Biological Effects of Transforming Growth Factor Beta in Human Cholangiocytes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Cell Culture and Treatment
2.3. Cell Counting Assay
2.4. Cytotoxicity Assay
2.5. Cell Cycle Analysis by Flow Cytometry
2.6. Wound-Healing Assay
2.7. Sample Preparation for Mass Spectrometry-Based Proteomics
2.8. MS Acquisitions: IDA and SWATH-MS
2.9. Data Analysis
3. Results
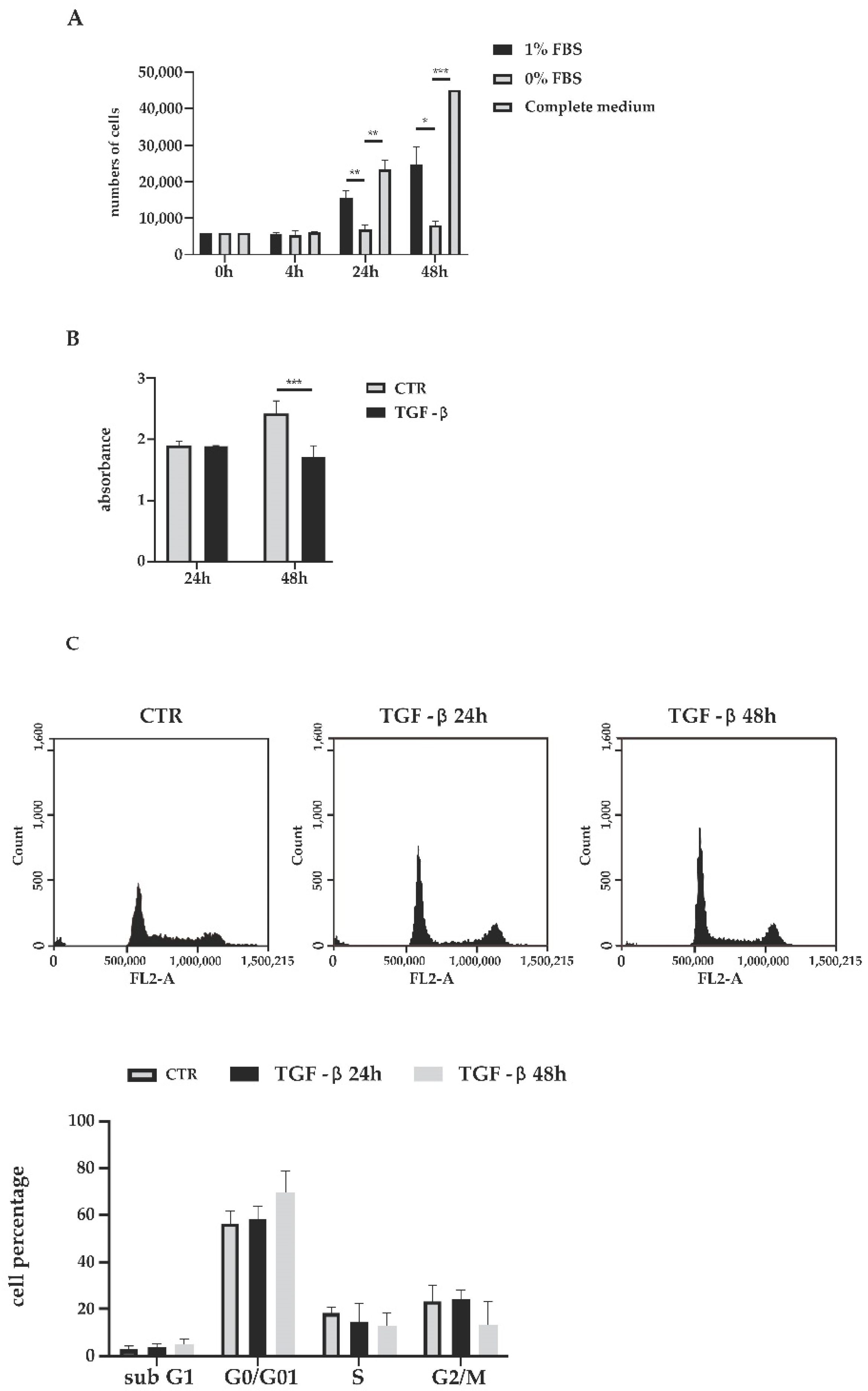
3.1. Biological Effects of TGF-β Treatment
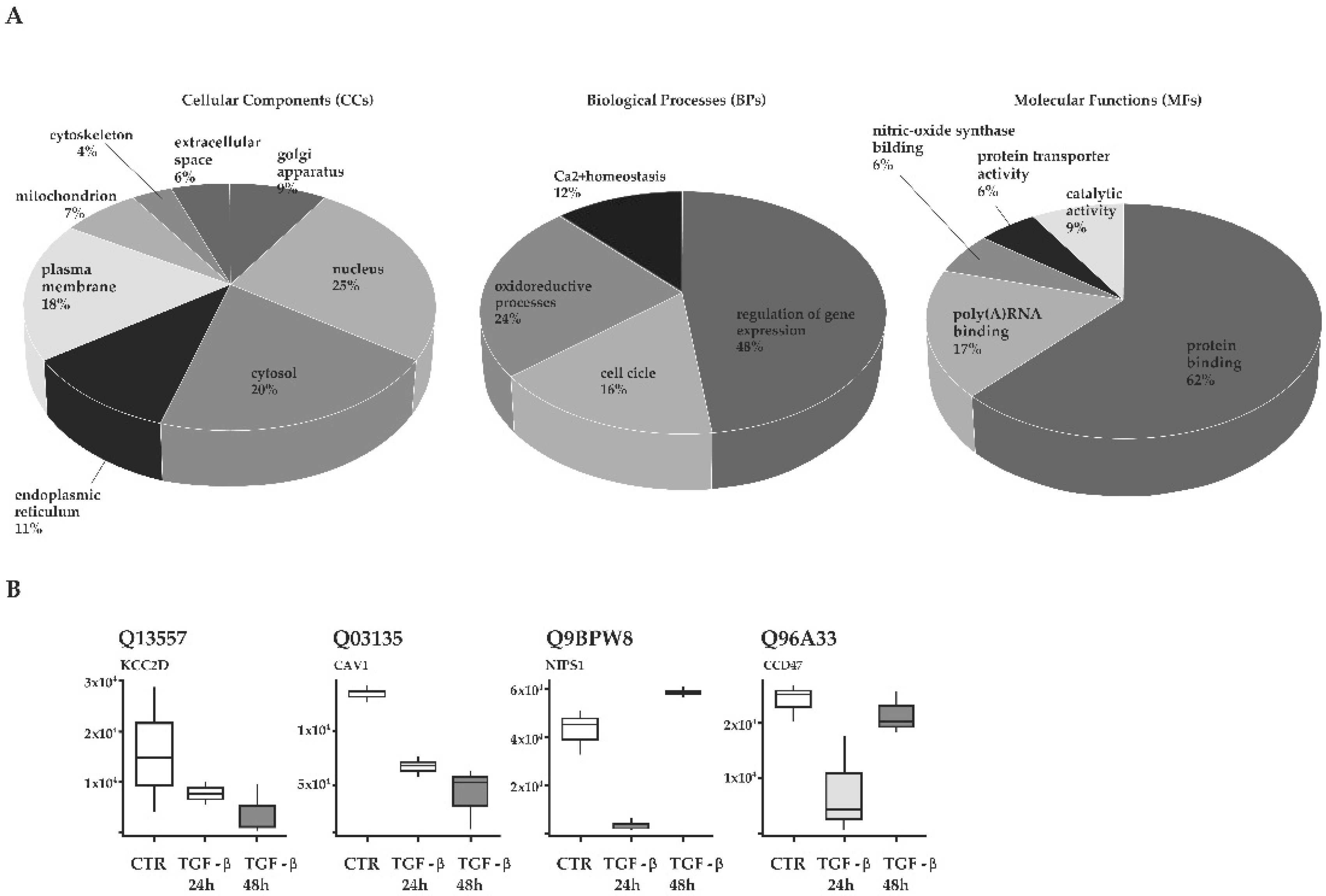
3.2. Hypothesis Free Proteomics of TGF-β-Treated Cholangiocytes
3.3. Functional Annotation of Proteins Enriched in TGF-β-Treated Cholangiocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Alexander, P.B.; Wang, X.F. TGF-β Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harbor Perspect. Biol. 2017, 9, a022145. [Google Scholar] [CrossRef]
- Zhu, Q.; Pearson-White, S.; Luo, K. Requirement for the SnoN Oncoprotein in Transforming Growth Factor β-Induced Oncogenic Transformation of Fibroblast Cells. Mol. Cell. Biol. 2005, 25, 10731–10744. [Google Scholar] [CrossRef][Green Version]
- Bulut, K.; Meier, J.J.; Ansorge, N.; Felderbauer, P.; Schmitz, F.; Hoffmann, P.; Schmidt, W.E.; Gallwitz, B. Glucagon-like Peptide 2 Improves Intestinal Wound Healing through Induction of Epithelial Cell Migration in Vitro—Evidence for a TGF-β-Mediated Effect. Regul. Pept. 2004, 121, 137–143. [Google Scholar] [CrossRef]
- Giehl, K.; Imamichi, Y.; Menke, A. Smad4-Independent TGF-β Signaling in Tumor Cell Migration. Cells Tissues Organs 2007, 185, 123–130. [Google Scholar] [CrossRef]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; ten Dijke, P.; The IT-LIVER Consortium. TGF-β Signalling and Liver Disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef]
- Meng, X.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The Master Regulator of Fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Santos-Laso, A.; Munoz-Garrido, P.; Felipe-Agirre, M.; Bujanda, L.; Banales, J.M.; Perugorria, M.J. New Advances in the Molecular Mechanisms Driving Biliary Fibrosis and Emerging Molecular Targets. Curr. Drug Targets 2017, 18, 908–920. [Google Scholar] [CrossRef]
