An Anaerobic Environment Drives the Harboring of Helicobacter pylori within Candida Yeast Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Conditions
2.2. Growth of H. pylori Strains and Candida Strains Cultured under Aerobic, Microaerobic, or Anaerobic Conditions
2.3. Co-Cultures of H. pylori Strains with Candida Strains
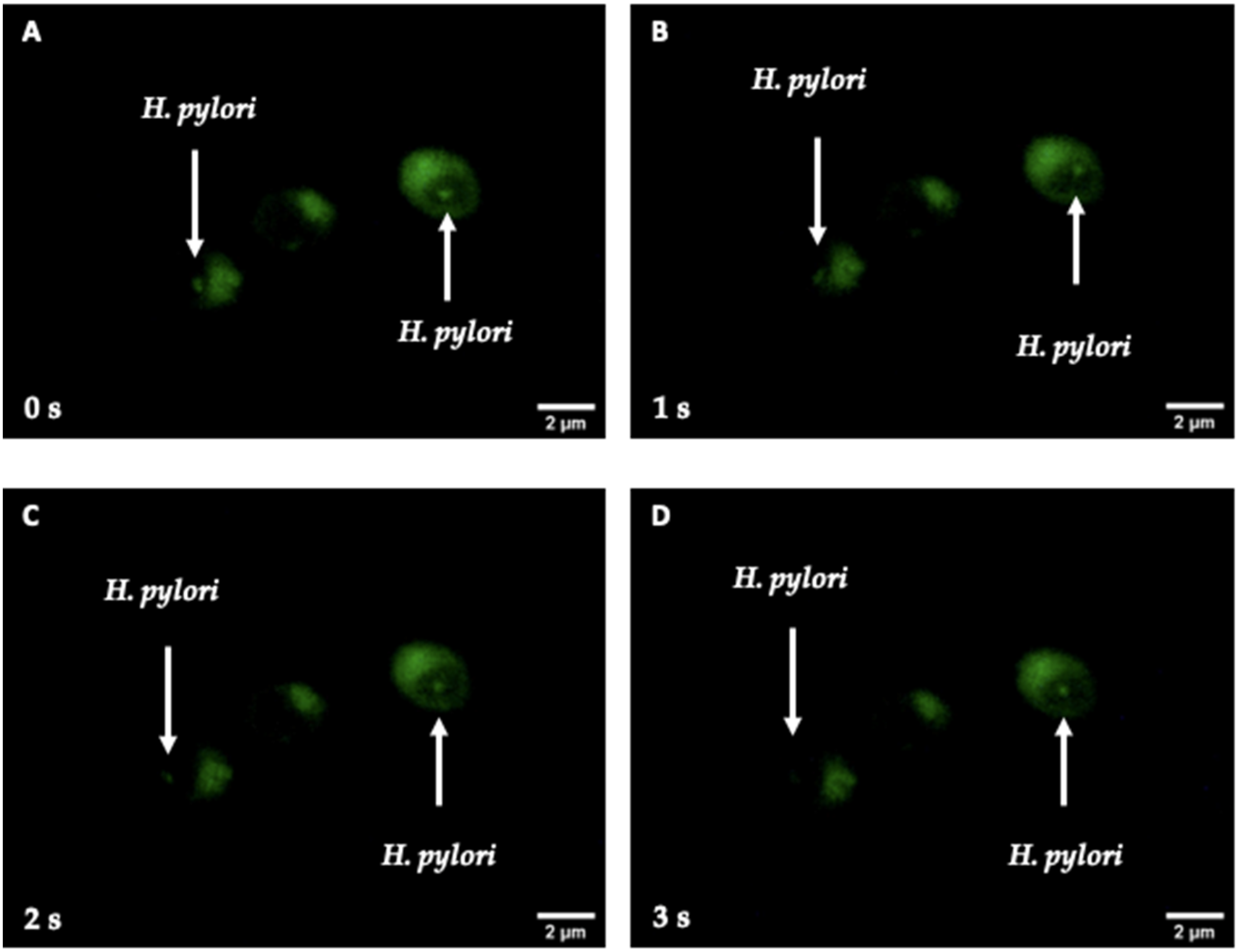
2.4. Search for Intra-Yeast Bacteria-like Bodies (BLBs)
2.5. Identification of Intra-Yeast BLBs Using the FISH Technique
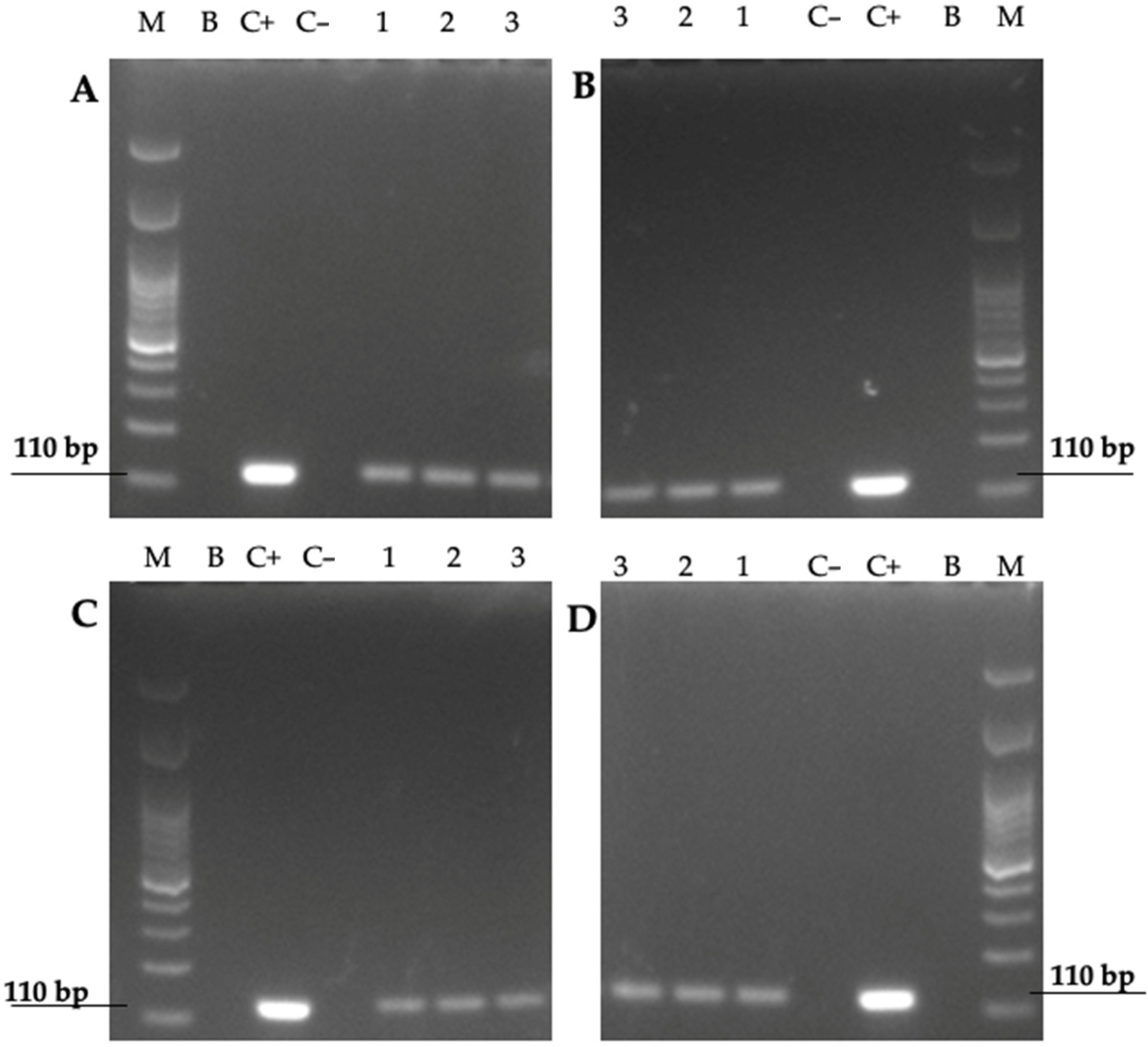
2.6. Detection of the 16S rRNA Gene of H. pylori in the Total DNA of Yeast Cells
2.7. H. pylori Viability Assay
2.8. Statistical Analysis
3. Results
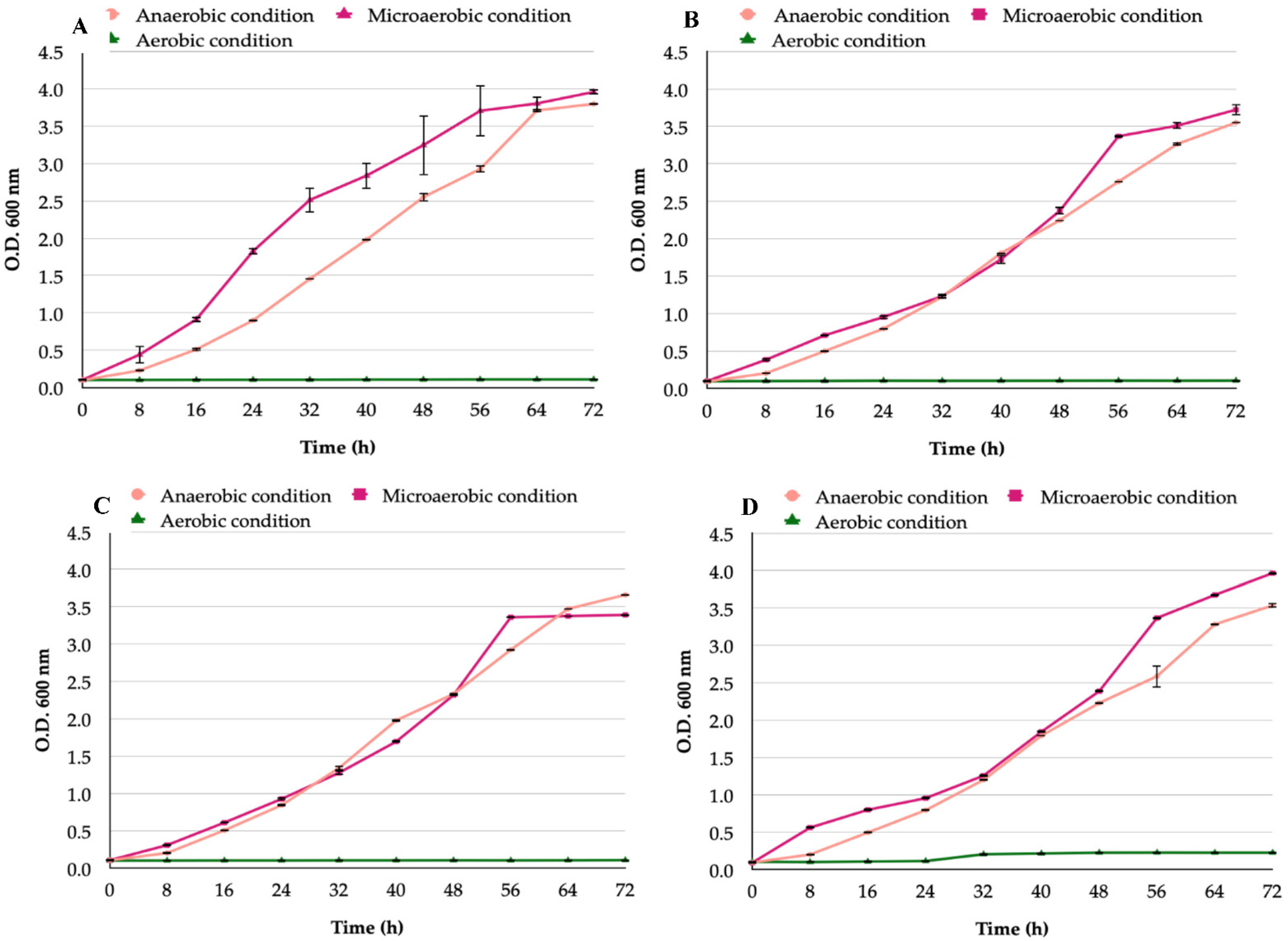
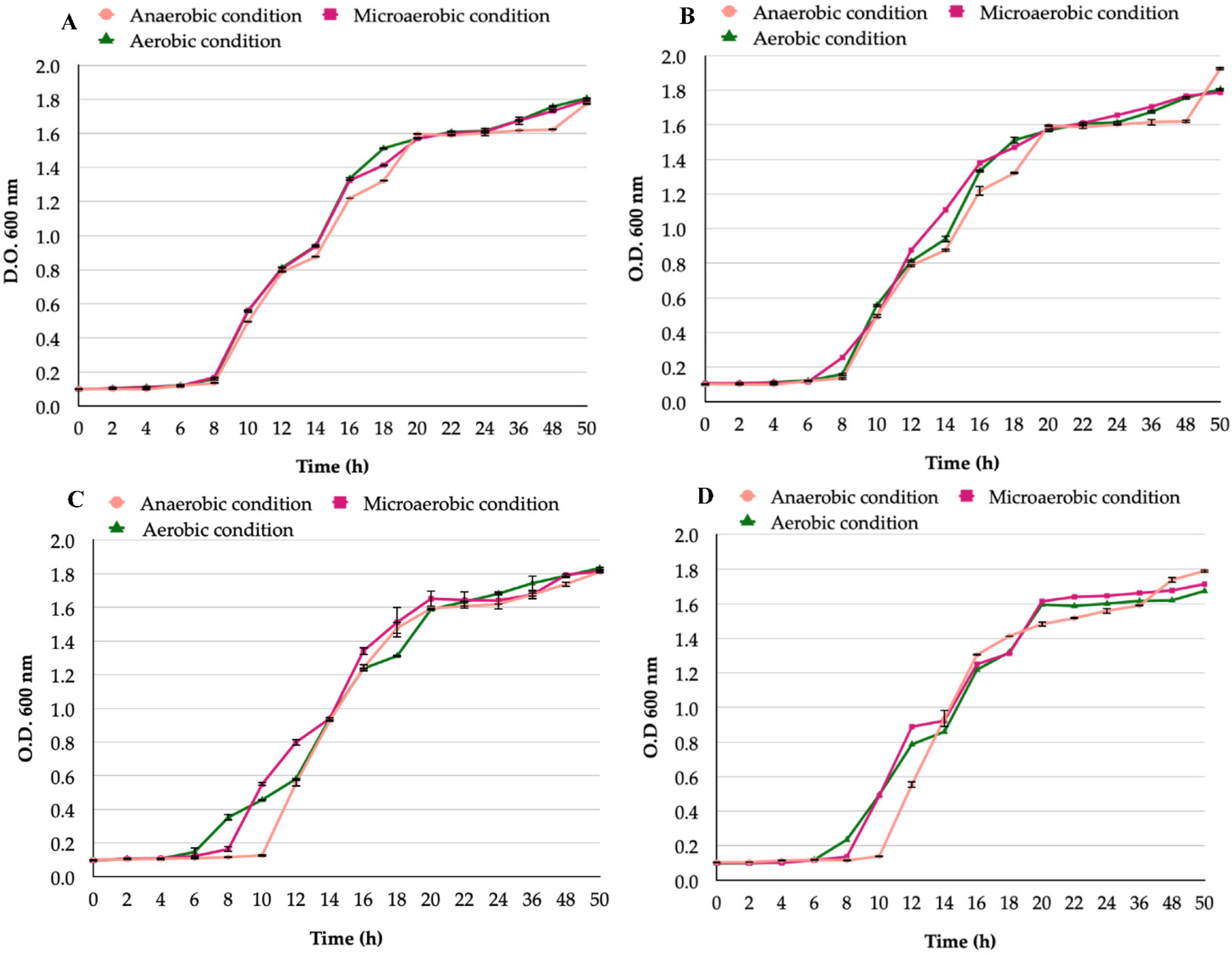
3.1. Growth Curves of H. pylori and Candida Strains Cultured under Aerobic, Microaerobic, or Anaerobic Conditions
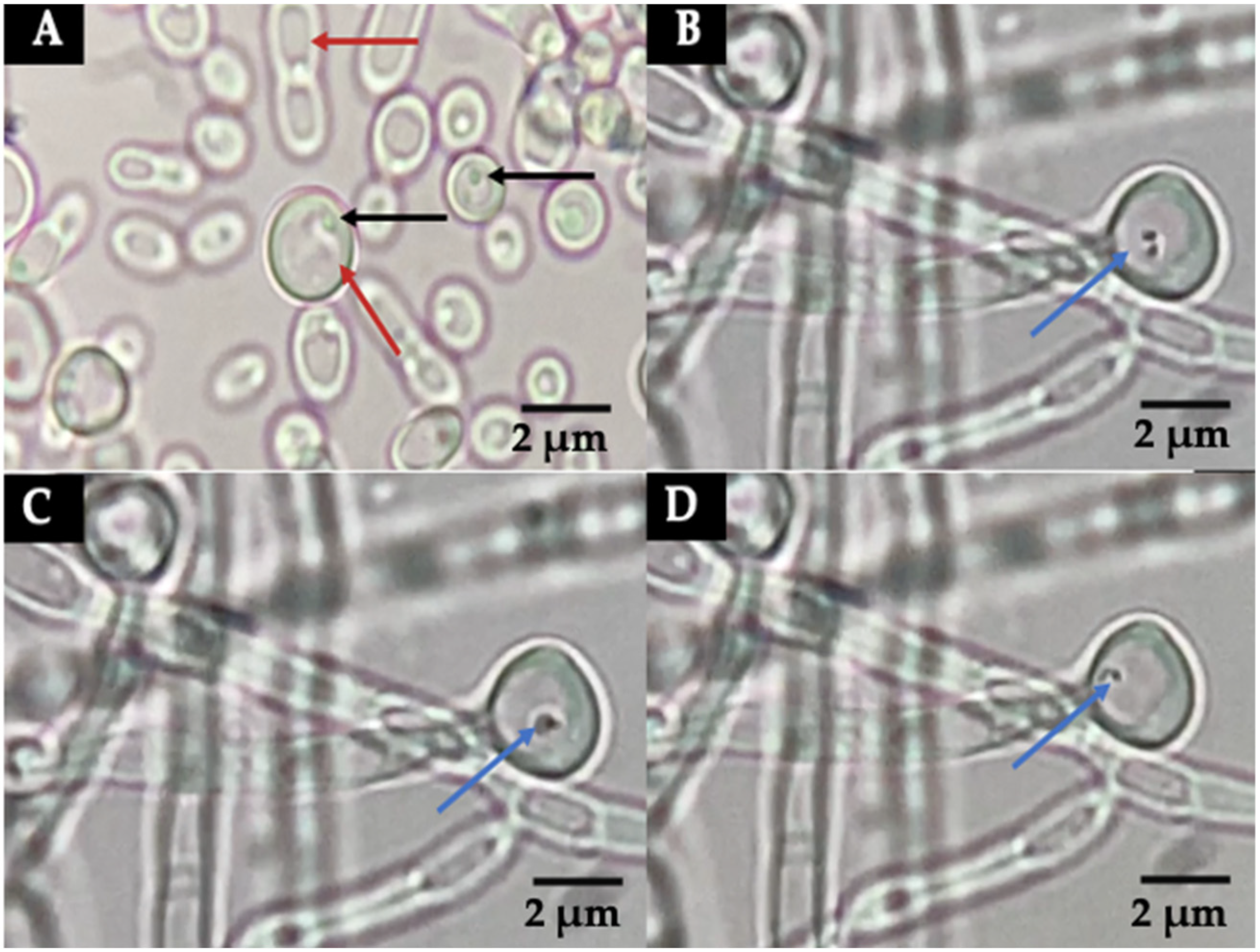
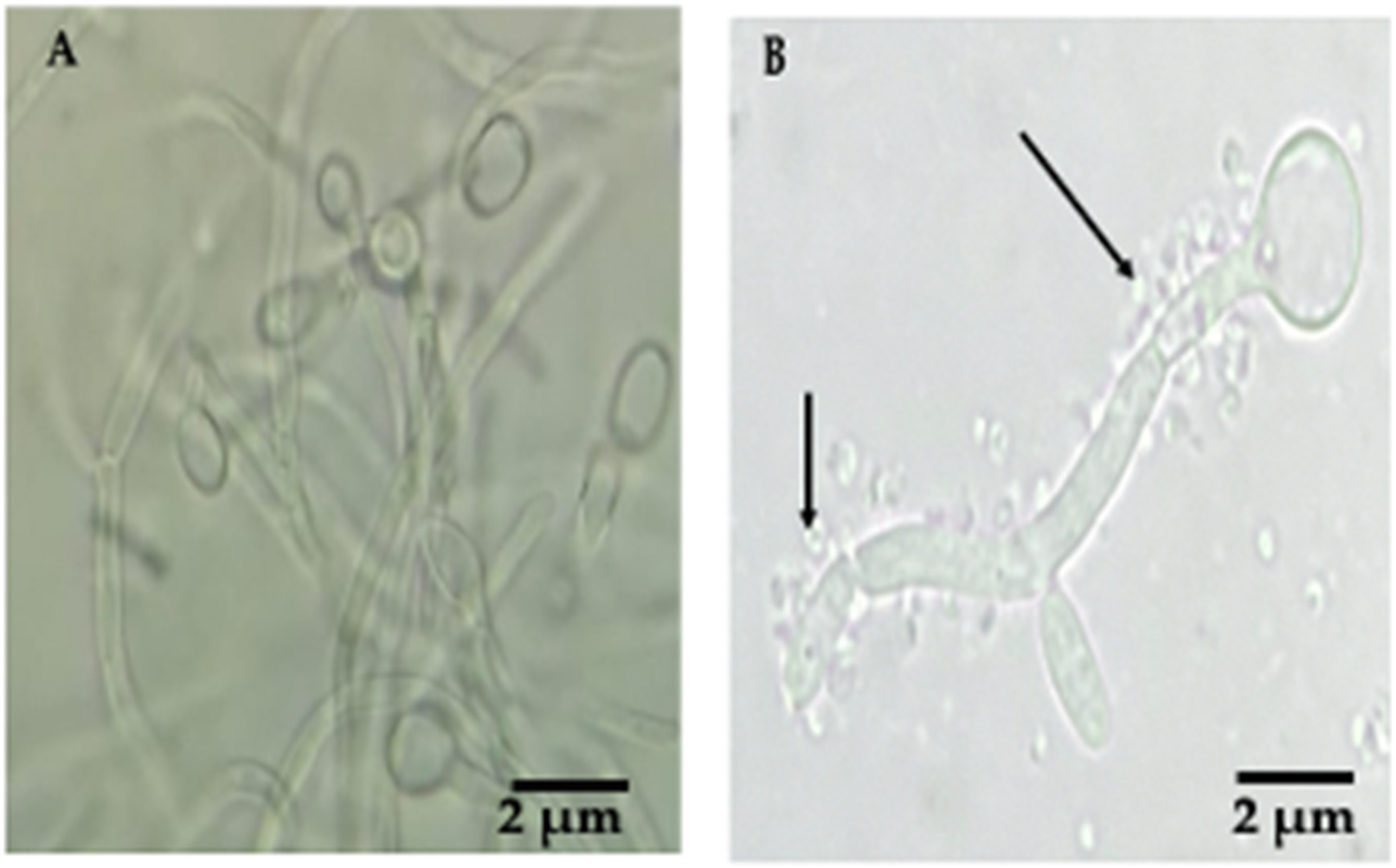
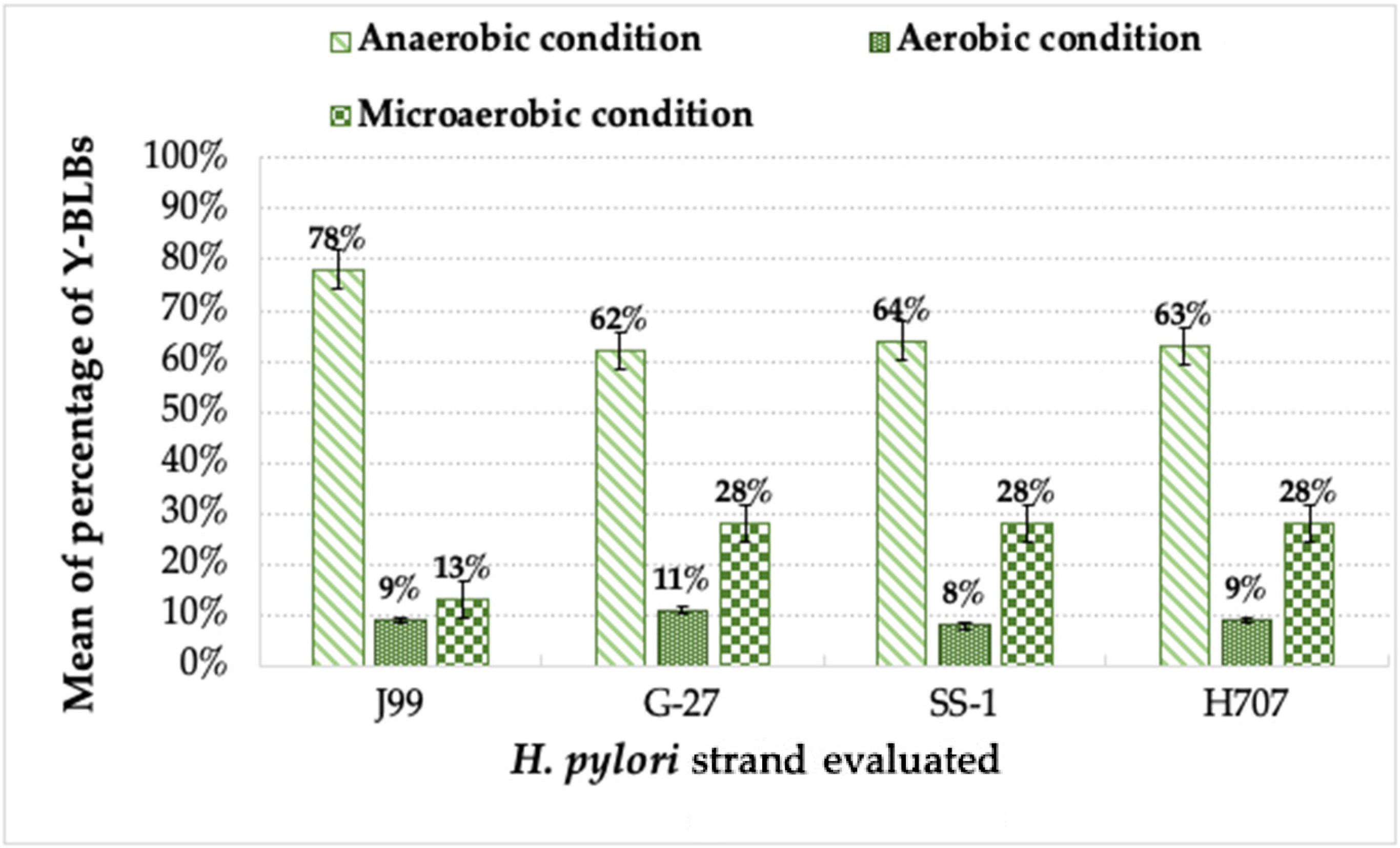
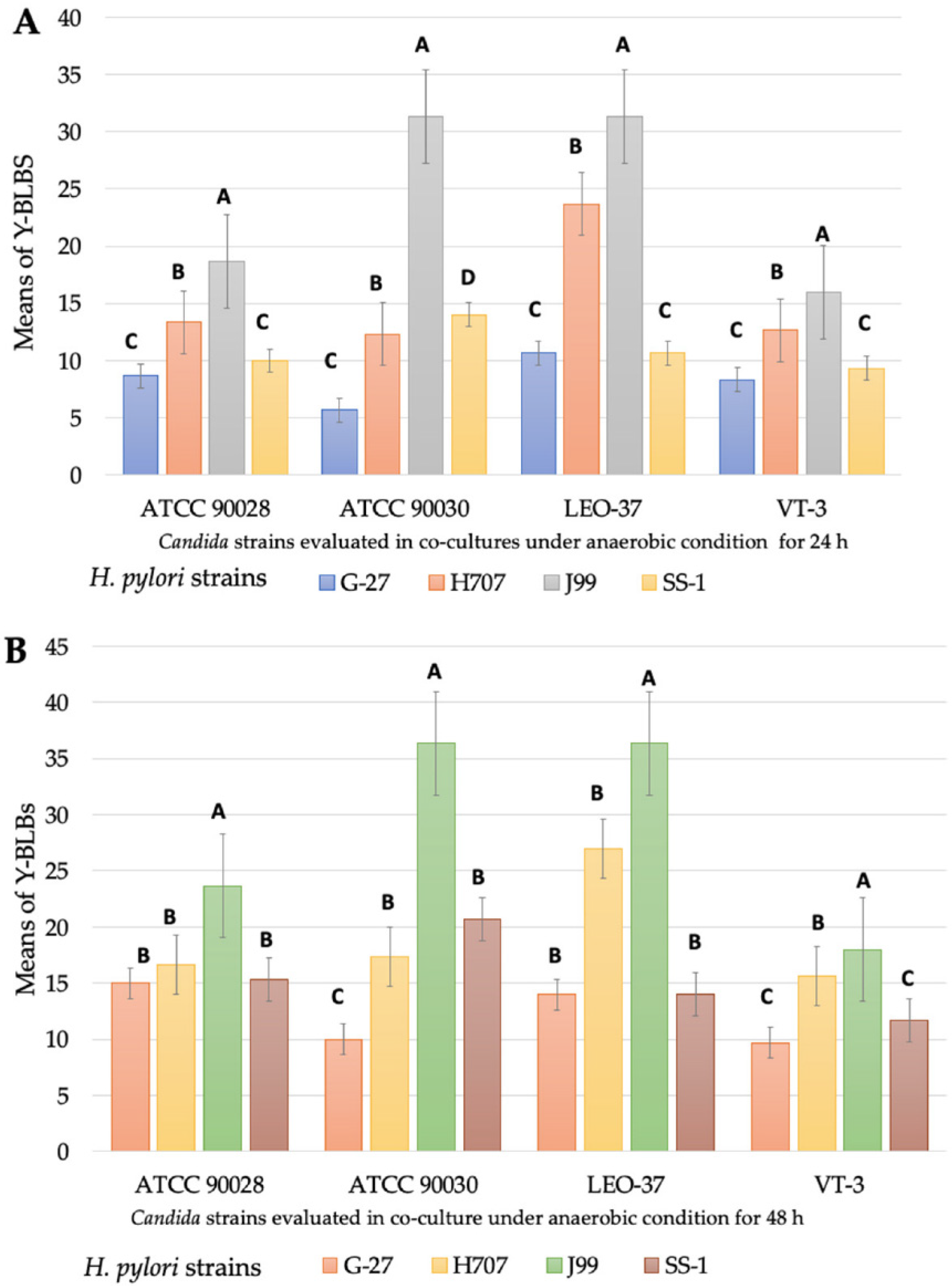
3.2. Detection of Bacteria-like Bodies (Y-BLBs) within Yeasts
3.3. Identification, at the Species Level, of Intra-Yeast Bacteria-like Bodies (BLBs)
3.4. Viability Assessment of H. pylori Cells Harboring in Yeast Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdi, Y.M.; Young, K.A.; Rampton, D.S.; Hardie, J.M.; Feldman, R.A.; Banatvala, N. Comparison of the Effects of Anaerobic and Micro-Aerophilic Incubation on Resistance of Helicobacter pylori to Metronidazole. J. Med. Microbiol. 1999, 48, 407–410. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bravo, D.; Hoare, A.; Soto, C.; Valenzuela, M.A.; Quest, A.F. Helicobacter pylori in Human Health and Disease: Mechanisms for Local Gastric and Systemic Effects. World J. Gastroenterol. 2018, 24, 3071–3089. [Google Scholar] [CrossRef] [PubMed]
