What Does the Brain Have to Keep Working at Its Best? Resilience Mechanisms Such as Antioxidants and Brain/Cognitive Reserve for Counteracting Alzheimer’s Disease Degeneration
Abstract
:Simple Summary
Abstract
1. Introduction: Population Aging and Dementia Emergency
2. From Normal Aging to Alzheimer’s Disease
3. Brain and Cognitive Reserve as Resilience Mechanisms to Brain Deterioration
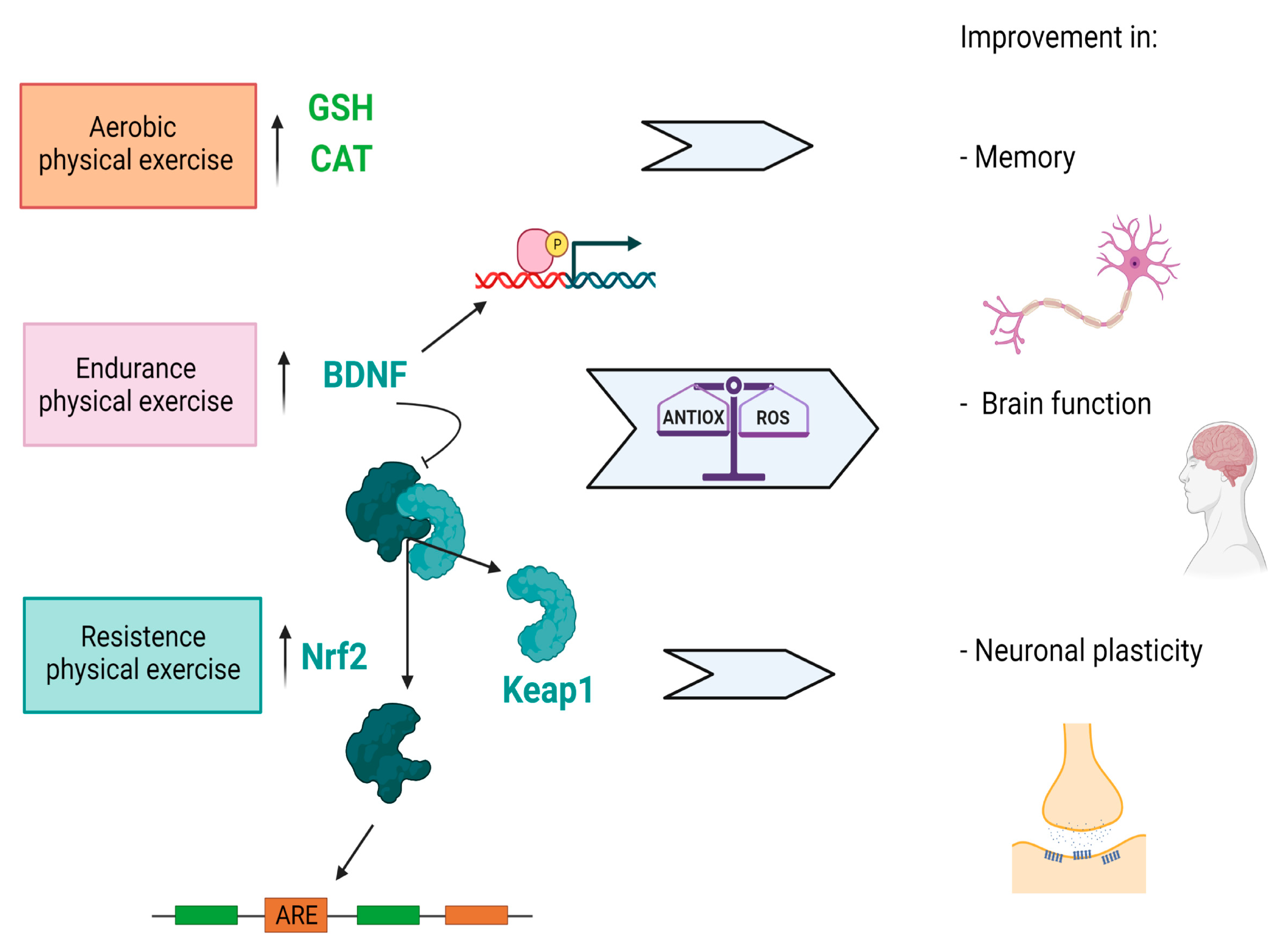
4. Antioxidants as Resilience Mechanisms to Brain Degeneration
5. Additional Role of Nutrition and Physical Activity
6. Discussion: Antioxidants Capacity and Increased BR/CR
7. Conclusions: Depicting the Trajectory of Cognitive Decline
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations; Department of Economic and Social Affairs; Population Division. World Population Ageing 2019: Highlights (ST/ESA/SER.A/430); United Nations: New York, NY, USA, 2019; pp. 5–10. [Google Scholar]
- Sengoku, R. Aging and Alzheimer’s disease pathology. Neuropathology 2020, 40, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Lobo, A.; Launer, L.J.; Fratiglioni, L.; Andersen, K.; Di Carlo, A.; Breteler, M.M.; Copeland, J.R.; Dartigues, J.F.; Jagger, C.; Martinez-Lange, J.; et al. Prevalence of dementia and major subtypes In Europe: A collaborative study of population based cohorts. Neurology 2000, 54, S4–S9. [Google Scholar] [PubMed]
- Galimberti, D.; Scarpini, E. Treatment of Alzheimers Disease: Symptomatic and Disease-Modifying Approaches. Curr. Aging Sci. 2010, 3, 46–56. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Mechanisms of disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [Green Version]
- Kivipelto, M.; Rovio, S.; Ngandu, T.; Kåreholt, I.; Eskelinen, M.; Winblad, B.; Hachinski, V.; Minguez, A.C.; Soininen, H.; Tuomiletho, J.; et al. Apolipoprotein E ɛ4 magnifies lifestyle risks for dementia: A population-based study. J. Cell. Mol. Med. 2008, 12, 2762–2771. [Google Scholar] [CrossRef] [Green Version]
- Kloppenborg, R.P.; van den Berg, E.; Kappelle, L.J.; Biessels, G.J. Diabetes and other vascular risk factors for dementia: Which factor matters most? A systematic review. Eur. J. Pharmacol. 2008, 585, 97–108. [Google Scholar] [CrossRef]
- Kang, S.; Lee, Y.H.; Lee, J.E. Metabolism-centric overview of the pathogenesis of Alzheimer’s disease. Yonsei Med. J. 2017, 58, 479–488. [Google Scholar] [CrossRef]
- Ferreira-Silva, M.V.F.; Loures, C.D.M.G.; Alves, L.C.V.; de Souza, L.C.; Borges, K.B.G.; das Graças Carvalho, M. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 1–11. [Google Scholar]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Campbell, N.L.; Unverzagt, F.; LaMantia, M.A.; Khan, B.A.; Boustani, M.A. Risk factors for the progression of mild cognitive impairment to dementia. Clin. Geriatr. Med. 2013, 29, 873–893. [Google Scholar] [CrossRef] [Green Version]
- Rabin, L.A.; Smart, C.M.; Amariglio, R.E. Subjective cognitive decline in preclinical Alzheimer’s disease. Ann. Rev. Clin. Psychol. 2017, 13, 369–396. [Google Scholar] [CrossRef]
