Comparative Testing of Two Ligature-Induced Periodontitis Models in Rats: A Clinical, Histological and Biochemical Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Blood Sampling
2.3. Anesthesia Protocol
2.4. Ligature-Induced Periodontitis Protocol
2.5. Analytical Methods
2.5.1. Clinical Investigation
2.5.2. Laboratory Investigation
2.6. Histological Investigation
2.7. Statistical Investigation
3. Results
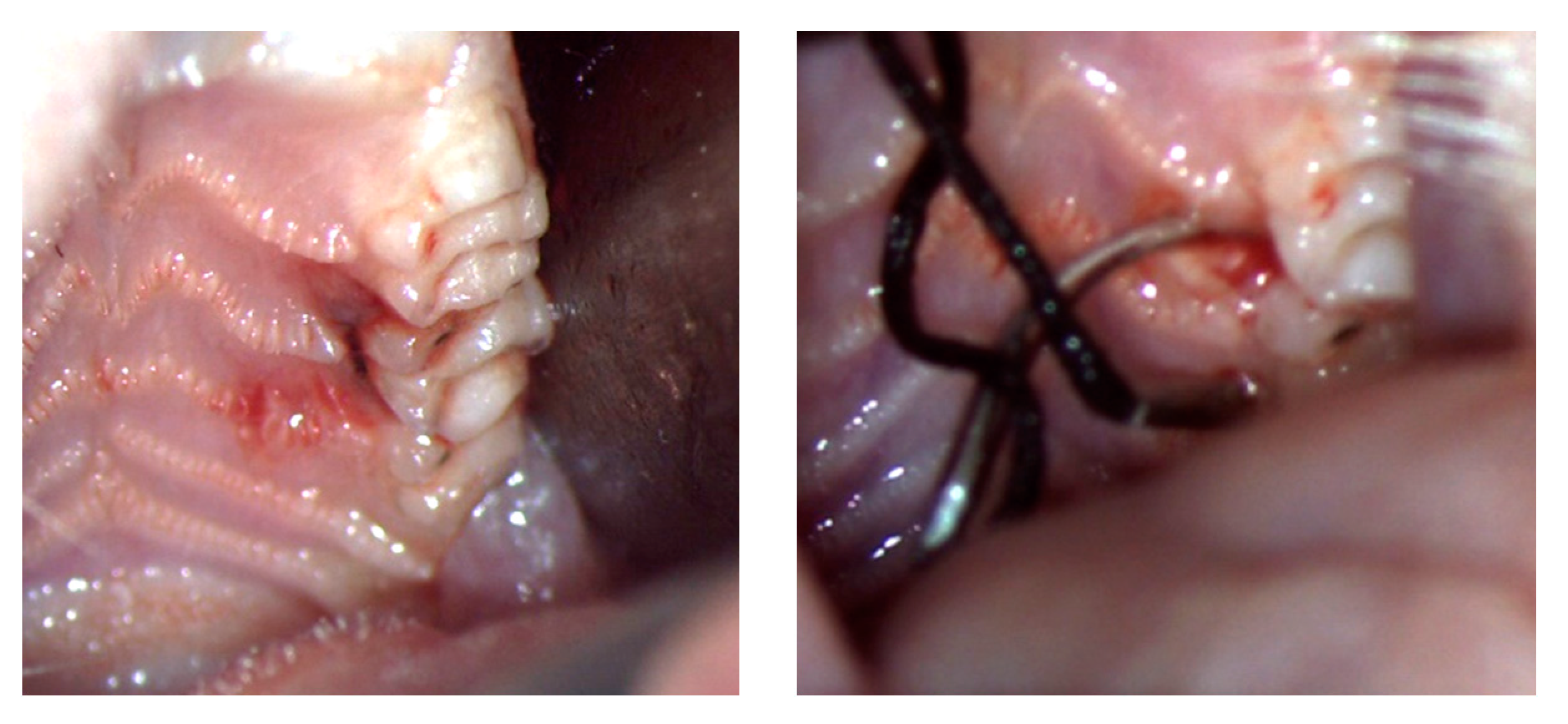
3.1. Clinical Oral Characteristics
3.2. Biochemical Characteristics
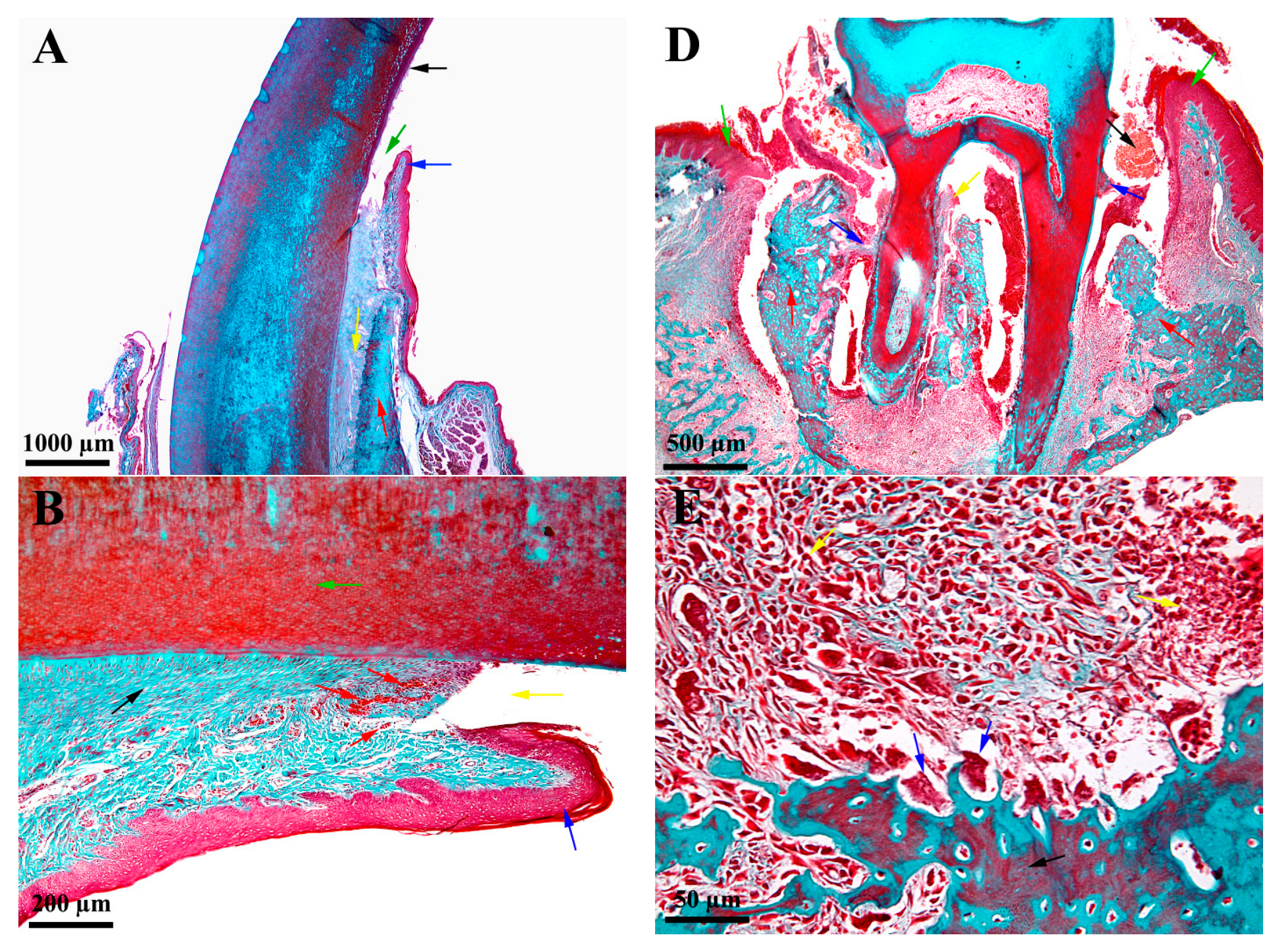
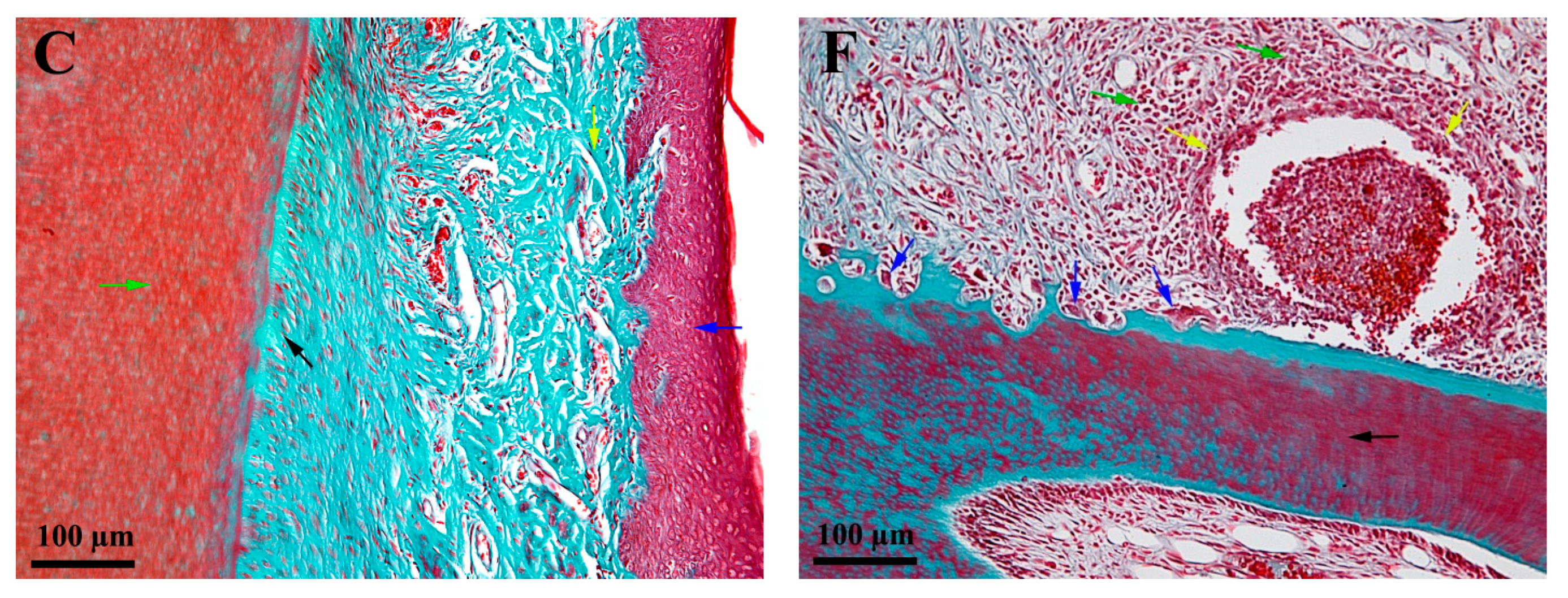
3.3. Histological Characteristics—Descriptive Histology
4. Discussion
4.1. Clinical Oral Aspects
4.2. Biochemical Aspects
4.3. Histological Aspectss—Descriptive Histology
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferreira, M.C.; Dias-Pereira, A.C.; Branco-de-Almeida, L.S.; Martins, C.C.; Paiva, S.M. Impact of Periodontal Disease on Quality of Life: A Systematic Review. J. Periodontal Res. 2017, 52, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.A.; Martins, T.M.; de Almeida, J.M.; Theodoro, L.H.; Garcia, V.G. Radiographic Assessment of Photodynamic Therapy as an Adjunctive Treatment on Induced Periodontitis in Immunosuppressed Rats. J. Appl. Oral Sci. Rev. FOB 2010, 18, 237–243. [Google Scholar] [CrossRef] [PubMed]
- De Souza, D.M.; Ricardo, L.H.; de Prado, M.A.; de Prado, F.A.; da Rocha, R.F. The Effect of Alcohol Consumption on Periodontal Bone Support in Experimental Periodontitis in Rats. J. Appl. Oral Sci. Rev. FOB 2006, 14, 443–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petruţiu, Ş.A.; Stratul, S.-I.; Soancă, A.; Roman, A.; Băciuţ, M.; Kasaj, A.; Bocşan, I.S. The Impact of Some Behavioral Aspects on Periodontal Disease in a Group of Romanian Students—An Epidemiological Survey. Rev. Epidemiol. Sante Publique 2014, 62, 367–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionel, A.; Lucaciu, O.; Tăbăran, F.; Berce, C.; Toader, S.; Hurubeanu, L.; Bondor, C.; Câmpian, R.S. Histopathological and Clinical Expression of Periodontal Disease Related to the Systemic Inflammatory Response. Histol. Histopathol. 