- Araújo, G.R.L.; Marques, H.S.; Santos, M.L.C.; da Silva, F.A.F.; da Brito, B.B.; Correa Santos, G.L.; de Melo, F.F. Helicobacter pylori Infection: How Does Age Influence the Inflammatory Pattern? World J. Gastroenterol. 2022, 28, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Bury-Moné, S.; Kaakoush, N.O.; Asencio, C.; Mégraud, F.; Thibonnier, M.; De Reuse, H.; Mendz, G.L. Is Helicobacter pylori a True Microaerophile? Helicobacter 2006, 11, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef]
- Atherton, J.C.; Cao, P.; Peek, R.M.; Tummuru, M.K.; Blaser, M.J.; Cover, T.L. Mosaicism in Vacuolating Cytotoxin Alleles of Helicobacter pylori. Association of Specific VacA Types with Cytotoxin Production and Peptic Ulceration. J. Biol. Chem. 1995, 270, 17771–17777. [Google Scholar] [CrossRef]
- Gilani, A.; Razavilar, V.; Rokni, N.; Rahimi, E. VacA and CagA Genotypes Status and Antimicrobial Resistance Properties of Helicobacter pylori Strains Isolated from Meat Products in Isfahan Province, Iran. Iran. J. Vet. Res. 2017, 18, 97–102. [Google Scholar]
- Sharndama, H.C.; Mba, I.E. Helicobacter pylori: An up-to-Date Overview on the Virulence and Pathogenesis Mechanisms. Braz. J. Microbiol. 2022, 53, 33–50. [Google Scholar] [CrossRef]
- Robinson, K.; Atherton, J.C. The Spectrum of Helicobacter-Mediated Diseases. Annu. Rev. Pathol. 2021, 16, 123–144. [Google Scholar] [CrossRef]
- Franceschi, F.; Covino, M.; Baudron, C.R. Review: Helicobacter pylori and Extragastric Diseases. Helicobacter 2019, 24, e12636. [Google Scholar] [CrossRef]
- Guerra-Valle, M.; Orellana-Palma, P.; Petzold, G. Plant-Based Polyphenols: Anti-Helicobacter pylori Effect and Improvement of Gut Microbiota. Antioxidants 2022, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Merino, J.S.; García, A.; Pastene, E.; Salas, A.; Saez, K.; González, C.L. Lactobacillus fermentum UCO-979C Strongly Inhibited Helicobacter pylori SS1 in Meriones Unguiculatus. Benef. Microbes 2018, 9, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Salas-Jara, M.J.; Sanhueza, E.A.; Retamal-Díaz, A.; González, C.; Urrutia, H.; García, A. Probiotic Lactobacillus fermentum UCO-979C Biofilm Formation on AGS and Caco-2 Cells and Helicobacter pylori Inhibition. Biofouling 2016, 32, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Navarro, K.; Sanhueza, E.; Pineda, S.; Pastene, E.; Quezada, M.; Henríquez, K.; Karlyshev, A.; Villena, J.; González, C. Characterization of Lactobacillus fermentum UCO-979C, a Probiotic Strain with a Potent Anti-Helicobacter pylori Activity. Electron. J. Biotechnol. 2017, 25, 75–83. [Google Scholar] [CrossRef]
- Baker, D.A. Plants against Helicobacter pylori to Combat Resistance: An Ethnopharmacological Review. Biotechnol. Rep. Amst. Neth. 2020, 26, e00470. [Google Scholar] [CrossRef] [PubMed]
- Bonifácio, B.V.; dos Santos Ramos, M.A.; da Silva, P.B.; Bauab, T.M. Antimicrobial Activity of Natural Products against Helicobacter Pylori: A Review. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 54. [Google Scholar] [CrossRef]
- Kayali, S.; Manfredi, M.; Gaiani, F.; Bianchi, L.; Bizzarri, B.; Leandro, G.; Di Mario, F.; De’ Angelis, G.L. Helicobacter pylori, Transmission Routes and Recurrence of Infection: State of the Art. Acta Bio-Med. Atenei Parm. 2018, 89, 72–76. [Google Scholar] [CrossRef]
- Olivera-Severo, D.; Uberti, A.F.; Marques, M.S.; Pinto, M.T.; Gomez-Lazaro, M.; Figueiredo, C.; Leite, M.; Carlini, C.R. A New Role for Helicobacter pylori Urease: Contributions to Angiogenesis. Front. Microbiol. 2017, 8, 1883. [Google Scholar] [CrossRef]
- Hazell, S.L.; Evans, D.J.; Graham, D.Y. Helicobacter pylori Catalase. J. Gen. Microbiol. 1991, 137, 57–61. [Google Scholar] [CrossRef]
- Mori, M.; Suzuki, H.; Suzuki, M.; Kai, A.; Miura, S.; Ishii, H. Catalase and Superoxide Dismutase Secreted from Helicobacter pylori. Helicobacter 1997, 2, 100–105. [Google Scholar] [CrossRef]
- Nilsson, H.-O.; Blom, J.; Al-Soud, W.A.; Ljungh, Å.; Andersen, L.P.; Wadström, T. Effect of Cold Starvation, Acid Stress, and Nutrients on Metabolic Activity of Helicobacter pylori. Appl. Environ. Microbiol. 2002, 68, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Mouery, K.; Rader, B.A.; Gaynor, E.C.; Guillemin, K. The Stringent Response Is Required for Helicobacter pylori Survival of Stationary Phase, Exposure to Acid, and Aerobic Shock. J. Bacteriol. 