1) |
| Parameter | Abbreviation | Unit of Measure | Species | Age Stage | Median | Mean | S. D | S. E | Difference between Means | 95% Reference Interval | Confidence Interval (95%) | Test | Data Processing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total protein | TPO | g/L | Coho salmon | Smolt | 40.00 | 39.33 | 10.00 | 0.64 | b | 20.03–59.61 | 18.21–61.17 | ANOVA, Tukey | Normal |
| Postsmolt | 43.90 | 43.42 | 6.46 | 0.60 | a | 30.69–56.50 | 28.88–58.06 | ANOVA, Tukey | Normal | ||||
| Adult | 41.20 | 42.24 | 7.63 | 0.41 | a | 26.58–56.92 | 25.32–58.30 | ANOVA, Tukey | Normal | ||||
| Atlantic salmon | Presmolt | 43.80 | 44.97 | 8.61 | 0.64 | b | 27.38–61.79 | 25.34–63.69 | ANOVA, Tukey | Normal | |||
| Smolt | 45.50 | 45.39 | 9.96 | 0.52 | b | 25.67–64.90 | 24.35–66.29 | ANOVA, Tukey | Normal | ||||
| Postsmolt | 44.50 | 44.60 | 7.61 | 0.33 | a | 29.60–59.54 | 28.67–60.44 | ANOVA, Tukey | Normal | ||||
| Adult | 49.80 | 48.29 | 7.90 | 0.41 | b | 33.75–65.39 | 32.27–66.60 | ANOVA, Tukey | Normal | ||||
| Rainbow trout | Presmolt | 33.00 | 34.42 | 10.09 | 1.84 | c | 13.05–55.80 | 7.44–63.16 | ANOVA, Tukey | Normal | |||
| Smolt | 41.00 | 40.15 | 7.63 | 0.45 | b | 25.11–55.38 | 23.86–56.61 | ANOVA, Tukey | Normal | ||||
| Postsmolt | 41.00 | 41.53 | 6.56 | 0.42 | a | 28.17–54.17 | 27.06–55.39 | ANOVA, Tukey | Normal | ||||
| Adult | 46.90 | 46.52 | 8.93 | 0.60 | b | 28.79–64.13 | 27.06–65.80 | ANOVA, Tukey | Normal | ||||
| Albumins | ALB | g/L | Coho salmon | Smolt | 15.70 | 15.77 | 2.73 | 0.18 | b | 10.21–21.0 | 9.70–21.56 | ANOVA, Tukey | log(x + 1) |
| Postsmolt | 16.60 | 16.73 | 2.75 | 0.26 | a | 11.28–22.26 | 10.58–22.91 | ANOVA, Tukey | log(x + 1) | ||||
| Adult | 16.20 | 16.35 | 2.38 | 0.13 | a | 11.40–20.80 | 11.00–21.30 | ANOVA, Tukey | log(x + 1) | ||||
| Atlantic salmon | Presmolt | 16.60 | 16.81 | 2.26 | 0.17 | b | 12.31–21.32 | 11.87–21.75 | ANOVA, Tukey | Normal | |||
| Smolt | 18.15 | 18.23 | 3.29 | 0.18 | a | 11.66–24.65 | 11.21–25.16 | ANOVA, Tukey | Normal | ||||
| Postsmolt | 17.90 | 18.03 | 2.44 | 0.11 | a | 13.09–22.70 | 12.77–23.03 | ANOVA, Tukey | Normal | ||||
| Adult | 18.60 | 18.47 | 2.67 | 0.14 | a | 13.45–23.97 | 13.01–24.33 | ANOVA, Tukey | Normal | ||||
| Rainbow trout | Presmolt | 15.45 | 15.49 | 2.87 | 0.17 | c | 9.81–21.11 | 9.34–21.60 | ANOVA, Tukey | Normal | |||
| Smolt | 16.50 | 16.49 | 2.28 | 0.15 | b | 11.91–20.91 | 11.50–21.35 | ANOVA, Tukey | Normal | ||||
| Postsmolt | 18.40 | 18.62 | 3.43 | 0.23 | a | 11.68–25.27 | 11.08–25.96 | ANOVA, Tukey | Normal | ||||
| Globulins | GLO | g/L | Coho salmon | Smolt | 26.20 | 25.61 | 7.00 | 0.46 | a | 11.83–39.57 | 10.61–40.69 | ANOVA, Tukey | Normal |
| Postsmolt | 26.70 | 26.68 | 4.43 | 0.41 | a | 17.71–35.36 | 16.64–36.54 | ANOVA, Tukey | Normal | ||||
| Adult | 25.50 | 25.91 | 6.08 | 0.33 | a | 13.60–37.60 | 12.70–38.59 | ANOVA, Tukey | Normal | ||||
| Atlantic salmon | Presmolt | 27.20 | 27.74 | 6.50 | 0.49 | b | 14.59–40.42 | 13.40–41.79 | Kruskal-Wallis, Dunn | ||||
| Smolt | 27.90 | 27.31 | 8.53 | 0.47 | b | 10.88–44.06 | 9.53–45.06 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 26.50 | 26.64 | 6.32 | 0.28 | b | 14.37–39.11 | 13.58–39.88 | Kruskal-Wallis, Dunn | |||||
| Adult | 30.75 | 29.83 | 6.02 | 0.31 | a | 18.78–42.34 | 17.75–43.21 | Kruskal-Wallis, Dunn | |||||
| Rainbow trout | Smolt | 25.00 | 24.77 | 5.56 | 0.33 | b | 13.71–35.68 | 12.80–36.59 | ANOVA, Tukey | Normal | |||
| Postsmolt | 24.60 | 25.04 | 5.58 | 0.36 | b | 13.67–35.77 | 12.69–36.91 | ANOVA, Tukey | Normal | ||||
| Adult | 28.00 | 27.95 | 6.61 | 0.45 | a | 14.95–41.06 | 13.74–42.24 | ANOVA, Tukey | Normal | ||||
| Total bilirubin | TBI | μmol/L | Coho salmon | Smolt | 2.48 | 2.79 | 0.55 | 0.08 | b | 1.7–3.87 | 1.60–3.97 | Kruskal-Wallis, Dunn | |
| Adult | 3.55 | 3.58 | 0.60 | 0.15 | a | 2.23–4.90 | 1.79–5.39 | Kruskal-Wallis, Dunn | |||||
| Atlantic salmon | Presmolt | 2.90 | 2.97 | 0.12 | 0.07 | a | 2.74–3.19 | 2.72–3.22 | ANOVA, Tukey | POWER BOX COX | |||
| Smolt | 2.90 | 2.96 | 0.32 | 0.06 | a | 2.22–3.58 | 1.90–3.88 | ANOVA, Tukey | POWER BOX COX | ||||
| Postsmolt | 2.95 | 3.03 | 0.40 | 0.08 | a | 2.16–3.88 | 1.93–4.08 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 2.80 | 3.06 | 0.72 | 0.18 | a | 1.11–4.37 | 0.19–5.46 | ANOVA, Tukey | POWER BOX COX | ||||
| Rainbow trout | Smolt | 3.00 | 3.18 | 0.58 | 0.16 | a | 2.11–3.92 | 1.76–4.33 | ANOVA, Tukey | POWER BOX COX | |||
| Postsmolt | 3.20 | 3.30 | 0.17 | 0.10 | a | 2.96–3.64 | 2.93–3.67 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 2.90 | 3.06 | 0.34 | 0.11 | a | 2.09–3.95 | 1.71–4.41 | ANOVA, Tukey | POWER BOX COX | ||||
| Direct bilirubin | DBI | μmol/L | Coho salmon | Smolt | 1.88 | 2.05 | 0.40 | 0.10 | a | 1.03–2.95 | 0.82–3.23 | ANOVA, Tukey | BOX COX |
| Adult | 1.90 | 1.98 | 0.40 | 0.07 | a | 1.10–2.80 | 0.78–3.04 | ANOVA, Tukey | BOX COX | ||||
| Atlantic salmon | Presmolt | 1.80 | 1.83 | 0.26 | 0.13 | ab | 1.31–2.34 | 1.26–2.39 | ANOVA, Tukey | POWER BOX COX | |||
| Smolt | 1.80 | 1.88 | 0.32 | 0.05 | b | 1.31–2.29 | 1.20–2.40 | ANOVA, Tukey | POWER BOX COX | ||||
