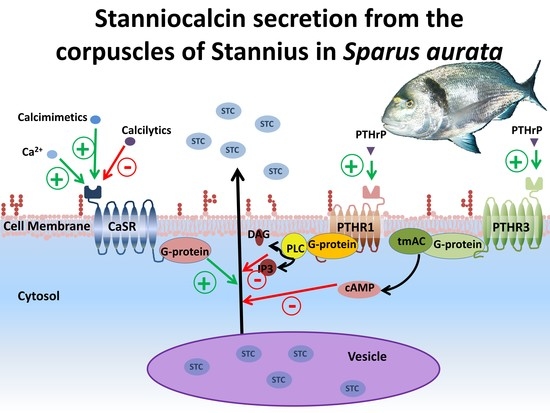
Regulation of Stanniocalcin Secretion by Calcium and PTHrP in Gilthead Seabream (Sparus aurata)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Maintenance
2.2. Sampling
2.3. Development of an ELISA Method
2.4. Ex Vivo Incubation of CS
2.5. CaSR and PTHrP Receptors RT-PCR
2.6. Statistics
3. Results
3.1. ELISA
3.2. STC Secretion and CS Content
3.3. CaSR and PTHrP Receptors
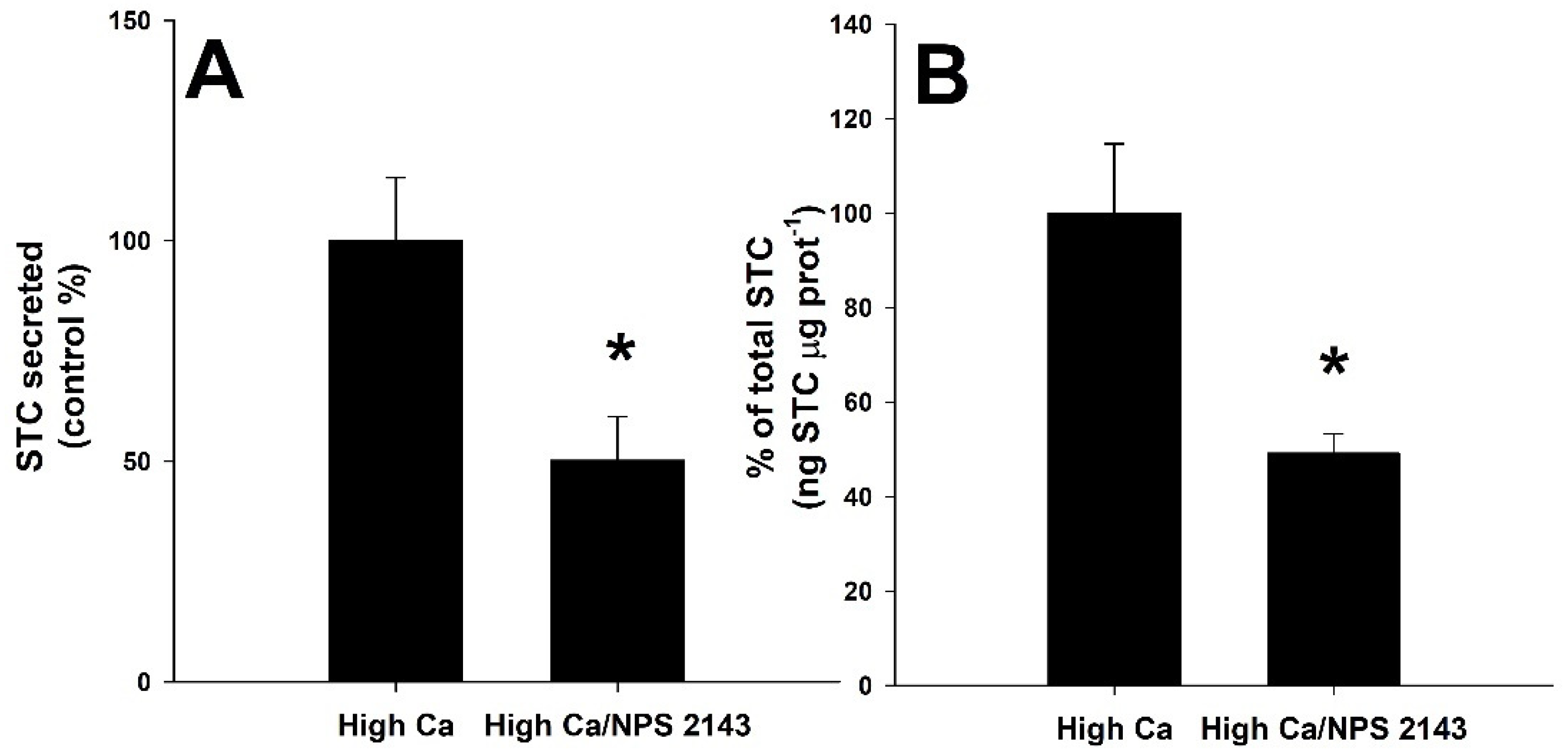
3.4. Effect of CaSR Agonist and Antagonist Molecules
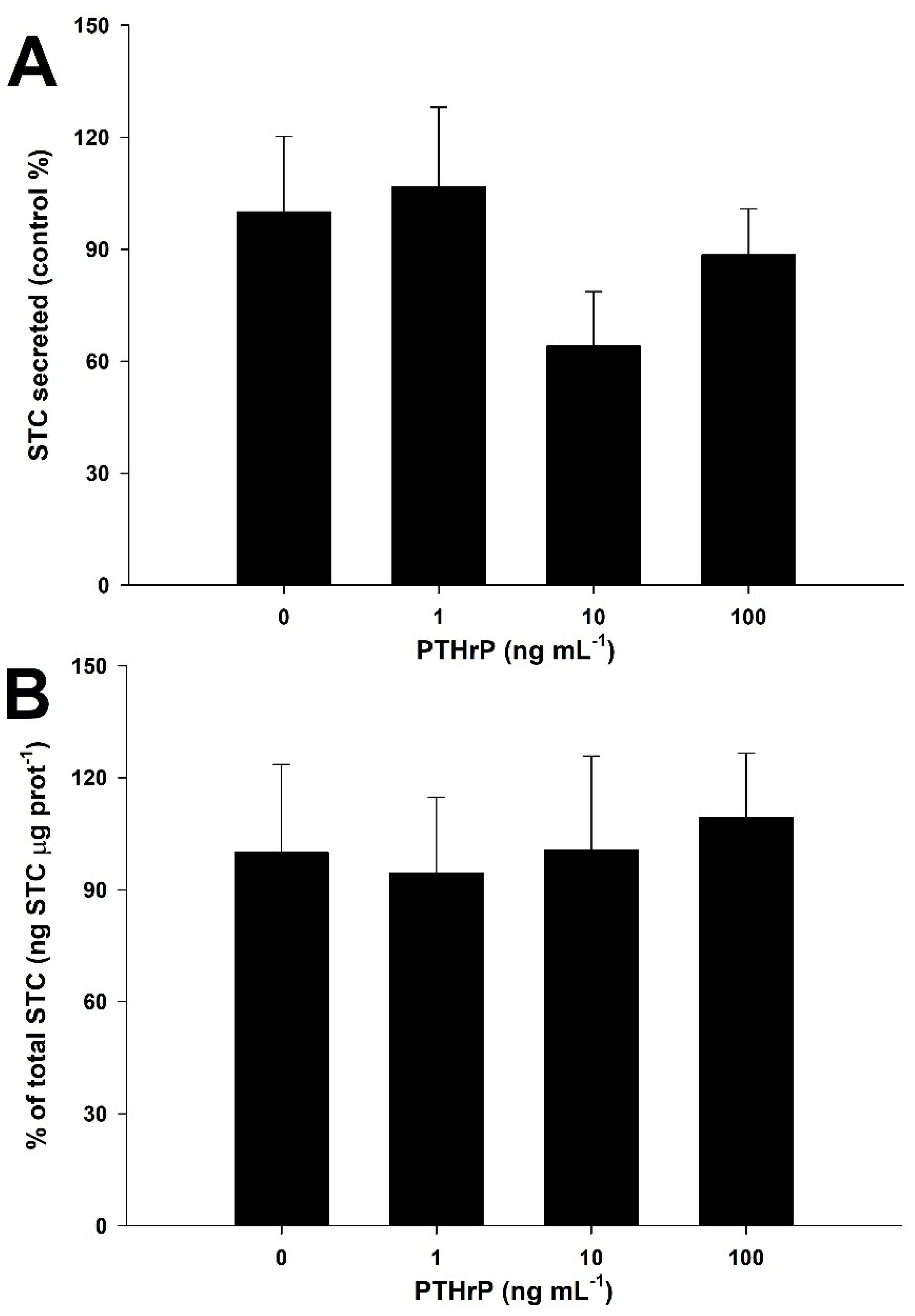
3.5. PTHrP Downregulates STC Secretion
3.6. Combined Effects of High Calcium and PTHrP
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riccardi, D. Cell surface, Ca2+(cation)-sensing receptor(s): One or many? Cell Calcium. 1999, 26, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Abou-Samra, A.B.; Juppner, H.; Force, T.; Freeman, M.W.; Kong, X.F.; Schipani, E.; Urena, P.; Richards, J.; Bonventre, J.V.; Potts, J.T., Jr.; et al. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: A single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc. Natl. Acad. Sci. USA 1992, 89, 2732–2736. [Google Scholar] [PubMed]
- Fuentes, J.; Power, D.M.; Canario, A.V. Parathyroid hormone-related protein-stanniocalcin antagonism in regulation of bicarbonate secretion and calcium precipitation in a marine fish intestine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R150–R158. [Google Scholar] [CrossRef]
- Wagner, G.F.; Hampong, M.; Park, C.M.; Copp, D.H. Purification, characterization, and bioassay of teleocalcin, a glycoprotein from salmon corpuscles of Stannius. Gen. Comp. Endocrinol. 1986, 63, 481–491. [Google Scholar] [CrossRef]
- Abbink, W.; Flik, G. Parathyroid hormone-related protein in teleost fish. Gen. Comp. Endocrinol. 2007, 152, 243–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yeung, B.H.; Law, A.Y.; Wong, C.K. Evolution and roles of stanniocalcin. Mol. Cell Endocrinol. 2012, 349, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.F.; Milliken, C.; Friesen, H.G.; Copp, D.H. Studies on the regulation and characterization of plasma stanniocalcin in rainbow trout. Mol. Cell Endocrinol. 1991, 79, 129–138. [Google Scholar] [CrossRef]
- Wagner, G.F.; Guiraudon, C.C.; Milliken, C.; Copp, D.H. Immunological and biological evidence for a stanniocalcin-like hormone in human kidney. Proc. Natl. Acad. Sci. USA 1995, 92, 1871–1875. [Google Scholar] [CrossRef]
