The Dual Role of Innate Immune Response in Acetaminophen-Induced Liver Injury
Abstract
Simple Summary
Abstract
1. Introduction
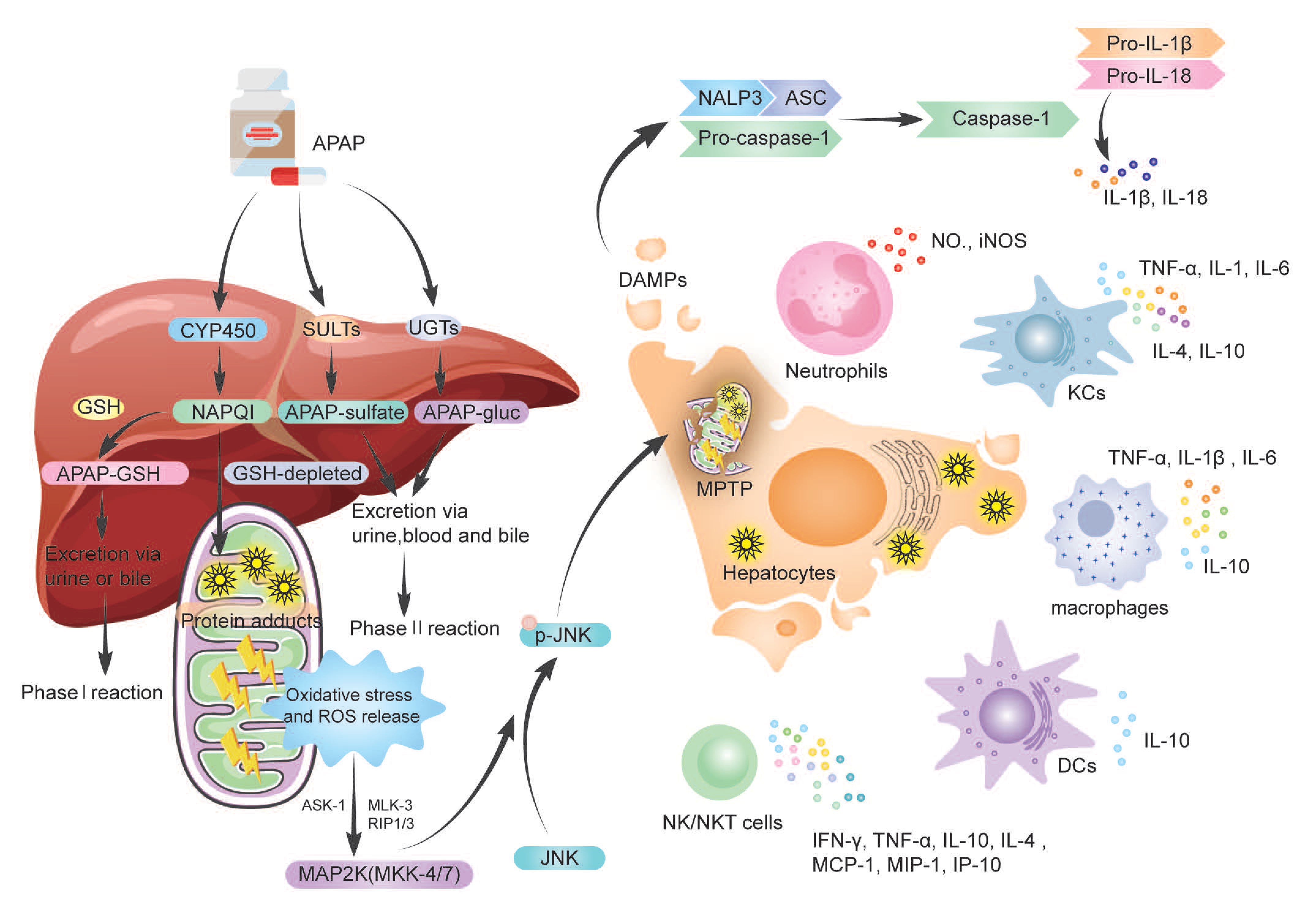
2. Metabolism of APAP
3. The Dual Role of Immune Cells and Cytokines
3.1. Neutrophils in AILI
| Proinflammatory | No Effect or Pro-Regenerative | |
|---|---|---|
| Neutrophils | Depleted neutrophils could protect against AILI via reduced FasL-expression, hepatocytotoxicity, and mitochondrial respiratory chain burst [32]. Blockade of neutrophil infiltration by anti-granulocyte receptor 1 depletion or combined CXCR2-FPR1 antagonism prevented liver injury [46] | No activation of circulating and liver neutrophils during AILI [42]. Neutrophil infiltration could be moving necrotic cell debris but not cause further damage, and CD-18-deficient mice were not protected [43]. gp91phox−/− did not protect [44]. Anti-Ly6G, genetic knockout in granulocyte-colony-stimulating factor, or genetic deletion in Nox2 did not protect against APAP overdose, promoting the phenotypic conversion of proinflammatory macrophages to pro-resolving macrophages, and promoting liver repair [45] |
| KCs | Depletion of KCs can restrain APAP injury [50,51]. Mincle deletion (or KCs depletion) may reduce APAP hepatotoxicity [52]. | EPO promotes the proliferation and function of KCs, ameliorating AILI [53]. Depletion of KCs can lead to liver injury aggravation [54,55,56]. KCs against AILI by secreting cytokines [57,58,59,60] |
| MoMFs | The activated MoMFs produce O2.-, NO., and peroxynitrite, promoting AILI progression [51], and upregulating proinflammatory gene expressions [61,62]. | Upregulate endocytosis- and apoptotic-cell-clearance-related proteins which promote liver repair [63]. Promotes macrophage differentiation [64,65,66,67]. |
| DCs | Prevent NKs cell activation and induce neutrophil apoptosis to reveal a protective role [68]. | |
| NK/NKT cells | Amplified the immune response, upregulated proinflammatory cytokine expressions, and increased neutrophil accumulation [41]. DMSO activated NK/NKT cells [69]. NKT-cell-deficient (Jα18−/−) mice could be resistant to AILI [70]. | NKT cell-deficient mice (CD1d−/− and Jα18−/−) were more vulnerable to AILI [71]. Reduce the release of inflammatory cytokines [72]. |
| γδT cells | Depletion of γδT cells reduced IL-17A production and attenuated liver injury [34]. HIF attenuated abnormal γδT cell recruitment and alleviated AILI [73]. |
3.2. Macrophages in AILI
3.2.1. Kupffer Cells (KCs)
3.2.2. Monocyte-Derived Macrophages (MoMFs)
3.3. Dendritic Cells (DCs) in AILI
3.4. Natural Killer Cells (NK Cells) and NKT Cells in AILI
3.5. γδT Cells in AILI
3.6. Cytokine Storm in AILI
4. Inflammasomes in AILI
5. Other Immune Cells in AILI
6. Conclusions and Research Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chalasani, N.P.; Maddur, H.; Russo, M.W.; Wong, R.J.; Reddy, K.R. Practice Parameters Committee of the American College of Gas-troenterology. ACG Clinical Guideline: Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am. J. Gastro-Enterol. 2021, 116, 878–898. [Google Scholar] [CrossRef] [PubMed]
- Real, M.; Barnhill, M.S.; Higley, C.; Rosenberg, J.; Lewis, J.H. Drug-Induced Liver Injury: Highlights of the Recent Literature. Drug Saf. 2019, 42, 365–387. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Liu, Y.; Shang, J.; Xie, Q.; Li, J.; Yan, M.; Xu, J.; Niu, J.; Liu, J.; Watkins, P.B.; et al. Incidence and Etiology of Drug-Induced Liver Injury in Mainland China. Gastroenterology. 2019, 156, 2230–2241. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. Electronic address: Easloffice@easloffice.eu; Clinical Practice Guideline Panel: Chair: Panel members; EASL Governing Board representative: EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cortes, M.; Robles-Diaz, M.; Stephens, C.; Ortega-Alonso, A.; Lucena, M.I.; Andrade, R.J. Drug induced liver injury: An up-date. Arch. Toxicol. 2020, 94, 3381–3407. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, L.; Wu, Z.; Li, L. Transplantation of mesenchymal stem cells and their derivatives effectively promotes liver regen-eration to attenuate acetaminophen-induced liver injury. Stem. Cell Res. 2020, 11, 88. [Google Scholar]
- Lee, W.M. Acetaminophen (APAP) hepatotoxicity-Isn’t it time for APAP to go away? J. Hepatol. 2017, 67, 1324–1331. [Google Scholar] [CrossRef]
- Goldberg, D.S.; Forde, K.A.; Carbonari, D.M.; Lewis, J.D.; Leidl, K.B.; Reddy, K.R.; Haynes, K.; Roy, J.; Sha, D.; Marks, A.R.; et al. Population-representative incidence of drug-induced acute liver failure based on an analysis of an integrated health care system. Gastroenterology 2015, 148, 1353–1361. [Google Scholar] [CrossRef]
