Past and Contemporaneous Otolith Fingerprints Reveal Potential Anthropogenic Interferences and Allows Refinement of the Population Structure of Isopisthus parvipinnis in the South Brazil Bight
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Sampling and Otolith Preparation
2.2. Otolith Elemental Analysis
2.3. Statistical Analysis
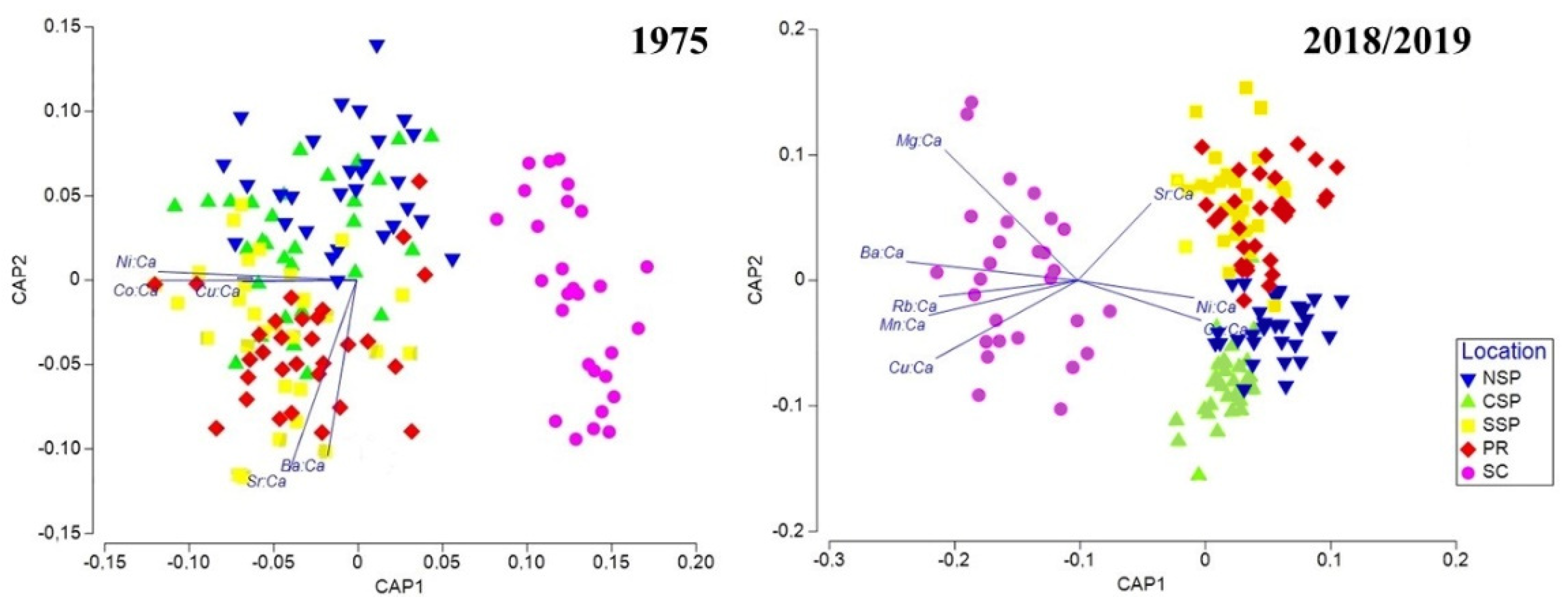
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Begg, G.; Brown, R. Stock identification of Haddock Melanogrammus aeglefinus on Georges Bank based on otolith shape analysis. Trans. Am. Fish. Soc. 2000, 129, 935–945. [Google Scholar] [CrossRef]
- Galli, O.; Norbis, W. Morphometric and meristic spatial differences and mixed groups of the whitemouth croaker (Micropo-gonias furnieri (Demearest, 1823)) during the spawning season: Implications for management. J. Appl. Ichthyol. 2013, 29, 782–788. [Google Scholar] [CrossRef]
- Kerr, L.; Hintzen, N.; Cadrin, S.; Clausen, L.; Worsoedickey-Collas, M.; Goethel, D.; Hatfield, E.; Kritzer, J.; Nash, R. Lessons learned from practical approaches to reconcile mismatches between biological population structure and stock units of ma-rine fish. ICES J. Mar. Sci. 2017, 74, 1708–1722. [Google Scholar] [CrossRef]
- Poulin, R.; Kamiya, T. Parasites as biological tags of fish stocks: A meta-analysis of their discriminatory power. Parasitology 2013, 142, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Moreira, C.; Froufe, E.; Vaz-Pires, P.; Triay-Portella, R.; Correia, A.T. Landmark-based geometric morphometrics analysis of body shape variation among populations of the blue jack mackerel, Trachurus picturatus, from the North-East Atlantic. J. Sea Res. 2020, 163, 101926. [Google Scholar] [CrossRef]
- Moura, A.; Muniz, A.A.; Mullins, R.; Wilson, J.M.; Vieira, R.P.; Almeida, A.A.; Pinto, E.; Brummer, G.J.A.; Gaever, P.V.; Gon-çalves, J.M.S.; et al. Population structure and dynamics of the Atlantic mackerel (Scomber scombrus) in the North At-lantic inferred from otolith chemical and shape signatures. Fish. Res. 2020, 230, 105621. [Google Scholar] [CrossRef]
- Daros, F.A.; Spach, H.L.; Sial, A.N.; Correia, A.T. Otolith fingerprints of the coral reef fish Stegastes fuscus in southeast Brazil: A useful tool for population and connectivity studies. Reg. Stud. Mar. Sci. 2016, 3, 262–272. [Google Scholar] [CrossRef]
- Adelir-Alves, J.; Daros, F.; Spach, H.L.; Soeth, M.; Correia, A.T. Otoliths as a tool to study reef fish population structure from coastal islands of South Brazil. Mar. Biol. Res. 2018, 14, 973–988. [Google Scholar] [CrossRef]
- Soeth, M.; Spach, H.L.; Daros, F.; Adelir-Alves, J.; de Almeida, A.C.O.; Correia, A.T. Stock structure of Atlantic spadefish Chaetodipterus faber from Southwest Atlantic Ocean inferred from otolith elemental and shape signatures. Fish. Res. 2019, 211, 81–90. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Sarmiento, J.L.; Dunne, J.P.; Frölicher, T.L.; Lam, V.W.Y.; Deng Palomares, M.L.; Watson, R.; Pauly, D. Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat. Clim. Change 2013, 3, 254–258. [Google Scholar] [CrossRef]
- Wheeler, S.; Russell, A.; Fehrenbacher, J.; Morgan, S. Evaluating chemical signatures in a coastal upwelling region to reconstruct water mass associations of settlement-stage rockfishes. Mar. Ecol. Prog. Ser. 2016, 550, 191–206. [Google Scholar] [CrossRef]