- Chang, A.C.; Janosi, J.; Hulsbeek, M.; de Jong, D.; Jeffrey, K.J.; Noble, J.R.; Reddel, R.R. A novel human cDNA highly homologous to the fish hormone stanniocalcin. Mol. Cell Endocrinol. 1995, 112, 241–247. [Google Scholar] [CrossRef]
- Madsen, K.L.; Tavernini, M.M.; Yachimec, C.; Mendrick, D.L.; Alfonso, P.J.; Buergin, M.; Olsen, H.S.; Antonaccio, M.J.; Thomson, A.B.; Fedorak, R.N. Stanniocalcin: A novel protein regulating calcium and phosphate transport across mammalian intestine. Am. J. Physiol. 1998, 274, G96–G102. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wagner, G.F.; Renfro, J.L. Stanniocalcin stimulates phosphate reabsorption by flounder renal proximal tubule in primary culture. Am. J. Physiol. 1994, 267, R1356–R1362. [Google Scholar] [CrossRef]
- Pena, C.; Cespedes, M.V.; Lindh, M.B.; Kiflemariam, S.; Mezheyeuski, A.; Edqvist, P.H.; Hagglof, C.; Birgisson, H.; Bojmar, L.; Jirstrom, K.; et al. STC1 expression by cancer-associated fibroblasts drives metastasis of colorectal cancer. Cancer Res. 2013, 73, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Yoshiko, Y.; Aubin, J.E. Stanniocalcin 1 as a pleiotropic factor in mammals. Peptides 2004, 25, 1663–1669. [Google Scholar] [CrossRef] [PubMed]
- Chou, M.Y.; Lin, C.H.; Chao, P.L.; Hung, J.C.; Cruz, S.A.; Hwang, P.P. Stanniocalcin-1 controls ion regulation functions of ion-transporting epithelium other than calcium balance. Int. J. Biol. Sci. 2015, 11, 122–132. [Google Scholar] [CrossRef]
- Flik, G.; Labedz, T.; Neelissen, J.A.; Hanssen, R.G.; Wendelaar Bonga, S.E.; Pang, P.K. Rainbow trout corpuscles of Stannius: Stanniocalcin synthesis in vitro. Am. J. Physiol. 1990, 258, R1157–R1164. [Google Scholar] [CrossRef]
- Wagner, G.F.; Jaworski, E. Calcium regulates stanniocalcin mRNA levels in primary cultured rainbow trout corpuscles of stannius. Mol. Cell Endocrinol. 1994, 99, 315–322. [Google Scholar] [CrossRef]
- Greenwood, M.P.; Flik, G.; Wagner, G.F.; Balment, R.J. The corpuscles of Stannius, calcium-sensing receptor, and stanniocalcin: Responses to calcimimetics and physiological challenges. Endocrinology 2009, 150, 3002–3010. [Google Scholar] [CrossRef]
- Radman, D.P.; McCudden, C.; James, K.; Nemeth, E.M.; Wagner, G.F. Evidence for calcium-sensing receptor mediated stanniocalcin secretion in fish. Mol. Cell Endocrinol. 2002, 186, 111–119. [Google Scholar] [CrossRef]