| Postsmolt | 2.10 | 2.16 | 0.36 | 0.06 | a | 1.47–2.76 | 1.31–2.90 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 1.80 | 1.87 | 0.27 | 0.05 | b | 1.26–2.43 | 1.12–2.60 | ANOVA, Tukey | POWER BOX COX | ||||
| Rainbow trout | Smolt | 1.70 | 1.78 | 0.28 | 0.06 | a | 1.09–2.29 | 0.89–2.56 | ANOVA, Tukey | POWER BOX COX | |||
| Postsmolt | 1.60 | 1.77 | 0.29 | 0.17 | a | 1.20–2.33 | 1.15–2.38 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 1.60 | 1.57 | 0.09 | 0.03 | a | 1.39–1.75 | 1.37–1.77 | ANOVA, Tukey | POWER BOX COX | ||||
| Creatinine | CRE | μmol/L | Coho salmon | Smolt | 24.64 | 26.67 | 9.15 | 1.08 | a | 6.63–44.29 | 3.61–48.26 | Kruskal-Wallis, Dunn | |
| Postsmolt | 23.87 | 21.51 | 4.87 | 2.81 | a | 15.91–24.75 | 15.91–24.75 | Kruskal-Wallis, Dunn | |||||
| Adult | 22.10 | 24.21 | 8.04 | 1.02 | a | 5.56–39.03 | 2.30–43.24 | Kruskal-Wallis, Dunn | |||||
| Atlantic salmon | Presmolt | 23.90 | 26.91 | 10.27 | 1.00 | b | 6.79–47.04 | 4.78–49.05 | ANOVA, Tukey | log(x + 1) | |||
| Smolt | 25.64 | 26.72 | 7.67 | 0.51 | b | 11.09–41.70 | 9.67–43.21 | ANOVA, Tukey | log(x + 1) | ||||
| Postsmolt | 31.90 | 31.28 | 8.80 | 0.85 | a | 13.94–49.13 | 11.50–51.25 | ANOVA, Tukey | log(x + 1) | ||||
| Adult | 23.90 | 24.32 | 5.57 | 0.92 | b | 11.90–34.83 | 9.09–38.17 | ANOVA, Tukey | log(x + 1) | ||||
| Rainbow trout | Presmolt | 61.44 | 59.63 | 12.95 | 2.90 | a | 33.25–89.47 | 22.86–101.49 | ANOVA, Tukey | POWER BOX COX | |||
| Smolt | 28.68 | 32.01 | 13.77 | 1.44 | b | 5.03–58.99 | 2.77–61.26 | ANOVA, Tukey | POWER BOX COX | ||||
| Postsmolt | 26.52 | 27.77 | 8.18 | 0.99 | b | 10.60–43.93 | 8.00–46.86 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 26.08 | 27.35 | 8.74 | 1.15 | b | 8.60–44.26 | 4.00–48.23 | ANOVA, Tukey | POWER BOX COX | ||||
| Glucose | GLU | mmol/L | Coho salmon | Smolt | 4.29 | 4.65 | 1.51 | 0.13 | a | 1.34–7.51 | 0.94–7.98 | ANOVA, Tukey | log(x + 1) |
| Postsmolt | 4.58 | 4.61 | 0.76 | 0.07 | a | 3.11–6.12 | 2.92–6.31 | ANOVA, Tukey | log(x + 1) | ||||
| Adult | 2.85 | 3.07 | 1.47 | 0.15 | b | 1.10–5.96 | 1.02–5.99 | ANOVA, Tukey | log(x + 1) | ||||
| Atlantic salmon | Presmolt | 5.40 | 5.40 | 1.85 | 0.13 | b | 1.77–9.02 | 1.40–9.39 | Kruskal-Wallis, Dunn | ||||
| Smolt | 6.10 | 6.33 | 1.68 | 0.09 | a | 2.91–9.60 | 2.68–9.87 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 4.10 | 4.06 | 1.65 | 0.08 | c | 0.76–7.06 | 0.57–7.29 | Kruskal-Wallis, Dunn | |||||
| Adult | 5.50 | 5.70 | 0.81 | 0.05 | b | 4.0–7.30 | 3.86–7.43 | Kruskal-Wallis, Dunn | |||||
| Rainbow trout | Presmolt | 2.79 | 2.76 | 0.56 | 0.10 | c | 1.60–3.93 | 1.36–4.16 | Kruskal-Wallis, Dunn | ||||
| Smolt | 2.76 | 3.13 | 1.82 | 0.12 | c | 1.08–7.48 | 1.06–8.46 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 4.08 | 4.32 | 2.13 | 0.18 | b | 0.15–8.49 | 8.11–8.88 | Kruskal-Wallis, Dunn | |||||
| Adult | 5.24 | 5.04 | 2.05 | 0.17 | a | 0.92–9.12 | 0.51–9.60 | Kruskal-Wallis, Dunn | |||||
| Lactate | LAC | mmol/L | Coho salmon | Smolt | 4.69 | 5.44 | 3.20 | 0.23 | b | 1.25–13.17 | 1.12–13.81 | Kruskal-Wallis, Dunn | |
| Postsmolt | 5.15 | 5.47 | 1.78 | 0.16 | a | 1.67–8.84 | 1.21–9.43 | Kruskal-Wallis, Dunn | |||||
| Adult | 5.63 | 5.81 | 1.85 | 0.11 | b | 1.90–9.22 | 1.59–9.59 | Kruskal-Wallis, Dunn | |||||
| Atlantic salmon | Presmolt | 4.98 | 5.60 | 2.21 | 0.17 | a | 3.03–11.44 | 3.0–12.59 | Kruskal-Wallis, Dunn | ||||
| Smolt | 5.10 | 5.77 | 2.16 | 0.13 | a | 3.10–11.29 | 3.0–13.18 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 4.80 | 5.25 | 2.33 | 0.11 | b | 1.56–11.35 | 1.40–11.74 | Kruskal-Wallis, Dunn | |||||
| Adult | 3.80 | 3.84 | 0.94 | 0.06 | a | 1.97–5.69 | 1.82–5.85 | Kruskal-Wallis, Dunn | |||||
| Rainbow trout | Presmolt | 11.77 | 12.27 | 2.84 | 0.52 | c | 8.05–19.26 | 8.05–19.26 | Kruskal-Wallis, Dunn | ||||
| Smolt | 6.55 | 7.00 | 3.54 | 0.22 | c | 2.27–14.18 | 2.0–14.97 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 6.00 | 6.23 | 2.38 | 0.15 | b | 1.38–10.84 | 0.96–11.29 | Kruskal-Wallis, Dunn | |||||
| Adult | 4.72 | 4.88 | 1.99 | 0.14 | a | 0.77–8.65 | 0.41–9.11 | Kruskal-Wallis, Dunn | |||||
| Urea | URE | mmol/L | Coho salmon | Smolt | 1.80 | 1.76 | 0.27 | 0.03 | a | 1.21–2.30 | 1.13–2.39 | ANOVA, Tukey | Normal |
| Postsmolt | 0.90 | 0.91 | 0.20 | 0.02 | b | 0.52–1.31 | 0.45–1.39 | ANOVA, Tukey | Normal | ||||
| Adult | 1.60 | 1.60 | 0.35 | 0.06 | c | 0.89–2.33 | 0.70–2.51 | ANOVA, Tukey | Normal | ||||
| Atlantic salmon | Presmolt | 1.45 | 1.40 | 0.26 | 0.03 | a | 0.87–1.94 | 0.78–2.03 | ANOVA, Tukey | BOX COX | |||
| Smolt | 1.10 | 1.14 | 0.30 | 0.04 | b | 0.51–1.76 | 0.37–1.88 | ANOVA, Tukey | BOX COX | ||||
| Postsmolt | 1.50 | 1.50 | 0.19 | 0.03 | a | 1.09–1.86 | 0.97–1.98 | ANOVA, Tukey | BOX COX | ||||
| Adult | 1.10 | 1.09 | 0.30 | 0.05 | b | 0.46–1.70 | 0.32–1.86 | ANOVA, Tukey | BOX COX | ||||
| Rainbow trout | Presmolt | 1.20 | 1.20 | 0.09 | 0.02 | a | 1.0–1.38 | 0.94–1.45 | ANOVA, Tukey | BOX COX | |||
| Smolt | 1.00 | 1.07 | 0.19 | 0.02 | b | 0.65–1.42 | 0.59–1.48 | ANOVA, Tukey | BOX COX | ||||