- Wei, G.; Bergquist, A.; Broomé, U.; Lindgren, S.; Wallerstedt, S.; Almer, S.; Sangfelt, P.; Danielsson, A.; Sandberg-Gertzén, H.; Lööf, L.; et al. Acute liver failure in Sweden: Etiology and outcome. J. Intern. Med. 2007, 262, 393–401. [Google Scholar] [CrossRef]
- Bernal, W.; Wendon, J. Acute liver failure. N. Engl. J. Med. 2014, 370, 1170–1171. [Google Scholar]
- Larson, A.M. Acetaminophen hepatotoxicity. Clin. Liver Dis. 2007, 11, 525–548. [Google Scholar] [CrossRef]
- Hinson, J.A.; Roberts, D.W.; James, L.P. Mechanisms of acetaminophen-induced liver necrosis. Handb. Exp. Pharmacol. 2010, 196, 369–405. [Google Scholar]
- Ramachandran, A.; Jaeschke, H. Acetaminophen Toxicity: Novel Insights into Mechanisms and Future Perspectives. Gene Expr. 2018, 18, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Mazaleuskaya, L.L.; Sangkuhl, K.; Thorn, C.F.; FitzGerald, G.A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Pathways of aceta-minophen metabolism at the therapeutic versus toxic doses. Pharm. Genom. 2015, 25, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Xie, Y.; McGill, M.R.; Jaeschke, H. Pathophysiological significance of c-jun N-terminal kinase in acetaminophen hepato-toxicity. Expert Opin. Drug Metab. Toxicol 2015, 11, 1769–1779. [Google Scholar] [CrossRef]
- Win, S.; Than, T.A.; Han, D.; Petrovic, L.M.; Kaplowitz, N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acet-aminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J. Biol. Chem. 2011, 286, 35071–35078. [Google Scholar] [CrossRef] [PubMed]
- Win, S.; Than, T.A.; Min, R.W.; Aghajan, M.; Kaplowitz, N. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial. Src. Hepatol. 2016, 63, 1987–2003. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Akakpo, J.Y.; Umbaugh, D.S.; Ramachandran, A. Novel Therapeutic Approaches Against Acetaminophen-induced Liver Injury and Acute Liver Failure. Toxicol. Sci. 2020, 174, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Min, R.W.M.; Le, K.; Zhou, S.; Aghajan, M.; Than, T.A.; Win, S.; Kaplowitz, N. The role of MAP2 kinases and p38 kinase in acute murine liver injury models. Cell Death Dis. 2017, 8, e2903. [Google Scholar] [CrossRef]
- Zhang, Y.F.; He, W.; Zhang, C.; Liu, X.J.; Lu, Y.; Wang, H.; Zhang, Z.H.; Chen, X.; Xu, D.X. Role of receptor interacting protein (RIP)1 on apoptosis-inducing factor-mediated necroptosis during acetaminophen-evoked acute liver failure in mice. Toxicol Lett. 2014, 225, 445–453. [Google Scholar] [CrossRef]
- Yang, R.; Tonnesseen, T.I. DAMPs and sterile inflammation in drug hepatotoxicity. Hepatol. Int. 2019, 13, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Woolbright, B.L.; Jaeschke, H. Role of the inflammasome in acetaminophen-induced liver injury and acute liver failure. J. Hepa-Tol. 2017, 66, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Chen, C.J.; Ontiveros, F.; Rock, K.L. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J. Clin. Investig. 2010, 120, 1939–1949. [Google Scholar] [CrossRef]
- Amaral, S.S.; Oliveira, A.G.; Marques, P.E.; Pires, D.A.; Resende, R.R.; Sousa, B.R.; Pinto, M.A.; Russo, R.C.; Andrade, L.M.; Leite, M.F.; et al. Altered responsiveness to extracellular ATP enhances acetaminophen hepatotox-icity. Cell Commun. Signal. 2013, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chen, S.; Xie, M.; Zhou, C.; Zheng, M. The complex roles of neutrophils in APAP-induced liver injury. Cell Prolif. 2021, 54, e13040. [Google Scholar] [CrossRef]
- Shen, K.; Chang, W.; Gao, X.; Wang, H.; Niu, W.; Song, L.; Qin, X. Depletion of activated hepatic stellate cell correlates with severe liver damage and abnormal liver regeneration in acetaminophen-induced liver injury. Acta Biochim. Biophys. Sinica 2021, 43, 307–315. [Google Scholar] [CrossRef]
- Lotze, M.T.; Tracey, K.J. High-mobility group box 1 protein (HMGB1): Nuclear weapon in the immune arsenal. Nat. Rev. Im-Munol. 2005, 5, 331–342. [Google Scholar] [CrossRef]
- Mossanen, J.C.; Krenkel, O.; Ergen, C.; Govaere, O.; Liepelt, A.; Puengel, T.; Heymann, F.; Kalthoff, S.; Lefebvre, E.; Eulberg, D.; et al. Chemokine (C-C motif) receptor 2-positive monocytes aggravate the early phase of acetaminophen-induced acute liver injury. Hepatology 2016, 64, 1667–1682. [Google Scholar] [CrossRef]
- Barman, P.K.; Mukherjee, R.; Prusty, B.K.; Suklabaidya, S.; Senapati, S.; Ravindran, B. Chitohexaose protects against acetaminophen-induced hepatotoxicity in mice. Cell Death Dis. 2016, 7, e2224. [Google Scholar] [CrossRef]