2017, 32, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Meusel, D.R.D.Z.; Ramacciato, J.C.; Motta, R.H.L.; Brito Júnior, R.B.; Flório, F.M. Impact of the Severity of Chronic Periodontal Disease on Quality of Life. J. Oral Sci. 2015, 57, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Struillou, X.; Boutigny, H.; Soueidan, A.; Layrolle, P. Experimental Animal Models in Periodontology: A Review. Open Dent. J. 2010, 4, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Tomina, D.; Roman, A.; Condor, D.; Dinu, C.; Petruțiu, S.A. Experimental Rat Model—Is It Still Used?—Review Article. HVM Bioflux 2017, 9, 130–136. [Google Scholar]
- Rovin, S.; Costich, E.R.; Gordon, H.A. The Influence of Bacteria and Irritation in the Initiation of Periodontal Disease in Germfree and Conventional Rats. J. Periodontal Res. 1966, 1, 193–204. [Google Scholar] [CrossRef]
- Sallay, K.; Sanavi, F.; Ring, I.; Pham, P.; Behling, U.H.; Nowotny, A. Alveolar Bone Destruction in the Immunosuppressed Rat. J. Periodontal Res. 1982, 17, 263–274. [Google Scholar] [CrossRef]
- Azeez, S.H.; Gaphor, S.M.; Sha, A.M.; Garib, B.T. Effect of Pistacia Atlantica Subsp. Kurdica Gum in Experimental Periodontitis Induced in Wistar Rats by Utilization of Osteoclastogenic Bone Markers. Molecules 2020, 25, 5819. [Google Scholar] [CrossRef]
- Giménez-Siurana, A.; Gómez García, F.; Pagan Bernabeu, A.; Lozano-Pérez, A.A.; Aznar-Cervantes, S.D.; Cenis, J.L.; López-Jornet, P. Chemoprevention of Experimental Periodontitis in Diabetic Rats with Silk Fibroin Nanoparticles Loaded with Resveratrol. Antioxidants 2020, 9, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionel, A.; Lucaciu, O.; Moga, M.; Buhatel, D.; Ilea, A.; Catoi, C.; Berce, C.; Toader, S.; Campian, R.S. Periodontal Disease Induced in Wistar Rats—Experimental Study. Int. J. Bioflux Soc. 2015, 7, 6. [Google Scholar]