2006, 188, 5494–5500. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hathroubi, S.; Servetas, S.L.; Windham, I.; Merrell, D.S.; Ottemann, K.M. Helicobacter pylori Biofilm Formation and Its Potential Role in Pathogenesis. Microbiol. Mol. Biol. Rev. 2018, 82, e00001-18. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.P.; Rasmussen, L. Helicobacter pylori-Coccoid Forms and Biofilm Formation. FEMS Immunol. Med. Microbiol. 2009, 56, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Aktas, D.; Bagirova, M.; Allahverdiyev, A.M.; Abamor, E.S.; Safarov, T.; Kocazeybek, B.S. Resuscitation of the Helicobacter pylori Coccoid Forms by Resuscitation Promoter Factor Obtained from Micrococcus luteus. Curr. Microbiol. 2020, 77, 2093–2103. [Google Scholar] [CrossRef]
- Moreno-Mesonero, L.; Moreno, Y.; Alonso, J.L.; Ferrús, M.A. Detection of Viable Helicobacter pylori inside Free-Living Amoebae in Wastewater and Drinking Water Samples from Eastern Spain. Environ. Microbiol. 2017, 19, 4103–4112. [Google Scholar] [CrossRef]
- Matamala-Valdés, L.; Sánchez-Alonzo, K.; Parra, C.; Sáez, K.; Aguayo-Reyes, A.; García, A.; Matamala-Valdés, L.; Sánchez-Alonzo, K.; Parra, C.; Sáez, K.; et al. Detection of Intracellular Helicobacter pylori in Candida spp. from Neonate Oral Swabs. Rev. Assoc. Méd. Bras. 2018, 64, 928–935. [Google Scholar] [CrossRef]
- Salmanian, A.-H.; Siavoshi, F.; Akbari, F.; Afshari, A.; Malekzadeh, R. Yeast of the Oral Cavity Is the Reservoir of Helicobacter pylori. J. Oral Pathol. Med. 2008, 37, 324–328. [Google Scholar] [CrossRef]
- Siavoshi, F.; Salmanian, A.H.; Akbari, F.; Kbari, F.A.; Malekzadeh, R.; Massarrat, S. Detection of Helicobacter pylori-Specific Genes in the Oral Yeast. Helicobacter 2005, 10, 318–322. [Google Scholar] [CrossRef]
- Sánchez-Alonzo, K.; Parra-Sepúlveda, C.; Vergara, L.; Bernasconi, H.; García-Cancino, A.; Sánchez-Alonzo, K.; Parra-Sepúlveda, C.; Vergara, L.; Bernasconi, H.; García-Cancino, A. Detection of Helicobacter pylori in Oral Yeasts from Students of a Chilean University. Rev. Assoc. Med. Bras. 2020, 66, 1509–1514. [Google Scholar] [CrossRef]