| Postsmolt | 1.30 | 1.27 | 0.16 | 0.03 | a | 0.92–1.58 | 0.83–1.69 | ANOVA, Tukey | BOX COX | ||||
| Uric acid | UAC | μmol/L | Coho salmon | Smolt | 1.80 | 1.76 | 0.27 | 0.03 | a | 1.21–2.30 | 1.12–2.39 | ANOVA, Tukey | Normal |
| Postsmolt | 0.90 | 0.91 | 0.20 | 0.02 | c | 0.52–1.31 | 0.45–1.38 | ANOVA, Tukey | Normal | ||||
| Adult | 1.60 | 1.60 | 0.35 | 0.06 | b | 0.89–2.33 | 0.71–2.51 | ANOVA, Tukey | Normal | ||||
| Atlantic salmon | Presmolt | 1.45 | 1.40 | 0.26 | 0.03 | a | 0.87–1.94 | 0.78–2.33 | ANOVA, Tukey | log(x + 1) | |||
| Smolt | 1.10 | 1.13 | 0.28 | 0.04 | b | 0.55–1.70 | 0.44–1.8 | ANOVA, Tukey | log(x + 1) | ||||
| Postsmolt | 1.50 | 1.49 | 0.16 | 0.02 | a | 1.15–1.80 | 1.08–1.87 | ANOVA, Tukey | log(x + 1) | ||||
| Adult | 1.10 | 1.09 | 0.30 | 0.05 | b | 0.46–1.70 | 0.32–1.86 | ANOVA, Tukey | log(x + 1) | ||||
| Rainbow trout | Presmolt | 1.20 | 1.20 | 0.09 | 0.02 | a | 1.0–1.38 | 0.94–1.46 | Kruskal-Wallis, Dunn | ||||
| Smolt | 1.00 | 1.06 | 0.17 | 0.02 | b | 0.68–1.39 | 0.63–1.44 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 1.20 | 1.24 | 0.12 | 0.02 | a | 0.97–1.51 | 0.93–1.56 | Kruskal-Wallis, Dunn | |||||
| Ammonia | NH3 | mmol/L | Coho salmon | Smolt | 2.92 | 2.75 | 0.60 | 0.08 | a | 1.58–4.09 | 1.29–4.32 | Kruskal-Wallis, Dunn | |
| Postsmolt | 1.07 | 0.98 | 0.34 | 0.03 | b | 0.36–1.76 | 0.24–1.83 | Kruskal-Wallis, Dunn | |||||
| Adult | 2.62 | 2.51 | 0.50 | 0.10 | a | 1.53–3.66 | 1.18–4.06 | Kruskal-Wallis, Dunn | |||||
| Atlantic salmon | Presmolt | 1.92 | 1.83 | 0.34 | 0.05 | b | 1.22–2.63 | 1.02–2.75 | Kruskal-Wallis, Dunn | ||||
| Smolt | 1.85 | 1.51 | 0.68 | 0.09 | b | 0.19–2.84 | 0.09–2.94 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 1.83 | 1.88 | 0.22 | 0.03 | b | 1.38–2.30 | 1.25–2.44 | Kruskal-Wallis, Dunn | |||||
| Adult | 1.24 | 1.13 | 0.36 | 0.07 | a | 0.36–1.95 | 0.16–2.11 | Kruskal-Wallis, Dunn | |||||
| Rainbow trout | Presmolt | 1.71 | 1.73 | 0.13 | 0.02 | a | 1.46–1.99 | 1.40–2.05 | Kruskal-Wallis, Dunn | ||||
| Smolt | 1.63 | 1.62 | 0.32 | 0.03 | a | 0.97–2.27 | 0.83–2.41 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 1.69 | 1.72 | 0.24 | 0.04 | a | 1.21–2.20 | 1.10–2.34 | Kruskal-Wallis, Dunn | |||||
| Total Cholesterol | TCH | mmol/L | Coho salmon | Smolt | 7.14 | 7.76 | 2.95 | 0.19 | c | 1.41–13.30 | 0.99–13.94 | Kruskal-Wallis, Dunn | |
| Postsmolt | 9.04 | 8.86 | 1.67 | 0.16 | a | 5.62–12.29 | 5.11–12.77 | Kruskal-Wallis, Dunn | |||||
| Adult | 8.09 | 8.13 | 2.84 | 0.16 | b | 2.46–13.65 | 1.96–14.11 | Kruskal-Wallis, Dunn | |||||
| Atlantic salmon | Presmolt | 12.32 | 12.78 | 2.74 | 0.20 | a | 7.17–18.17 | 6.67–18.72 | Kruskal-Wallis, Dunn | ||||
| Smolt | 11.10 | 11.44 | 3.66 | 0.20 | b | 4.08–18.56 | 3.62–19.09 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 7.50 | 7.69 | 2.63 | 0.11 | d | 2.39–12.78 | 2.05–13.11 | Kruskal-Wallis, Dunn | |||||
| Adult | 8.80 | 9.06 | 1.90 | 0.10 | c | 5.05–12.59 | 4.69–12.96 | Kruskal-Wallis, Dunn | |||||
| Rainbow trout | Presmolt | 5.34 | 6.08 | 2.44 | 0.45 | b | 2.07–12.07 | 2.07–12.07 | ANOVA, Tukey | POWER BOX COX | |||
| Smolt | 8.94 | 9.08 | 3.19 | 0.19 | a | 2.54–15.14 | 1.96–15.73 | ANOVA, Tukey | POWER BOX COX | ||||
| Postsmolt | 6.78 | 6.87 | 2.56 | 0.17 | b | 1.65–11.79 | 1.21–12.29 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 5.91 | 6.34 | 2.85 | 0.20 | b | 0.74–11.93 | 0.21–12.47 | ANOVA, Tukey | POWER BOX COX | ||||
| Triglycerides | TRG | mmol/L | Coho salmon | Smolt | 2.84 | 2.90 | 1.31 | 0.09 | a | 0.34–5.47 | 0.11–5.70 | ANOVA, Tukey | POWER BOX COX |
| Postsmolt | 2.73 | 3.12 | 1.26 | 0.12 | a | 1.57–6.94 | 1.48–7.58 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 2.77 | 2.91 | 1.24 | 0.07 | a | 0.34–5.24 | 0.13–5.45 | ANOVA, Tukey | POWER BOX COX | ||||
| Atlantic salmon | Presmolt | 4.25 | 4.54 | 1.42 | 0.11 | c | 1.4–7.15 | 0.98–7.60 | Kruskal-Wallis, Dunn | ||||
| Smolt | 4.10 | 4.19 | 1.71 | 0.09 | a | 0.54–7.29 | 0.29–7.60 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 2.80 | 3.01 | 1.39 | 0.06 | b | 0.27–5.74 | 0.10–5.91 | Kruskal-Wallis, Dunn | |||||
| Adult | 2.75 | 3.20 | 1.59 | 0.09 | b | 0.31–6.19 | 0.60–6.46 | Kruskal-Wallis, Dunn | |||||
| Rainbow trout | Presmolt | 6.73 | 7.40 | 2.62 | 0.50 | a | 1.63–12.97 | 0.56–14.27 | ANOVA, Tukey | POWER BOX COX | |||
| Smolt | 5.00 | 4.99 | 1.85 | 0.11 | b | 1.26–8.55 | 1.0–8.89 | ANOVA, Tukey | POWER BOX COX | ||||
| Postsmolt | 4.20 | 4.70 | 2.09 | 0.14 | b | 0.59–8.80 | 0.22–9.18 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 4.33 | 4.73 | 2.11 | 0.15 | b | 0.6–8.86 | 0.20–9.26 | ANOVA, Tukey | POWER BOX COX | ||||
| High-density lipoprotein cholesterol | HDL | mmol/L | Coho salmon | Smolt | 3.39 | 3.49 | 1.49 | 0.20 | b | 0.57–6.40 | 0.31–6.66 | Kruskal-Wallis, Dunn | |
| Postsmolt | 5.65 | 5.45 | 1.62 | 0.16 | a | 2.26–8.76 | 1.82–9.20 | Kruskal-Wallis, Dunn | |||||
| Adult | 0.28 | 0.32 | 0.11 | 0.02 | c | 0.10–0.54 | 0.09–0.56 | Kruskal-Wallis, Dunn | |||||
| Atlantic salmon | Presmolt | 1.86 | 1.98 | 0.67 | 0.10 | c | 0.45–3.19 | 0.14–3.56 | ANOVA, Tukey | Normal | |||