- Banales, J.M.; Huebert, R.C.; Karlsen, T.; Strazzabosco, M.; LaRusso, N.F.; Gores, G.J. Cholangiocyte Pathobiology. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 269–281. [Google Scholar] [CrossRef]
- Duwaerts, C.C.; Maher, J.J. Mechanisms of Liver Injury in Non-Alcoholic Steatohepatitis. Curr. Hepatol. Rep. 2014, 13, 119–129. [Google Scholar] [CrossRef]
- Fabregat, I.; Caballero-Díaz, D. Transforming Growth Factor-β-Induced Cell Plasticity in Liver Fibrosis and Hepatocarcinogenesis. Front. Oncol. 2018, 8, 357. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, M.; Luo, Z.; Wen, Z.; Yan, X. The Dichotomous Role of TGF-β in Controlling Liver Cancer Cell Survival and Proliferation. J. Genet. Genom. 2020, 47, 497–512. [Google Scholar] [CrossRef]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, N. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis—Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef]
- Tang, L.-Y.; Heller, M.; Meng, Z.; Yu, L.-R.; Tang, Y.; Zhou, M.; Zhang, Y.E. Transforming Growth Factor-β (TGF-β) Directly Activates the JAK1-STAT3 Axis to Induce Hepatic Fibrosis in Coordination with the SMAD Pathway. J. Biol. Chem. 2017, 292, 4302–4312. [Google Scholar] [CrossRef]
- Maroni, L.; Haibo, B.; Ray, D.; Zhou, T.; Wan, Y.; Meng, F.; Marzioni, M.; Alpini, G. Functional and Structural Features of Cholangiocytes in Health and Disease. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 368–380. [Google Scholar] [CrossRef]
- Glaser, S.S.; Gaudio, E.; Miller, T.; Alvaro, D.; Alpini, G. Cholangiocyte Proliferation and Liver Fibrosis. Expert Rev. Mol. Med. 2009, 11, e7. [Google Scholar] [CrossRef]
- Novo, E.; Valfrè di Bonzo, L.; Cannito, S.; Colombatto, S.; Parola, M. Hepatic Myofibroblasts: A Heterogeneous Population of Multifunctional Cells in Liver Fibrogenesis. Int. J. Biochem. Cell Biol. 2009, 41, 2089–2093. [Google Scholar] [CrossRef]