- Sánchez-Alonzo, K.; Parra-Sepúlveda, C.; Vega, S.; Bernasconi, H.; Campos, V.L.; Smith, C.T.; Sáez, K.; García-Cancino, A. In Vitro Incorporation of Helicobacter pylori into Candida albicans Caused by Acidic PH Stress. Pathogens 2020, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alonzo, K.; Belmar, L.; Parra-Sepúlveda, C.; Bernasconi, H.; Campos, V.L.; Smith, C.T.; Sáez, K.; García-Cancino, A. Antibiotics as a Stressing Factor Triggering the Harboring of Helicobacter pylori J99 within Candida albicans ATCC10231. Pathogens 2021, 10, 382. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alonzo, K.; Silva-Mieres, F.; Arellano-Arriagada, L.; Parra-Sepúlveda, C.; Bernasconi, H.; Smith, C.T.; Campos, V.L.; García-Cancino, A. Nutrient Deficiency Promotes the Entry of Helicobacter pylori Cells into Candida Yeast Cells. Biology 2021, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alonzo, K.; Arellano-Arriagada, L.; Castro-Seriche, S.; Parra-Sepúlveda, C.; Bernasconi, H.; Benavidez-Hernández, H.; Campos, V.L.; Sáez, K.; Smith, C.T.; García-Cancino, A. Temperatures Outside the Optimal Range for Helicobacter pylori Increase Its Harboring within Candida Yeast Cells. Biology 2021, 10, 915. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.P.; Wadström, T. Basic Bacteriology and Culture. In Helicobacter pylori: Physiology and Genetics; Mobley, H.L., Mendz, G.L., Hazell, S.L., Eds.; ASM Press: Washington, DC, USA, 2001; ISBN 978-1-55581-213-3. [Google Scholar]
- Olver, W.J.; Stafford, J.; Cheetham, P.; Boswell, T.C. Comparison of Candida ID Medium with Sabouraud-Chloramphenicol Agar for the Isolation of Yeasts from Clinical Haematology Surveillance Specimens. J. Med. Microbiol. 2002, 51, 221–224. [Google Scholar] [CrossRef]
- Böckelmann, U.; Manz, W.; Neu, T.R.; Szewzyk, U. Investigation of Lotic Microbial Aggregates by a Combined Technique of Fluorescent In Situ Hybridization and Lectin-Binding-Analysis. J. Microbiol. Methods 2002, 49, 75–87. [Google Scholar] [CrossRef]