| Smolt | 5.41 | 5.60 | 1.27 | 0.17 | b | 2.94–8.14 | 2.49–8.61 | ANOVA, Tukey | Normal | ||||
| Postsmolt | 6.61 | 6.23 | 1.54 | 0.21 | a | 3.18–9.61 | 2.47–10.11 | ANOVA, Tukey | Normal | ||||
| Adult | 6.88 | 6.90 | 1.21 | 0.27 | a | 4.36–9.59 | 3.48–10.39 | ANOVA, Tukey | Normal | ||||
| Rainbow trout | Presmolt | 1.63 | 1.62 | 0.44 | 0.08 | c | 0.69–2.53 | 0.48–2.74 | ANOVA, Tukey | Normal | |||
| Smolt | 4.46 | 4.26 | 1.64 | 0.18 | b | 0.99–7.61 | 0.54–8.04 | ANOVA, Tukey | Normal | ||||
| Postsmolt | 2.53 | 2.50 | 0.90 | 0.17 | a | 0.65–4.40 | 0.15–4.86 | ANOVA, Tukey | Normal | ||||
| Low-density lipoprotein cholesterol | LDL | mmol/L | Coho salmon | Smolt | 1.20 | 1.34 | 0.95 | 0.12 | c | 0.15–4.25 | 0.30–5.30 | ANOVA, Tukey | BOX COX |
| Postsmolt | 1.57 | 1.60 | 0.51 | 0.05 | b | 0.57–2.59 | 0.40–2.75 | ANOVA, Tukey | BOX COX | ||||
| Adult | 2.10 | 2.20 | 1.02 | 0.19 | a | 0.19–4.20 | 0.04–4.36 | ANOVA, Tukey | BOX COX | ||||
| Atlantic salmon | Presmolt | 2.35 | 2.52 | 0.90 | 0.16 | a | 0.75–4.29 | 0.57–4.47 | ANOVA, Tukey | BOX COX | |||
| Smolt | 2.42 | 2.40 | 1.00 | 0.13 | a | 0.33–4.40 | 0.04–4.77 | ANOVA, Tukey | BOX COX | ||||
| Postsmolt | 1.55 | 1.62 | 0.56 | 0.07 | b | 0.39–2.66 | 0.16–2.93 | ANOVA, Tukey | BOX COX | ||||
| Adult | 0.90 | 0.97 | 0.33 | 0.06 | c | 0.22–1.63 | 0.04–1.87 | ANOVA, Tukey | BOX COX | ||||
| Rainbow trout | Presmolt | 1.45 | 1.46 | 0.60 | 0.11 | b | 0.50–3.10 | 0.50–3.10 | ANOVA, Tukey | BOX COX | |||
| Smolt | 1.89 | 1.98 | 0.96 | 0.12 | a | 0.41–4.74 | 0.60–4.88 | ANOVA, Tukey | BOX COX | ||||
| Postsmolt | 2.40 | 2.50 | 1.20 | 0.22 | a | 0.36–4.79 | 0.94–4.79 | ANOVA, Tukey | BOX COX |
| Parameter | Abbreviation | Unit of Measure | Species | Age Stage | Median | Mean | S. D | S. E | Difference between Means | 95% Reference Interval | Confidence Interval (95%) | Test | Data Processing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alkaline phosphatase | ALP | U/L | Coho salmon | Smolt | 43.00 | 48.74 | 25.86 | 1.85 | b | 18.93–115.23 | 15.0–122.0 | Kruskal-Wallis, Dunn | |
| Postsmolt | 67.00 | 65.80 | 22.58 | 2.18 | a | 20.56–110.74 | 15.04–116.44 | Kruskal-Wallis, Dunn | |||||
| Adult | 39.00 | 46.68 | 26.40 | 1.65 | c | 13.00–106.2 | 11.0–255.49 | Kruskal-Wallis, Dunn | |||||
| Atlantic salmon | Presmolt | 177.00 | 179.77 | 35.12 | 2.73 | a | 107.55–247.14 | 99.80–255.49 | Kruskal-Wallis, Dunn | ||||
| Smolt | 176.00 | 176.56 | 38.76 | 2.18 | a | 99.25–252.11 | 93.88–258.23 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 156.00 | 153.59 | 41.11 | 2.10 | b | 70.50–232.67 | 64.90–238.67 | Kruskal-Wallis, Dunn | |||||
| Adult | 171.00 | 170.62 | 34.77 | 2.10 | a | 101.98–239.18 | 96.24–244.93 | Kruskal-Wallis, Dunn | |||||
| Rainbow trout | Presmolt | 164.00 | 161.00 | 27.71 | 5.54 | a | 101.37–218.62 | 84.47–234.88 | Kruskal-Wallis, Dunn | ||||
| Smolt | 83.00 | 90.14 | 41.69 | 2.74 | c | 8.42–171.85 | 1.57–178.71 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 99.00 | 104.74 | 46.80 | 3.09 | b | 7.95–194.24 | 0.57–204.26 | Kruskal-Wallis, Dunn | |||||
| Adult | 65.50 | 74.77 | 36.68 | 2.53 | d | 22.28–157.73 | 20.0–162.0 | Kruskal-Wallis, Dunn | |||||
| Alanine transaminase | ALT | U/L | Coho salmon | Smolt | 15.90 | 15.86 | 7.41 | 0.59 | b | 1.35–30.38 | 0.05–31.68 | ANOVA, Tukey | POWER BOX COX |
| Postsmolt | 10.30 | 10.91 | 3.61 | 0.36 | a | 3.13–17.73 | 1.98–18.91 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 14.80 | 15.38 | 5.39 | 0.35 | c | 4.31–25.73 | 3.54–26.78 | ANOVA, Tukey | POWER BOX COX | ||||
| Atlantic salmon | Presmolt | 19.95 | 19.70 | 7.81 | 0.64 | c | 4.62–35.63 | 2.70–37.38 | ANOVA, Tukey | POWER BOX COX | |||
| Smolt | 15.20 | 16.88 | 7.41 | 0.41 | b | 1.04–30.98 | 0.09–32.38 | ANOVA, Tukey | POWER BOX COX | ||||
| Postsmolt | 14.10 | 14.47 | 6.75 | 0.36 | a | 1.25–27.69 | 0.44–28.50 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 12.65 | 12.73 | 4.68 | 0.30 | a | 3.28–21.74 | 2.45–22.68 | ANOVA, Tukey | POWER BOX COX | ||||
| Rainbow trout | Presmolt | 6.60 | 7.44 | 1.70 | 0.37 | c | 3.00–11.08 | 1.74–12.27 | ANOVA, Tukey | POWER BOX COX | |||
| Smolt | 14.50 | 15.87 | 7.83 | 0.48 | a | 5.40–33.90 | 5.37–34.67 | ANOVA, Tukey | POWER BOX COX | ||||
| Postsmolt | 10.90 | 11.61 | 4.84 | 0.37 | b | 1.47–20.85 | 0.39–22.15 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 8.20 | 10.33 | 4.95 | 0.38 | d | 5.50–25.79 | 5.14–29.30 | ANOVA, Tukey | POWER BOX COX | ||||
| Aspartate aminotransferase | AST | U/L | Coho salmon | Smolt | 245.50 | 269.36 | 134.75 | 9.11 | b | 52.0–582.85 | 38.10–601.50 | ANOVA, Tukey | POWER BOX COX |
| Postsmolt | 271.30 | 293.45 | 92.50 | 8.70 | a | 86.95–465.02 | 52.98–500.03 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 259.30 | 291.12 | 125.28 | 6.91 | c | 45.57–536.67 | 26.50–555.74 | ANOVA, Tukey | POWER BOX COX | ||||
| Atlantic salmon | Presmolt | 346.00 | 353.18 | 92.48 | 7.96 | c | 160.02–528.15 | 136.88–555.85 | ANOVA, Tukey | POWER BOX COX | |||