- Oz, H.S.; Puleo, D.A. Animal Models for Periodontal Disease. J. Biomed. Biotechnol. 2011, 2011, 754857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graves, D.T.; Kang, J.; Andriankaja, O.; Wada, K.; Rossa, C. Animal Models to Study Host-Bacteria Interactions Involved in Periodontitis. Front. Oral Biol. 2012, 15, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Mester, A.; Ciobanu, L.; Taulescu, M.; Apostu, D.; Lucaciu, O.; Filip, G.A.; Feldrihan, V.; Licarete, E.; Ilea, A.; Piciu, A.; et al. Periodontal Disease May Induce Liver Fibrosis in an Experimental Study on Wistar Rats. J. Periodontol. 2019, 90, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.V.; Albassam, M.A. Susceptibility of Rats to Corneal Lesions after Injectable Anesthesia. Comp. Med. 2005, 55, 175–182. [Google Scholar] [PubMed]
- Xu, Y.; Wei, W. A Comparative Study of Systemic Subantimicrobial and Topical Treatment of Minocycline in Experimental Periodontitis of Rats. Arch. Oral Biol. 2006, 51, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, N.; Liu, N.; Zhou, X.; Dong, Z.; Wen, X.; Liu, L. Effects of Systemic Ornidazole, Systemic and Local Compound Ornidazole and Pefloxacin Mesylate on Experimental Periodontitis in Rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2012, 18, BR95–BR102. [Google Scholar] [CrossRef] [Green Version]
- Xie, R.; Kuijpers-Jagtman, A.M.; Maltha, J.C. Inflammatory Responses in Two Commonly Used Rat Models for Experimental Tooth Movement: Comparison with Ligature-Induced Periodontitis. Arch. Oral Biol. 2011, 56, 159–167. [Google Scholar] [CrossRef]