- Fernández-Blázquez, M.A.; Ávila-Villanueva, M.; Maestú, F.; Medina, M. Specific features of subjective cognitive decline predict faster conversion to mild cognitive impairment. J. Alzheimer′s Dis. 2016, 52, 271–281. [Google Scholar] [CrossRef]
- Jessen, F.; Amariglio, R.E.; Buckley, R.F.; van der Flier, W.M.; Han, Y.; Molinuevo, J.L.; Rabin, L.; Rentz, D.M.; Rodriguez-Gomez, O.; Sajkin, A.J.; et al. The characterisation of subjective cognitive decline. Lancet Neurol. 2020, 19, 271–278. [Google Scholar] [CrossRef]
- Jessen, F.; Wiese, B.; Bachmann, C.; Eifflaender-Gorfer, S.; Haller, F.; Kölsch, H.; Luck, T.; Mösch, E.; van den Bussche, H.; Wagner, M.; et al. German Study on Aging, Cognition and Dementia in Primary Care Patients Study Groupet al. Prediction of dementia by subjective memory impairment effects of severity and temporal association with cognitive impairment. Arch. Gen. Psychiatry 2010, 67, 414–422. [Google Scholar] [CrossRef]
- van Harten, A.C.; Mielke, M.M.; Swenson-Dravis, D.M.; Hagen, C.E.; Edwards, K.K.; Roberts, R.O.; Geda, Y.E.; Knopman, D.S.; Petersen, R.C. Subjective cognitive decline and risk of MCI: The Mayo Clinic Study of Aging. Neurology 2018, 91, e300–e312. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Stern, Y. What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 2002, 8, 448–460. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef]
- Jones, R.N.; Fong, T.G.; Metzger, E.; Tulebaev, S.; Yang, F.M.; Alsop, D.C.; Marcantonio, E.R.; Cupples, L.A.; Gottieb, G.; Inouye, S.K. Aging, brain disease, and reserve: Implications for delirium. Am. J. Geriatr. Psychiatry 2010, 18, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Pettigrew, C.; Soldan, A. Defining cognitive reserve and implications for cognitive aging. Curr. Neurol. Neurosci. Rep. 2019, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Krivanek, T.J.; Gale, S.A.; McFeeley, B.M.; Nicastri, C.M.; Daffner, K.R. Promoting Successful Cognitive Aging: A Ten-Year Update. J. Alzheimer′s Dis. 2021, 81, 871–920. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P.S.; Valenzuela, M. Brain and cognitive reserve. Am. J. Geriatr. Psychiatry 2009, 17, 175–178. [Google Scholar] [CrossRef] [Green Version]
- Moga, D.C.; Beech, B.F.; Abner, E.L.; Schmitt, F.A.; El Khouli, R.H.; Martinez, A.I.; Eckmann, L.; Huffmyer, M.; George, R.; Jicha, G.A. INtervention for Cognitive Reserve Enhancement in delaying the onset of Alzheimer’s Symptomatic Expression (INCREASE), a randomized controlled trial: Rationale, study design, and protocol. Trials 2019, 20, 806. [Google Scholar] [CrossRef] [PubMed]
- Pratico, D. Oxidative stress hypothesis in Alzheimer’s disease: A reappraisal. Trends Pharmacol. Sci. 2008, 29, 609–615. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxidative Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buccellato, F.R.; D’Anca, M.; Fenoglio, C.; Scarpini, E.; Galimberti, D. Role of oxidative damage in alzheimer’s disease and neurodegeneration: From pathogenic mechanisms to biomarker discovery. Antioxidants 2021, 10, 1353. [Google Scholar] [CrossRef]