- Aseem, S.O.; Jalan-Sakrikar, N.; Chi, C.; Navarro-Corcuera, A.; De Assuncao, T.M.; Hamdan, F.H.; Chowdhury, S.; Banales, J.M.; Johnsen, S.A.; Shah, V.H.; et al. Epigenomic Evaluation of Cholangiocyte Transforming Growth Factor-β Signaling Identifies a Selective Role for Histone 3 Lysine 9 Acetylation in Biliary Fibrosis. Gastroenterology 2021, 160, 889–905. [Google Scholar] [CrossRef]
- Liu, B. Aberrant TGF-Β1 Signaling Contributes to the Development of Primary Biliary Cirrhosis in Murine Model. World J. Gastroenterol. 2013, 19, 5828. [Google Scholar] [CrossRef]
- Martinez, O.M.; Villanueva, J.C.; Gershwin, M.E.; Krams, S.M. Cytokine Patterns and Cytotoxic Mediators in Primary Biliary Cirrhosis. Hepatology 1995, 21, 113–119. [Google Scholar]
- Neuman, M.; Angulo, P.; Malkiewicz, I.; Jorgensen, R.; Shear, N.; Dickson, E.R.; Haber, J.; Katz, G.; Lindor, K. Tumor Necrosis Factor-α and Transforming Growth Factor-β Reflect Severity of Liver Damage in Primary Biliary Cirrhosis. J. Gastroenterol. Hepatol. 2002, 17, 196–202. [Google Scholar] [CrossRef]
- Lahn, M.; Herbertz, S.; Sawyer, J.S.; Stauber, A.J.; Gueorguieva, I.; Driscoll, K.E.; Estrem, S.T.; Cleverly, A.L.; Desaiah, D.; Guba, S.C.; et al. Clinical Development of Galunisertib (LY2157299 Monohydrate), a Small Molecule Inhibitor of Transforming Growth Factor-Beta Signaling Pathway. Drug Des. Devel. Ther. 2015, 9, 4479. [Google Scholar] [CrossRef]
- Hillege, M.; Galli Caro, R.; Offringa, C.; de Wit, G.; Jaspers, R.; Hoogaars, W. TGF-β Regulates Collagen Type I Expression in Myoblasts and Myotubes via Transient Ctgf and Fgf-2 Expression. Cells 2020, 9, 375. [Google Scholar] [CrossRef]
- Barrett, C.S.X.; Millena, A.C.; Khan, S.A. TGF-β Effects on Prostate Cancer Cell Migration and Invasion Require FosB: FosB Is Required for Prostate Cancer Cell Migration and Invasion. Prostate 2017, 77, 72–81. [Google Scholar] [CrossRef]
- Kim, B.N.; Ahn, D.H.; Kang, N.; Yeo, C.D.; Kim, Y.K.; Lee, K.Y.; Kim, T.-J.; Lee, S.H.; Park, M.S.; Yim, H.W.; et al. TGF-β Induced EMT and Stemness Characteristics Are Associated with Epigenetic Regulation in Lung Cancer. Sci. Rep. 2020, 10, 10597. [Google Scholar] [CrossRef]