| Smolt | 352.10 | 367.40 | 106.57 | 6.20 | b | 134.57–558.92 | 115.90–584.67 | ANOVA, Tukey | POWER BOX COX | ||||
| Postsmolt | 267.10 | 272.41 | 107.76 | 4.99 | a | 49.99–474.44 | 34.97–490.75 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 278.10 | 286.64 | 112.45 | 6.16 | a | 52.49–496.83 | 35.13–518.01 | ANOVA, Tukey | POWER BOX COX | ||||
| Rainbow trout | Presmolt | 346.65 | 362.46 | 112.69 | 20.57 | c | 110.81–584.47 | 51.37–653.85 | ANOVA, Tukey | POWER BOX COX | |||
| Smolt | 349.20 | 348.92 | 124.85 | 7.80 | a | 99.19–591.83 | 73.07–615.53 | ANOVA, Tukey | POWER BOX COX | ||||
| Postsmolt | 276.45 | 290.74 | 110.98 | 7.25 | b | 54.44–496.13 | 31.02–519.56 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 307.00 | 316.66 | 129.73 | 9.00 | d | 50.78–565.15 | 24.77–594.71 | ANOVA, Tukey | POWER BOX COX | ||||
| Total amylase | TAM | U/L | Coho salmon | Smolt | 990.50 | 972.68 | 502.26 | 44.39 | c | 216.13–2366.88 | 163.50–2662.95 | ANOVA, Tukey | BOX COX |
| Postsmolt | 1539.00 | 1593.84 | 417.81 | 39.30 | a | 711.29–2388.73 | 599.84–3517.48 | ANOVA, Tukey | BOX COX | ||||
| Adult | 1281.50 | 1267.71 | 544.66 | 34.18 | b | 143.61–2294.0 | 53.19–2404.72 | ANOVA, Tukey | BOX COX | ||||
| Atlantic salmon | Presmolt | 609.00 | 656.76 | 303.19 | 22.85 | a | 62.53–1250.99 | 3.15–1310.37 | Kruskal-Wallis, Dunn | ||||
| Smolt | 880.00 | 924.00 | 303.60 | 16.61 | b | 306.71–1515.83 | 264.88–1562.42 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 967.90 | 987.36 | 381.63 | 17.91 | b | 208.88–1712.55 | 140.99–1777.90 | Kruskal-Wallis, Dunn | |||||
| Adult | 1177.00 | 1180.59 | 341.57 | 21.22 | c | 491.25–1832.17 | 422.24–1898.45 | Kruskal-Wallis, Dunn | |||||
| Rainbow trout | Presmolt | 445.00 | 493.08 | 220.81 | 43.30 | c | 160.0–1050.0 | 160.0–1050.0 | Kruskal-Wallis, Dunn | ||||
| Smolt | 876.00 | 887.59 | 445.30 | 27.78 | b | 155.60–1583.10 | 135.45–2414.80 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 934.00 | 991.61 | 399.42 | 25.89 | a | 160.73–1754.25 | 79.31–1836.25 | Kruskal-Wallis, Dunn | |||||
| Adult | 1120.00 | 1069.92 | 352.98 | 26.02 | a | 357.87–1768.88 | 276.34–1844.43 | Kruskal-Wallis, Dunn | |||||
| Lipase | LIP | U/L | Coho salmon | Smolt | 5.40 | 5.53 | 0.83 | 0.07 | b | 4.04–6.89 | 3.88–7.06 | ANOVA, Tukey | BOX COX |
| Postsmolt | 5.70 | 5.69 | 0.40 | 0.04 | ab | 4.91–6.50 | 4.79–6.64 | ANOVA, Tukey | BOX COX | ||||
| Adult | 5.90 | 5.85 | 0.88 | 0.05 | a | 4.19–7.59 | 4.05–7.72 | ANOVA, Tukey | BOX COX | ||||
| Atlantic salmon | Presmolt | 6.20 | 6.02 | 0.86 | 0.06 | a | 4.35–7.83 | 4.05–8.12 | Kruskal-Wallis, Dunn | ||||
| Smolt | 5.90 | 5.63 | 1.21 | 0.06 | b | 3.39–8.26 | 3.14–8.48 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 5.20 | 5.33 | 1.70 | 0.08 | c | 2.01–8.46 | 1.81–8.68 | Kruskal-Wallis, Dunn | |||||
| Adult | 5.60 | 5.42 | 1.44 | 0.08 | c | 2.83–8.12 | 2.59–8.34 | Kruskal-Wallis, Dunn | |||||
| Rainbow trout | Presmolt | 6.60 | 6.50 | 0.57 | 0.13 | a | 5.23–7.70 | 4.79–8.14 | Kruskal-Wallis, Dunn | ||||
| Smolt | 5.30 | 5.33 | 0.74 | 0.04 | c | 3.83–6.76 | 3.70–6.91 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 5.60 | 5.62 | 0.81 | 0.05 | b | 3.96–7.15 | 3.80–7.30 | Kruskal-Wallis, Dunn | |||||
| Adult | 5.60 | 5.47 | 1.34 | 0.09 | b | 2.88–8.21 | 2.59–8.44 | Kruskal-Wallis, Dunn | |||||
| Creatine kinase total | CKT | U/L | Coho salmon | Smolt | 5212.50 | 5980.72 | 4527.46 | 377.29 | b | 23.0–16,269.25 | 16.0–16,967.75 | ANOVA, Tukey | POWER BOX COX |
| Postsmolt | 7926.00 | 8324.64 | 6839.67 | 687.41 | a | 22.0–23,723.00 | 22.0–26,210.0 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 5181.00 | 7207.93 | 5716.01 | 340.99 | c | 457.0–22,267.10 | 293.0–23,667.0 | ANOVA, Tukey | POWER BOX COX | ||||
| Atlantic salmon | Presmolt | 7548.00 | 8670.21 | 4816.54 | 455.12 | c | 1398.98–20,848.55 | 1014.0–21,725.0 | ANOVA, Tukey | POWER BOX COX | |||
| Smolt | 7029.50 | 8638.65 | 4422.61 | 351.84 | b | 2827.35–18,632.50 | 2522.90–21,403.50 | ANOVA, Tukey | POWER BOX COX | ||||
| Postsmolt | 4637.00 | 4787.97 | 1878.53 | 114.11 | a | 883.94–8317.86 | 552.21–8674.11 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 5670.50 | 6144.78 | 3025.80 | 198.65 | a | 1921.18–14,481.05 | 1347.0–16,082.0 | ANOVA, Tukey | POWER BOX COX | ||||
| Rainbow trout | Presmolt | 2705.00 | 3414.33 | 2052.56 | 374.74 | c | 1380.0–9180.0 | 1380.0–9180.0 | ANOVA, Tukey | POWER BOX COX | |||
| Smolt | 5294.00 | 6660.63 | 5120.90 | 368.61 | b | 265.75–20,177.30 | 24.0–20,617.0 | ANOVA, Tukey | POWER BOX COX | ||||
| Postsmolt | 5250.00 | 7235.51 | 6037.84 | 390.56 | ab | 751.95–23,438.70 | 421.0–23,963.0 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 5965.00 | 7875.02 | 5099.61 | 386.60 | a | 1553.48–21,792.40 | 1273.0–22,850.0 | ANOVA, Tukey | POWER BOX COX | ||||
| Cardiac creatine kinase isoenzyme | CK-MB | U/L | Coho salmon | Smolt | 3419.50 | 3628.47 | 1410.47 | 190.19 | b | 626.15–6386.21 | 45.34–6982.88 | Kruskal-Wallis, Dunn | |
| Postsmolt | 9707.00 | 10,268.18 | 5107.02 | 484.74 | a | 1706.40–22,365.60 | 1248.0–23,554.0 | Kruskal-Wallis, Dunn | |||||