- Araújo, F.G.; Teixeira, T.P.; Guedes, A.P.P.; de Azevedo, M.C.C.; Pessanha, A.L.M. Shifts in the abundance and distribution of shallow water fish fauna on the southeastern Brazilian coast: A response to climate change. Hydrobiologia 2018, 814, 205–218. [Google Scholar] [CrossRef]
- Albuquerque, C.Q.; Miekeley, N.; Muelbert, J.H.; Walther, B.D.; Jaureguizar, A.J. Estuarine dependency in a marine fish evaluated with otolith chemistry. Mar. Biol. 2012, 159, 2229–2239. [Google Scholar] [CrossRef]
- Daros, F.A.; Spach, H.L.; Correia, A.T. Habitat residency and movement patterns of Centropomus parallelus juveniles in a subtropical estuarine complex. J. Fish Biol. 2016, 88, 1796–1810. [Google Scholar] [CrossRef]
- Carvalho, B.M.; Pupo, D.V.; Volpedo, A.V.; Pisonero, J.; Méndez, A.; Avigliano, E. Spatial environmental variability of nat-ural markers and habitat use of Cathorops spixii in a neotropical estuary from otolith chemistry. J. Mar. Biol. Assoc. UK 2020, 100, 783–793. [Google Scholar] [CrossRef]
- Soeth, M.; Spach, H.L.; Daros, F.A.; Jorge Pisonero, J.; Correia, A.T. Use of otolith elemental signatures to unravel lifetime movements patterns of Atlantic spadefish, Chaetodipterus faber, in the Southwest Atlantic Ocean. J. Sea Res. 2020, 158, 101873. [Google Scholar] [CrossRef]
- Maciel, T.R.; Avigliano, E.; de Carvalho, B.M.; Miller, N.; Vianna, M. Population structure and habitat connectivity of Genidens genidens (Siluriformes) in tropical and subtropical coasts from Southwestern Atlantic. Estuar. Coast. Shelf Sci. 2020, 242, 106839. [Google Scholar] [CrossRef]
- Nelson, J.S. Fishes of the World, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Menezes, N. Checklist dos peixes marinhos do Estado de São Paulo, Brasil. Biota Neotrop. 2011, 11, 33–46. [Google Scholar] [CrossRef]
- Spier, D.; Gerum, H.L.N.; Bornatowski, H.; Contente, R.; Mattos, N.A.S.; Vilar, C.C.; Spach, H.L. Ichthyofauna of the inner shelf of Paraná, Brazil: Checklist, geographic distribution, economic importance and conservation status. Biota Neotropi. 2018, 18, e20170385. [Google Scholar] [CrossRef]
- Gianinni, R.; Paiva Filho, A.M. Os Sciaenidae (Teleostei: Perciformes) da Baía de Santos (SP), Brasil. Bolm. Inst. Oceanogr. 1990, 38, 69–86. [Google Scholar] [CrossRef][Green Version]
- Haimovici, M.; Ignacio, J.M. Pesca da corvina no sul do Brasil. In Análise das Principais Pescarias Comerciais da Região Sudeste-Sul do Brasil: Dinâmica Populacional das Espécies em Exploração, Série Documentos Revizee-Score Sul; Rossi-Wongtschowski, C.L.B., Cergole, M.C., Avila-da-Silva, A.O., Eds.; IOUSP: São Paulo, Brazil, 2005; pp. 101–107. [Google Scholar]
- Souza, U.P.; Costa, R.C.; Martins, I.A.; Fransozo, A. Associações entre as biomassas de peixes Sciaenidae (Teleostei: Perci-formes) e de camarões Penaeoidea (Decapoda: Dendrobranchiata) no litoral norte do Estado de São Paulo. Biota Neotrop. 2008, 8, 83–92. [Google Scholar] [CrossRef]
- Aguilera Socorro, O. 2020. Isopisthus parvipinnis. The IUCN Red List of Threatened Species 2020: e.T47147702A82679809. Available online: https://www.iucnredlist.org/species/47147702/82679809 (accessed on 3 June 2022).