- Luan, Q.; Desta, T.; Chehab, L.; Sanders, V.J.; Plattner, J.; Graves, D.T. Inhibition of Experimental Periodontitis by a Topical Boron-Based Antimicrobial. J. Dent. Res. 2008, 87, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Fine, D.; Teng, Y.-T.A.; Van Dyke, T.E.; Hajishengallis, G. The Use of Rodent Models to Investigate Host-Bacteria Interactions Related to Periodontal Diseases. J. Clin. Periodontol. 2008, 35, 89–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, M.-J.; Kim, K.; Lee, H.; Cha, S.; Seo, T.; Park, H.-J.; Lee, J.-S.; Kim, T.-I. Development of Animal Experimental Periodontitis Models. J. Periodontal Implant Sci. 2013, 43, 147–152. [Google Scholar] [CrossRef]
- Newman and Carranza’s Clinical Periodontology—13th Edition. Available online: https://www.elsevier.com/books/newman-and-carranzas-clinical-periodontology/newman/978-0-323-52300-4 (accessed on 3 March 2022).
- Lindhe, J.; Lang, N.P.; Karring, T. Clinical Periodontology and Implant Dentistry; John Wiley and Sons Ltd.: Chicester, UK, 2009; ISBN 978-1-4443-1304-8. [Google Scholar]
- Behmanesh, M. Expression of Suppressor of Cytokine Signaling 1 and 3 in Ligature-Induced Periodontitis in Rats. Arch. Oral Biol. 2011, 56, 1120–1128. [Google Scholar]
- Jacob, S.P.; Nath, S. Rat Gingival Model for Testing Drugs Influencing Inflammation. IeJSME 2013, 7, 8–16. [Google Scholar]
- Lau, D.C.W.; Dhillon, B.; Yan, H.; Szmitko, P.E.; Verma, S. Adipokines: Molecular Links between Obesity and Atheroslcerosis. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2031–H2041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pepys, M.B.; Hirschfield, G.M. C-Reactive Protein: A Critical Update. J. Clin. Investig. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Nicklin, M.J.; Weith, A.; Duff, G.W. A Physical Map of the Region Encompassing the Human Interleukin-1 Alpha, Interleukin-1 Beta, and Interleukin-1 Receptor Antagonist Genes. Genomics 1994, 19, 382–384. [Google Scholar] [CrossRef]