- Beard, E.; Lengacher, S.; Dias, S.; Magistretti, P.; Finsterwald, C. Astrocytes as Key Regulators of Brain Energy Metabolism: New Therapeutic Perspectives. Front. Physiol. 2021, 12, 825816. [Google Scholar] [CrossRef]
- Manninen, T.; Saudargiene, A.; Linne, M.L. Astrocyte-mediated spiketiming-dependent long-term depression modulates synaptic properties in the developing cortex. PLoS Comput. Biol. 2020, 16, e1008360. [Google Scholar] [CrossRef]
- Takahashi, S. Neuroprotective Function of High Glycolytic Activity in Astrocytes: Common Roles in Stroke and Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 6568. [Google Scholar] [CrossRef]
- Takahashi, S. Metabolic compartmentalization between astroglia and neurons in physiological and pathophysiological conditions of the neurovascular unit. Neuropathology 2020, 40, 121–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, S. Lactate and ketone bodies act as energy substrates as well as signal molecules in the brain. In Psychology and Paho-Physiological Outcomes of Eating; Takada, A., Himmerich, H., Eds.; InTech Open: Rijeka, Croatia, 2021; pp. 1–20. [Google Scholar]
- Asanuma, M.; Miyazaki, I. Glutathione and Related Molecules in Parkinsonism. Int. J. Mol. Sci. 2021, 22, 8689. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Park, H.Y.; Ali, T.; Kim, M.O. Melatonin as a Potential Regulator of Oxidative Stress, and Neuroinflammation: Mechanisms and Implications for the Management of Brain Injury-Induced Neurodegeneration. J. Inflamm. Res. 2021, 14, 6251–6264. [Google Scholar] [CrossRef] [PubMed]
- Anghel, L.; Baroiu, L.; Popazu, C.R.; Pătraș, D.; Fotea, S.; Nechifor, A.; Ciubara, A.; Nechita, L.; Mușat, C.L.; Stefanopol, I.A.; et al. Benefits and adverse events of melatonin use in the elderly. Exp. Ther. Med. 2022, 23, 219. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a naturalally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, Y. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Aly, H.F.; Rizk, M.Z. Melatonin and Its Indisputable Effects on the Health State. In Melatonin—Molecular Biology, Clinical and Pharmaceutical Approaches, 5th ed.; Drăgoi, C.M., Nicolae, A.C., Eds.; InTech Open: London, UK, 2018; pp. 87–103. [Google Scholar]
- Liu, F.; Zhang, X.; Zhao, B.; Tan, X.; Wang, L.; Liu, X. Role of Food Phytochemicals in the Modulation of Circadian Clocks. J. Agric. Food Chem. 2019, 67, 8735–8739. [Google Scholar] [CrossRef]
- Khapre, R.V.; Kondratova, A.A.; Susova, O.; Kondratov, R.V. Circadian clock protein BMAL1 regulates cellular senescence in vivo. Cell Cycle 2011, 10, 4162–4169. [Google Scholar] [CrossRef] [Green Version]
- Yasuno, F.; Tanimukai, S.; Sasaki, M.; Ikejima, C.; Yamashita, F.; Kodama, C.; Mizukami, K.; Asada, T. Combination of antioxidant supplements improved cognitive function in the elderly. J. Alzheimer′s Dis. 2012, 32, 895–903. [Google Scholar] [CrossRef]