- Freire, K.M.F.; Pauly, D. Fisheries catch reconstructions for Brazil’s mainland and oceanic islands. Fish. Cent. Res. Rep. 2015, 23, 47. [Google Scholar]
- Graça Lopes, R.; Tomás, A.; Tutui, S.; Rodrigues, E.; Puzzi, A. Fauna acompanhante da pesca camaroeira no litoral do estado de são paulo, Brasil. Bol. Inst. Pesca 2002, 28, 173–188. [Google Scholar]
- Rossi-Wongtschowski, C.L.B.; Paes, E. Padrões espaciais e temporais da comunidade de peixes demersais do litoral norte do Estado de São Paulo—Ubatuba, Brasil. Publção esp. Inst. Oceanogr. 1993, 10, 169–188. [Google Scholar]
- Soares, L.S.H. Alimentação de Isopisthus parvipinnis (Teleostei: Sciaenidae) na Baía de Santos, São Paulo. Bolm. Inst. Oceanogr. 1989, 37, 95–105. [Google Scholar] [CrossRef][Green Version]
- Santos, M.N.; Rocha, G.R.A.; Freire, K.M.F. Diet composition for three sciaenids caught off northeastern Brazil. Rev. Biol. Mar. Oceanogr. 2016, 51, 493–504. [Google Scholar] [CrossRef]
- Chaves, P.; Rickli, A.; Bouchereau, J. Stratégie d’occupation de la mangrove de la baie de Guaratuba (Brésil) par le Sciaeni-dae prédateur Isopisthus parvipinnis (Teleostei, Pisces). Cah. Biol. Mar. 1998, 39, 63–71. [Google Scholar]
- Souza, L.; Chaves, P. Atividade reprodutiva de peixes (Teleostei) e o defeso da pesca de arrasto no litoral norte de Santa Catarina, Brasil. Rev. Bras. Zool. 2007, 24, 1113–1121. [Google Scholar] [CrossRef]
- Romero, R.; Moraes, L.; Santos, M.; Rocha, G.; Cetra, M. Biology of Isopisthus parvipinnis: An abundant sciaenid species cap-tured bycatch during sea-bob shrimp fishery in Brazil. Neotrop. Ichtyol. 2008, 6, 67–74. [Google Scholar] [CrossRef]
- Hoff, N.T.; Dias, J.F.; Zani-Teixeira, M.D.L.; Correia, A.T. Spatio-temporal evaluation of the population structure of the bigtooth corvina Isopisthus parvipinnis from Southwest Atlantic Ocean using otolith shape signatures. J. Appl. Ichthyol. 2020, 36, 439–450. [Google Scholar] [CrossRef]
- Hoff, N.T.; Dias, J.F.; Zani-Teixeira, M.L.; Soeth, M.; Correia, A.T. Population structure of the bigtooth corvina Isopisthus par-vipinnis from the Southwest Atlantic Ocean as determined by whole body morphology. Reg. Stud. Mar. Sci. 2020, 39, 101379. [Google Scholar] [CrossRef]
- Lamas, R.A.; Soares, L.S.H. Isopisthus parvipinnis (Cuvier, 1830) at the continental shelf of the Southeastern Brazilian Bight. In Growth of Fisheries Resources from the Southwestern Atlantic, 1st ed.; Vaz-dos-Santos, A.M., Rossi-Wongtschowski, C.L.D.B., Eds.; IOUSP: São Paulo, Brazil, 2019; pp. 133–135. [Google Scholar]
- Campana, S.; Chouinard, G.; Hanson, J.; Fréchet, A.; Brattey, J. Otolith elemental fingerprints as biological tracers of fish stocks. Fish. Res. 2000, 46, 343–357. [Google Scholar] [CrossRef]
- Menezes, N.; Figueiredo, J. Manual de Peixes Marinhos do Sudeste do Brasil. IV. Actinopterygii (3); Museu de Zoologia da Universidade de São Paulo: São Paulo, Brasil, 1980. [Google Scholar]
- Rooker, J.R.; Zdanowicz, V.S.; Secor, D.H. Chemistry of tuna otoliths: Assessment of base composition and postmortem handling effects. Mar. Biol. 2001, 139, 35–43. [Google Scholar]
- Patterson, H.M.; Thorrold, S.R.; Shenker, J. Analysis of otolith chemistry in Nassau grouper ( Epinephelus striatus ) from the Bahamas and Belize using solution-based ICP-MS. Coral Reefs 1999, 18, 171–178. [Google Scholar] [CrossRef]
- Correia, A.; Moura, A.; Triay-Portella, R.; Santos, P.; Pinto, E.; Almeida, A.; Sial, A.; Muniz, A. Population structure of the chub mackerel (Scomber colias) in the NE Atlantic inferred from otolith elemental and isotopic signatures. Fish. Res. 2021, 234, 105785. [Google Scholar] [CrossRef]
- Moreira, C.; Froufe, E.; Sial, A.; Caeiro, A.; Vaz-Pires, P.; Correia, A. Population structure of the blue jack mackerel (Trachurus picturatus) in the NE Atlantic inferred from otolith microchemistry. Fish. Res. 2018, 197, 113–122. [Google Scholar] [CrossRef]
- Higgins, R.; Isidro, E.; Menezes, G.; Correia, A. Otolith elemental signatures indicate population separation in deep-sea rockfish, Helicolenus dactylopterus and Pontinus kuhlii, from the Azores. J. Sea Res. 2013, 83, 202–208. [Google Scholar] [CrossRef]