- Mantovani, A.; Cassatella, M.A.; Costantini, C.; Jaillon, S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011, 11, 519–531. [Google Scholar] [CrossRef]
- Jaillon, S.; Galdiero, M.R.; Del Prete, D.; Cassatella, M.A.; Garlanda, C.; Mantovani, A. Neutrophils in innate and adaptive immunity. Semin. Immunopathol. 2013, 35, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; Han, D.; Gunawan, B.; Kaplowitz, N. Neutrophil depletion protects against murine acetaminophen hepatotoxicity. Hepatology 2006, 43, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Foureau, D.M.; Walling, T.L.; Maddukuri, V.; Anderson, W.; Culbreath, K.; Kleiner, D.E.; Ahrens, W.A.; Jacobs, C.; Watkins, P.B.; Fontana, R.J.; et al. Comparative analysis of portal hepatic infiltrating leucocytes in acute drug-induced liver injury, idiopathic autoimmune and viral hepatitis. Clin. Exp. Immunol. 2015, 180, 40–51. [Google Scholar] [CrossRef]
- Wang, X.; Sun, R.; Wei, H.; Tian, Z. High-mobility group box 1 (HMGB1)-Toll-like receptor (TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced damage-associated lethal hepatitis: Interaction of γδ T cells with macrophages. Hepatology 2013, 57, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, M.; Jin, Q.; Wu, Y.L.; Cui, Z.Y.; Cui, B.W.; Shang, Y.; Zhan, Z.Y.; Lin, Y.C.; Jiao, J.Y.; et al. Modulation of HMGB1 Release in APAP-Induced Liver Injury: A Possible Strategy of Chikusetsusaponin V Targeting NETs Formation. Front. Pharmacol. 2021, 12, 723881. [Google Scholar] [CrossRef]
- Lundbäck, P.; Lea, J.D.; Sowinska, A.; Ottosson, L.; Fürst, C.M.; Steen, J.; Aulin, C.; Clarke, J.I.; Kipar, A.; Klevenvall, L.; et al. A novel high mobility group box 1 neutralizing chimeric antibody attenuates drug-induced liver injury and postinjury inflammation in mice. Hepatology 2016, 64, 1699–1710. [Google Scholar] [CrossRef] [PubMed]
- Ayata, C.K.; Ganal, S.C.; Hockenjos, B.; Willim, K.; Vieira, R.P.; Grimm, M.; Robaye, B.; Boeynaems, J.M.; Di Virgilio, F.; Pellegatti, P.; et al. Purinergic P2Y₂ receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury. Gastroenterology 2012, 143, 1620–1629. [Google Scholar] [CrossRef]
- He, Y.; Feng, D.; Li, M.; Gao, Y.; Ramirez, T.; Cao, H.; Kim, S.J.; Yang, Y.; Cai, Y.; Ju, C.; et al. Hepatic mitochondrial DNA/Toll-like receptor 9/MicroRNA-223 forms a negative feedback loop to limit neutrophil overactivation and acetaminophen hepatotoxicity in mice. Hepatology 2017, 66, 220–234. [Google Scholar] [CrossRef]
- Volmering, S.; Block, H.; Boras, M.; Lowell, C.A.; Zarbock, A. The Neutrophil Btk Signalosome Regulates Integrin Activation during Sterile Inflammation. Immunity. 2016, 44, 73–87. [Google Scholar] [CrossRef]
- Cover, C.; Liu, J.; Farhood, A.; Malle, E.; Waalkes, M.P.; Bajt, M.L.; Jaeschke, H. Pathophysiological role of the acute inflammatory response during acetaminophen hepa-totoxicity. Toxicol. Appl. Pharm. 2006, 216, 98–107. [Google Scholar] [CrossRef]
- Liu, Z.X.; Govindarajan, S.; Kaplowitz, N. Innate immune system plays a critical role in determining the progression and sever-ity of acetaminophen hepatotoxicity. Gastroenterology 2004, 127, 1760–1774. [Google Scholar] [CrossRef] [PubMed]
- Lawson, J.A.; Farhood, A.; Hopper, R.D.; Bajt, M.L.; Jaeschke, H. The hepatic inflammatory response after acetaminophen over-dose: Role of neutrophils. Toxicol. Sci. 2000, 54, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.D.; Bajt, M.L.; Farhood, A.; Jaeschke, H. Acetaminophen-induced hepatic neutrophil accumulation and inflammatory liver injury in CD18-deficient mice. Liver Int. 2010, 30, 1280–1292. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.D.; Bajt, M.L.; Sharpe, M.R.; McGill, M.R.; Farhood, A.; Jaeschke, H. Neutrophil activation during acetaminophen hepato-toxicity and repair in mice and humans. Toxicol. Appl. Pharm. 2014, 275, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tao, Y.; Wu, Y.; Zhao, X.; Ye, W.; Zhao, D.; Fu, L.; Tian, C.; Yang, J.; He, F.; et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orches-trate liver repair. Nat. Commun. 2019, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Marques, P.E.; Amaral, S.S.; Pires, D.A.; Nogueira, L.L.; Soriani, F.M.; Lima, B.H.; Lopes, G.A.; Russo, R.C.; Avila, T.V.; Melgaço, J.G.; et al. Chemokines and mitochondrial products activate neutrophils to amplify organ in-jury during mouse acute liver failure. Hepatology 2012, 56, 1971–1982. [Google Scholar] [CrossRef]