- March, C.J.; Mosley, B.; Larsen, A.; Cerretti, D.P.; Braedt, G.; Price, V.; Gillis, S.; Henney, C.S.; Kronheim, S.R.; Grabstein, K. Cloning, Sequence and Expression of Two Distinct Human Interleukin-1 Complementary DNAs. Nature 1985, 315, 641–647. [Google Scholar] [CrossRef]
- Bankers-Fulbright, J.L.; Kalli, K.R.; McKean, D.J. Interleukin-1 Signal Transduction. Life Sci. 1996, 59, 61–83. [Google Scholar] [CrossRef]
- Dinarello, C.A. Induction of Interleukin-1 and Interleukin-1 Receptor Antagonist. Semin. Oncol. 1997, 24, S9-81–S9-93. [Google Scholar] [PubMed]
- Locksley, R.M.; Killeen, N.; Lenardo, M.J. The TNF and TNF Receptor Superfamilies: Integrating Mammalian Biology. Cell 2001, 104, 487–501. [Google Scholar] [CrossRef] [Green Version]
- Bezerra, M.M.; de Lima, V.; Alencar, V.B.; Vieira, I.B.; Brito, G.A.; Ribeiro, R.A.; Rocha, F.A. Selective Cyclooxygenase-2 Inhibition Prevents Alveolar Bone Loss in Experimental Periodontitis in Rats. J. Periodontol. 2000, 71, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Samejima, Y.; Ebisu, S.; Okada, H. Effect of Infection with Eikenella Corrodens on the Progression of Ligature-Induced Periodontitis in Rats. J. Periodontal Res. 1990, 25, 308–315. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, X.; Zhang, J.; Xia, X.; Li, H.; Qiu, C.; Liao, Y.; Chen, H.; He, Z.; Song, Z.; et al. Activated STAT3 Signaling Pathway by Ligature-Induced Periodontitis Could Contribute to Neuroinflammation and Cognitive Impairment in Rats. J. Neuroinflamm. 2021, 18, 80. [Google Scholar] [CrossRef]
- Seymour, G.J.; Ford, P.J.; Cullinan, M.P.; Leishman, S.; Yamazaki, K. Relationship between Periodontal Infections and Systemic Disease. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2007, 13 (Suppl. S4), 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freire, M.O.; Van Dyke, T.E. Natural Resolution of Inflammation. Periodontology 2000 2013, 63, 149–164. [Google Scholar] [CrossRef] [Green Version]