- Xu, Q.; Norman, J.T.; Shrivastav, S.; Lucio-Cazana, J.; Kopp, J.B. In Vitro Models of TGF-β-Induced Fibrosis Suitable for High-Throughput Screening of Antifibrotic Agents. Am. J. Physiol. Ren. Physiol. 2007, 293, F631–F640. [Google Scholar] [CrossRef]
- Yu, L.; Border, W.A.; Huang, Y.; Noble, N.A. TGF-β Isoforms in Renal Fibrogenesis. Kidney Int. 2003, 64, 844–856. [Google Scholar] [CrossRef]
- Pan, X.; Chen, Z.; Huang, R.; Yao, Y.; Ma, G. Transforming Growth Factor Β1 Induces the Expression of Collagen Type I by DNA Methylation in Cardiac Fibroblasts. PLoS ONE 2013, 8, e60335. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics Enrichment Tools: Paths toward the Comprehensive Functional Analysis of Large Gene Lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Melzer, C.; von der Ohe, J.; Hass, R.; Ungefroren, H. TGF-β-Dependent Growth Arrest and Cell Migration in Benign and Malignant Breast Epithelial Cells Are Antagonistically Controlled by Rac1 and Rac1b. Int. J. Mol. Sci. 2017, 18, 1574. [Google Scholar] [CrossRef]
- Iordanskaia, T.; Nawshad, A. Mechanisms of Transforming Growth Factor β Induced Cell Cycle Arrest in Palate Development. J. Cell. Physiol. 2011, 226, 1415–1424. [Google Scholar] [CrossRef]
- Mukherjee, P.; Winter, S.L.; Alexandrow, M.G. Cell Cycle Arrest by Transforming Growth Factor Β1 near G1/S Is Mediated by Acute Abrogation of Prereplication Complex Activation Involving an Rb-MCM Interaction. Mol. Cell. Biol. 2010, 30, 845–856. [Google Scholar] [CrossRef]
- Postlethwaite, A.E.; Keski-Oja, J.; Moses, H.L.; Kang, A.H. Stimulation of the Chemotactic Migration of Human Fibroblasts by Transforming Growth Factor Beta. J. Exp. Med. 1987, 165, 251–256. [Google Scholar] [CrossRef]
- Alevizopoulos, A.; Dusserre, Y.; Rüegg, U.; Mermod, N. Regulation of the Transforming Growth Factor β-Responsive Transcription Factor CTF-1 by Calcineurin and Calcium/ Calmodulin-Dependent Protein Kinase IV. J. Biol. Chem. 1997, 272, 23597–23605. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kolb, M.R.J.; Duan, F.; Janssen, L.J. Transforming Growth Factor–β Evokes Ca2+ Waves and Enhances Gene Expression in Human Pulmonary Fibroblasts. Am. J. Respir. Cell Mol. Biol. 2012, 46, 757–764. [Google Scholar] [CrossRef]