- Graubardt, N.; Vugman, M.; Mouhadeb, O.; Caliari, G.; Pasmanik-Chor, M.; Reuveni, D.; Zigmond, E.; Brazowski, E.; David, E.; Chappell-Maor, L.; et al. Ly6Chi Monocytes and Their Macrophage Descendants Regulate Neutrophil Function and Clearance in Acetaminophen-Induced Liver Injury. Front. Immunol. 2017, 8, 626. [Google Scholar] [CrossRef]
- Jaeschke, H.; Farhood, A.; Smith, C.W. Neutrophil-induced liver cell injury in endotoxin shock is a CD11b/CD18-dependent mechanism. Am. J. Physiol. 1991, 261, G1051–G1056. [Google Scholar] [CrossRef]
- Gong, L.; Liao, L.; Dai, X.; Xue, X.; Peng, C.; Li, Y. The dual role of immune response in acetaminophen hepatotoxicity: Implication for immune pharmacological targets. Toxicol. Lett. 2021, 351, 37–52. [Google Scholar] [CrossRef]
- Fisher, J.E.; McKenzie, T.J.; Lillegard, J.B.; Yu, Y.; Juskewitch, J.E.; Nedredal, G.I.; Brunn, G.J.; Yi, E.S.; Malhi, H.; Smyrk, T.C.; et al. Role of Kupffer cells and toll-like receptor 4 in acetaminophen-induced acute liver failure. J. Surg. Res. 2013, 180, 147–155. [Google Scholar] [CrossRef]
- Michael, S.L.; Pumford, N.R.; Mayeux, P.R.; Niesman, M.R.; Hinson, J.A. Pretreatment of mice with macrophage inactivators de-creases acetaminophen hepatotoxicity and forms reactive oxygen and nitrogen species. Hepatology 1999, 30, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Kim, J.W.; Zhou, Z.; Qi, J.; Tian, W.; Lim, C.W.; Han, K.M.; Kim, B. Macrophage-Inducible C-Type Lectin Signaling Exacer-bates Acetaminophen-Induced Liver Injury by Promoting Kupffer Cell Activation in Mice. Mol. Pharmacol. 2021, 99, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Gilboa, D.; Haim-Ohana, Y.; Deshet-Unger, N.; Ben-Califa, N.; Hiram-Bab, S.; Reuveni, D.; Zigmond, E.; Gassmann, M.; Gabet, Y.; Varol, C.; et al. Erythropoietin enhances Kupffer cell number and activity in the chal-lenged liver. Sci. Rep. 2017, 7, 10379. [Google Scholar] [CrossRef] [PubMed]
- Campion, S.N.; Johnson, R.; Aleksunes, L.M.; Goedken, M.J.; van Rooijen, N.; Scheffer, G.L.; Cherrington, N.J.; Manautou, J.E. Hepatic Mrp4 induction following acetaminophen exposure is dependent on Kupffer cell function. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G294–G304. [Google Scholar] [CrossRef]
- Ju, C.; Reilly, T.P.; Bourdi, M.; Radonovich, M.F.; Brady, J.N.; George, J.W.; Pohl, L.R. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem. Res. Toxicol. 2002, 15, 1504–1513. [Google Scholar] [CrossRef]
- Knight, T.R.; Jaeschke, H. Peroxynitrite formation and sinusoidal endothelial cell injury during acetaminophen-induced hepa-totoxicity in mice. Comp. Hepatol. 2004, 3, S46. [Google Scholar] [CrossRef]
- Jaeschke, H.; Ramachandran, A. Mechanisms and pathophysiological significance of sterile inflammation during acetamino-phen hepatotoxicity. Food Chem. Toxicol. 2020, 138, 111240. [Google Scholar] [CrossRef]
- Gao, R.Y.; Wang, M.; Liu, Q.; Feng, D.; Wen, Y.; Xia, Y.; Colgan, S.P.; Eltzschig, H.K.; Ju, C. Hypoxia-Inducible Factor-2α Reprograms Liver Macrophages to Protect Against Acute Liver Injury Through the Production of Interleukin-6. Hepatology 2020, 71, 2105–2117. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, L.; Pan, B.; Chen, Y.; Zhang, T.; Tang, N. Interleukin 33 mediates hepatocyte autophagy and innate immune re-sponse in the early phase of acetaminophen-induced acute liver injury. Toxicology 2021, 456, 152788. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Umbaugh, D.S.; Sanchez-Guerrero, G.; Ramachandran, A.; Jaeschke, H. Kupffer cells regulate liver recovery through induction of chemokine receptor CXCR2 on hepatocytes after acetaminophen overdose in mice. Arch. Toxicol. 2022, 96, 305–320. [Google Scholar] [CrossRef]
- Zigmond, E.; Samia-Grinberg, S.; Pasmanik-Chor, M.; Brazowski, E.; Shibolet, O.; Halpern, Z.; Varol, C. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J. Immunol. 2014, 193, 344–353. [Google Scholar] [CrossRef] [PubMed]
- You, Q.; Holt, M.; Yin, H.; Li, G.; Hu, C.J.; Ju, C. Role of hepatic resident and infiltrating macrophages in liver repair after acute injury. Biochem. Pharmacol. 2013, 86, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhao, X.; Tao, Y.; Wu, Y.; He, F.; Tang, L. Proteomic analysis reveals a protective role of specific macrophage subsets in liver repair. Sci. Rep. 2019, 9, 2953. [Google Scholar] [CrossRef] [PubMed]
- Stutchfield, B.M.; Antoine, D.J.; Mackinnon, A.C.; Gow, D.J.; Bain, C.C.; Hawley, C.A.; Hughes, M.J.; Francis, B.; Wojtacha, D.; Man, T.Y.; et al. CSF1 Restores Innate Immunity after Liver Injury in Mice and Serum Levels Indicate Outcomes of Patients with Acute Liver Failure. Gastroenterology 2015, 149, 1896–1909. [Google Scholar] [CrossRef]