| Group | Variables | Values | p-Value | |
|---|---|---|---|---|
| Initial | Final | |||
| TEST 1 | Mobility | 1 | 1.9 ± 0.32 | 0.0440 × 10−6 |
| Inflammation | 0 | 2.9 ± 0.74 | 0.0286 × 10−8 | |
| Weight (grams) | 223.4 ± 22.98 | 231.6 ± 16.93 | 0.3756 | |
| TEST 2 | Mobility | 0.00 ± 0.032 | 3.2 ± 0.42 | 0.034 × 10−11 |
| Inflammation | 0 | 4.80 ± 0.42 | 0.0317 × 10−16 | |
| Weight (grams) | 217 ± 29.47 | 211.90 ± 24.06 | 0.5947 | |
| CONTROL | Mobility | 0 | 0 | - |
| Inflammation | 0 | 0 | - | |
| Weight (grams) | 236.63 ± 23.14 | 253.88 ± 22.36 | 0.17 | |
| Group | Variables | Values | p-Value | |
|---|---|---|---|---|
| Initial | Final | |||
| CONTROL | hsCRP (pg/mL) | 46.7 ± 7.1 | 47.9 ± 4.58 | 0.65 |
| TNF-α (pg/mL) | 42.4 ± 6.53 | 43.3 ± 6.39 | 0.75 | |
| IL1-α (pg/mL) | 47.5 ± 8.61 | 50.1 ± 3.81 | 0.39 | |
| TEST 1 | hsCRP(pg/mL) | 46.4 ± 10.81 | 86.8 ± 9.97 | 0.0748 × 10−6 |
| TNF-α (pg/mL) | 43.6 ± 8.93 | 64.5 ± 8.19 | 0.035 × 10−3 | |
| IL1-α (pg/mL) | 36 ± 15.74 | 51 ± 24.51 | 0.12 | |
| TEST 2 | hsCRP (pg/mL) | 52.1 ± 9.55 | 117.2 ± 15.73 | 0.015 × 10−7 |
| TNF-α (pg/mL) | 54.5 ± 11.38 | 106.5 ± 16.72 | 0.019 × 10−5 | |
| IL1-α (pg/mL) | 38 ± 13.88 | 65.8 ± 14.34 | 0.03 × 10−2 | |
| Parameter | TEST 1 | TEST 2 | p-Value |
|---|---|---|---|
| hsCRP (pg/mL) | 86.8 ± 9.97 | 117.2 ± 15.73 | 0.065 × 10−3 |
| TNF—α (pg/mL) | 64.5 ± 8.19 | 106.5 ± 16.72 | 0.012 × 10−4 |
| IL1-α (pg/mL) | 51 ± 24.51 | 65.8 ± 14.34 | 0.05 |
| Groups | Variables | Values | p-Value | |
|---|---|---|---|---|
| Initial | Final | |||
| CONTROL | Leukocytes [103/µL] | 10.12 ± 1.85 | 11.37 ± 1.34 | 0.098 |
| Neutrophils (%) | 16.4 ± 0.96 | 16.01 ± 0.94 | 0.37 | |
| Eosinophils (%) | 1.62 ± 0.78 | 1.61 ± 0.62 | 0.97 | |
| Lymphocytes (%) | 75.23 ± 0.7 | 75.24 ± 2.35 | 0.98 | |
| Monocytes (%) | 6.49 ± 0.86 | 6.16 ± 0.98 | 0.43 | |
| Platelets [103/µL] | 1137.1 ± 109.47 | 1162.6 ± 225.77 | 0.75 | |