- Muldoon, L.L.; Rodland, K.D.; Magun, B.E. Transforming Growth Factor Beta and Epidermal Growth Factor Alter Calcium Influx and Phosphatidylinositol Turnover in Rat-1 Fibroblasts. J. Biol. Chem. 1988, 263, 18834–18841. [Google Scholar] [CrossRef]
- Kahl, C.R.; Means, A.R. Regulation of Cell Cycle Progression by Calcium/Calmodulin-Dependent Pathways. Endocr. Rev. 2003, 24, 719–736. [Google Scholar] [CrossRef]
- Salm, S.N.; Burger, P.E.; Coetzee, S.; Goto, K.; Moscatelli, D.; Wilson, E.L. TGF-β Maintains Dormancy of Prostatic Stem Cells in the Proximal Region of Ducts. J. Cell Biol. 2005, 170, 81–90. [Google Scholar] [CrossRef]
- Gao, H.; Chakraborty, G.; Lee-Lim, A.P.; Mo, Q.; Decker, M.; Vonica, A.; Shen, R.; Brogi, E.; Brivanlou, A.H.; Giancotti, F.G. The BMP Inhibitor Coco Reactivates Breast Cancer Cells at Lung Metastatic Sites. Cell 2012, 150, 764–779. [Google Scholar] [CrossRef]
- Bragado, P.; Estrada, Y.; Parikh, F.; Krause, S.; Capobianco, C.; Farina, H.G.; Schewe, D.M.; Aguirre-Ghiso, J.A. TGF-Β2 Dictates Disseminated Tumour Cell Fate in Target Organs through TGF-β-RIII and P38α/β Signalling. Nat. Cell Biol. 2013, 15, 1351–1361. [Google Scholar] [CrossRef]
- Zhang, M.; Yamazaki, T.; Yazawa, M.; Treves, S.; Nishi, M.; Murai, M.; Shibata, E.; Zorzato, F.; Takeshima, H. Calumin, a Novel Ca2+-Binding Transmembrane Protein on the Endoplasmic Reticulum. Cell Calcium. 2007, 42, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Schoeber, J.P.H.; Topala, C.N.; Lee, K.P.; Lambers, T.T.; Ricard, G.; van der Kemp, A.W.C.M.; Huynen, M.A.; Hoenderop, J.G.J.; Bindels, R.J.M. Identification of Nipsnap1 as a Novel Auxiliary Protein Inhibiting TRPV6 Activity. Pflugers Arch. Eur. J. Physiol. 2008, 457, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Konno, M.; Shirakawa, H.; Miyake, T.; Sakimoto, S.; Nakagawa, T.; Kaneko, S. Calumin, a Ca2+-binding protein on the endoplasmic reticulum, alters the ion permeability of Ca2+ release-activated Ca2+ (CRAC) channels. Biochem. Biophys. Res. Commun. 2012, 417, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Trampert, D.C.; Nathanson, M.H. Regulation of Bile Secretion by Calcium Signaling in Health and Disease. Biochim. Biophys. Acta BBA Mol. Cell Res. 2018, 1865, 1761–1770. [Google Scholar] [CrossRef]