- Zagórska, A.; Través, P.G.; Lew, E.D.; Dransfield, I.; Lemke, G. Diversification of TAM receptor tyrosine kinase function. Nat. Immunol. 2014, 15, 920–928. [Google Scholar] [CrossRef]
- Triantafyllou, E.; Pop, O.T.; Possamai, L.A.; Wilhelm, A.; Liaskou, E.; Singanayagam, A.; Bernsmeier, C.; Khamri, W.; Petts, G.; Dargue, R.; et al. MerTK expressing hepatic macrophages promote the resolution of inflamma-tion in acute liver failure. Gut 2018, 67, 333–347. [Google Scholar] [CrossRef]
- Campana, L.; Lewis, P.J.S.; Pellicoro, A.; Aucott, R.L.; Man, J.; O’Duibhir, E.; Mok, S.E.; Ferreira-Gonzalez, S.; Livingstone, E.; Greenhalgh, S.N.; et al. The STAT3-IL-10-IL-6 Pathway Is a Novel Regulator of Macrophage Ef-ferocytosis and Phenotypic Conversion in Sterile Liver Injury. J. Immunol. 2018, 200, 1169–1187. [Google Scholar] [CrossRef]
- Connolly, M.K.; Ayo, D.; Malhotra, A.; Hackman, M.; Bedrosian, A.S.; Ibrahim, J.; Cieza-Rubio, N.E.; Nguyen, A.H.; Henning, J.R.; Dorvil-Castro, M.; et al. Dendritic cell depletion exacerbates acetaminophen hepatotoxicity. Hepatology 2011, 54, 959–968. [Google Scholar] [CrossRef]
- Masson, M.J.; Carpenter, L.D.; Graf, M.L.; Pohl, L.R. Pathogenic role of natural killer T and natural killer cells in acetaminophen-induced liver injury in mice is dependent on the presence of dimethyl sulfoxide. Hepatology 2008, 48, 889–897. [Google Scholar] [CrossRef]
- Downs, I.; Aw, T.Y.; Liu, J.; Adegboyega, P.; Ajuebor, M.N. Vα14iNKT cell deficiency prevents acetaminophen-induced acute liver failure by enhancing hepatic glutathione and altering APAP metabolism. Biochem. Biophys. Res. Commun. 2012, 428, 245–251. [Google Scholar] [CrossRef][Green Version]
- Martin-Murphy, B.V.; Kominsky, D.J.; Orlicky, D.J.; Donohue, T.M., Jr.; Ju, C. Increased susceptibility of natural killer T-cell-deficient mice to acetaminophen-induced liver injury. Hepatology 2013, 57, 1575–1584. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J.; Won, Y.S.; Park, O.; Feng, D.; Gao, B. Opposing effects of prednisolone treatment on T/NKT cell- and hepatotoxin-mediated hepatitis in mice. Hepatology 2014, 59, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Minagawa, S.; Yamazaki, T.; Arai, T.; Kanai, M.; Shinjo, S.; Goda, N. Loss of hypoxia inducible factor-1α aggravates γδ T-cell-mediated inflammation during acetaminophen-induced liver injury. Hepatol. Commun. 2018, 2, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F. Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 2017, 66, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.; Tacke, F. Liver Macrophages: Old Dogmas and New Insights. Hepatol. Commun. 2019, 3, 730–743. [Google Scholar] [CrossRef]
- Kubes, P.; Jenne, C. Immune Responses in the Liver. Annu. Rev. Immunol. 2018, 36, 247–277. [Google Scholar] [CrossRef]
- Weston, C.J.; Zimmermann, H.W.; Adams, D.H. The Role of Myeloid-Derived Cells in the Progression of Liver Disease. Front. Immunol. 2019, 10, 893. [Google Scholar] [CrossRef]
- Zwicker, C.; Bujko, A.; Scott, C.L. Hepatic Macrophage Responses in Inflammation, a Function of Plasticity, Heterogeneity or Both? Front. Immunol. 2021, 12, 690813. [Google Scholar] [CrossRef]
- Tsutsui, H.; Nishiguchi, S. Importance of Kupffer cells in the development of acute liver injuries in mice. Int. J. Mol. Sci. 2014, 15, 7711–7730. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Federici, S.; Zmora, N.; Mohapatra, G.; Dori-Bachash, M.; Hornstein, S.; Leshem, A.; Reuveni, D.; Zigmond, E.; Tobar, A.; et al. Acute liver failure is regulated by MYC- and microbiome-dependent pro-grams. Nat. Med. 2020, 26, 1899–1911. [Google Scholar] [CrossRef]
- Cohen, K.; Mouhadeb, O.; Ben Shlomo, S.; Langer, M.; Neumann, A.; Erez, N.; Moshkovits, I.; Pelet, R.; Kedar, D.J.; Brazowski, E.; et al. COMMD10 is critical for Kupffer cell survival and controls Ly6Chi monocyte differentiation and inflammation in the injured liver. Cell Rep. 2021, 37, 110026. [Google Scholar] [CrossRef]
- Liu, P.; McGuire, G.M.; Fisher, M.A.; Farhood, A.; Smith, C.W.; Jaeschke, H. Activation of Kupffer cells and neutrophils for reactive oxygen formation is responsible for endotoxin-enhanced liver injury after hepatic ischemia. Shock 1995, 3, 56–62. [Google Scholar] [CrossRef]
- Blériot, G.; Barreby, E.; Dunsmore, G.; Ballaire, R.; Chakarov, S.; Ficht, X.; De Simone, G.; Andreata, F.; Fumagalli, V.; Guo, W.; et al. A subset of Kupffer cells regulates metabolism through the expression of CD36. Immunity 2021, 54, 2101–2116. [Google Scholar] [CrossRef]