| Adult | 3811.00 | 4445.89 | 2760.02 | 235.80 | b | 1244.57–13,392.15 | 1059.78–19,625.45 | Kruskal-Wallis, Dunn | |||||
| Atlantic salmon | Presmolt | 11,277.00 | 11,012.54 | 6265.61 | 934.02 | c | 169.67–22,306.20 | 17.31–22,362.0 | Kruskal-Wallis, Dunn | ||||
| Smolt | 16,970.00 | 16,911.02 | 4926.72 | 703.82 | b | 6946.41–26,975.05 | 4820.19–29,099.54 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 3951.50 | 4361.31 | 2036.19 | 176.56 | a | 370.44–8352.18 | 126.15–8596.47 | Kruskal-Wallis, Dunn | |||||
| Adult | 3812.90 | 4352.02 | 2502.36 | 347.02 | a | 428.48–12,130.70 | 174.0–12,743.0 | Kruskal-Wallis, Dunn | |||||
| Rainbow trout | Presmolt | 2285.00 | 2713.33 | 1799.22 | 328.49 | b | 790.0–7500.0 | 790.0–7500.0 | Kruskal-Wallis, Dunn | ||||
| Smolt | 3939.00 | 3890.86 | 2372.30 | 199.78 | a | 597.90–9265.55 | 351.80–12,833.83 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 3306.85 | 3443.51 | 1808.86 | 137.92 | a | 578.61–7795.09 | 347.0–8608.0 | Kruskal-Wallis, Dunn | |||||
| Adult | 4206.60 | 4258.64 | 2108.70 | 274.53 | a | 651.70–8941.30 | 189.0–8948.60 | Kruskal-Wallis, Dunn | |||||
| Lactate dehydrogenase | LDH | U/L | Coho salmon | Smolt | 775.00 | 781.61 | 284.63 | 25.46 | a | 194.81–1324.83 | 87.63–1424.18 | Kruskal-Wallis, Dunn | |
| Postsmolt | 619.50 | 686.95 | 301.19 | 28.21 | b | 330.0–1572.38 | 292.0–1626.0 | Kruskal-Wallis, Dunn | |||||
| Adult | 848.00 | 823.44 | 309.42 | 19.23 | c | 181.70–1405.37 | 113.53–1465.71 | Kruskal-Wallis, Dunn | |||||
| Atlantic salmon | Presmolt | 980.00 | 1007.72 | 248.13 | 25.46 | b | 492.44–1488.65 | 407.86–1571.51 | Kruskal-Wallis, Dunn | ||||
| Smolt | 1018.00 | 1122.48 | 446.28 | 39.76 | b | 431.75–2342.0 | 296.25–2496.50 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 873.00 | 869.34 | 364.41 | 17.03 | a | 131.86–1564.84 | 60.42–1629.83 | Kruskal-Wallis, Dunn | |||||
| Adult | 952.00 | 954.57 | 309.14 | 17.97 | c | 343.34–1559.91 | 258.42–1639.88 | Kruskal-Wallis, Dunn | |||||
| Rainbow trout | Presmolt | 1130.00 | 1215.77 | 539.96 | 105.89 | c | 400.0–2140.0 | 400.0–2140.0 | Kruskal-Wallis, Dunn | ||||
| Smolt | 733.00 | 835.04 | 392.65 | 27.03 | a | 65.46–1604.61 | 0.87–1669.21 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 615.00 | 696.93 | 342.99 | 23.45 | a | 233.13–1615.0 | 174.0–1788.38 | Kruskal-Wallis, Dunn | |||||
| Adult | 870.00 | 882.25 | 308.17 | 23.03 | d | 219.87–1440.16 | 155.0–1529.68 | Kruskal-Wallis, Dunn |
| Parameter | Abbreviation | Unit of Measure | Species | Age Stage | Median | Mean | S. D | S. E | Difference between Means | 95% Reference Interval | Confidence Interval (95%) | Test | Data Processing |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sodium | Na | mmol/L | Coho salmon | Smolt | 159.00 | 158.17 | 6.17 | 0.41 | c | 146.47–171.04 | 145.12–172.25 | Kruskal-Wallis, Dunn | |
| Postsmolt | 171.05 | 170.99 | 4.16 | 0.39 | a | 162.67–179.22 | 160.93–181.0 | Kruskal-Wallis, Dunn | |||||
| Adult | 166.00 | 164.66 | 9.26 | 0.54 | b | 147.67–184.12 | 145.97–185.55 | Kruskal-Wallis, Dunn | |||||
| Atlantic salmon | Presmolt | 160.00 | 159.63 | 6.57 | 0.48 | a | 147.05–173.05 | 144.74–175.17 | Kruskal-Wallis, Dunn | ||||
| Smolt | 170.00 | 167.33 | 8.83 | 0.48 | b | 150.02–186.37 | 148.58–187.42 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 173.00 | 173.32 | 10.36 | 0.47 | c | 153.43–194.23 | 152.04–195.49 | Kruskal-Wallis, Dunn | |||||
| Adult | 180.00 | 176.93 | 10.62 | 0.57 | d | 158.55–202.07 | 156.05–203.90 | Kruskal-Wallis, Dunn | |||||
| Rainbow trout | Presmolt | 179.90 | 179.40 | 3.30 | 0.62 | a | 173.09–187.06 | 170.64–188.46 | Kruskal-Wallis, Dunn | ||||
| Smolt | 157.00 | 156.01 | 10.45 | 0.63 | c | 135.89–177.28 | 133.89–179.17 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 156.00 | 156.12 | 13.10 | 0.85 | cb | 130.37–182.11 | 127.89–184.51 | Kruskal-Wallis, Dunn | |||||
| Adult | 168.00 | 164.61 | 12.61 | 0.87 | b | 142.03–193.58 | 138.60–197.61 | Kruskal-Wallis, Dunn | |||||
| Potassium | K | mmol/L | Coho salmon | Smolt | 3.40 | 4.08 | 2.69 | 0.18 | b | 1.17–10.49 | 1.0–12.30 | Kruskal-Wallis, Dunn | |
| Postsmolt | 0.99 | 1.17 | 0.72 | 0.07 | c | 0.53–3.85 | 0.45–5.21 | Kruskal-Wallis, Dunn | |||||
| Adult | 4.40 | 5.02 | 2.86 | 0.17 | a | 1.20–13.28 | 1.10–14.26 | Kruskal-Wallis, Dunn | |||||
| Atlantic salmon | Presmolt | 3.70 | 3.90 | 1.95 | 0.14 | b | 0.83–8.76 | 0.45–9.29 | Kruskal-Wallis, Dunn | ||||
| Smolt | 3.60 | 3.69 | 1.48 | 0.08 | b | 0.59–6.45 | 0.29–6.75 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 3.00 | 3.37 | 1.89 | 0.09 | a | 0.87–8.32 | 0.80–9.60 | Kruskal-Wallis, Dunn | |||||
| Adult | 4.30 | 4.50 | 2.25 | 0.12 | c | 1.07–9.73 | 0.90–10.60 | Kruskal-Wallis, Dunn | |||||
| Rainbow trout | Presmolt | 2.80 | 2.78 | 1.23 | 0.23 | bc | 0.76–6.02 | 0.76–6.02 | Kruskal-Wallis, Dunn | ||||
| Smolt | 2.30 | 2.81 | 1.95 | 0.12 | c | 0.70–8.57 | 0.70–8.88 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 3.90 | 4.29 | 2.06 | 0.13 | a | 1.20–9.50 | 1.10–10.28 | Kruskal-Wallis, Dunn | |||||