- Dropmann, A.; Dooley, S.; Dewidar, B.; Hammad, S.; Dediulia, T.; Werle, J.; Hartwig, V.; Ghafoory, S.; Woelfl, S.; Korhonen, H.; et al. TGF-Β2 Silencing to Target Biliary-Derived Liver Diseases. Gut 2020, 69, 1677–1690. [Google Scholar] [CrossRef]
- Tam, P.K.H.; Yiu, R.S.; Lendahl, U.; Andersson, E.R. Cholangiopathies—Towards a Molecular Understanding. EBioMedicine 2018, 35, 381–393. [Google Scholar] [CrossRef]

| Protein Name | Protein ID | CTRL | TGF-β 24 h | TGF-β 48 h | GF-β 24 h vs. CTRL | TGF-β 48 h vs. CTRL |
|---|---|---|---|---|---|---|
| Importin subunit alpha-1 | P52292 | 77,882.7 | 310,000.0 | 70,184.0 | 3.980 | 0.901 |
| Pre-mRNA-splicing factor SRP55 | Q13247 | 598,000.0 | 580,333.3 | 148,666.7 | 0.970 | 0.249 |
| Cornifin-A | P35321 | 4,276,666.7 | 744,333.3 | 900,666.7 | 0.174 | 0.211 |
| CaMK-II subunit delta | Q13557 | 15,669.6 | 7554.6 | 3596.0 | 0.482 | 0.229 |
| Heme oxygenase 1 | P09601 | 64,398.3 | 282,000.0 | 74,248.0 | 4.379 | 1.153 |
| Parathymosin | P20962 | 4206.5 | 13,311.4 | 27,348.3 | 3.165 | 6.501 |
| Caveolin-1 | Q03135 | 133,000.0 | 65,955.0 | 41,126.8 | 0.496 | 0.309 |
| Prolyl 4-hydroxylase subunit alpha-1 | P13674 | 3170.9 | 18,216.3 | 7815.8 | 5.745 | 2.465 |
| Thimet oligopeptidase | P52888 | 3589.1 | 4644.1 | 17,872.7 | 1.294 | 4.980 |
| Protein NipSnap homolog 1 | Q9BPW8 | 42,351.3 | 3074.1 | 58,310.7 | 0.073 | 1.377 |
| AP-1 complex subunit beta-1 | Q10567 | 31,677.3 | 4524.2 | 28,842.3 | 0.143 | 0.911 |
| Albumin | P02768 | 18,434.3 | 101,405.3 | 27,261.3 | 5.501 | 1.479 |
| Proline-rich and coiled-coil-containing protein 1 | Q96M27 | 3901.5 | 13,124.0 | 26,563.7 | 3.364 | 6.809 |
| Glutaredoxin-related protein 5 | Q86SX6 | 44,413.3 | 13,184.3 | 24,104.3 | 0.297 | 0.543 |
| Eukaryotic initiation factor 4A-II | Q14240 | 38,164.7 | 37,378.0 | 6906.5 | 0.979 | 0.181 |