- Castro, B.M.; Lorenzzetti, J.A.; Silveira, I.C.A.; Miranda, L.B. Estrutura termohalina e circulação na região entre o Cabo de São Tomé (RJ) e o Chuí (RS). In O Ambiente Oceanográfico da Plataforma Continental e do Talude na Região Sudeste-Sul do Brasil; Rossi-Wongtschowski, C.L.B., Madureira, L.S.P., Eds.; Edusp: São Paulo, Brazil, 2006; pp. 11–120. [Google Scholar]
- Campos, E.J.D.; Lorenzzetti, J.A.; Stevenson, M.R.; Stech, J.L.; Souza, R.B. Penetration of waters from the Brazil-Malvinas Confluence region along the South American Continental Shelf up to 23° S. An. Acad. Bras. Cienc 1996, 68, 49–58. [Google Scholar]
- Campos, E.J.D.; Lentini, C.A.D.; Miller, J.L.; Piola, A.R. Interannual variability of the sea surface temperature in the South Brazil Bight. Geophys. Res. Lett. 1999, 26, 2061–2064. [Google Scholar] [CrossRef]
- Souza, R.B.; Robinson, I.S. Lagrangian and satellite observations of the Brazilian Coastal Current. Cont. Shelf Res. 2004, 24, 241–262. [Google Scholar] [CrossRef]
- Simonassi, J.C.; Hennemann, M.C.; Talgatti, D.; Marques, A.N., Jr. Nutrient variations and coastal water quality of Santa Ca-tarina Island, Brazil. Biotemas 2010, 23, 211–223. [Google Scholar]
- Spach, H.; Yamaguti, N. Variação geográfica de Cynoscion jamaicensis (Pisces: Sciaenidae) entre as latitudes 20°18′S (Vitória, ES)—32°10′S (Barra do Rio Grande, RS). II—Caracteres morfométricos. Rev. Nerítica 1989, 4, 77–104. [Google Scholar]
- Spach, H.; Yamaguti, N. Variação geográfica de Cynoscion jamaicensis (Pisces: Sciaenidae) entre as latitudes 20°18′S (Vitória, ES)—32°10′S (Barra do Rio Grande, RS). III—Otólito Sagitta. Rev. Nerítica 1989, 4, 105–117. [Google Scholar]
- Yamaguti, N. Diferenciação geográfica de Macrodon ancylodon (Bloch & Schneider,1801) na costa brasileira, entre as latitudes 18o36’S e 32o10’S. Etapa I. Bol. Inst. Oceanogr. 1979, 28, 53–118. [Google Scholar]
- Vazzoler, A. Diversidade fisiológica e morfológica de Micropogon furnieri (Desmarest, 1822) ao sul de Cabo Frio, Brasil. Bol. Inst. Oceanogr. 1971, 20, 1–70. [Google Scholar] [CrossRef]
- Filho, A.M.P.; Cergole, M.C. Diferenciação geográfica de Nebris microps (Cuvier, 1830), na costa sudeste do Brasil. Bolm. Inst. Oceanogr. 1988, 36, 37–45. [Google Scholar] [CrossRef][Green Version]
- Biolé, F.G.; Thompson, G.A.; Vargas, C.V.; Leisen, M.; Barra, F.; Volpedo, A.; Avigliano, E. Fish stocks of Urophycis brasiliensis revealed by otolith fingerprint and shape in the Southwestern Atlantic Ocean. Estuar. Coast. Shelf Sci. 2019, 229, 106406. [Google Scholar] [CrossRef]
- Mazzini, P.L.F.; Barth, J.A. A comparison of mechanisms generating vertical transport in the Brazilian coastal upwelling regions. J. Geophys. Res. Oceans 2013, 118, 5977–5993. [Google Scholar] [CrossRef]
- Castro, B.M. Summer/winter stratification variability in the central part of the South Brazil Bight. Cont. Shelf Res. 2014, 89, 15–23. [Google Scholar] [CrossRef]
- Marone, E.; Machado, E.C.; Lopes, R.M.; Silva, E.T. Land-ocean fluxes in the Paranaguá Bay estuarine system, southern Brazil. Braz. J. Oceanogr. 2005, 53, 169–181. [Google Scholar] [CrossRef]
- De Mahiques, M.M.; Burone, L.; Figueira, R.; Lavenére-Wanderley, A.A.D.O.; Capellari, B.; Rogacheski, C.E.; Barroso, C.P.; Dos Santos, L.A.S.; Cordero, L.M.; Cussioli, M.C. Anthropogenic influences in a lagoonal environment: A multiproxy approach at the valo grande mouth, Cananéia-Iguape system (SE Brazil). Braz. J. Oceanogr. 2009, 57, 325–337. [Google Scholar] [CrossRef]
- Elsdon, T.; Wells, B.; Campana, S.; Gillanders, B.; Jones, C.; Limburg, K.; Secor, D.; Thorrold, S.; Walther, B. Otolith chemis-try to describe movements and life-history parameters of fishes: Hypotheses, assumptions, limitations and inferences. Oceanogr. Mar. Biol. Annu. Rev. 2008, 46, 297–330. [Google Scholar]
- Sturrock, A.M.; Hunter, E.; Milton, J.A.; EIMF; Johnson, R.C.; Waring, C.P.; Trueman, C.N. Quantifying physiological influences on otolith microchemistry. Methods Ecol. Evol. 2015, 6, 806–816. [Google Scholar] [CrossRef]