- Kwong, R.W.; Auprix, D.; Perry, S.F. Involvement of the calcium-sensing receptor in calcium homeostasis in larval zebrafish exposed to low environmental calcium. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R211–R221. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Su, C.H.; Hwang, P.P. Calcium-sensing receptor mediates Ca(2+) homeostasis by modulating expression of PTH and stanniocalcin. Endocrinology 2014, 155, 56–67. [Google Scholar] [CrossRef]
- Brown, E.M. The extracellular Ca2+-sensing receptor: Central mediator of systemic calcium homeostasis. Annu. Rev. Nutr. 2000, 20, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, S.F.; Carvalho, E.S.; Campinho, M.A.; Power, D.M.; Canario, A.V.; Fuentes, J. Endocrine regulation of carbonate precipitate formation in marine fish intestine by stanniocalcin and PTHrP. J. Exp. Biol. 2014, 217, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.M.; Gamba, G.; Riccardi, D.; Lombardi, M.; Butters, R.; Kifor, O.; Sun, A.; Hediger, M.A.; Lytton, J.; Hebert, S.C. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature 1993, 366, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, S.F.; Fuentes, J. Regulation of bicarbonate secretion in marine fish intestine by the calcium-sensing receptor. Int. J. Mol. Sci. 2018, 19, 1072. [Google Scholar] [CrossRef] [PubMed]
- Magno, A.L.; Ward, B.K.; Ratajczak, T. The calcium-sensing receptor: A molecular perspective. Endocr. Rev. 2011, 32, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Saidak, Z.; Brazier, M.; Kamel, S.; Mentaverri, R. Agonists and allosteric modulators of the calcium-sensing receptor and their therapeutic applications. Mol. Pharmacol. 2009, 76, 1131–1144. [Google Scholar] [CrossRef] [PubMed]
- Norskov-Lauritsen, L.; Thomsen, A.R.; Brauner-Osborne, H. G protein-coupled receptor signaling analysis using homogenous time-resolved Forster resonance energy transfer (HTRF(R)) technology. Int. J. Mol. Sci. 2014, 15, 2554–2572. [Google Scholar] [CrossRef]
- Gardella, T.J.; Juppner, H. Molecular properties of the PTH/PTHrP receptor. Trends Endocrinol. Metab. 2001, 12, 210–217. [Google Scholar] [CrossRef]
- Hoare, S.R.; Rubin, D.A.; Juppner, H.; Usdin, T.B. Evaluating the ligand specificity of zebrafish parathyroid hormone (PTH) receptors: Comparison of PTH, PTH-related protein, and tuberoinfundibular peptide of 39 residues. Endocrinology 2000, 141, 3080–3086. [Google Scholar] [CrossRef][Green Version]