- Rüssmann, H.; Kempf, V.A.J.; Koletzko, S.; Heesemann, J.; Autenrieth, I.B. Comparison of Fluorescent In Situ Hybridization and Conventional Culturing for Detection of Helicobacter pylori in Gastric Biopsy Specimens. J. Clin. Microbiol. 2001, 39, 304–308. [Google Scholar] [CrossRef]
- Yang, I.; Nell, S.; Suerbaum, S. Survival in Hostile Territory: The Microbiota of the Stomach. FEMS Microbiol. Rev. 2013, 37, 736–761. [Google Scholar] [CrossRef]
- Ranjbar, R.; Khamesipour, F.; Jonaidi-Jafari, N.; Rahimi, E. Helicobacter pylori Isolated from Iranian Drinking Water: VacA, CagA, IceA, OipA and BabA2 Genotype Status and Antimicrobial Resistance Properties. FEBS Open Bio 2016, 6, 433–441. [Google Scholar] [CrossRef]
- Zamani, M.; Vahedi, A.; Maghdouri, Z.; Shokri-Shirvani, J. Role of Food in Environmental Transmission of Helicobacter pylori. Casp. J. Intern. Med. 2017, 8, 146–152. [Google Scholar] [CrossRef]
- Saeidi, E.; Sheikhshahrokh, A. VacA Genotype Status of Helicobacter pylori Isolated from Foods with Animal Origin. BioMed Res. Int. 2016, 2016, 8701067. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Jithendra, K.D. Presence of Helicobacter pylori in Subgingival Plaque of Periodontitis Patients with and without Dyspepsia, Detected by Polymerase Chain Reaction and Culture. J. Indian Soc. Periodontol. 2012, 16, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hu, J.Y. Assessment of the Extent of Bacterial Growth in Reverse Osmosis System for Improving Drinking Water Quality. J. Environ. Sci. Health Part A 2010, 45, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Donelli, G.; Matarrese, P.; Fiorentini, C.; Dainelli, B.; Taraborelli, T.; Di Campli, E.; Di Bartolomeo, S.; Cellini, L. The Effect of Oxygen on the Growth and Cell Morphology of Helicobacter pylori. FEMS Microbiol. Lett. 1998, 168, 9–15. [Google Scholar] [CrossRef]