- Walther, B.D. The art of otolith chemistry: Interpreting patterns by integrating perspectives. Mar. Freshw. Res. 2019, 70, 1643. [Google Scholar] [CrossRef]
- Figueira, R.C.L.; Tessler, M.G.; Mahiques, M.M.; Cunha, I.I.L. Distribution of 137Cs, 238Pb and 239+240Pu in sediments of the southeastern Brazilian shelf-SW Atlantic margin. Sci. Total Environ. 2006, 357, 146–159. [Google Scholar] [CrossRef]
- Garcia, M.R.; Cattani, A.P.; Lana, P.D.C.; Figueira, R.C.L.; Martins, C.C. Petroleum biomarkers as tracers of low-level chronic oil contamination of coastal environments: A systematic approach in a subtropical mangrove. Environ. Pollut. 2019, 249, 1060–1070. [Google Scholar] [CrossRef]
- De Mahiques, M.M.; Hanebuth, T.; Martins, C.D.C.; Montoya-Montes, I.; Alcántara-Carrió, J.; Figueira, R.; Bicego, M. Mud depocentres on the continental shelf: A neglected sink for anthropogenic contaminants from the coastal zone. Environ. Earth Sci. 2016, 75, 44. [Google Scholar] [CrossRef]
- Geffen, A.J.; Pearce, N.J.G.; Perkins, W.T. Metal concentrations in fish otoliths in relation to body composition after labora-tory exposure to mercury and lead. Mar. Ecol. Progr. Ser. 1998, 165, 235–245. [Google Scholar] [CrossRef][Green Version]
- Avigliano, E.; Saez, M.B.; Rico, R.; Volpedo, A.V. Use of otolith strontium:calcium and zinc:calcium ratios as an indicator of the habitat of Percophis brasiliensis Quoy & Gaimard, 1825 in the southwestern Atlantic Ocean. Neotrop. Ichthyol. 2015, 13, 187–194. [Google Scholar] [CrossRef]
- Sturrock, A.M.; Hunter, E.; Milton, J.A.; Trueman, C.N. Analysis methods and reference concentrations of 12 minor and trace elements in fish blood plasma. J. Trace Elem. Med. Biol. 2013, 27, 273–285. [Google Scholar] [CrossRef]
- Hüssy, K.; Limburg, K.E.; de Pontual, H.; Thomas, O.R.B.; Cook, P.K.; Heimbrand, Y.; Blass, M.; Sturrock, A.M. Trace Element Patterns in Otoliths: The Role of Biomineralization. Rev. Fish. Sci. Aquac. 2020, 29, 445–477. [Google Scholar] [CrossRef]
- Hamer, P.A.; Jenkins, G.P. Comparison of spatial variation in otolith chemistry of two fish species and relationships with water chemistry and otolith growth. J. Fish Biol. 2007, 71, 1035–1055. [Google Scholar] [CrossRef]
- Barnes, T.C.; Gillanders, B.M. Combined effects of extrinsic and intrinsic factors on otolith chemistry: Implications for en-vironmental reconstructions. Can. J. Fish. Aquat. Sci. 2013, 70, 1159–1166. [Google Scholar] [CrossRef]
- Marriott, C.S.; Henderson, G.M.; Crompton, R.; Staubwasser, M.; Shaw, S. Effect of mineralogy, salinity, and temperature on Li/Ca and Li isotope composition of calcium carbonate. Chem. Geol. 2004, 212, 5–15. [Google Scholar] [CrossRef]
- Hicks, A.S.; Closs, G.P.; Swearer, S.E. Otolith microchemistry of two amphidromous galaxiids across an experimental salin-ity gradient: A multi-element approach for tracking diadromous migrations. J. Exp. Mar. Biol. Ecol. 2010, 394, 86–97. [Google Scholar] [CrossRef]
- Grammer, G.L.; Morrongiello, J.R.; Izzo, C.; Hawthorne, P.J.; Middleton, J.F.; Gillanders, B.M. Coupling biogeochemical tracers with fish growth reveals physiological and environmental controls on otolith chemistry. Ecol. Monogr. 2017, 87, 487–507. [Google Scholar] [CrossRef]
- Pan, X.; Ye, Z.; Xu, B.; Jiang, T.; Yang, J.; Tian, Y. Population connectivity in a highly migratory fish, Japanese Spanish mackerel (Scomberomorus niphonius), along the Chinese coast, implications from otolith chemistry. Fish. Res. 2020, 231, 105690. [Google Scholar] [CrossRef]
- Thomas, O.R.B.; Ganio, K.; Roberts, B.R.; Swearer, S.E. Trace elemento-protein interactions in endolymph from the inner ear of fish: Implications for environmental reconstructions using fish otolith chemistry. Metallomics 2017, 9, 239–249. [Google Scholar] [CrossRef]
- Sturrock, A.; Trueman, C.; Darnaude, A.; Hunter, E. Can otolith elemental chemistry retrospectively track migrations in fully marine fishes? J. Fish Biol. 2012, 81, 766–795. [Google Scholar] [CrossRef]
- Thomas, O.R.B.; Swearer, S.; Kapp, E.A.; Peng, P.; Tonkin-Hill, G.Q.; Papenfuss, A.; Roberts, A.; Bernard, P.; Roberts, B.R. The inner ear proteome of fish. FEBS J. 2019, 286, 66–81. [Google Scholar] [CrossRef]