| Adult | 3.70 | 3.62 | 1.58 | 0.11 | b | 0.80–8.17 | 0.70–8.80 | Kruskal-Wallis, Dunn | |||||
| Chloride | Cl | mmol/L | Coho salmon | Smolt | 130.85 | 130.38 | 11.12 | 0.73 | c | 108.90–152.87 | 106.78–154.61 | Kruskal-Wallis, Dunn | |
| Postsmolt | 141.25 | 141.92 | 4.38 | 0.41 | a | 132.66–150.27 | 130.68–152.12 | Kruskal-Wallis, Dunn | |||||
| Adult | 139.25 | 134.26 | 13.13 | 0.75 | b | 109.23–164.61 | 106.51–166.66 | Kruskal-Wallis, Dunn | |||||
| Atlantic salmon | Presmolt | 119.00 | 120.09 | 6.91 | 0.50 | a | 105.85–133.50 | 104.30–135.09 | Kruskal-Wallis, Dunn | ||||
| Smolt | 125.70 | 127.38 | 12.08 | 0.67 | a | 101.58–149.61 | 99.85–152.02 | Kruskal-Wallis, Dunn | |||||
| Postsmolt | 134.05 | 138.07 | 12.43 | 0.57 | c | 111.44–162.83 | 110.17–164.46 | Kruskal-Wallis, Dunn | |||||
| Adult | 143.10 | 143.17 | 12.57 | 0.67 | d | 118.62–168.17 | 116.8–169.81 | Kruskal-Wallis, Dunn | |||||
| Rainbow trout | Presmolt | 141.80 | 142.16 | 2.04 | 0.39 | a | 138.16–146.16 | 137.12–147.21 | ANOVA, Tukey | BOX COX | |||
| Smolt | 120.60 | 122.58 | 12.47 | 0.76 | c | 97.30–147.14 | 94.96–149.08 | ANOVA, Tukey | BOX COX | ||||
| Postsmolt | 119.00 | 120.26 | 9.23 | 0.60 | c | 101.03–137.83 | 99.28–139.80 | ANOVA, Tukey | BOX COX | ||||
| Adult | 131.25 | 129.03 | 15.07 | 1.04 | b | 99.68–159.91 | 96.72–162.55 | ANOVA, Tukey | BOX COX | ||||
| Calcium | Ca | mmol/L | Coho salmon | Smolt | 8.75 | 8.91 | 1.58 | 0.21 | a | 5.66–12.08 | 5.14–12.64 | ANOVA, Tukey | Normal |
| Postsmolt | 12.25 | 12.12 | 1.56 | 0.15 | c | 9.31–15.52 | 8.81–16.02 | ANOVA, Tukey | Normal | ||||
| Adult | 13.56 | 13.25 | 2.96 | 0.51 | b | 8.64–18.98 | 7.34–20.22 | ANOVA, Tukey | Normal | ||||
| Atlantic salmon | Presmolt | 14.45 | 14.78 | 1.66 | 0.26 | a | 11.34–17.53 | 10.57–18.45 | ANOVA, Tukey | POWER BOX COX | |||
| Smolt | 11.60 | 11.68 | 1.44 | 0.19 | b | 8.61–14.41 | 8.06–15.04 | ANOVA, Tukey | POWER BOX COX | ||||
| Postsmolt | 11.96 | 12.13 | 2.14 | 0.29 | b | 8.22–16.33 | 7.60–17.08 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 12.09 | 12.04 | 1.40 | 0.26 | b | 9.09–14.87 | 8.16–15.69 | ANOVA, Tukey | POWER BOX COX | ||||
| Rainbow trout | Presmolt | 16.99 | 15.78 | 2.83 | 0.57 | a | 9.73–22.72 | 7.89–24.36 | ANOVA, Tukey | BOX COX | |||
| Smolt | 13.32 | 13.33 | 1.96 | 0.21 | b | 9.33–17.15 | 8.83–17.80 | ANOVA, Tukey | BOX COX | ||||
| Magnesium | Mg | mmol/L | Coho salmon | Smolt | 4.60 | 4.56 | 1.12 | 0.15 | a | 2.26–6.81 | 1.89–7.22 | ANOVA, Tukey | BOX COX |
| Postsmolt | 1.31 | 1.33 | 0.32 | 0.03 | b | 0.68–1.97 | 0.60–2.05 | ANOVA, Tukey | BOX COX | ||||
| Adult | 1.06 | 1.15 | 0.26 | 0.04 | c | 0.51–1.63 | 0.39–1.80 | ANOVA, Tukey | BOX COX | ||||
| Atlantic salmon | Presmolt | 3.46 | 3.56 | 0.61 | 0.11 | b | 2.17–4.71 | 1.73–5.14 | ANOVA, Tukey | BOX COX | |||
| Smolt | 6.25 | 5.96 | 1.12 | 0.15 | a | 3.70–8.39 | 3.27–8.77 | ANOVA, Tukey | BOX COX | ||||
| Postsmolt | 5.36 | 5.75 | 1.45 | 0.22 | a | 2.58–8.75 | 1.59–9.43 | ANOVA, Tukey | BOX COX | ||||
| Adult | 2.94 | 3.24 | 0.81 | 0.15 | b | 1.40–5.02 | 0.99–5.44 | ANOVA, Tukey | BOX COX | ||||
| Rainbow trout | Presmolt | 3.70 | 3.81 | 0.85 | 0.16 | a | 2.0–5.62 | 1.66–5.98 | ANOVA, Tukey | BOX COX | |||
| Smolt | 3.31 | 3.33 | 0.44 | 0.05 | b | 2.43–4.21 | 2.26–4.36 | ANOVA, Tukey | BOX COX | ||||
| Postsmolt | 1.57 | 1.57 | 0.23 | 0.04 | c | 1.10–2.05 | 0.97–2.17 | ANOVA, Tukey | BOX COX | ||||
| Iron | Fe | μmol/L | Coho salmon | Smolt | 72.50 | 108.86 | 73.56 | 10.62 | a | 26.95–244.50 | 26.50–244.40 | ANOVA, Tukey | POWER BOX COX |
| Postsmolt | 58.30 | 64.27 | 25.30 | 2.41 | c | 25.12–131.99 | 17.30–170.0 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 103.00 | 106.99 | 30.76 | 5.20 | b | 45.58–169.08 | 31.28–186.60 | ANOVA, Tukey | POWER BOX COX | ||||
| Atlantic salmon | Presmolt | 125.15 | 122.42 | 26.37 | 4.81 | c | 87.09–159.23 | 76.95–171.46 | ANOVA, Tukey | POWER BOX COX | |||
| Smolt | 114.50 | 119.62 | 31.60 | 4.08 | b | 51.04–180.10 | 37.43–193.84 | ANOVA, Tukey | POWER BOX COX | ||||
| Postsmolt | 75.60 | 70.81 | 19.87 | 2.61 | a | 34.31–111.09 | 27.83–116.32 | ANOVA, Tukey | POWER BOX COX | ||||
| Rainbow trout | Presmolt | 6.93 | 8.19 | 4.26 | 0.78 | b | 1.97–17.09 | 1.97–17.09 | ANOVA, Tukey | Normal | |||
| Smolt | 13.45 | 13.43 | 4.06 | 0.44 | a | 5.37–21.59 | 4.02–22.97 | ANOVA, Tukey | Normal | ||||
| Postsmolt | 10.67 | 11.34 | 3.07 | 0.57 | a | 4.58–17.72 | 2.85–19.49 | ANOVA, Tukey | Normal | ||||
| Phosphorus | P | mmol/L | Coho salmon | Smolt | 29.00 | 29.38 | 3.65 | 0.48 | a | 21.90–36.76 | 20.77–38.09 | ANOVA, Tukey | sqrt(x) |
| Postsmolt | 16.39 | 16.71 | 2.35 | 0.22 | c | 11.90–21.34 | 11.32–21.96 | ANOVA, Tukey | sqrt(x) | ||||
| Adult | 20.86 | 20.11 | 3.42 | 0.59 | b | 13.05–27.53 | 11.29–28.99 | ANOVA, Tukey | sqrt(x) | ||||
| Atlantic salmon | Presmolt | 21.58 | 21.37 | 2.85 | 0.37 | c | 15.86–27.40 | 14.65–28.32 | ANOVA, Tukey | POWER BOX COX | |||
| Smolt | 28.25 | 26.67 | 7.45 | 1.01 | b | 11.54–42.48 | 8.83–45.66 | ANOVA, Tukey | POWER BOX COX | ||||