- Biswas, S.K.; Chaffin, W.L. Anaerobic Growth of Candida albicans Does Not Support Biofilm Formation under Similar Conditions Used for Aerobic Biofilm. Curr. Microbiol. 2005, 51, 100–104. [Google Scholar] [CrossRef]
- Dumitru, R.; Hornby, J.M.; Nickerson, K.W. Defined Anaerobic Growth Medium for Studying Candida albicans Basic Biology and Resistance to Eight Antifungal Drugs. Antimicrob. Agents Chemother. 2004, 48, 2350–2354. [Google Scholar] [CrossRef]
- Rosa, E.A.R.; Rached, R.N.; Ignácio, S.A.; Rosa, R.T.; José da Silva, W.; Yau, J.Y.Y.; Samaranayake, L.P. Phenotypic Evaluation of the Effect of Anaerobiosis on Some Virulence Attributes of Candida albicans. J. Med. Microbiol. 2008, 57, 1277–1281. [Google Scholar] [CrossRef]
- Cottier, F.; Mühlschlegel, F.A. Sensing the Environment: Response of Candida albicans to the X Factor. FEMS Microbiol. Lett. 2009, 295, 1–9. [Google Scholar] [CrossRef][Green Version]
- Liu, H.; Semino-Mora, C.; Dubois, A. Mechanism of H. pylori Intracellular Entry: An In Vitro Study. Front. Cell. Infect. Microbiol. 2012, 2, 13. [Google Scholar] [CrossRef]
- Rathore, A.S.; Gupta, R.D. Chitinases from Bacteria to Human: Properties, Applications, and Future Perspectives. Available online: https://www.hindawi.com/journals/er/2015/791907/ (accessed on 9 March 2021).
- Rehman, S.; Grigoryeva, L.S.; Richardson, K.H.; Corsini, P.; White, R.C.; Shaw, R.; Portlock, T.J.; Dorgan, B.; Zanjani, Z.S.; Fornili, A.; et al. Structure and Functional Analysis of the Legionella Pneumophila Chitinase ChiA Reveals a Novel Mechanism of Metal-Dependent Mucin Degradation. PLoS Pathog. 2020, 16, e1008342. [Google Scholar] [CrossRef]
- Ansorg, R.; Schmid, E.N. Adhesion of Helicobacter pylori to Yeast Cells. Zentralbl. Bakteriol. Int. J. Med. Microbiol. 1998, 288, 501–508. [Google Scholar] [CrossRef]
- Ipcho, S.; Sundelin, T.; Erbs, G.; Kistler, H.C.; Newman, M.-A.; Olsson, S. Fungal Innate Immunity Induced by Bacterial Microbe-Associated Molecular Patterns (MAMPs). G3 Genes Genomes Genet. 2016, 6, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Shirtliff, M.E.; Peters, B.M.; Jabra-Rizk, M.A. Cross-Kingdom Interactions: Candida albicans and Bacteria. FEMS Microbiol. Lett. 2009, 299, 1–8. [Google Scholar] [CrossRef]