- Evans, D.H. Teleost fish osmoregulation: What have we learned since August Krogh, Homer Smith, and Ancel Keys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R704–R713. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.H. A brief history of the study of fish osmoregulation: The central role of the Mt. Desert Island Biological Laboratory. Front. Physiol. 2010, 1, 13. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, L.A.; Halden, N.M. Determining Exposure History of Northern Pike and Walleye to Tailings Effluence Using Trace Metal Uptake in Otoliths. Environ. Sci. Technol. 2010, 44, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Mager, E.M. Lead. Fish Physiol. 2011, 31, 185–236. [Google Scholar]
- Wood, C.M. An introduction to metals in fish physiology and toxicology: Basic principles. Fish Physiol. 2011, 31, 1–51. [Google Scholar] [CrossRef]
- Angulo, R.; Soares, C.; Marone, E.; Souza, M.; Odreski, L.; Noernberg, M. Paraná. In Erosão e Progradação do Litoral Brasileiro; Muehe, D., Ed.; Ministério do Meio Ambiente (MMA): Governo Federal, Brasília, 2006; pp. 347–400. [Google Scholar]
- Klein, A.; Menezes, J.; Diehl, F.; Abreu, J.; Polette, M.; Sperb, R.; Sperb, R.; Horn, N. Santa Catarina. In Erosão e Progradação do Litoral Brasileiro; Muehe, D., Ed.; Ministério do Meio Ambiente (MMA): Brasília, Brazil, 2006; pp. 401–436. [Google Scholar]
- Tessler, M.; Goya, S.; Yoshikawa, P.; Hurtado, S. São Paulo. In Erosão e Progradação do Litoral Brasileiro; Muehe, D., Ed.; Ministério do Meio Ambiente (MMA): Brasília, Brazil, 2006; pp. 297–346. [Google Scholar]
- Azevedo, J.S.; Fernandez, W.S.; Farias, L.A.; Fávaro, D.T.I.; Braga, E.S. Use of Cathrops spixii as bioindicator of pollution of trace metals in the Santos Bay, Brazil. Ecotoxicology 2009, 18, 577–586. [Google Scholar] [CrossRef]
- Moura, A.M.M. Trajetória da política ambiental federal no Brasil. In Governança Ambiental no Brasil: Instituições, Atores e Políticas Públicas; Moura, A.M.M., Ed.; Ipea: Brasília, Brazil, 2016; pp. 13–44. [Google Scholar]
- Mahiques, M.M.; Figueira, R.C.L.; Salaroli, A.B.; Alves, D.P.V.; Gonçalves, C. 150 years of anthropogenic metal input in a Biosphere Reserve: The case study of the Cananéia Iguape coastal system, Southeastern Brazil. Environ. Earth Sci. 2013, 68, 1073–1087. [Google Scholar] [CrossRef]
- Conrad, S.R.; Sanders, C.J. Influence of anthropogenic activities on trace metal accumulation in Brazilian mangrove sedi-ments. Rev. Virtual Quím. 2017, 9, 2017–2031. [Google Scholar] [CrossRef]
- Jesus, M.S.D.S.D.; Frontalini, F.; Bouchet, V.M.; Yamashita, C.; Sartoretto, J.R.; Figueira, R.C.; Sousa, S.H.D.M.E. Reconstruction of the palaeo-ecological quality status in an impacted estuary using benthic foraminifera: The Santos Estuary (São Paulo state, SE Brazil). Mar. Environ. Res. 2020, 162, 105121. [Google Scholar] [CrossRef]
- Spencer, K.; Shafer, D.J.; Gauldie, R.W.; DeCarlo, E.H. Stable lead isotope ratios from distinct anthropogenic sources in fish otoliths: A potential nursery ground stock marker. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2000, 127, 273–284. [Google Scholar] [CrossRef]
- Geffen, A.J.; Jarvis, K.; Thorpe, J.P.; Leah, R.T.; Nash, R.D.M. Spatial differences in the trace element concentrations of Irish Sea plaice Pleuronectes platessa and whiting Merlangius merlangus otoliths. J. Sea Res. 2003, 50, 245–254. [Google Scholar] [CrossRef]
- Morales-Nin, B.; Geffen, A.; Cardona, F.; Kruber, C.; Saborido-Rey, F. The effect of Prestige oil ingestion on the growth and chemical composition of turbot otoliths. Mar. Pollut. Bull. 2007, 54, 1732–1741. [Google Scholar] [CrossRef] [PubMed]
- López-Duarte, P.C.; Fodrie, F.J.; Jensen, O.P.; Whitehead, A.; Galvez, F.; Dubansky, B.; Able, K.W. Is exposure to Ma-condo Oil reflected in the otolith chemistry of marsh-resident fish? PLoS ONE 2016, 11, e0162699. [Google Scholar] [CrossRef] [PubMed]
- Vrdoljak, D.; Matic-Skoko, S.; Peharda, M.; Uvanovic, H.; Markulin, K.; Mertz-Kraus, R. Otolith fingerprints reveals poten-tial pollution exposure of newly settled juvenile Sparus aurata. Mar. Pollut. Bull. 2020, 160, 111695. [Google Scholar] [CrossRef]