- Suh, S.W.; Kim, H.S.; Han, J.H.; Bae, J.B.; Oh, D.J.; Han, J.W.; Kim, K.W. Efficacy of vitamins on cognitive function of non-demented people: A systematic review and meta-analysis. Nutrients 2020, 12, 1168. [Google Scholar] [CrossRef] [Green Version]
- Grodstein, F.; Kang, J.H.; Glynn, R.J.; Cook, N.R.; Gaziano, J.M. A randomized trial of beta carotene supplementation and cognitive function in men: The Physicians’ Health Study II. Arch. Intern. Med. 2007, 167, 2184–2190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.H.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Albert, C.M.; Grodstein, F. Vitamin E, vitamin C, beta carotene, and cognitive function among women with or at risk of cardiovascular disease: The Women’s Antioxidant and Cardiovascular Study. Circulation 2009, 119, 2772–2780. [Google Scholar] [CrossRef] [PubMed]
- Guyonnet, S.G.; Van Kan, G.A.; Andrieu, S.; Gateau, P.B.; Berr, C.; Bonnefoy, M.; Dartigues, J.F.; de Groot, L.; Ferry, M.; Galan, P.; et al. IANA task force on nutrition and cognitive decline with aging. J. Nutr. Health Aging 2007, 11, 132. [Google Scholar]
- Dysken, M.W.; Sano, M.; Asthana, S.; Vertrees, J.E.; Pallaki, M.; Llorente, M.; Love, S.; Schellenberg, G.D.; McCarten, J.R.; Malphurs, J.; et al. Effect of vitamin E and memantine on functional decline in Alzheimer disease: The TEAM-AD VA cooperative randomized trial. JAMA 2014, 311, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Thomas, R.G.; Grundman, M.; Bennett, D.; Doody, R.; Ferris, S.; Galasko, D.; Jin, S.; Kaye, J.; Levey, A.; et al. Alzheimer’s Disease Cooperative Study Group; Vitamin E and donepezil for the treatment of mild cognitive impairment. N. Engl. J. Med. 2005, 352, 2379–2388. [Google Scholar] [CrossRef] [Green Version]
- Ito, N.; Saito, H.; Seki, S.; Ueda, F.; Asada, T. Effects of Composite Supplement Containing Astaxanthin and Sesamin on Cognitive Functions in People with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Alzheimer′s Dis. 2018, 62, 1767–1775. [Google Scholar] [CrossRef] [Green Version]
- Mori, J.; Yokoyama, H.; Sawada, T.; Miyashita, Y.; Nagata, K. Anti-oxidative properties of astaxanthin and related compounds. Mol. Cryst. Liq. Cryst. 2013, 580, 52–57. [Google Scholar] [CrossRef]
- Guo, H.; Tian, J.; Wang, X.; Tian, Z.; Li, X.; Yang, L.; Zhao, M.; Liu, S. Neuroprotection of sesamin against cerebral ischemia in-vivo and N-Methyl-D-Aspartate-induced apoptosis in-vitro. Biochem. Pharmacol. 2015, 4, 185. [Google Scholar]
- Liu, X.; Yamashita, T.; Shang, J.; Shi, X.; Morihara, R.; Huang, Y.; Sato, K.; Takemoto, M.; Hishinkawa, N.; Ohta, N.; et al. Clinical and Pathological Benefit of Twendee X in Alzheimer’s Disease Transgenic Mice with Chronic Cerebral Hypoperfusion. J. Stroke Cerebrovasc. Dis. 2019, 28, 1993–2002. [Google Scholar] [CrossRef]
- Tadokoro, K.; Morihara, R.; Ohta, Y.; Hishikawa, N.; Kawano, S.; Sasaki, R.; Abe, K. Clinical benefits of antioxidative supplement twendee X for mild cognitive impairment: A multicenter, randomized, double-blind, and placebo-controlled prospective interventional study. J. Alzheimer′s Dis. 2019, 71, 1063–1069. [Google Scholar] [CrossRef]