| TEST 1 | Leukocytes [103/µL] | 9.10 ± 3.02 | 13.63 ± 3.5 | 0.006 |
| Neutrophils (%) | 18.03 ± 7.27 | 22.62 ± 9.2 | 0.23 | |
| Eosinophils (%) | 1.78 ± 0.83 | 1.15 ± 0.3 | 0.03 | |
| Lymphocytes (%) | 70.8 ± 7.69 | 76.74 ± 7.92 | 0.1 | |
| Monocytes (%) | 9.79 ± 1.94 | 10.88 ± 1.78 | 0.2 | |
| Platelets [103/µL] | 1141.8 ± 88.73 | 1255.4 ± 97.65 | 0.01 | |
| TEST 2 | Leukocytes [103/µL] | 10.27 ± 2.14 | 13.14 ± 1.12 | 0.001 |
| Neutrophils (%) | 19.33 ± 3.31 | 22.13 ± 3.49 | 0.04 | |
| Eosinophils (%) | 2.01 ± 1.2 | 1.4 ± 0.72 | 0.09 | |
| Lymphocytes (%) | 70.02 ± 3.71 | 74.91 ± 4.76 | 0.01 | |
| Monocytes (%) | 8.05 ± 1.95 | 8.38 ± 2.07 | 0.71 | |
| Platelets [103/µL] | 1009.00 ± 182.71 | 1227.70 ± 167.35 | 0.01 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomina, D.C.; Petruțiu, Ș.A.; Dinu, C.M.; Crișan, B.; Cighi, V.S.; Rațiu, I.A. Comparative Testing of Two Ligature-Induced Periodontitis Models in Rats: A Clinical, Histological and Biochemical Study. Biology 2022, 11, 634. https://doi.org/10.3390/biology11050634
Tomina DC, Petruțiu ȘA, Dinu CM, Crișan B, Cighi VS, Rațiu IA. Comparative Testing of Two Ligature-Induced Periodontitis Models in Rats: A Clinical, Histological and Biochemical Study. Biology. 2022; 11(5):634. https://doi.org/10.3390/biology11050634
Chicago/Turabian StyleTomina, Darius C., Ștefan A. Petruțiu, Cristian M. Dinu, Bogdan Crișan, Vasile S. Cighi, and Ioana A. Rațiu. 2022. "Comparative Testing of Two Ligature-Induced Periodontitis Models in Rats: A Clinical, Histological and Biochemical Study" Biology 11, no. 5: 634. https://doi.org/10.3390/biology11050634
APA StyleTomina, D. C., Petruțiu, Ș. A., Dinu, C. M., Crișan, B., Cighi, V. S., & Rațiu, I. A. (2022). Comparative Testing of Two Ligature-Induced Periodontitis Models in Rats: A Clinical, Histological and Biochemical Study. Biology, 11(5), 634. https://doi.org/10.3390/biology11050634