- Pinheiro, P.L.; Cardoso, J.C.; Power, D.M.; Canario, A.V. Functional characterization and evolution of PTH/PTHrP receptors: Insights from the chicken. BMC Evol. Biol. 2012, 12, 110. [Google Scholar] [CrossRef]
- Rubin, D.A.; Juppner, H. Zebrafish express the common parathyroid hormone/parathyroid hormone-related peptide receptor (PTH1R) and a novel receptor (PTH3R) that is preferentially activated by mammalian and fugufish parathyroid hormone-related peptide. J. Biol. Chem. 1999, 274, 28185–28190. [Google Scholar] [CrossRef]
- Rotllant, J.; Guerreiro, P.M.; Redruello, B.; Fernandes, H.; Apolonia, L.; Anjos, L.; Canario, A.V.; Power, D.M. Ligand binding and signalling pathways of PTH receptors in sea bream (Sparus auratus) enterocytes. Cell Tissue Res. 2006, 323, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kenakin, T. Functional selectivity and biased receptor signaling. J. Pharmacol. Exp. Ther. 2011, 336, 296–302. [Google Scholar] [CrossRef]
- Mayer-Gostan, N.; Flik, G.; Pang, P.T. An enzyme-linked immunosorbent assay for stanniocalcin, a major hypocalcemic hormone in teleost. Gen. Comp. Endocrinol. 1992, 86, 10–19. [Google Scholar] [CrossRef]
- Fuentes, J.; Brinca, L.; Guerreiro, P.M.; Power, D.M. PRL and GH synthesis and release from the sea bream (Sparus auratus L.) pituitary gland in vitro in response to osmotic challenge. Gen. Comp. Endocrinol. 2010, 168, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.; Guerreiro, P.M.; Modesto, T.; Rotllant, J.; Canario, A.V.; Power, D.M. A PTH/PTHrP receptor antagonist blocks the hypercalcemic response to estradiol-17beta. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R956–R960. [Google Scholar] [CrossRef]
- Marquez, L.; Fuentes, J. In vitro characterization of acid secretion in the gilthead sea bream (Sparus aurata) stomach. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 167, 52–58. [Google Scholar] [CrossRef]
- Casare, F.; Milan, D.; Fernandez, R. Stimulation of calcium-sensing receptor increases biochemical H(+)-ATPase activity in mouse cortex and outer medullary regions. Can. J. Physiol. Pharmacol. 2014, 92, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Armato, U.; Chiarini, A.; Chakravarthy, B.; Chioffi, F.; Pacchiana, R.; Colarusso, E.; Whitfield, J.F.; Dal Pra, I. Calcium-sensing receptor antagonist (calcilytic) NPS 2143 specifically blocks the increased secretion of endogenous Abeta42 prompted by exogenous fibrillary or soluble Abeta25-35 in human cortical astrocytes and neurons-therapeutic relevance to Alzheimer’s disease. Biochim. Biophys Acta 2013, 1832, 1634–1652. [Google Scholar] [CrossRef]