- Yang, P.; Wu, Q.; Sun, L.; Fang, P.; Liu, L.; Ji, Y.; Park, J.Y.; Qin, X.; Yang, X.; Wang, H. Adaptive Immune Response Signaling Is Suppressed in Ly6Chigh Monocyte but Upregulated in Monocyte Subsets of ApoE−/− Mice - Functional Implication in Atherosclerosis. Front Immunol. 2021, 12, 809208. [Google Scholar] [CrossRef]
- Van der Heide, D.; Weiskirchen, R.; Bansal, R. Therapeutic Targeting of Hepatic Macrophages for the Treatment of Liver Dis-eases. Front. Immunol. 2019, 10, 2852. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Lambrecht, J.; Ju, C.; Tacke, F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and thera-peutic opportunities. Cell Mol. Immunol. 2021, 18, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Heymann, F.; Tacke, F. Immunology in the liver—From homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 88–110. [Google Scholar] [CrossRef]
- Ginhoux, F.; Schultze, J.L.; Murray, P.J.; Ochando, J.; Biswas, S.K. New insights into the multidimensional concept of macrophage ontogeny, activation, and function. Nat. Immunol. 2016, 17, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Holt, M.P.; Cheng, L.; Ju, C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J. Leukoc. Biol. 2008, 84, 1410–1421. [Google Scholar] [CrossRef]
- Lewis, P.S.; Campana, L.; Aleksieva, N.; Cartwright, J.A.; Mackinnon, A.; O’Duibhir, E.; Kendall, T.; Vermeren, M.; Thomson, A.; Gadd, V.; et al. Alternatively activated macrophages promote resolution of necrosis fol-lowing acute liver injury. J. Hepatol. 2020, 73, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, B.J.; Macmillan, M.T.; Brennan, P.N.; Forbes, S.J. Cell therapy for advanced liver diseases: Repair or rebuild. J. Hepatol. 2021, 74, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Banchereau, J. Taking dendritic cells into medicine. Nature 2007, 449, 419–426. [Google Scholar] [CrossRef]
- Jung, H.E.; Kim, T.H.; Lee, H.K. Contribution of Dendritic Cells in Protective Immunity against Respiratory Syncytial Virus In-fection. Viruses 2020, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Plitas, G.; Burt, B.M.; Stableford, J.A.; Nguyen, H.M.; Welles, A.P.; DeMatteo, R.P. Dendritic cells are required for effective cross-presentation in the murine liver. Hepatology 2008, 47, 1343–1351. [Google Scholar] [CrossRef]
- Pillarisetty, V.G.; Shah, A.B.; Miller, G.; Bleier, J.I.; DeMatteo, R.P. Liver dendritic cells are less immunogenic than spleen dendritic cells because of differences in subtype composition. J. Immunol. 2004, 172, 1009–1017. [Google Scholar] [CrossRef]
- Xia, S.; Guo, Z.; Xu, X.; Yi, H.; Wang, Q.; Cao, X. Hepatic microenvironment programs hematopoietic progenitor differentiation into regulatory dendritic cells, maintaining liver tolerance. Blood 2008, 112, 3175–3185. [Google Scholar] [CrossRef]
- Connolly, M.K.; Bedrosian, A.S.; Malhotra, A.; Henning, J.R.; Ibrahim, J.; Vera, V.; Cieza-Rubio, N.E.; Hassan, B.U.; Pachter, H.L.; Cohen, S.; et al. In hepatic fibrosis, liver sinusoidal endothelial cells acquire enhanced immu-nogenicity. J. Immunol. 2010, 185, 2200–2208. [Google Scholar] [CrossRef]
- Peng, H.; Wisse, E.; Tian, Z. Liver natural killer cells: Subsets and roles in liver immunity. Cell Mol. Immunol. 2016, 13, 328–336. [Google Scholar] [CrossRef]
- Liu, W.; Zeng, X.; Liu, Y.; Liu, J.; Li, C.; Chen, L.; Chen, H.; Ouyang, D. The Immunological Mechanisms and Immune-Based Biomarkers of Drug-Induced Liver Injury. Front. Pharmacol. 2021, 12, 723940. [Google Scholar] [CrossRef]
- Peng, H.; Jiang, X.; Chen, Y.; Sojka, D.K.; Wei, H.; Gao, X.; Sun, R.; Yokoyama, W.M.; Tian, Z. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Investig. 2013, 123, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Wallace, K.L.; Marshall, M.A.; Ramos, S.I.; Lannigan, J.A.; Field, J.J.; Strieter, R.M.; Linden, J. NKT cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of IFN-gamma and CXCR3 chemokines. Blood 2009, 114, 667–676. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fasbender, F.; Obholzer, M.; Metzler, S.; Stöber, R.; Hengstler, J.G.; Watzl, C. Enhanced activation of human NK cells by drug-exposed hepatocytes. Arch. Toxicol. 2020, 94, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Constant, P.; Davodeau, F.; Peyrat, M.A.; Poquet, Y.; Puzo, G.; Bonneville, M.; Fournié, J.J. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science 1994, 264, 267–270. [Google Scholar] [CrossRef]
- Kabelitz, D. Gamma Delta T Cells (γδ T Cells) in Health and Disease: In Memory of Professor Wendy Havran. Cells 2020, 9, 2564. [Google Scholar] [CrossRef]