- Barnett, T.P.; Pierce, D.W.; Schnur, R. Detection of Anthropogenic Climate Change in the World’s Oceans. Science 2001, 292, 270–274. [Google Scholar] [CrossRef]
- Pörtner, H.-O. Ecosystem effects of ocean acidification in times of ocean warming: A physiologist’s view. Mar. Ecol. Prog. Ser. 2008, 373, 203–217. [Google Scholar] [CrossRef]
- IPCC. Summary for Policymakers. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Man-agement, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Shukla, P.R., Skea, J., Calvo Buendia, E., Masson-Delmotte, V., Pörtner, H.-O., Roberts, D.C., Zhai, P., Slade, R., Connors, S., van Diemen, R., et al., Eds.; IPCC: Geneva, Switzerland, 2020. [Google Scholar]
- Lumpkin, R.; Garzoli, S. Interannual to decadal changes in the western South Atlantic’s surface circulation. J. Geophys. Res. Earth Surf. 2011, 116, C01014. [Google Scholar] [CrossRef]
- Koenigstein, S.; Mark, F.; Gößling-Reisemann, S.; Reuter, H.; Poertner, H.-O. Modelling climate change impacts on marine fish populations: Process-based integration of ocean warming, acidification and other environmental drivers. Fish Fish. 2016, 17, 972–1004. [Google Scholar] [CrossRef]
- Kuczynski, L.; Chevalier, M.; Laffaille, P.; Legrand, M.; Grenouillet, G. Indirect effect of temperature on fish population abundances through phenological changes. PLoS ONE 2017, 12, e0175735. [Google Scholar] [CrossRef]
- Heuer, R.M.; Grosell, M. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am. J. Physiol. Integr. Comp. Physiol. 2014, 307, R1061–R1084. [Google Scholar] [CrossRef]
- Moreira, C.; Froufe, E.; Vaz-Pires, P.; Correia, A. Otolith shape analysis as a tool to infer the population structure of the blue jack mackerel, Trachurus picturatus, in the NE Atlantic. Fish. Res. 2019, 209, 40–48. [Google Scholar] [CrossRef]
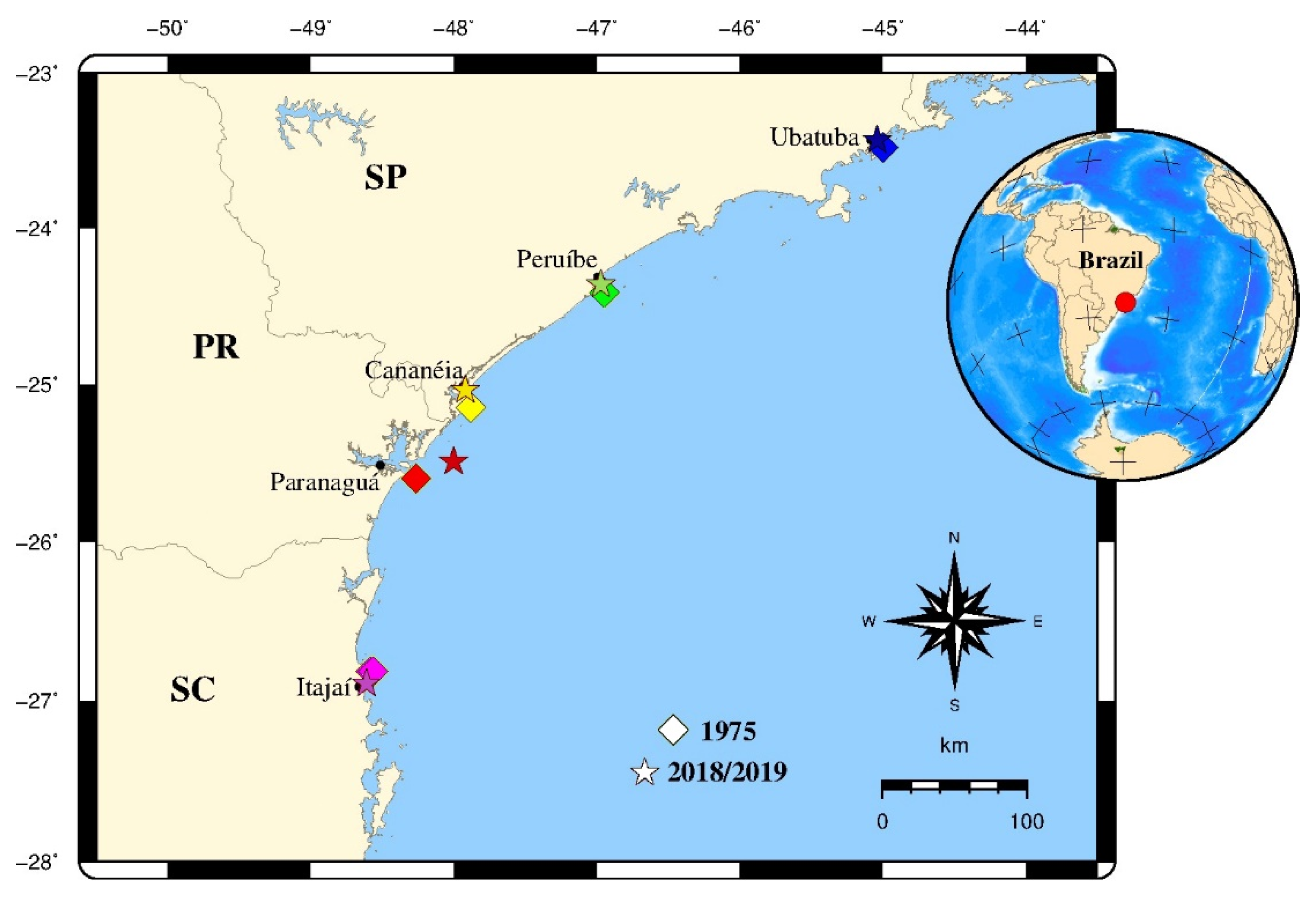

| States | Location | Code | 1975 | 2018/2019 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Period | n | TL (mm) | OM (mg) | Period | n | TL (mm) | OM (mg) | |||
| São Paulo | Ubatuba | NSP | Sep. and Nov. | 30 | 109 ± 12 | 26.39 ± 6.64 | Nov. | 30 | 110 ± 11 | 30.74 ± 7.38 |
| Peruíbe | CSP | Sep. and Nov. | 30 | 108 ± 14 | 27.89 ± 8.14 | Nov. | 30 | 134 ± 5 | 46.29 ± 4.03 | |
| Cananéia | SSP | Sep. and Nov. | 30 | 109 ± 14 | 28.97 ± 8.67 | Oct. | 30 | 113 ± 8 | 32.95 ± 4.95 | |
| Paraná | Paranaguá | PR | Sep. and Nov. | 30 | 109 ± 14 | 30.06 ± 9.22 | Sep. | 30 | 112 ± 11 | 32.83 ± 7.30 |
| Santa Catarina | Itajaí | SC | Sep. and Nov. | 29 | 111 ± 14 | 29.45 ± 8.77 | May | 28 | 76 ± 10 | 11.74 ± 4.40 |
| Ba:Ca | Co:Ca | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DF | SS | MS | F | p | DF | SS | MS | F | p | |
| Year | 1 | 29,477 | 29,477 | 174.20 | <0.000 | 1 | 0.0205 | 0.0205 | 0.3514 | 0.5538 |
| Location | 4 | 17,719 | 4429.7 | 26.180 | <0.000 | 4 | 29.862 | 7.4654 | 128.20 | <0.000 |
| Interaction | 4 | 14,975 | 3743.7 | 22.130 | <0.000 | 4 | 2.8357 | 0.7089 | 12.180 | <0.000 |
| Within | 287 | 48,560 | 169.20 | 287 | 16.706 | 0.0582 | ||||
| Total | 296 | 110,568 | 296 | 49.435 | ||||||
| Cu:Ca | Li:Ca | |||||||||
| DF | SS | MS | F | p | DF | SS | MS | F | p | |
| Year | 1 | 6.8509 | 6.8509 | 45.080 | <0.000 | 1 | 0.2463 | 0.246 | 31.740 | <0.000 |
| Location | 4 | 4.2744 | 1.0686 | 7.0320 | <0.000 | 4 | 0.3217 | 0.080 | 10.370 | <0.000 |
| Interaction | 4 | 24.288 | 6.0720 | 39.960 | <0.000 | 4 | 0.1510 | 0.038 | 4.8650 | <0.000 |
| Within | 271 | 41.184 | 0.1520 | 286 | 2.2191 | 0.008 | ||||
| Total | 280 | 77.142 | 295 | 2.9420 | ||||||
| Mg:Ca | Mn:Ca | |||||||||
| DF | SS | MS | F | p | DF | SS | MS | F | p | |
| Year | 1 | 9181.7 | 9181.7 | 284.800 | <0.000 | 1 | 1851.8 | 1851.8 | 101.40 | <0.000 |
| Location | 4 | 9145.9 | 2286.5 | 70.910 | <0.000 | 4 | 2494.5 | 623.62 | 34.160 | <0.000 |
| Interaction | 4 | 5683.1 | 1420.8 | 44.060 | <0.000 | 4 | 1708.1 | 427.03 | 23.390 | <0.000 |
| Within | 287 | 9253.7 | 32.243 | 287 | 5239.6 | 18.256 | ||||