| Postsmolt | 30.39 | 30.79 | 3.14 | 0.41 | a | 24.01–36.78 | 22.92–38.16 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 11.72 | 11.69 | 1.81 | 0.33 | d | 7.87–15.36 | 6.65–16.34 | ANOVA, Tukey | POWER BOX COX | ||||
| Rainbow trout | Presmolt | 23.15 | 23.86 | 3.68 | 0.67 | a | 15.85–31.51 | 13.91–33.41 | ANOVA, Tukey | POWER BOX COX | |||
| Smolt | 21.28 | 21.85 | 4.35 | 0.46 | b | 12.69–30.22 | 11.22–31.78 | ANOVA, Tukey | POWER BOX COX | ||||
| Postsmolt | 24.68 | 25.07 | 3.17 | 0.58 | a | 18.29–31.59 | 16.75–33.38 | ANOVA, Tukey | POWER BOX COX | ||||
| Bicarbonate ion concentration | HCO3− | mmol/L | Coho salmon | Postsmolt | 7.43 | 7.43 | 1.12 | 0.14 | NA | 5.15–9.65 | 4.78–10.05 | NA | |
| Atlantic salmon | Presmolt | 10.71 | 10.86 | 1.37 | 0.18 | a | 7.86–13.44 | 7.21–14.12 | ANOVA, Tukey | log(x + 1) | |||
| Postsmolt | 8.41 | 8.53 | 0.90 | 0.13 | b | 6.60–10.28 | 6.31–10.69 | ANOVA, Tukey | log(x + 1) | ||||
| Rainbow trout | Presmolt | 10.92 | 11.24 | 1.39 | 0.26 | a | 8.17–14.15 | 7.57–14.89 | ANOVA, Tukey | Normal | |||
| Smolt | 7.72 | 7.61 | 1.35 | 0.26 | b | 4.86–10.55 | 4.01–11.35 | ANOVA, Tukey | Normal | ||||
| Partial pressure of carbon dioxide | pCO2 | mmHg | Coho salmon | Postsmolt | 11.90 | 12.50 | 2.31 | 0.28 | NA | 7.32–16.92 | 6.65–17.86 | NA | |
| Atlantic salmon | Presmolt | 22.55 | 22.76 | 2.67 | 0.36 | a | 17.33–28.19 | 16.41–29.08 | Kruskal-Wallis, Dunn | ||||
| Postsmolt | 9.20 | 7.85 | 3.58 | 0.53 | b | 1.67–12.88 | 1.62–13.0 | Kruskal-Wallis, Dunn | |||||
| Rainbow trout | Presmolt | 20.80 | 20.74 | 1.72 | 0.33 | a | 17.19–24.43 | 16.23–25.23 | ANOVA, Tukey | Normal | |||
| Smolt | 16.50 | 16.94 | 1.57 | 0.31 | b | 13.41–20.29 | 12.53–21.18 | ANOVA, Tukey | Normal | ||||
| Hydrogen potential | pH | Coho salmon | Postsmolt | 7.39 | 7.39 | 0.06 | 0.01 | NA | 7.28–7.54 | 7.28–7.55 | NA | ||
| Atlantic salmon | Presmolt | 7.29 | 7.29 | 0.08 | 0.01 | a | 7.12–7.45 | 7.09–7.48 | ANOVA, Tukey | Normal | |||
| Postsmolt | 7.32 | 7.32 | 0.12 | 0.02 | a | 7.08–7.55 | 7.04–7.60 | ANOVA, Tukey | Normal | ||||
| Rainbow trout | Presmolt | 7.34 | 7.34 | 0.07 | 0.01 | a | 7.20–7.48 | 7.17–7.52 | ANOVA, Tukey | Normal | |||
| Smolt | 7.28 | 7.26 | 0.10 | 0.02 | b | 7.06–7.48 | 7.0–7.53 | ANOVA, Tukey | Normal | ||||
| Cortisol | COR | ng/mL | Coho salmon | Smolt | 34.69 | 27.23 | 26.6 | 1.80 | b | 8.22–92.97 | 4.71–117.17 | ANOVA, Tukey | POWER BOX COX |
| Postsmolt | 77.65 | 77.64 | 37.3 | 5.63 | a | 13.35–129.91 | 3.32–134.37 | ANOVA, Tukey | POWER BOX COX | ||||
| Adult | 30.90 | 27.68 | 15.1 | 0.90 | b | 10.52–64.0 | 10.07–69.17 | ANOVA, Tukey | POWER BOX COX | ||||
| Atlantic salmon | Presmolt | 23.82 | 19.20 | 15.8 | 1.76 | c | 9.60–99.39 | 2.17–71.74 | Kruskal-Wallis, Dunn | Not normal | |||
| Smolt | 39.28 | 37.85 | 20.5 | 1.17 | b | 11.44–114.71 | 7.90–93.26 | Kruskal-Wallis, Dunn | Not normal | ||||
| Postsmolt | 55.60 | 50.40 | 31.3 | 1.54 | a | 6.28–105.12 | 11.28–140.83 | Kruskal-Wallis, Dunn | Not normal | ||||
| Adult | 59.10 | 56.60 | 28.2 | 1.52 | a | 4.23–59.49 | 10.4–118.80 | Kruskal-Wallis, Dunn | Not normal | ||||
| Rainbow trout | Presmolt | 37.81 | 34.94 | 15.4 | 2.82 | a | 6.90–75.18 | 7.28–72.75 | Kruskal-Wallis, Dunn | Not normal | |||
| Smolt | 39.17 | 35.48 | 22.0 | 1.51 | a | 7.28–72.75 | 7.47–100.47 | Kruskal-Wallis, Dunn | Not normal | ||||
| Postsmolt | 43.26 | 40.76 | 19.1 | 2.25 | a | 4.19–134.14 | 8.53–102.17 | Kruskal-Wallis, Dunn | Not normal | ||||
| Adult | 30.33 | 22.57 | 21.42 | 1.83 | b | 10.87–88.47 | 6.52–94.49 | Kruskal-Wallis, Dunn | Not normal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozas-Serri, M.; Correa, R.; Walker-Vergara, R.; Coñuecar, D.; Barrientos, S.; Leiva, C.; Ildefonso, R.; Senn, C.; Peña, A. Reference Intervals for Blood Biomarkers in Farmed Atlantic Salmon, Coho Salmon and Rainbow Trout in Chile: Promoting a Preventive Approach in Aquamedicine. Biology 2022, 11, 1066. https://doi.org/10.3390/biology11071066
Rozas-Serri M, Correa R, Walker-Vergara R, Coñuecar D, Barrientos S, Leiva C, Ildefonso R, Senn C, Peña A. Reference Intervals for Blood Biomarkers in Farmed Atlantic Salmon, Coho Salmon and Rainbow Trout in Chile: Promoting a Preventive Approach in Aquamedicine. Biology. 2022; 11(7):1066. https://doi.org/10.3390/biology11071066
Chicago/Turabian StyleRozas-Serri, Marco, Rodolfo Correa, Romina Walker-Vergara, Darling Coñuecar, Soraya Barrientos, Camila Leiva, Ricardo Ildefonso, Carolina Senn, and Andrea Peña. 2022. "Reference Intervals for Blood Biomarkers in Farmed Atlantic Salmon, Coho Salmon and Rainbow Trout in Chile: Promoting a Preventive Approach in Aquamedicine" Biology 11, no. 7: 1066. https://doi.org/10.3390/biology11071066
APA StyleRozas-Serri, M., Correa, R., Walker-Vergara, R., Coñuecar, D., Barrientos, S., Leiva, C., Ildefonso, R., Senn, C., & Peña, A. (2022). Reference Intervals for Blood Biomarkers in Farmed Atlantic Salmon, Coho Salmon and Rainbow Trout in Chile: Promoting a Preventive Approach in Aquamedicine. Biology, 11(7), 1066. https://doi.org/10.3390/biology11071066