- Rotllant, J.; Guerreiro, P.M.; Anjos, L.; Redruello, B.; Canario, A.V.; Power, D.M. Stimulation of cortisol release by the N terminus of teleost parathyroid hormone-related protein in interrenal cells in vitro. Endocrinology 2005, 146, 71–76. [Google Scholar] [CrossRef]
- Anjos, L.; Rotllant, J.; Guerreiro, P.M.; Hang, X.; Canario, A.V.; Balment, R.; Power, D.M. Production and characterisation of gilthead sea bream (Sparus auratus) recombinant parathyroid hormone related protein. Gen. Comp. Endocrinol. 2005, 143, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Anjos, L.; Gomes, A.S.; Redruello, B.; Reinhardt, R.; Canario, A.V.; Power, D.M. PTHrP-induced modifications of the sea bream (Sparus auratus) vertebral bone proteome. Gen. Comp. Endocrinol. 2013, 191, 102–112. [Google Scholar] [CrossRef]
- Fange, R. Regulation of blood and body fluids in primitive fish groups. In Evolutionary Biology of Primitive Fishes; Foreman, R.E., Gorbman, A., Dodd, J.M., Olsson, R., Eds.; Plenum Press: New York, NY, USA; London, UK, 1985; pp. 253–273. [Google Scholar]
- Yildiz, H.Y.; Altunay, S. Physiological stress and innate immune response in gilthead sea bream (Sparus aurata) and sea bass (Dicentrarchus labrax) exposed to combination of trimethoprim and sulfamethoxazole (TMP-SMX). Fish. Physiol. Biochem. 2011, 37, 401–409. [Google Scholar] [CrossRef]
- Gu, J.; Li, J.W.; Tse, W.K.; Chan, T.F.; Lai, K.P.; Wong, C.K. Transcriptomic responses of corpuscle of Stannius gland of Japanese eels (Anguilla japonica) to changes in water salinity. Sci. Rep. 2015, 5, 9836. [Google Scholar] [CrossRef]
- Hanssen, R.G.; Aarden, E.M.; van der Venne, W.P.; Pang, P.K.; Wendelaar Bonga, S.E. Regulation of secretion of the teleost fish hormone stanniocalcin: Effects of extracellular calcium. Gen. Comp. Endocrinol. 1991, 84, 155–163. [Google Scholar] [CrossRef]
- Rotllant, J.; Worthington, G.P.; Fuentes, J.; Guerreiro, P.M.; Teitsma, C.A.; Ingleton, P.M.; Balment, R.J.; Canario, A.V.; Power, D.M. Determination of tissue and plasma concentrations of PTHrP in fish: Development and validation of a radioimmunoassay using a teleost 1-34 N-terminal peptide. Gen. Comp. Endocrinol. 2003, 133, 146–153. [Google Scholar] [CrossRef]