- Cammisuli, D.M.; Innocenti, A.; Franzoni, F.; Pruneti, C. Aerobic exercise effects upon cognition in Mild Cognitive Impairment: A systematic review of randomized controlled trials. Arch. Ital. Biol. 2017, 155, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Fang, R.; Li, B.Y.; Chen, S.D. Exercise related changes of networks in aging and mild cognitive impairment brain. Front. Aging Neurosci. 2017, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.N.; Deuster, P.A. Biological Mechanisms Underlying the Role of Physical Fitness in Health and Resilience. Interface Focus 2014, 4, 20140040. [Google Scholar] [CrossRef] [Green Version]
- Radak, Z.; Ihasz, F.; Koltai, E.; Goto, S.; Taylor, A.W.; Boldogh, I. The Redox-Associated Adaptive Response of Brain to Physical Exercise. Free Radic. Res. 2014, 48, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Onanong, M.-I.; Zhao, Z.-W.; Kuo, Y.M. Physical Exercise Inhibits Inflammation and Microglial Activation. Cells 2019, 8, E691. [Google Scholar]
- Um, H.S.; Eun, B.K.; Yea, H.L.; In, H.C.; Chun, H.Y.; Kab, R.C.; Dae, Y.H.; Youn, J.Y.C. Exercise Training Acts as a Therapeutic Strategy for Reduction of the Pathogenic Phenotypes for Alzheimer’s Disease in an NSE/APPsw-Transgenic Model. Int. J. Mol. Med. 2008, 22, 529–539. [Google Scholar] [PubMed]
- Aderbal Silva, A.; Duzzioni, M.; Remor, A.P.; Massafera Tristão, F.S.; Matheus, F.C.; Raisman-Vozari, R.; Latini, A.; Prediger, R.D. Moderate-Intensity Physical Exercise Protects Against Experimental 6-Hydroxydopamine-Induced Hemiparkinsonism Through Nrf2-Antioxidant Response Element Pathway. Neurochem. Res. 2016, 41, 64–72. [Google Scholar]
- Pinho, R.A.; Aguiar, A.S.; Radák, Z. Effects of Resistance Exercise on Cerebral Redox Regulation and Cognition: An Interplay Between Muscle and Brain. Antioxidants 2019, 8, E529. [Google Scholar] [CrossRef] [Green Version]
- Tetsuro, I.; Mann, G.E. When and How Does Brain-Derived Neurotrophic Factor Activate Nrf2 in Astrocytes and Neurons? Neural. Regen. Res. 2018, 13, 803–804. [Google Scholar]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 Regulatory Network Provides an Interface between Redox and Intermediary Metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef]
- Mats, S.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-Regulation in Brain Health and Disease: Implication of Cerebral Inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar]
- Sperling, R. The potential of functional MRI as a biomarker in early Alzheimer’s disease. Neurobiol. Aging 2011, 32, S37–S43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benton, D. Neurodevelopment and neurodegeneration: Are there critical stages for nutritional intervention? Nutr. Rev. 2010, 68 (Suppl. 1), S6–S10. [Google Scholar] [CrossRef]
- Middleton, L.E.; Yaffe, K. Promising strategies for the prevention of dementia. Arch. Neurol. 2009, 66, 1210–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Song, R.; Qi, X.; Xu, H.; Yang, W.; Kivipelto, M.; Bennet, D.; Xu, W. Influence of Cognitive Reserve on Cognitive Trajectories: Role of Brain Pathologies. Neurology 2021, 97, e1695–e1706. [Google Scholar] [CrossRef]