| Replication protein A 14 kDa subunit | P35244 | 153,937.5 | 26,327.8 | 103,743.7 | 0.171 | 0.674 |
| Calumin | Q96A33 | 23,889.7 | 7707.2 | 21,445.7 | 0.323 | 0.898 |
| Squalene monooxygenase | Q14534 | 6788.0 | 29,325.3 | 2011.4 | 4.320 | 0.296 |
| RNA helicase aquarius | O60306 | 14,418.7 | 41,652.0 | 44,619.3 | 2.889 | 3.095 |
| ELKS/Rab6-interacting/CAST family member 1 | Q8IUD2 | 6478.8 | 1507.8 | 10,435.0 | 0.233 | 1.611 |
| U4/U6 small nuclear ribonucleoprotein Prp31 | Q8WWY3 | 2117.3 | 20,569.7 | 16,382.3 | 9.715 | 7.738 |
| Histidine triad nucleotide-binding protein 1 | P49773 | 9938.9 | 3275.4 | 15,506.0 | 0.330 | 1.560 |
| Influenza virus NS1A-binding protein | Q9Y6Y0 | 2593.6 | 22,095.7 | 6731.7 | 8.519 | 2.596 |
| Palladin | Q8WX93 | 8038.7 | 36,757.3 | 35,210.3 | 4.573 | 4.380 |
| Cysteine and glycine-rich protein 2 | Q16527 | 8382.2 | 19,592.7 | 26,170.7 | 2.337 | 3.122 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccherini, E.; Di Giorgi, N.; Michelucci, E.; Signore, G.; Tedeschi, L.; Vozzi, F.; Rocchiccioli, S.; Cecchettini, A. Biological Effects of Transforming Growth Factor Beta in Human Cholangiocytes. Biology 2022, 11, 566. https://doi.org/10.3390/biology11040566
Ceccherini E, Di Giorgi N, Michelucci E, Signore G, Tedeschi L, Vozzi F, Rocchiccioli S, Cecchettini A. Biological Effects of Transforming Growth Factor Beta in Human Cholangiocytes. Biology. 2022; 11(4):566. https://doi.org/10.3390/biology11040566
Chicago/Turabian StyleCeccherini, Elisa, Nicoletta Di Giorgi, Elena Michelucci, Giovanni Signore, Lorena Tedeschi, Federico Vozzi, Silvia Rocchiccioli, and Antonella Cecchettini. 2022. "Biological Effects of Transforming Growth Factor Beta in Human Cholangiocytes" Biology 11, no. 4: 566. https://doi.org/10.3390/biology11040566
APA StyleCeccherini, E., Di Giorgi, N., Michelucci, E., Signore, G., Tedeschi, L., Vozzi, F., Rocchiccioli, S., & Cecchettini, A. (2022). Biological Effects of Transforming Growth Factor Beta in Human Cholangiocytes. Biology, 11(4), 566. https://doi.org/10.3390/biology11040566