| Total | 296 | 33,363 | 296 | 11,323 | ||||||
| Na:Ca | Ni:Ca | |||||||||
| DF | SS | MS | F | p | DF | SS | MS | F | p | |
| Year | 1 | 2.58 × 105 | 2.58 × 105 | 1.4630 | 0.2275 | 1 | 7.3276 | 7.3276 | 0.6257 | 0.4296 |
| Location | 4 | 6.40 × 106 | 1.60 × 106 | 9.0870 | <0.000 | 4 | 3935.9 | 983.97 | 84.020 | <0.000 |
| Interaction | 4 | 6.60 × 106 | 1.65 × 106 | 9.3770 | <0.000 | 4 | 241.86 | 60.465 | 5.1630 | <0.000 |
| Within | 287 | 5.05 × 107 | 1.76 × 105 | 287 | 3361.3 | 11.712 | ||||
| Total | 296 | 6.38 × 107 | 296 | 7548.7 | ||||||
| Pb:Ca | Rb:Ca | |||||||||
| DF | SS | MS | F | p | DF | SS | MS | F | p | |
| Year | 1 | 3.3757 | 3.3757 | 788.8 | <0.000 | 1 | 0.0010 | 0.0010 | 2.4540 | 0.1183 |
| Location | 4 | 0.4424 | 0.1106 | 25.84 | <0.000 | 4 | 0.0298 | 0.0074 | 17.910 | <0.000 |
| Interaction | 4 | 0.0370 | 0.0093 | 2.163 | 0.0732 | 4 | 0.0060 | 0.0015 | 3.5890 | <0.000 |
| Within | 287 | 1.2283 | 0.0043 | 287 | 0.1192 | 0.0004 | ||||
| Total | 296 | 5.0685 | 296 | 0.1560 | ||||||
| Sr:Ca | ||||||||||
| DF | SS | MS | F | p | ||||||
| Year | 1 | 7.18 × 106 | 7.18 × 106 | 53.850 | <0.000 | |||||
| Location | 4 | 1.97 × 107 | 4.92 × 106 | 36.890 | <0.000 | |||||
| Interaction | 4 | 3.37 × 106 | 8.42 × 105 | 6.3160 | <0.000 | |||||
| Within | 287 | 3.83 × 107 | 1.33 × 105 | |||||||
| Total | 296 | 6.86 × 107 | ||||||||
| Overall PERMANOVA | Pairwise PERMANOVA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source | SS | DF | MS | F | p | NSP | CSP | SSP | PR | SC | ||
| Year | 9.99 × 106 | 1 | 9.99 × 106 | 30.100 | 0.0001 | NSP | 0.0152 | 0.0001 | 0.0001 | 0.0001 | 1975 | |
| Location | 2.74 × 107 | 4 | 6.86 ×106 | 20.676 | 0.0001 | CSP | 0.0001 | 0.0001 | 0.0002 | 0.0028 | ||
| Interaction | 8.23 × 106 | 4 | 2.06 × 106 | 6.1994 | 0.0001 | SSP | 0.0001 | 0.0002 | 0.9031 | 0.0001 | ||
| Residual | 9.54 × 107 | 287 | 3.32 × 105 | PR | 0.0001 | 0.0001 | 0.9367 | 0.0001 | ||||
| Total | 1.40 × 108 | 296 | SC | 0.0005 | 0.0001 | 0.0001 | 0.0001 | |||||
| 2018/2019 | ||||||||||||
| 1975 | ||||||
|---|---|---|---|---|---|---|
| Original Location | Predicted Location | % Correct | ||||
| NSP | CSP | SSP | PR | SC | ||
| NSP | 21 | 7 | 0 | 2 | 0 | 70 |
| CSP | 12 | 9 | 5 | 4 | 0 | 30 |
| SSP | 0 | 7 | 13 | 10 | 0 | 43 |
| PR | 3 | 1 | 11 | 15 | 0 | 50 |
| SC | 0 | 0 | 0 | 0 | 29 | 100 |
| Total | 58 | |||||
| 2018/2019 | ||||||
| Original Location | Predicted Location | % Correct | ||||
| NSP | CSP | SSP | PR | SC | ||
| NSP | 29 | 0 | 0 | 1 | 0 | 97 |
| CSP | 4 | 26 | 0 | 0 | 0 | 87 |
| SSP | 0 | 2 | 18 | 10 | 0 | 60 |
| PR | 1 | 2 | 10 | 17 | 0 | 57 |
| SC | 0 | 0 | 0 | 0 | 28 | 100 |
| Total | 80 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoff, N.T.; Dias, J.F.; Pinto, E.; Almeida, A.; Schroeder, R.; Correia, A.T. Past and Contemporaneous Otolith Fingerprints Reveal Potential Anthropogenic Interferences and Allows Refinement of the Population Structure of Isopisthus parvipinnis in the South Brazil Bight. Biology 2022, 11, 1005. https://doi.org/10.3390/biology11071005
Hoff NT, Dias JF, Pinto E, Almeida A, Schroeder R, Correia AT. Past and Contemporaneous Otolith Fingerprints Reveal Potential Anthropogenic Interferences and Allows Refinement of the Population Structure of Isopisthus parvipinnis in the South Brazil Bight. Biology. 2022; 11(7):1005. https://doi.org/10.3390/biology11071005
Chicago/Turabian StyleHoff, Natasha Travenisk, June Ferraz Dias, Edgar Pinto, Agostinho Almeida, Rafael Schroeder, and Alberto Teodorico Correia. 2022. "Past and Contemporaneous Otolith Fingerprints Reveal Potential Anthropogenic Interferences and Allows Refinement of the Population Structure of Isopisthus parvipinnis in the South Brazil Bight" Biology 11, no. 7: 1005. https://doi.org/10.3390/biology11071005
APA StyleHoff, N. T., Dias, J. F., Pinto, E., Almeida, A., Schroeder, R., & Correia, A. T. (2022). Past and Contemporaneous Otolith Fingerprints Reveal Potential Anthropogenic Interferences and Allows Refinement of the Population Structure of Isopisthus parvipinnis in the South Brazil Bight. Biology, 11(7), 1005. https://doi.org/10.3390/biology11071005