- Song, S.; Stern, Y.; Gu, Y. Modifiable lifestyle factors and cognitive reserve: A systemic review of current evidence. Ageing Res. Rev. 2021, 74, 101551. [Google Scholar] [CrossRef]
- Foubert-Samier, A.; Catheline, G.; Amieva, H.; Dilharreguy, B.; Helmer, C.; Allard, M.; Dartigues, J.F. Education, occupation, leisure activities, and brain reserve: A population-based study. Neurobiol. Aging 2012, 33. [Google Scholar] [CrossRef]
- Kivimäki, M.; Walker, K.A.; Pentti, J.; Nyberg, S.T.; Mars, N.; Vahtera, J.; Suominen, S.B.; Lallukka, T.; Rahkonen, O.; Pietiläinen, O.; et al. Cognitive stimulation in the workplace, plasma proteins, and risk of dementia: Three analyses of population cohort studies. BMJ 2021, 374, n1804. [Google Scholar] [CrossRef]
- Verghese, J.; Lipton, R.B.; Katz, M.J.; Hall, C.B.; Derby, C.A.; Kuslansky, G.; Ambrose, A.F.; Sliwinski, M.; Buschke, H. Leisure activities and the risk of dementia in the elderly. N. Engl. J. Med. 2003, 348, 2508–2516. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.; Bajgai, J.; Fadriquela, A.; Sharma, S.; Trinh, T.T.; Akter, R.; Jeong, Y.J.; Goh, S.H.; Lee, K.J. Therapeutic Potential of Natural Products in Treating Neurodegenerative Disorders and Their Future Prospects and Challenges. Molecules 2021, 26, 5327. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cammisuli, D.M.; Franzoni, F.; Scarfò, G.; Fusi, J.; Gesi, M.; Bonuccelli, U.; Daniele, S.; Martini, C.; Castelnuovo, G. What Does the Brain Have to Keep Working at Its Best? Resilience Mechanisms Such as Antioxidants and Brain/Cognitive Reserve for Counteracting Alzheimer’s Disease Degeneration. Biology 2022, 11, 650. https://doi.org/10.3390/biology11050650
Cammisuli DM, Franzoni F, Scarfò G, Fusi J, Gesi M, Bonuccelli U, Daniele S, Martini C, Castelnuovo G. What Does the Brain Have to Keep Working at Its Best? Resilience Mechanisms Such as Antioxidants and Brain/Cognitive Reserve for Counteracting Alzheimer’s Disease Degeneration. Biology. 2022; 11(5):650. https://doi.org/10.3390/biology11050650
Chicago/Turabian StyleCammisuli, Davide Maria, Ferdinando Franzoni, Giorgia Scarfò, Jonathan Fusi, Marco Gesi, Ubaldo Bonuccelli, Simona Daniele, Claudia Martini, and Gianluca Castelnuovo. 2022. "What Does the Brain Have to Keep Working at Its Best? Resilience Mechanisms Such as Antioxidants and Brain/Cognitive Reserve for Counteracting Alzheimer’s Disease Degeneration" Biology 11, no. 5: 650. https://doi.org/10.3390/biology11050650
APA StyleCammisuli, D. M., Franzoni, F., Scarfò, G., Fusi, J., Gesi, M., Bonuccelli, U., Daniele, S., Martini, C., & Castelnuovo, G. (2022). What Does the Brain Have to Keep Working at Its Best? Resilience Mechanisms Such as Antioxidants and Brain/Cognitive Reserve for Counteracting Alzheimer’s Disease Degeneration. Biology, 11(5), 650. https://doi.org/10.3390/biology11050650