- Vantourout, P.; Hayday, A. Six-of-the-best: Unique contributions of γδ T cells to immunology. Nat. Rev. Immunol. 2013, 13, 88–100. [Google Scholar] [CrossRef]
- Ferrara, J.L.; Abhyankar, S.; Gilliland, D.G. Cytokine storm of graft-versus-host disease: A critical effector role for interleukin-1. Transpl. Proc. 1993, 25, 1216–1217. [Google Scholar]
- Morgan, R.A.; Yang, J.C.; Kitano, M.; Dudley, M.E.; Laurencot, C.M.; Rosenberg, S.A. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 2010, 18, 843–851. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- Chang, W.; Song, L.; Chang, X.; Ji, M.; Wang, H.; Qin, X.; Niu, W. Early activated hepatic stellate cell-derived paracrine molecules modulate acute liver injury and regeneration. Lab Investig. 2017, 97, 318–328. [Google Scholar] [CrossRef]
- Pires, D.A.; Marques, P.E.; Pereira, R.V.; David, B.A.; Gomides, L.F.; Dias, A.C.; Nunes-Silva, A.; Pinho, V.; Cara, D.C.; Vieira, L.Q.; et al. Interleukin-4 deficiency protects mice from acetaminophen-induced liver injury and inflammation by pre-vention of glutathione depletion. Inflamm. Res. 2014, 63, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Yee, S.B.; Bourdi, M.; Masson, M.J.; Pohl, L.R. Hepatoprotective role of endogenous interleukin-13 in a murine model of aceta-minophen-induced liver disease. Chem. Res. Toxicol. 2007, 20, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Liao, C.C.; Day, Y.J.; Liou, J.T.; Li, A.H.; Liu, F.C. IL-17 deficiency attenuates acetaminophen-induced hepatotoxicity in mice. Toxicol. Lett. 2018, 292, 20–30. [Google Scholar] [CrossRef]
- Jiang, J.; Messner, S.; Kelm, J.M.; van Herwijnen, M.; Jennen, D.G.J.; Kleinjans, J.C.; de Kok, T.M. Human 3D multicellular microtis-sues: An upgraded model for the in vitro mechanistic investigation of inflammation-associated drug toxicity. Toxicol. Lett. 2019, 312, 34–44. [Google Scholar] [CrossRef]
- Imaeda, A.B.; Watanabe, A.; Sohail, M.A.; Mahmood, S.; Mohamadnejad, M.; Sutterwala, F.S.; Flavell, R.A.; Mehal, W.Z. Acetamino-phen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J. Clin. Investig. 2009, 119, 305–314. [Google Scholar] [PubMed]
- Liao, Y.; Yang, Y.; Wang, X.; Wei, M.; Guo, Q.; Zhao, L. Oroxyloside ameliorates acetaminophen-induced hepatotoxicity by inhibiting JNK related apoptosis and necroptosis. J. Ethnopharmacol. 2020, 258, 112917. [Google Scholar] [CrossRef]
- Viswanathan, P.; Sharma, Y.; Jaber, F.L.; Tchaikovskaya, T.; Gupta, S. Transplanted hepatocytes rescue mice in acetaminophen-induced acute liver failure through paracrine signals for hepatic ATM and STAT3 pathways. FASEB J. 2021, 35, e21471. [Google Scholar] [CrossRef]
- Jiang, W.P.; Deng, J.S.; Huang, S.S.; Wu, S.H.; Chen, C.C.; Liao, J.C.; Chen, H.Y.; Lin, H.Y.; Huang, G.J. Sanghuangporus sanghuangMyceli-um Prevents Paracetamol-Induced Hepatotoxicity through Regulating the MAPK/NF-κB, Keap1/Nrf2/HO-1, TLR4/PI3K/Akt, and CaMKKβ/LKB1/AMPK Pathways and Suppressing Oxidative Stress and Inflammation. Antioxidants 2021, 10, 897. [Google Scholar] [CrossRef]
- Jiang, L.; Ke, M.; Yue, S.; Xiao, W.; Yan, Y.; Deng, X.; Ying, Q.L.; Li, J.; Ke, B. Blockade of Notch signaling promotes acetaminophen-induced liver injury. Immunol. Res. 2017, 65, 739–749. [Google Scholar] [CrossRef]
- Li, C.; Ming, Y.; Hong, W.; Tang, Y.; Lei, X.; Li, X.; Mao, Y. Comparison of hepatic transcriptome profiling between acute liver inju-ry and acute liver failure induced by acetaminophen in mice. Toxicol. Lett. 2018, 283, 69–76. [Google Scholar] [CrossRef]
- Bourdi, M.; Masubuchi, Y.; Reilly, T.P.; Amouzadeh, H.R.; Martin, J.L.; George, J.W.; Shah, A.G.; Pohl, L.R. Protection against aceta-minophen-induced liver injury and lethality by interleukin 10: Role of inducible nitric oxide synthase. Hepatology 2002, 35, 289–298. [Google Scholar] [CrossRef] [PubMed]
- James, L.P.; Kurten, R.C.; Lamps, L.W.; McCullough, S.; Hinson, J.A. Tumour necrosis factor receptor 1 and hepatocyte regenera-tion in acetaminophen toxicity: A kinetic study of proliferating cell nuclear antigen and cytokine expression. Basic Clin. Pharma-Col. Toxicol. 2005, 97, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Chen, C.J.; Kono, H.; Golenbock, D.; Reed, G.; Akira, S.; Rock, K.L. Identification of a key pathway required for the sterile in-flammatory response triggered by dying cells. Nat. Med. 2007, 13, 851–856. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, J.; Du, J.; Zhuo, Z.; Yang, S.; Zhang, W.; Wang, W.; Zhang, S.; Iwakura, Y.; Meng, G.; et al. Macrophage-derived IL-1α promotes sterile inflammation in a mouse model of acetamino-phen hepatotoxicity. Cell Mol. Immunol. 2018, 15, 973–982. [Google Scholar] [CrossRef]