- Deveau, A.; Brulé, C.; Palin, B.; Champmartin, D.; Rubini, P.; Garbaye, J.; Sarniguet, A.; Frey-Klett, P. Role of Fungal Trehalose and Bacterial Thiamine in the Improved Survival and Growth of the Ectomycorrhizal Fungus Laccaria Bicolor S238N and the Helper Bacterium Pseudomonas fluorescens BBc6R8. Environ. Microbiol. Rep. 2010, 2, 560–568. [Google Scholar] [CrossRef]
- Wang, G.; Alamuri, P.; Maier, R.J. The Diverse Antioxidant Systems of Helicobacter pylori. Mol. Microbiol. 2006, 61, 847–860. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Alonzo, K.; Arellano-Arriagada, L.; Bernasconi, H.; Parra-Sepúlveda, C.; Campos, V.L.; Silva-Mieres, F.; Sáez-Carrillo, K.; Smith, C.T.; García-Cancino, A. An Anaerobic Environment Drives the Harboring of Helicobacter pylori within Candida Yeast Cells. Biology 2022, 11, 738. https://doi.org/10.3390/biology11050738
Sánchez-Alonzo K, Arellano-Arriagada L, Bernasconi H, Parra-Sepúlveda C, Campos VL, Silva-Mieres F, Sáez-Carrillo K, Smith CT, García-Cancino A. An Anaerobic Environment Drives the Harboring of Helicobacter pylori within Candida Yeast Cells. Biology. 2022; 11(5):738. https://doi.org/10.3390/biology11050738
Chicago/Turabian StyleSánchez-Alonzo, Kimberly, Luciano Arellano-Arriagada, Humberto Bernasconi, Cristian Parra-Sepúlveda, Víctor L. Campos, Fabiola Silva-Mieres, Katia Sáez-Carrillo, Carlos T. Smith, and Apolinaria García-Cancino. 2022. "An Anaerobic Environment Drives the Harboring of Helicobacter pylori within Candida Yeast Cells" Biology 11, no. 5: 738. https://doi.org/10.3390/biology11050738
APA StyleSánchez-Alonzo, K., Arellano-Arriagada, L., Bernasconi, H., Parra-Sepúlveda, C., Campos, V. L., Silva-Mieres, F., Sáez-Carrillo, K., Smith, C. T., & García-Cancino, A. (2022). An Anaerobic Environment Drives the Harboring of Helicobacter pylori within Candida Yeast Cells. Biology, 11(5), 738. https://doi.org/10.3390/biology11050738