- Abbink, W.; Bevelander, G.S.; Rotllant, J.; Canario, A.V.; Flik, G. Calcium handling in Sparus auratus: Effects of water and dietary calcium levels on mineral composition, cortisol and PTHrP levels. J. Exp. Biol. 2004, 207, 4077–4084. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gensure, R.C.; Ponugoti, B.; Gunes, Y.; Papasani, M.R.; Lanske, B.; Bastepe, M.; Rubin, D.A.; Juppner, H. Identification and characterization of two parathyroid hormone-like molecules in zebrafish. Endocrinology 2004, 145, 1634–1639. [Google Scholar] [CrossRef]
- Aida, K.; Nishioka, R.S.; Bern, H.A. Changes in the corpuscles of Stannius of coho salmon (Oncorhynchus kisutch) during smoltification and seawater adaptation. Gen. Comp. Endocrinol. 1980, 41, 296–304. [Google Scholar] [CrossRef]
- Aida, K.; Nishioka, R.S.; Bern, H.A. Degranulation of the stannius corpuscles of coho salmon (Oncorhynchus kisutch) in response to ionic changes in vitro. Gen. Comp. Endocrinol. 1980, 41, 305–313. [Google Scholar] [CrossRef]





| Gene | Sequence (5′–3′) | Ta (°C) | Amplicon (bp) | Accession nº |
|---|---|---|---|---|
| Casra | Fw AGAGTTCTTACAGCACGTCCAAC | 60 | 131 | AJ289717 |
| Rv CTAGTGCTGCCATCTCACTTTC | ||||
| pth1rb | Fw TCACCAACGTCACTGCCAGAGGA | 55 | 142 | AJ619024 |
| Rv GTCCCGACGAGGGTATCGAGTT | ||||
| pth3rb | Fw ACATCCACATTCACTTCTTCAC | 53 | 250 | AY547261 |
| Rv GATGAGGGCCACAGGTAGT- | ||||
| 18s | Fw AACCAGACAAATCGCTCCAC | 60 | 139 | AY993930 |
| Rv CCTGCGGCTTAATTTGACTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Jarabo, I.; Gregório, S.F.; Fuentes, J. Regulation of Stanniocalcin Secretion by Calcium and PTHrP in Gilthead Seabream (Sparus aurata). Biology 2022, 11, 863. https://doi.org/10.3390/biology11060863
Ruiz-Jarabo I, Gregório SF, Fuentes J. Regulation of Stanniocalcin Secretion by Calcium and PTHrP in Gilthead Seabream (Sparus aurata). Biology. 2022; 11(6):863. https://doi.org/10.3390/biology11060863
Chicago/Turabian StyleRuiz-Jarabo, Ignacio, Silvia F. Gregório, and Juan Fuentes. 2022. "Regulation of Stanniocalcin Secretion by Calcium and PTHrP in Gilthead Seabream (Sparus aurata)" Biology 11, no. 6: 863. https://doi.org/10.3390/biology11060863
APA StyleRuiz-Jarabo, I., Gregório, S. F., & Fuentes, J. (2022). Regulation of Stanniocalcin Secretion by Calcium and PTHrP in Gilthead Seabream (Sparus aurata). Biology, 11(6), 863. https://doi.org/10.3390/biology11060863