- Hu, J.; Yan, D.; Gao, J.; Xu, C.; Yuan, Y.; Zhu, R.; Xiang, D.; Weng, S.; Han, W.; Zang, G.; et al. rhIL-1Ra reduces hepatocellular apoptosis in mice with acetaminophen-induced acute liver fail-ure. Lab Investig. 2010, 90, 1737–1746. [Google Scholar] [CrossRef]
- Cai, C.; Huang, H.; Whelan, S.; Liu, L.; Kautza, B.; Luciano, J.; Wang, G.; Chen, G.; Stratimirovic, S.; Tsung, A.; et al. Benzyl alcohol attenuates acetaminophen-induced acute liver injury in a Toll-like receptor-4-dependent pattern in mice. Hepatology 2014, 60, 990–1002. [Google Scholar] [CrossRef]
- Williams, C.D.; Farhood, A.; Jaeschke, H. Role of caspase-1 and interleukin-1beta in acetaminophen-induced hepatic inflamma-tion and liver injury. Toxicol. Appl. Pharmacol. 2010, 247, 169–178. [Google Scholar] [CrossRef]
- Hoque, R.; Sohail, M.A.; Salhanick, S.; Malik, A.F.; Ghani, A.; Robson, S.C.; Mehal, W.Z. P2X7 receptor-mediated purinergic signaling promotes liver injury in acetamino-phen hepatotoxicity in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1171–G1179. [Google Scholar] [CrossRef]
- Xie, Y.; Williams, C.D.; McGill, M.R.; Lebofsky, M.; Ramachandran, A.; Jaeschke, H. Purinergic receptor antagonist A438079 protects against acetaminophen-induced liver injury by inhibiting p450 isoenzymes, not by inflammasome activation. Toxicol. Sci. 2013, 131, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shan, S.; Kang, K.; Zhang, C.; Kou, R.; Song, F. The cross-talk of NLRP3 inflammasome activation and necroptotic hepato-cyte death in acetaminophen-induced mice acute liver injury. Hum. Exp. Toxicol. 2021, 40, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Kita, H. Eosinophils: Multifaceted biological properties and roles in health and disease. Immunol. Rev. 2011, 242, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Pham, B.N.; Bemuau, J.; Durand, F.; Sauvanet, A.; Degott, C.; Prin, L.; Janin, A. Eotaxin expression and eosinophil infiltrate in the liver of patients with drug-induced liver disease. J. Hepatol. 2001, 34, 537–547. [Google Scholar] [CrossRef]
- Xu, L.; Yang, Y.; Jiang, J.; Wen, Y.; Jeong, J.M.; Emontzpohl, C.; Atkins, C.L.; Kim, K.; Elizabeth, J.A.; Wang, H.; et al. Eosinophils protect against acetaminophen-induced liver injury through cyclooxygenase-mediated IL-4/IL-13 production. Hepatology, 2022; Accepted Author Manuscript. [Google Scholar] [CrossRef]
- Khamri, W.; Abeles, R.D.; Hou, T.Z.; Anderson, A.E.; El-Masry, A.; Triantafyllou, E.; Bernsmeier, C.; Larsen, F.S.; Singanayagam, A.; Kudo, N.; et al. Increased Expression of Cytotoxic T-Lymphocyte-Associated Protein 4 by T Cells, In-duced by B7 in Sera, Reduces Adaptive Immunity in Patients With Acute Liver Failure. Gastroenterology 2017, 153, 263–276. [Google Scholar] [CrossRef]
- Numata, K.; Kubo, M.; Watanabe, H.; Takagi, K.; Mizuta, H.; Okada, S.; Kunkel, S.L.; Ito, T.; Matsukawa, A. Overexpression of suppressor of cytokine signaling-3 in T cells exacerbates acetamino-phen-induced hepatotoxicity. J. Immunol. 2007, 178, 3777–3785. [Google Scholar] [CrossRef]
- Swain, S.L.; McKinstry, K.K.; Strutt, T.M. Expanding roles for CD4⁺ T cells in immunity to viruses. Nat. Rev. Immunol. 2012, 12, 136–148. [Google Scholar] [CrossRef]
- Wang, X.; Sun, R.; Chen, Y.; Lian, Z.X.; Wei, H.; Tian, Z. Regulatory T cells ameliorate acetaminophen-induced immune-mediated liver injury. Int. Immunopharmacol. 2015, 25, 293–301. [Google Scholar] [CrossRef]
- Zhu, X.; Uetrecht, J. A novel T(H)17-type cell is rapidly increased in the liver in response to acetaminophen-induced liver injury: T(H)17 cells and the innate immune response. J. Immunotoxicol. 2013, 10, 287–291. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Wang, H.; Wang, X.; Li, J.; Jiang, L. The Dual Role of Innate Immune Response in Acetaminophen-Induced Liver Injury. Biology 2022, 11, 1057. https://doi.org/10.3390/biology11071057
Yang T, Wang H, Wang X, Li J, Jiang L. The Dual Role of Innate Immune Response in Acetaminophen-Induced Liver Injury. Biology. 2022; 11(7):1057. https://doi.org/10.3390/biology11071057
Chicago/Turabian StyleYang, Tao, Han Wang, Xiao Wang, Jun Li, and Longfeng Jiang. 2022. "The Dual Role of Innate Immune Response in Acetaminophen-Induced Liver Injury" Biology 11, no. 7: 1057. https://doi.org/10.3390/biology11071057
APA StyleYang, T., Wang, H., Wang, X., Li, J., & Jiang, L. (2022). The Dual Role of Innate Immune Response in Acetaminophen-Induced Liver Injury. Biology, 11(7), 1057. https://doi.org/10.3390/biology11071057







